Abstract
T cells are exhausted and overexpress inhibitory molecules in chronic hepatitis C virus (HCV) infection. It is unclear whether this is the cause or consequence of HCV persistence. By studying serial blood and liver samples of chimpanzees during acute infection, we demonstrate that the early expression of the memory precursor marker CD127 on HCV-specific T cells, but not the expression of inhibitory molecules on those T cells or their ligands in the liver, predicts the outcome of acute infection.
TEXT
Acute hepatitis C virus (HCV) infection is spontaneously cleared by a minority (20 to 30%) of infected patients, and clearance typically occurs in the context of vigorous HCV-specific T-cell responses (1–5). In contrast, HCV-specific T cells are impaired in chronic HCV infection, as evidenced by decreased proliferation, cytokine production, and cytolytic activity (6, 7). This impaired phenotype has been attributed to a variety of inhibitory mechanisms, which include increased levels of regulatory T cells (Tregs) and inhibitory cytokines (8–12) as well as T-cell exhaustion due to upregulation of inhibitory molecules such as programmed cell death 1 (PD-1) (13–19), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (16, 17), and T-cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) (14, 15). In vitro blockade of these inhibitory receptors reverses functional exhaustion and restores proliferation and effector function of chronic phase CD8+ T cells (13, 15–19).
To date it is not clear whether the observed T-cell exhaustion is the cause or consequence of chronic HCV infection. High levels of PD-1 on HCV-specific T cells and PD-1 mRNA in the liver were reported in acutely infected patients (20) and chimpanzees (21) that later progressed to chronic hepatitis. In contrast, other studies demonstrated high PD-1 levels on HCV-specific T cells in acute HCV infection irrespective of the infection outcome (11, 22). These contradictory results may be due to heterogeneous study cohorts because the exact time point of infection was not known and the genotypes and sequences of the infecting virus differed among patients.
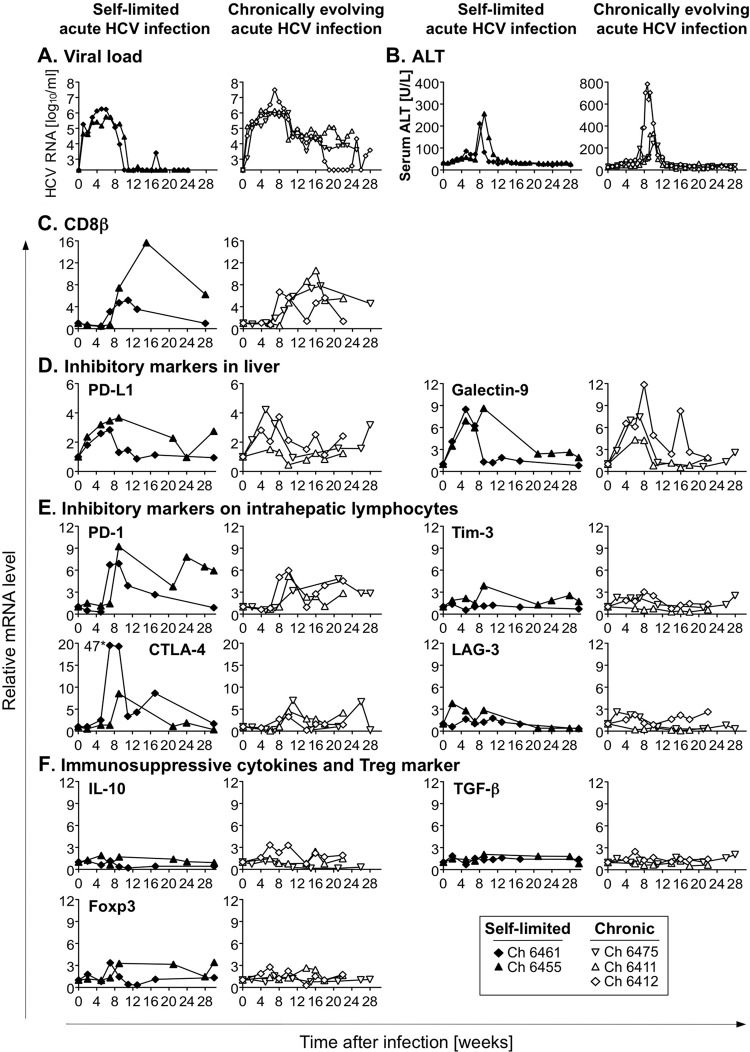
Using serial blood and liver biopsy samples from chimpanzees that had been infected with HCV genotype 1a in a previous study (23), we investigated whether the outcome of HCV infection can be predicted by the phenotype of HCV-specific CD8+ T cells at the earliest testable time point, i.e., when these T cells first appear in the blood. Chimpanzees Ch6461 and Ch6455 cleared HCV, whereas chimpanzees Ch6475, Ch6411, and Ch6412 developed persistent infection (Fig. 1A and B). Of note, Ch6412 temporarily controlled HCV at undetectable serum titers from week 19 to 23 prior to developing persistent viremia.
Fig 1.
Intrahepatic mRNA expression levels of T-cell-inhibitory molecules during acute HCV infection. (A to C) Five chimpanzees were intravenously challenged with serum containing 100 chimpanzee 50% infectious doses (CID50) of HCV genotype 1a in a protocol approved by the Public Health Service Interagency Model Committee (National Institutes of Health) and the Animal Care and Use Committee (Center for Biologics Evaluation and Research) at an Association for Assessment and Accreditation of Animal Care-accredited facility (23). Serum HCV RNA titers (A), alanine aminotransferase (ALT) levels (B), and intrahepatic CD8β mRNA levels (C) have previously been reported (23, 24) and are shown for reference purposes. (D to F) Serial liver biopsy specimens were analyzed for mRNA levels of PD-L1 and galectin-9 (D), of PD-1, Tim-3, CTLA-4, and LAG-3 (E), and of IL-10, TGF-β, and Foxp3 (F). Intrahepatic mRNA levels were normalized to mean levels of β-actin, GAPDH, and β7 mRNA as endogenous references and were expressed as fold increases over preinfection levels. The CTLA-4 mRNA value next to the asterisk specifies an off-scale value.
As previously described (24), CD8β mRNA levels increased in all chimpanzees during the acute phase of hepatitis, indicative of T-cell recruitment to the liver (Fig. 1C). To determine the intrahepatic mRNA level of T-cell surface markers, their ligands, and cytokines that are known to inhibit HCV-specific T cells, we extracted total RNA from serial liver biopsy specimens using the RNeasy minikit (Qiagen, Valencia, CA) and reverse transcribed it with the first-strand cDNA synthesis kit (Marligen Biosciences, Ijamsville, MD). mRNA levels of genes of interest were determined in duplicate with TaqMan gene expression assays (Applied Biosystems, Foster City, CA). The amount of specific mRNA was normalized to mean levels of β-actin, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), and β7 mRNA as endogenous references and is presented as fold increase over preinfection mRNA levels.
As shown in Fig. 1D, intrahepatic mRNA levels of PD-L1 and galectin-9, which are known to inhibit CD8 T cells, increased within 2 weeks of acute HCV infection in parallel with viremia. Thus, these ligands were upregulated prior to upregulation of mRNA levels of the PD-L1-receptor PD-1 and the galectin-9 receptor Tim-3, which are typically expressed on liver-infiltrating lymphocytes (Fig. 1E). In addition, mRNA levels of the lymphocyte inhibitory receptors CTLA-4 and lymphocyte activation gene 3 (LAG-3) increased (Fig. 1E). Importantly, expression levels of intrahepatic mRNA of these markers did not interfere with HCV clearance in the two chimpanzees with self-limited infection. Likewise, mRNA levels of immunosuppressive cytokines interleukin-10 (IL-10) and transforming growth factor β (TGF-β) and the Treg marker Foxp3 did not differ between the two groups of chimpanzees (Fig. 1F). These data indicate that acute phase levels of inhibitory molecules, which are typically increased in the chronic phase of HCV infection, do not differ among chimpanzees with differential outcomes of infection.
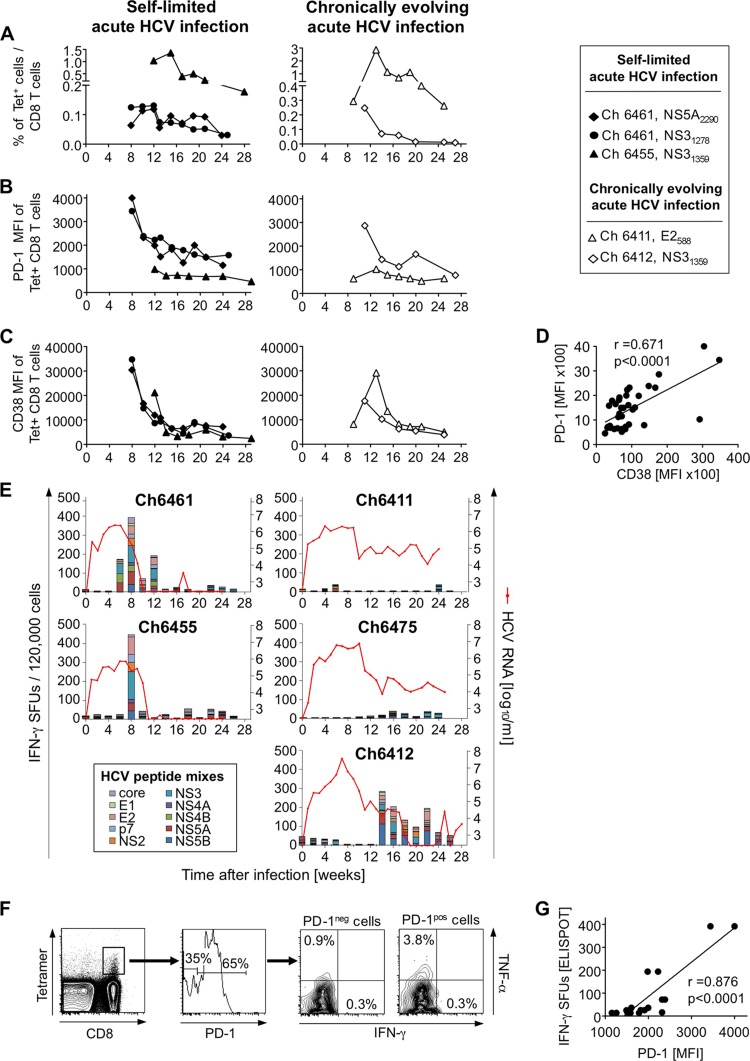
To characterize the quality and magnitude of the HCV-specific T-cell response, we used (i) Pan troglodytes class I tetramers (NIAID Tetramer Facility of the NIH AIDS Research and Reference Reagent Program) to analyze by flow cytometry HCV-specific T-cell responses against epitopes that we had previously mapped in these chimpanzees (24) and (ii) a set of 600 overlapping pentadecamer HCV peptides that matched the sequence of the infection virus (Mimotopes, Clayton, Australia) to analyze the total T-cell response in gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays. Due to the limited number of lymphocytes that can be isolated from liver biopsy specimens, these assays were performed with peripheral blood mononuclear cells (PBMCs). As shown in Fig. 2A, the frequency of tetramer+ CD8+ T cells in the blood was highest in the early acute phase of hepatitis C (weeks 8 to 14) and did not predict the outcome of acute HCV infection. Consistent with intrahepatic PD-1 mRNA levels, the mean fluorescence intensity (MFI) of PD-1 on tetramer+ T cells in the blood peaked at the two earliest time points with detectable tetramer+ T cells and decreased thereafter in both groups of chimpanzees (Fig. 2B). The MFI for CD38, a T-cell activation marker, tended to be higher on tetramer+ cells of chimpanzees with self-limited hepatitis C than on those of chimpanzees with chronically evolving hepatitis C at the first study time point (Fig. 2C).
Fig 2.
Characterization of HCV-specific T-cell responses in acute HCV infection. (A) The frequency of HCV-specific T cells in the blood was evaluated with P. troglodytes class I tetramers. (B to D) The levels of PD-1 (B) and CD38 (C) expression (MFIs) and the correlation between the CD38 MFI and the PD-1 MFI (D) were determined on tetramer+ CD8+ T cells during the first 6 months of HCV infection using previously described techniques (25). r, correlation coefficient, linear regression analysis. (E) Ex vivo IFN-γ ELISpot assays were performed in duplicate by stimulating PBMCs with 18 mixes of overlapping HCV peptides (600 peptides total) spanning the entire HCV polyprotein sequence using a previously described technique (35). HCV RNA titers were previously reported (23, 24) and are shown as red lines for reference purposes. (F) PD-1+ and PD-1− subsets of NS31278-stimulated tetramer+ CD8+ T cells (Ch6461, week 9 after infection) were assessed for IFN-γ and TNF-α secretion using a previously described technique (25). (G) The overall T-cell IFN-γ response against overlapping peptides (IFN-γ ELISpot) was correlated with the PD-1 MFIs of tetramer+ CD8+ T cells at time points throughout the course of infection (Ch6461) in a linear regression analysis. SFU, spot-forming unit.
Overall, there was a strong correlation between PD-1 and CD38 MFIs (r = 0.671, P < 0.0001) (Fig. 2D). Furthermore, the time points with the highest PD-1 and CD38 expression on tetramer+ CD8 T cells in self-limited acute HCV infection corresponded to the time points with strongest HCV-specific T-cell responses in the ELISpot assay (Fig. 2B and E). To confirm the functionality of tetramer+ CD8+ T cells in the context of their PD-1 status at the single-cell level, we assessed IFN-γ and tumor necrosis factor alpha (TNF-α) production of tetramer-stained peptide-stimulated PBMCs by flow cytometry at an early time point after infection using a previously described technique (25). As shown in Fig. 2F for Ch6461 at week 9 after infection, a greater percentage of PD-1+ than PD-1− T cells produced TNF-α. This was supported by the close correlation between PD-1 expression levels on tetramer+ T cells and the overall frequency of HCV-specific IFN-γ-producing T cells in this chimpanzee (r = 0.876, P < 0.0001) (Fig. 2G). Based on these findings we propose that PD-1 is an activation rather than exhaustion marker in acute HCV infection and that upregulation of PD-1 on HCV-specific T cells during the acute phase of infection does not interfere with HCV clearance. Indeed, as shown in other scenarios PD-1 expression can be transient on T-cell receptor (TCR)- and/or cytokine-stimulated T cells (26, 27). This may differ from chronic HCV infection, where PD-1 marks exhausted T cells with impaired function (13–19).
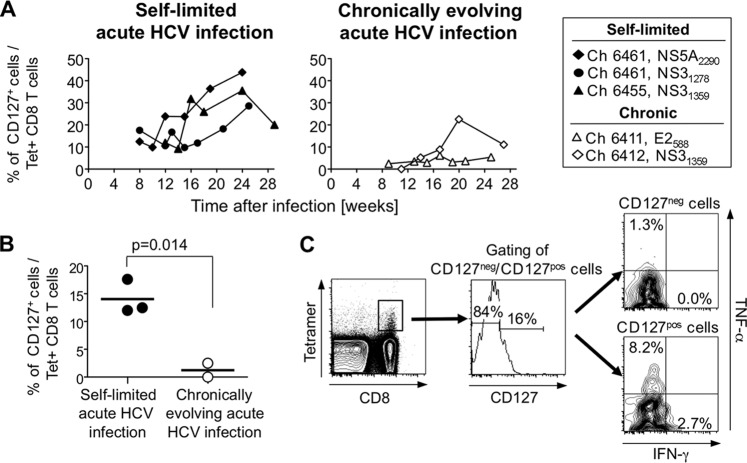
Next, we studied HCV-specific CD8+ T cells for the expression of CD127, a marker of memory precursor cells (28). While the overall frequency of CD127+ tetramer+ T cells was lower in HCV-infected chimpanzees than in previous studies on HCV-infected patients (29), differential CD127 expression was observed during the early phase of acute infection when virus was still detectable even in animals that subsequently cleared the infection. Indeed, at the time point when tetramer+ cells were first detectable in the blood, the frequency of CD127+ cells within the tetramer+ population was significantly higher in chimpanzees with self-limited acute infection than in chimpanzees with chronically evolving infection (P = 0.014, Student's t test) (Fig. 3B).
Fig 3.
Differential CD127 expression on HCV-specific T cells in acute HCV infection. (A) The frequency of CD127+ cells within the tetramer+ CD8+ T-cell population was determined during the first 6 months of HCV infection using previously described techniques (25). (B) The percentages of CD127+ cells within the tetramer+ CD8+ T-cell population were compared between chimpanzees with self-limited and chronically evolving courses at the time point at which tetramer+ T cells were first detected in the blood (unpaired Student t test). (C) CD127+ and CD127− subsets of NS31278-stimulated tetramer+ CD8+ T cells (Ch6461, week 9 after infection) were assessed for IFN-γ and TNF-α secretion using a previously described technique (25).
Because CD127 expression on T cells has been found to increase upon mutational escape of the targeted epitopes (9, 30), we sequenced HCV in all chimpanzees. A 5.2-kb HCV product was amplified by reverse transcription-PCR (RT-PCR) to sequence the E2 epitope with primer 5′-AYGTTCYGRTGRAGRTGGAT-3′ and the NS3 epitopes with primer 5′-ARCCRGTCATGAGRGCATC-3′ (31). A 0.8-kb HCV product was amplified to sequence the NS5A epitope based on a previous report (32) but using RT-PCR primer 5′-TTACGACCCCCCTTCTC-3′, first-round PCR primers 5′-ACACTCGCTGCCACTGTGG-3′ and 5′-TYGACCATGACCCGTCGC-3′, second-round PCR primers 5′-AGGAACATGTGGAGTGGG-3′ and 5′-GATTCRGTGAGGACCACC-3′, and sequencing primer 5′-GATTCRGTGAGGACCACC-3′. PCR conditions were 94°C (2 min) followed by 35 cycles of 94°C (20 s), 55°C (30 s), and 68°C (1 min 20 s), with a final extension at 70°C (10 min). The results from at least 17 molecular clones per sample confirmed that the observed differential CD127 expression in the early acute phase of HCV infection was not due to sequence variations in T-cell epitopes (Table 1). After the acute phase, the percentage of CD127+ cells gradually increased to 28 to 44% of the tetramer+ T-cell population in those chimpanzees that cleared HCV. The increase in CD127+ HCV-specific T-cell responses occurred while the bulk IFN-γ ELISpot response declined (Fig. 3A and 2E), which suggests an expansion of a small population of antigen-independent HCV-specific T cells, as observed during antiviral therapy. Expression of CD127, the IL-7 receptor, may enable these cells to respond effectively to minute amounts of IL-7 and to proliferate as antigen-independent memory precursors. This is consistent with the finding that CD127+ tetramer+ T cells mounted better TNF-α and IFN-γ responses upon stimulation with their cognate peptide than their CD127− counterparts (Fig. 3C) and that the higher frequency of CD127+ tetramer+ T cells in chimpanzees with self-limited hepatitis C in the early phase of acute infection corresponded to the early and strong induction of the total HCV-specific T-cell response observed in the IFN-γ ELISpot assay (Fig. 3A and 2E).
Table 1.
HCV epitope sequence during early acute and during chronic HCV infections
| Chimpanzee (infection outcome) | Epitope locationa | Wk after infectionb | Amino acid sequencec | No. of clones with indicated sequence/no. of clones sequenced |
|---|---|---|---|---|
| Ch6461 (self limited) | NS31278–1285 | 0 | GVDPNIRTGVRTITTGSP | |
| 8* | ------------------ | 17/17 | ||
| NS5A2290–2298 | 0 | LPVWARPDYNPPLVETWKK | ||
| 8* | ------------------- | 22/24 | ||
| -----Q------------- | 1/24 | |||
| ----------L-------- | 1/24 | |||
| Ch6455 (self limited) | NS31359–1367 | 0 | SVTVSHPNIEEVALSTTGE | |
| 8* | ------------------- | 16/18 | ||
| --I---------------- | 1/18 | |||
| ------------------R | 1/18 | |||
| Ch6411 (chronic) | E2588–596 | 0 | TDCFRKHPEATYSRCGSGP | |
| 9* | ------------------- | 22/23 | ||
| -------L----------- | 1/23 | |||
| 26 | ------------------- | 7/21 | ||
| ---------T--------- | 11/21 | |||
| ----------L-------- | 1/21 | |||
| ----------S-------- | 1/21 | |||
| -----T---T--------- | 1/21 | |||
| Ch6412 (chronic) | NS31359–1367 | 0 | SVTVSHPNIEEVALSTTGE | |
| 11* | ------------------- | 24/24 | ||
| 26 | ----P-------------- | 23/23 |
NS31278 and NS5A2290 are Patr-B*0301/02 restricted, NS31359 is Patr-B*1301 restricted, and E2588 is Patr-A*0401 restricted.
The HCV sequence that is indicated for the week 0 time point is the sequence of the HCV inoculum. *, first time point at which tetramer+ CD8 T cells were detected in the blood.
The epitope sequence is in boldface and underlined. Hyphens indicate amino acids identical to those at inoculation.
In contrast to results for chimpanzees with self-limited acute infection, the percentage of CD127+ HCV-specific T cells remained low in those chimpanzees with chronically evolving hepatitis (Fig. 3A). This is consistent with reports on decreased CD127 levels on HCV-specific T cells of patients who were studied late in the course of HCV infection (5, 9, 30, 33, 34). Solely Ch6412, which experienced transient viral control at weeks 19 to 23 after infection, displayed a transient increase in the CD127+ T-cell frequency (Fig. 3A). Reemergence of HCV to high titers at week 24 in this chimpanzee coincided with the failure to maintain an increasing frequency of CD127+ T cells. Again, this fluctuation in the size of the CD127+ HCV-specific T-cell population was not due to HCV sequence variations in the corresponding T-cell epitopes (Table 1).
Thus, the frequency of CD127+ cells in the HCV-specific CD8+ T-cell population in the early phase of acute hepatitis, when all chimpanzees were still viremic, rather than the expression of inhibitory molecules on HCV-specific T cells or the intrahepatic levels of their ligands or inhibitory cytokines correlated with the subsequent infection outcome.
ACKNOWLEDGMENTS
We thank the NIAID Tetramer Facility of the NIH AIDS Research and Reference Reagent Program for the generation of P. troglodytes tetramers.
This work was supported by grant AIRC-10266 to V.D.R. and by the NIDDK, NIH, intramural research program.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH, Reddy KR, McKeating JA, Chang KM. 2007. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology 132:654–666 [DOI] [PubMed] [Google Scholar]
- 2. Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. 2008. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J. Virol. 82:1827–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126–139 [DOI] [PubMed] [Google Scholar]
- 6. Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsacker F, Blum HE, Thimme R. 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42:828–837 [DOI] [PubMed] [Google Scholar]
- 7. Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447–3458 [DOI] [PubMed] [Google Scholar]
- 8. Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. 2004. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J. Clin. Invest. 113:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. 2010. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6:e1000947 doi:10.1371/journal.ppat.1000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. 2005. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 79:7860–7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasprowicz V, Schulze zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM. 2008. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J. Virol. 82:3154–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology 38:1437–1448 [DOI] [PubMed] [Google Scholar]
- 13. Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. 2007. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 81:9249–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. 2010. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J. Clin. Invest. 120:4546–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5:e1000313 doi:10.1371/journal.ppat.1000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. 2008. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 134:1927–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45:588–601 [DOI] [PubMed] [Google Scholar]
- 19. Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, Cox AL. 2008. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J. Immunol. 181:8215–8225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rollier CS, Paranhos-Baccala G, Verschoor EJ, Verstrepen BE, Drexhage JA, Fagrouch Z, Berland JL, Komurian-Pradel F, Duverger B, Himoudi N, Staib C, Meyr M, Whelan M, Whelan JA, Adams VC, Larrea E, Riezu JI, Lasarte JJ, Bartosch B, Cosset FL, Spaan WJ, Diepolder HM, Pape GR, Sutter G, Inchauspe G, Heeney JL. 2007. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology 45:602–613 [DOI] [PubMed] [Google Scholar]
- 22. Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, Feinstone SM. 2004. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 39:1709–1720 [DOI] [PubMed] [Google Scholar]
- 24. Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, Veerapu NS, Heller T, Feinstone SM, Rice CM, Rehermann B. 2011. Delayed induction, not impaired recruitment, of specific CD8 T cells causes the late onset of acute hepatitis C. Gastroenterology 141:686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SH, Shin EC, Capone S, Caggiari L, De Re V, Nicosia A, Folgori A, Rehermann B. 2012. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology 143:1048–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. 1996. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8:765–772 [DOI] [PubMed] [Google Scholar]
- 27. Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, Fauci AS. 2008. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J. Immunol. 181:6738–6746 [DOI] [PubMed] [Google Scholar]
- 28. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198 [DOI] [PubMed] [Google Scholar]
- 29. Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. 2008. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 82:10017–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kasprowicz V, Kang YH, Lucas M, Schulze zur Wiesch J, Kuntzen T, Fleming V, Nolan BE, Longworth S, Berical A, Bengsch B, Thimme R, Lewis-Ximenez L, Allen TM, Kim AY, Klenerman P, Lauer GM. 2010. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J. Virol. 84:1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. 2012. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J. Infect. Dis. 205:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuntzen T, Timm J, Berical A, Lewis-Ximenez LL, Jones A, Nolan B, Schulze zur Wiesch J, Li B, Schneidewind A, Kim AY, Chung RT, Lauer GM, Allen TM. 2007. Viral sequence evolution in acute hepatitis C virus infection. J. Virol. 81:11658–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsacker F, Blum HE, Pircher H, Thimme R. 2007. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 81:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Golden-Mason L, Burton JR, Jr, Castelblanco N, Klarquist J, Benlloch S, Wang C, Rosen HR. 2006. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology 44:1098–1109 [DOI] [PubMed] [Google Scholar]
- 35. Rahman F, Heller T, Sobao Y, Mizukoshi E, Nascimbeni M, Alter H, Herrine S, Hoofnagle J, Liang TJ, Rehermann B. 2004. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology 40:87–97 [DOI] [PubMed] [Google Scholar]