Abstract
Severe fever with thrombocytopenia syndrome virus (SFTSV) is a novel bunyavirus that recently emerged in China. Infection with SFTSV is associated with case-fatality rates of up to 30%, and neither antivirals nor vaccines are available at present. Development of antiviral strategies requires the elucidation of virus-host cell interactions. Here, we analyzed host cell entry of SFTSV. Employing lentiviral and rhabdoviral vectors, we found that the Gn/Gc glycoproteins (Gn/Gc) of SFTSV mediate entry into a broad range of human and animal cell lines, as well as human macrophages and dendritic cells. The Gn/Gc proteins of La Crosse virus (LACV) and Rift Valley Fever Virus (RVFV), other members of the bunyavirus family, facilitated entry into an overlapping but not identical range of cell lines, suggesting that SFTSV, LACV, and RVFV might differ in their receptor requirements. Entry driven by SFTSV Gn/Gc was dependent on low pH but did not require the activity of the pH-dependent endosomal/lysosomal cysteine proteases cathepsins B and L. Instead, the activity of a cellular serine protease was required for infection driven by SFTSV and LACV Gn/Gc. Sera from convalescent SFTS patients inhibited SFTSV Gn/Gc-driven host cell entry in a dose-dependent fashion, demonstrating that the vector system employed is suitable to detect neutralizing antibodies. Finally, the C-type lectin DC-SIGN was found to serve as a receptor for SFTSV Gn/Gc-driven entry into cell lines and dendritic cells. Our results provide initial insights into cell tropism, receptor usage, and proteolytic activation of SFTSV and will aid in the understanding of viral spread and pathogenesis.
INTRODUCTION
Bunyaviruses are enveloped viruses with a tripartite, single-stranded RNA genome and constitute the largest family of viruses (1). The genera Hantavirus, Orthobunyavirus, Phlebovirus, and Nairovirus comprise viruses pathogenic for humans. Viruses of the genus Hantavirus infect rodents, their natural reservoir, and are transmitted to humans via feces and urine. Hantaviruses circulating in Asia and Europe (Old World hantaviruses) can cause hemorrhagic fever with renal syndrome in humans, while American hantaviruses (New World hantaviruses) are the causative agents of hantavirus pulmonary syndrome (2). Members of the genera Orthobunyavirus, Phlebovirus, and Nairovirus are present in diverse animal reservoirs and are transmitted to humans via arthropod vectors. Infection of humans with Rift Valley fever virus (RVFV), a phlebovirus, can result in hemorrhagic fever or meningoencephalitis (3), and La Crosse virus (LACV) (genus Orthobunyavirus) infection can lead to encephalitis, primarily in children (4). Thus, zoonotic transmission of bunyaviruses to humans can be associated with severe disease, and at present, neither licensed vaccines nor specific antivirals are available to combat bunyavirus infection of humans (3, 5), with the exception of an inactivated hantavirus vaccine used in Asia.
In 2009, the emergence of a novel disease characterized by severe fever and thrombocytopenia was observed in central China. Gastrointestinal symptoms and neutropenia were also frequent in afflicted individuals, and 10 to 30% of the cases had a fatal outcome (6). Yu and colleagues demonstrated that severe fever with thrombocytopenia syndrome (SFTS) is associated with infection by a new phlebovirus, SFTS virus (SFTSV) (6). Phylogenetic analysis revealed that SFTSV is equally distant from the sandfly fever and the uukuniemi groups of phleboviruses and may thus be the first member of a third group of phleboviruses (6). SFTSV was detected in ticks, but not in mosquitoes (6), indicating that the virus is transmitted from a so far unknown reservoir species via ticks to humans. Initially, no evidence for person-to-person transmission of SFTSV was obtained (6). However, subsequent studies indicated that the virus can be transmitted between humans by personal contact (7–9). Collectively, these analyses indicate that SFTSV is an emerging threat against which countermeasures need to be developed (10).
The M segment of bunyaviruses encodes the viral surface glycoproteins, the sole targets for neutralizing antibodies. In addition, the M segment of some bunyaviruses contains the information for an NSm protein, but sequence analysis revealed that the SFTSV M segment does not encode such a protein (6, 11). The bunyavirus glycoproteins are synthesized as a single precursor polyprotein in the secretory compartments of infected cells, which are cotranslationally or posttranslationally processed into the subunits Gn and Gc (12). Mature Gn and Gc are targeted to the Golgi apparatus, the assembly site of bunyaviruses (13, 14), and heteromultimers of these proteins are incorporated into the viral envelope (15, 16), where they bind to host cell receptors and facilitate viral entry into target cells in a pH-dependent fashion (17). A recent study indicates that the lectin dendritic-cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) can facilitate entry of certain phleboviruses into dendritic cells (18). In addition, it has been proposed that pathogenic hantaviruses employ β3 integrins as receptors (19, 20) and decay-accelerating factor (DAF) as a coreceptor for host cell entry (21), while nonpathogenic viruses seem to have different receptor requirements. Deciphering the molecular interactions underlying SFTSV entry into host cells might thus yield important insights into viral tropism and pathogenesis and could provide valuable information for the design of vaccines and inhibitors. However, at present, the viral and cellular components essential for SFTSV entry are unknown, and the high level of biosafety containment required for analysis of authentic SFTSV impedes swift progress in this research field.
Here, we report the establishment of a rhabdoviral vector system suitable for safe and efficient analysis of SFTSV entry into host cells. Vectors pseudotyped with SFTSV glycoproteins (SFTSV Gn/Gc) infected human and animal cell lines in a pH-dependent fashion. Pseudotypes bearing the glycoproteins of SFTSV, LACV, and RVFV exhibited overlapping, but not identical, cell tropism, suggesting differences in receptor usage. Infection driven by bunyavirus glycoproteins was dependent on serine protease activity in host cells, suggesting that infectious entry depends on Gn/Gc protein activation in target cells. Entry of SFTSV Gn/Gc pseudotypes was neutralized in a dose-dependent fashion by sera from SFTS, but not control patients, demonstrating that our system is suitable to detect neutralizing antibodies. Finally, DC-SIGN expression was sufficient for SFTSV Gn/Gc-mediated entry into otherwise nonsusceptible cells. Thus, DC-SIGN is an entry receptor for SFTSV.
MATERIALS AND METHODS
Cell culture.
Adherent cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco or HyClone), and suspension cells were maintained in RPMI 1640 medium (PAA) supplemented with 5 to 10% fetal calf serum (FCS) (Biochrom or HyClone) and 1% penicillin-streptomycin sulfate. Raji B cells engineered for stable expression of DC-SIGN and DC-SIGN related (DC-SIGNR) have been described previously (22). Primary human foreskin fibroblasts (HFF) were prepared from freshly obtained human foreskin after circumcision and cultured in minimal essential medium (MEM) (Gibco). HFF were used between passages 8 and 12. Primary human macrophages and dendritic cells were generated from blood monocytes derived from peripheral blood mononuclear cells (PBMCs) purified by Ficoll gradient centrifugation. For generation of primary human monocyte-derived macrophages, freshly isolated PBMCs were cultured in monocyte adherence medium (RPMI 1640 medium with 7.5% human fibrin-depleted plasma) for 1 h. Thereafter, nonadherent cells were removed by washing, and adherent cells were cultivated in monocyte adherence medium for 24 h. Then, the adherent cells were detached and seeded in 96-well plates at a density of 7.5 × 104 cells/well using monocyte differentiation medium (X-Vivo 10 medium [Lonza] supplemented with 1% human fibrin-depleted plasma and 1% penicillin-streptomycin sulfate); every other day, the culture medium was changed. For generation of primary monocyte-derived dendritic cells, freshly isolated monocytes were allowed to adhere to 25-cm culture plates for 2 h, followed by seeding in 96-well plates at a density of 7.5 × 104 cells/well in RPMI 1640 medium supplemented with 20 ng/ml interleukin 4 (IL-4) and 25 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Cellgenix) and 10% FCS for 7 days, and the culture medium was replaced after 2 to 3 days. Fluorescence-activated cell sorter (FACS) analysis of the population revealed that at least 95% of the cells were positive for DC-SIGN (CD209). All cells were grown in a humidified atmosphere at 37°C and 5% CO2.
Plasmids and antibodies.
Expression plasmids for DC-SIGN and DC-SIGNR have been published (23). Expression vectors for the following glycoproteins were described previously: RVFV Gn/Gc (24), LACV Gn/Gc (25), and Zaire ebolavirus GP (EBOV GP), vesicular stomatitis virus (VSV) G, and murine leukemia virus (MLV) glycoprotein (26). A codon-optimized open reading frame for the SFTSV glycoprotein (SFTSV Gn/Gc) with a C-terminal V5 tag was synthesized as a consensus sequence representing the most frequent SFTSV sequences found in patients. The consensus sequence is identical to NCBI GenBank accession number ADZ04482.1, except for the amino acid changes F13L, S562G, and A501T, which are common in patient-derived SFTSV Gn/Gc sequences deposited in GenBank. The rationale for working with a consensus sequence instead of a sequence derived from a single patient was to cover different phenotypes potentially associated with diverse viral sequences. This SFTSV G consensus sequence was cloned into pMK-QR (Invitrogen), followed by subcloning into pCAGGS using Asp718 and XhoI; the resulting plasmid was termed pCAGGS-SFTSV-Gn/Gc V5. An untagged version of the SFTSV Gn/Gc sequence was obtained by removal of the C-terminal sequence using an internal MluI site, as well as XhoI, and replacement by a PCR product comprising the same sequence without the V5 tag (termed pCAGGS-SFTSV-Gn/Gc). For generation of lentiviral virus-like particles, plasmid p96ZM651gag-opt, encoding human immunodeficiency virus type 1 (HIV-1) Gag (p55), was employed (27). The V5-reactive monoclonal antibody was obtained from Invitrogen; HIV Gag proteins were detected using the hybridoma 183-H12-5C cell culture supernatant (NIH AIDS Reagent Program). A monoclonal antibody directed against the VSV M protein was obtained from Kerafast. The DC-SIGN/R-specific antibody 526 was described previously (28). Secondary antibodies were purchased from Dianova. SFTSV-reactive sera were isolated from blood samples from 4 SFTS patients in the convalescent phase. As controls, sera isolated from blood samples of healthy donors were employed.
Production of lentiviral pseudotypes.
Lentiviral pseudotypes were generated as described previously (26). In brief, 293T cells were calcium phosphate or Lipofectamine cotransfected with an expression plasmid encoding the glycoprotein of choice in combination with plasmid pNL4-3 E− R− (29). At 8 h posttransfection, the culture medium was replaced by fresh medium, and at 48 h posttransfection, supernatants were harvested, passed through 0.45-μm-pore-size filters, aliquoted, and stored at −80°C. Before use in entry experiments, pseudotypes were normalized for infectivity by infection of U373 or Vero cells with different dilutions of virus, followed by determination of luciferase activity in cell lysates, employing a commercially available kit (Promega). Alternatively, the 50% tissue culture infectious doses (TCID50s) of pseudotypes were determined (30). Normalization of the viral capsid protein content was performed using a commercially available HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) (NIH AIDS Reagent Program). To analyze viral cell tropism, target cell lines were infected as described for rhabdoviral pseudotypes.
Production and analysis of rhabdoviral pseudotypes.
Rhabdoviral pseudotypes were generated essentially as described previously (31). Briefly, 293T cells were transfected in T-25 culture flasks with 6 μg of glycoprotein expression plasmid. At 30 h posttransfection, the cells were infected with a replication-defective vesicular stomatitis virus vector (VSVΔG) encoding luciferase and green fluorescent protein (GFP) (32) and trans-complemented with the G protein of VSV. Infection was carried out at a multiplicity of infection (MOI) of 0.5, and targets cells were incubated with vector for 60 min at 37°C. Thereafter, the cells were washed with phosphate-buffered saline (PBS) and incubated with neutralizing polyclonal serum directed against VSV G for 60 min (33), in order to neutralize residual virus or cell-associated VSV G. The following day, rhabdoviral pseudotypes were collected from the culture supernatant, passed through 0.45-μm filters, and stored at −80°C. Infectivity was assessed on Vero cells by GFP fluorescence and luciferase assay. To analyze viral cell tropism, adherent cell lines were seeded in 96-well plates at a density of 3 × 104 cells/well. Monocytes were seeded and differentiated into either macrophages or dendritic cells in 96-well plates at a density of 7.5 × 104 cells/well. The cells were infected with 50 μl of infectivity-normalized pseudotyped virus. Human macrophages were infected by spin inoculation (spinoculation) at 2,000 rpm and 21°C for 2 h. Infection was quantified by determining luciferase activity in cell lysates at 24 h postinfection (p.i.) using a commercially available kit (Promega).
Neutralization assay.
Neutralization was measured by the reduction in luciferase reporter gene expression after preincubation of lentiviral pseudotypes with serum samples from SFTS patients. Briefly, 100 TCID50 of pseudotyped virus was incubated with different dilutions of positive serum samples (A, B, C, and D) in duplicate for 1 h at 37°C in a total volume of 150 μl of growth medium in 96-well flat-bottom culture plates (Corning-Costar), together with control wells without antibodies in quadruplicate. Freshly trypsinized 293T cells (104 cells in 50 μl of growth medium) were added to each well. After 48 h of incubation, the luciferase activities in cell lysates were measured.
Inhibition experiments.
The inhibitors mannan, bafilomycin A1, dynasore, wortmannin, CA074, CA074Me, CatL III, and AEBSF [4-(2-aminoethyl)-benzenesulfonylfluoride hydrochloride] (all Sigma-Aldrich) were diluted in solvent as recommended by the manufacturer and used in the indicated concentrations. The volume of solvent was kept constant within the experiments, and solvent without inhibitor was used as a control. Target cells were pretreated with the inhibitors for 30 to 60 min at 37°C and infected with infectivity-normalized pseudotypes in the presence of inhibitor. The inhibitor-containing medium was replaced by fresh culture medium without inhibitor at 8 to 12 h postinfection.
RESULTS
The SFTSV glycoprotein Gc is efficiently expressed in 293T cells and incorporated into a heterologous viral envelope.
For our analysis of SFTSV Gn/Gc-driven host cell entry, we used a codon-optimized SFTSV Gn/Gc sequence inserted into the plasmid pCAGGS. This plasmid is known to promote high expression of viral glycoproteins, most likely due to the presence of an intron upstream of the coding region, which might increase mRNA stability and protein translation (34). Transfection of the plasmid into 293T cells allowed robust expression of the SFTSV glycoprotein Gc. Thus, Western blot analysis with an antibody directed against a C-terminal V5 antigenic tag detected an approximately 58-kDa band (Fig. 1A), which corresponds to the predicted size of Gc (Fig. 1B). Furthermore, the Gc protein was detected in HIV-like particles generated in cells cotransfected with an SFTSV Gn/Gc expression plasmid, but not the corresponding empty plasmid (Fig. 1C). A similar observation was made when rhabdoviral pseudoparticles were analyzed (Fig. 1D). Thus, the expression of the SFTSV glycoprotein Gc in human cells is efficient, and the mature glycoprotein is incorporated into lentiviral and rhabdoviral particles. Although our approach was not designed to detect Gn, it is highly likely that Gn was also efficiently expressed and incorporated into particles, since Gn and Gc are produced from a single precursor protein and mature Gn and Gc associate (12).
Fig 1.
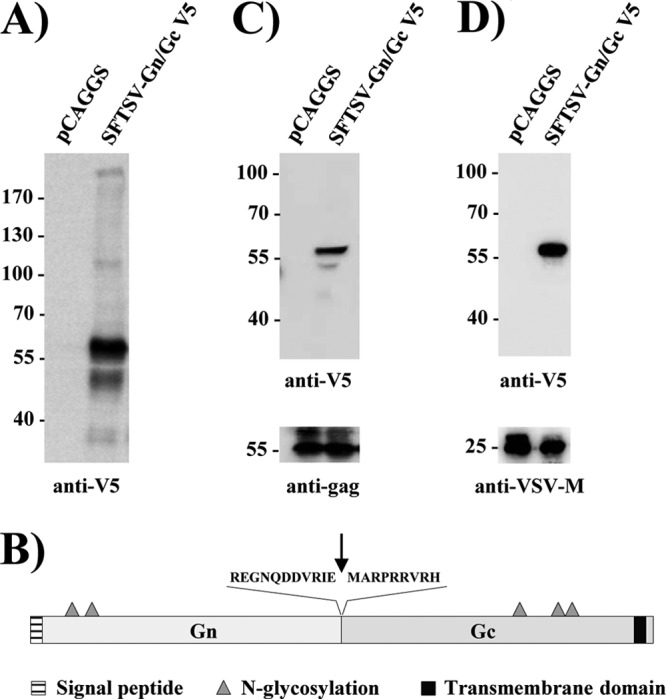
Expression of the SFTSV Gc glycoprotein and incorporation into virus-like particles. (A) 293T cells were transiently transfected with empty pCAGGS plasmid or pCAGGS encoding SFTSV Gn/Gc with a V5 antigenic tag. At 48 h posttransfection, cell lysates were prepared and analyzed for SFTSV Gn/Gc expression by Western blotting using a V5-specific monoclonal antibody. (B) Schematic overview of the SFTSV Gn/Gc protein. The signal peptide (striped) and the transmembrane domain (black) are shown. The putative proteolytic cleavage site is indicated by an arrow, and consensus signals for N-linked glycosylation are indicated by triangles. (C) Incorporation of SFTSV Gc into virus-like particles was analyzed by expression of pCAGGS SFTSV Gn/Gc V5 or empty plasmid in combination with p96ZM651gag-opt encoding human immunodeficiency virus type 1 Gag (p55), followed by Western blotting of particles released into the culture supernatant by a p55-specific antibody (bottom) or the V5-reactive antibody (top). (D) Rhabdoviral pseudotypes were harvested from the supernatant of 293T cells expressing SFTSV Gn/Gc V5 and a VSVΔG-GFP/luc genome and analyzed by Western blotting using a VSV M-specific antibody (bottom) or the V5-antibody (top). Molecular mass markers (kilodaltons) are indicated.
Cells from different organs and species are susceptible to infection driven by the glycoproteins of SFTSV.
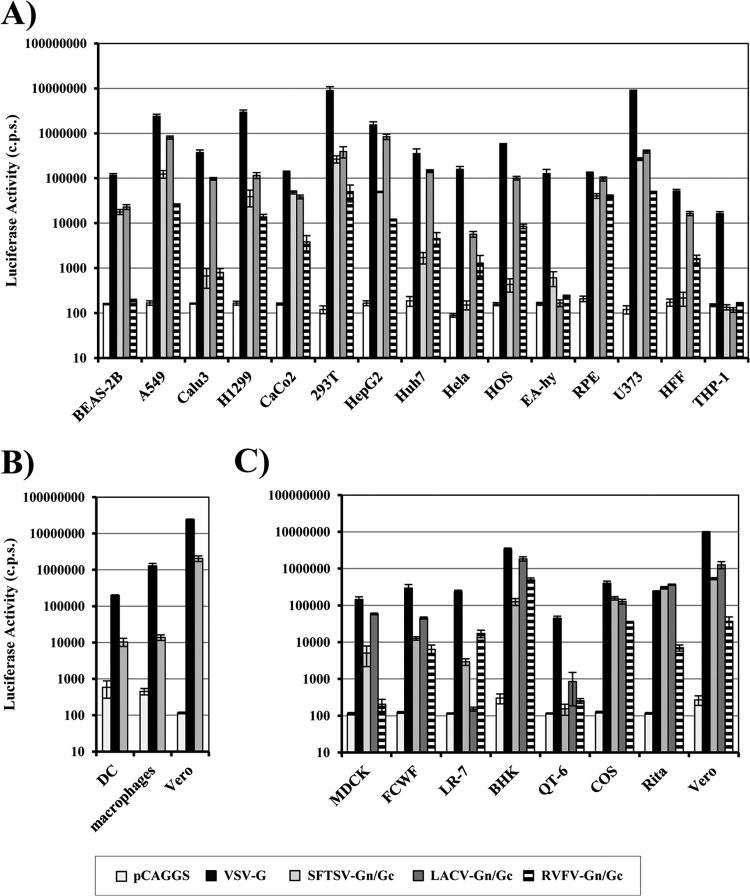
The range of cells susceptible to infectious SFTSV entry is currently unclear. We analyzed whether luciferase-encoding HIV particles pseudotyped with SFTSV Gn/Gc are suitable to determine the susceptibility of target cells to SFTSV Gn/Gc-driven infection. Infection of some cell lines by SFTSV Gn/Gc-bearing pseudotypes was observed, but infection was relatively inefficient (data not shown). Therefore, we asked whether a VSV-based vector system (33, 35) might be better suited for the functional analysis of the SFTSV Gn/Gc proteins. Cell tropism of SFTSV Gn/Gc-bearing VSV pseudotypes was compared with that of pseudotypes harboring LACV and RVFV Gn/Gc. The G protein of VSV served as a positive control, and particles without glycoproteins were used as negative controls. The infection of cells with particles bearing no glycoproteins resulted in luciferase activities close to background, while all target cells tested were efficiently infected with particles pseudotyped with VSV G, as expected (Fig. 2A). SFTSV Gn/Gc pseudotypes infected human lung (BEAS-2B, A549, and H1299), kidney (293T), liver (HepG2), colon (Caco-2), retinal epithelium (RPE), and glioblastoma (U373) cell lines, as well as human monocyte-derived dendritic cells, with high efficiency (Fig. 2A and B). In contrast, infection of human monocyte-derived macrophages was inefficient (data not shown), although infectious entry was readily observed upon spinoculation (36) (Fig. 2B). Monocytic (THP-1) cells, HFF, and cervical carcinoma (HeLa) cells were resistant to SFTSV Gn/Gc-driven entry, indicating that these cells lack expression of the appropriate receptor(s). Several cell lines were robustly infected by pseudotypes bearing SFTSV, LACV, and RVFV Gn/Gc, while some were susceptible to only one or two of the three pseudotypes tested. Thus, VSV-derived vectors are suitable to analyze SFTSV Gn/Gc-driven host cell entry, and the cell tropisms of particles bearing SFTSV, LACV, and RVFV Gn/Gc are overlapping but not identical, hinting at differences in receptor usage.
Fig 2.
Cellular entry of rhabdoviral pseudotypes bearing the glycoprotein of SFTSV. Rhabdoviral pseudotypes were generated by expression of VSV G or SFTSV, LACV, or RVFV Gn/Gc in 293T cells, followed by transduction with a VSVΔG-GFP/luc virus. Particles released into the supernatant were normalized for comparable infectivity and used for infection of the indicated human cell lines (A), primary human cells (B), or cell lines derived from nonhuman species (C); particles harboring no glycoprotein (pCAGGS) were used as a control. (B) Spinoculation was used for macrophage infection. After 24 h, the cells were lysed, and infectivity was determined by luciferase assay. The results of representative experiments performed in triplicate are shown and were confirmed in at least three independent experiments performed with different pseudotype preparations. The error bars indicate standard deviations (SD).
The detection of SFTSV in ticks indicates that the virus can be transmitted from an animal reservoir to humans via an arthropod vector. We therefore also analyzed SFTSV Gn/Gc-dependent infection of animal cell lines (Fig. 2C). Pseudotypes bearing SFTSV Gn/Gc robustly infected cells of dog (MDCK), cat (FCWF), mouse (LR-7), hamster (BHK), and monkey (COS, Rita, and Vero) origin, indicating that the SFTSV receptor(s) is conserved between species or that different species have equally functional receptors. The only exceptions were quail cells (QT6), which, like THP-1 cells, HFF, and HeLa cells, were refractory and thus might prove to be a useful tool for receptor identification (Fig. 2C). Finally, differences in cell tropism of SFTSV, LACV, and RVFV Gn/Gc-bearing pseudotypes observed for human cells also became apparent with animal cells, confirming potential differences in receptor usage. In sum, the Gn/Gc proteins of SFTSV can mediate entry into diverse cell lines of human and animal origin.
Cellular entry mediated by the glycoproteins of SFTSV is pH dependent.
Protonation of viral glycoproteins can be sufficient to trigger their membrane fusion activity. Entry of the respective viruses can therefore be inhibited with lysosomotropic agents, which elevate the low endosomal pH. In order to assess if SFTSV Gn/Gc-driven entry depends on low pH, we pretreated U373 target cells with ammonium chloride, which neutralizes the endosomal pH, and bafilomycin, which inhibits the endosomal V-ATPase. Pretreatment of target cells with these inhibitors blocked entry driven by VSV G (Fig. 3), which is known to depend on low pH (37). In contrast, entry driven by the glycoprotein of MLV was not inhibited (Fig. 3), in agreement with its ability to fuse the viral and the plasma membranes in a pH-independent fashion (38, 39). Finally, bafilomycin and ammonium chloride inhibited infection by pseudotypes bearing SFTSV Gn/Gc (Fig. 3), demonstrating that these proteins require low pH for cellular entry.
Fig 3.
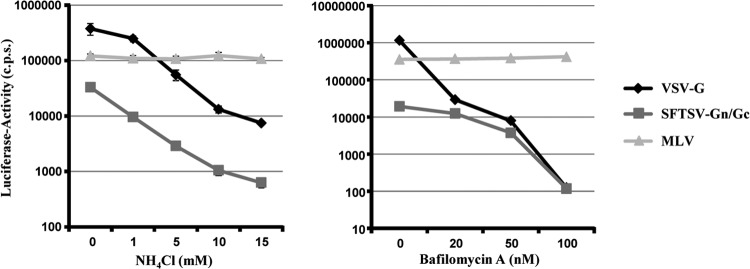
Entry driven by the SFTSV glycoproteins depends on low pH. Pseudotypes bearing the glycoproteins of VSV, MLV, and SFTSV were normalized for comparable infectivity and used for infection of U373 cells preincubated with the indicated concentrations of ammonium chloride (NH4Cl) (left), bafilomycin A (right), or solvent (0 mM), followed by transduction with the indicated pseudotypes. Infectivity was determined by luciferase activity in cell extracts. The results shown are representative of three independent experiments; the error bars indicate SD.
The activity of a cellular serine protease is required for host cell entry driven by the glycoproteins of SFTSV and LACV.
Infectious entry of Ebola virus and severe acute respiratory syndrome coronavirus (SARS-CoV) requires low pH (26, 40, 41). However, pH dependence is not due to protons triggering the membrane fusion activity of the viral glycoproteins. Instead, acidic pH is indispensable for the activity of cathepsins B and L, endosomal/lysosomal proteases that cleave the viral glycoproteins, and cleavage is a prerequisite for membrane fusion (42, 43). We therefore investigated if cathepsin activity is also required for entry of SFTSV Gn/Gc pseudotypes (Fig. 4). The cathepsin inhibitors CA074, CA074Me, and CatL III markedly inhibited entry into Vero cells driven by the EBOV GP but had no effect on entry mediated by VSV G, in accordance with published data (42, 44, 45). Similarly, cathepsin inhibitors did not decrease entry driven by SFTSV and LACV Gn/Gc, indicating that activity of cathepsins B and L is dispensable for cellular entry mediated by these glycoproteins (Fig. 4). In stark contrast, the serine protease inhibitor AEBSF efficiently reduced infection driven by SFTSV and LACV Gn/Gc, and similar observations were made with the human cell lines U373 and RPE (not shown), suggesting that a so-far-uncharacterized cellular serine protease activity might be required for infectious entry of SFTSV, LACV, and potentially other bunyaviruses.
Fig 4.
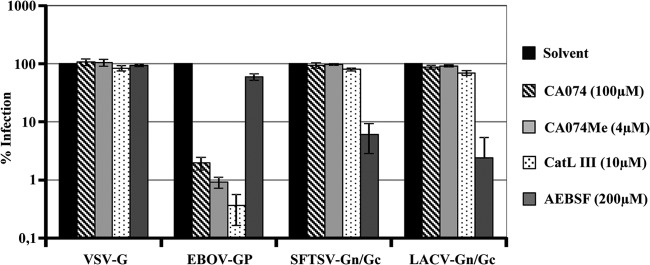
SFTSV glycoprotein-mediated entry is not dependent on endosomal cathepsin activity. Vero target cells were preincubated with the indicated concentrations of cathepsin B and L inhibitors (CA074, CA074Me, and CatL III) and a serine protease inhibitor (AEBSF) or solvent as a control. Thereafter, the cells were infected with pseudotypes bearing glycoproteins of VSV, Zaire EBOV, SFTSV, or LACV as indicated, normalized for comparable infectivity. Luciferase activity in cell lysates was measured at 24 h postinfection. The averages of four (three for LACV Gn/Gc) experiments are shown; the error bars indicate standard errors of the mean (SEM).
Host cell entry mediated by the SFTSV glycoproteins depends on dynamin.
A prerequisite for activation of SFTSV Gn/Gc by low pH is uptake of virions into host cell endosomes. Previous reports indicated that cellular uptake of bunyaviruses proceeds via clathrin-coated pits (46, 47). The small GTPase dynamin is essential for pinching off coated pits from the plasma membrane, and this process is inhibited by dynasore, a GTPase inhibitor that targets dynamin (48). To investigate if SFTSV Gn/Gc-facilitated entry depends on dynamin, we incubated target cells with dynasore before infection with pseudotypes. Pseudotypes bearing VSV G and EBOV GP were both inhibited by dynasore, in agreement with previous reports (49, 50), although inhibition of VSV G-dependent entry was observed only at the highest concentration of dynasore tested (Fig. 5A). Entry by SFTSV and LACV Gn/Gc was also blocked in a dose-dependent fashion, suggesting that entry driven by these glycoproteins depends on functional clathrin. EBOV GP employs a dynamin-dependent macropinocytosis pathway to mediate viral uptake into cells (49, 50). In order to investigate if macropinocytosis is also involved in SFTSV Gn/Gc-driven entry, we pretreated target cells with wortmannin, a covalent inhibitor of the macropinocytosis regulator phosphoinositide 3-kinase (51), prior to infection with pseudotypes (Fig. 5B). Wortmannin treatment markedly inhibited entry of EBOV GP-bearing pseudotypes, as expected (52), but did not diminish entry driven by VSV, SFTSV, and LACV Gn/Gc. These observations suggest that clathrin-mediated endocytosis is required for efficient SFTSV Gn/Gc-dependent host cell entry.
Fig 5.
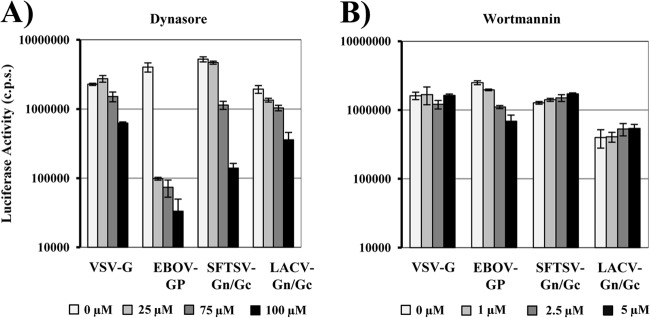
Dynamin activity is required for efficient cellular entry of pseudotypes bearing the glycoproteins of SFTSV. (A) Vero target cells were preincubated with the indicated concentrations of the dynamin inhibitor dynasore or solvent (0 μM), followed by inoculation with infectivity-normalized pseudotypes bearing the glycoproteins of VSV, Zaire EBOV, SFTSV, or LACV as indicated. (B) Vero target cells were pretreated with wortmannin at the indicated concentrations or with solvent (0 μM) and processed as described for panel A. At 24 h postinfection, luciferase activity was determined in cell extracts. Infections were performed in triplicate; the error bars indicate SD. The experiments shown are representative of three independent experiments.
Sera from convalescent SFTS patients inhibit host cell entry driven by the glycoproteins of SFTSV.
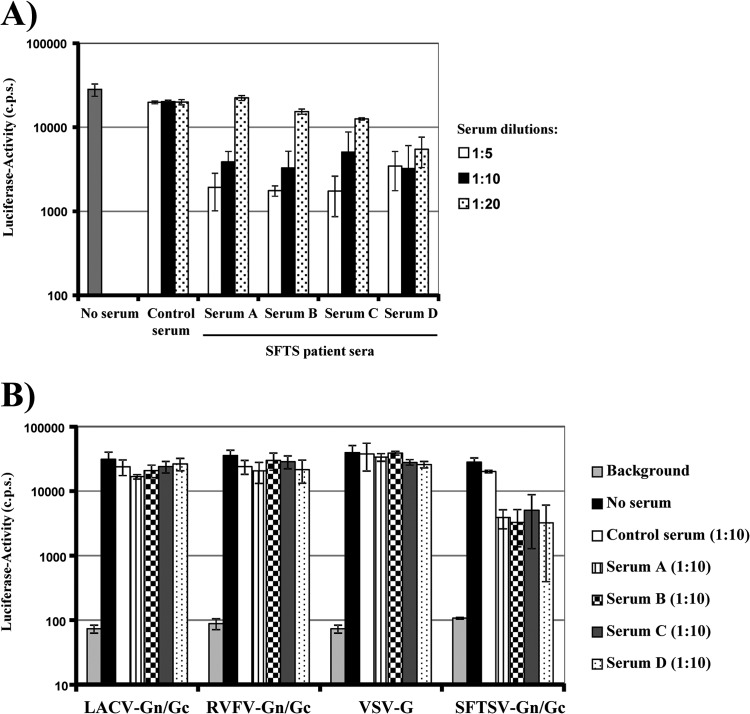
Studies with wild-type SFTSV revealed that sera from convalescent patients inhibited viral spread, indicating that neutralizing antibodies were generated (6). Indeed, sera from four SFTS patients inhibited SFTSV Gn/Gc-driven host cell entry in a dose-dependent fashion, while a similar activity was not observed with sera from healthy controls (Fig. 6A). The inhibitory activity was specific for SFTSV Gn/Gc, since infection driven by LACV and RVFV Gn/Gc was not inhibited (Fig. 6B). These results show that entry of SFTSV Gn/Gc pseudotypes is indeed dependent on the Gn/Gc protein and indicate that our assay is suitable to quantitatively detect the presence of neutralizing antibodies in human sera.
Fig 6.
Sera from convalescent SFTS patients neutralize infectivity of SFTSV glycoprotein-bearing pseudotypes. (A) One hundred TCID50 pseudotypes harboring the SFTSV Gn/Gc protein were preincubated with the indicated dilutions of serum from a healthy donor (control) or convalescent SFTS patients (sera A, B, C, and D) in duplicate. Thereafter, 293T target cells were infected with the pseudotypes in quadruplicate. Infectivity was determined after 48 h by luciferase assay. The results were confirmed in a separate experiment. The error bars indicate SD. (B) The experiment was carried out as in panel A, but pseudotypes bearing VSV G or LACV and RFVF Gn/Gc were included as controls, and a single serum dilution (1:10) was tested. Similar results were obtained in an independent experiment.
The glycoproteins of SFTSV can use the lectin DC-SIGN as a receptor for host cell entry.
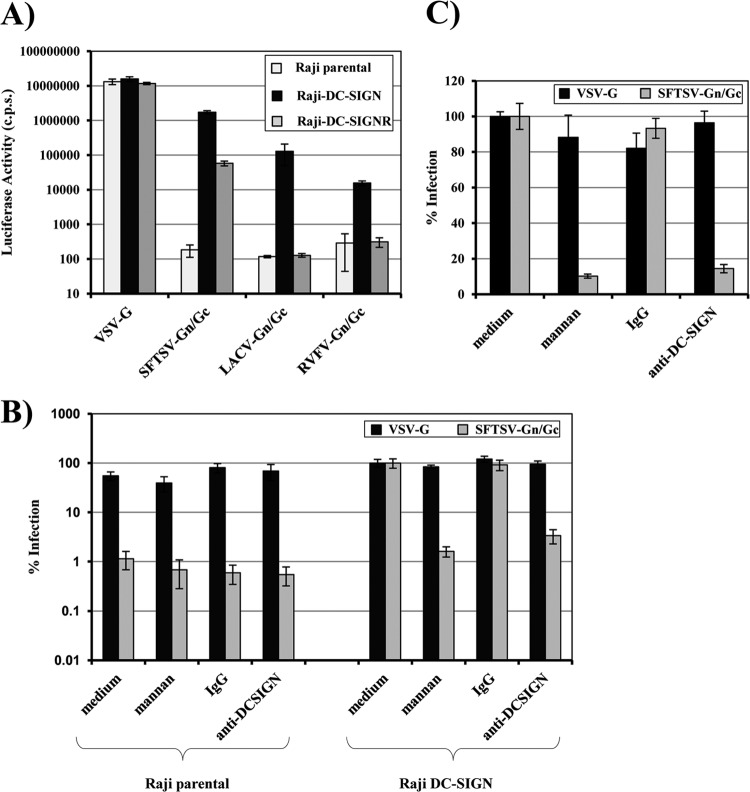
Cellular lectins involved in pathogen recognition and cell-cell adhesion can be hijacked by viruses for entry into host cells (53). We assessed if stable expression of the C-type lectins DC-SIGN (CD209) and DC-SIGNR (CD209L; L-SIGN) allows SFTSV Gn/Gc-driven entry into the B cell line Raji. Entry mediated by RVFV and LACV Gn/Gc was examined in parallel. The Raji cell lines analyzed were highly susceptible to VSV G-driven entry, and entry efficiency was not modulated by lectin expression (Fig. 7A), as expected from previous studies (22). In contrast, Raji cells were resistant to infection by SFTSV Gn/Gc pseudotypes. Expression of DC-SIGN was sufficient to render Raji cells susceptible to entry driven by SFTSV, RVFV, and LACV Gn/Gc, although infection of DC-SIGN-expressing cells by RVFV Gn/Gc pseudotypes was relatively inefficient (Fig. 7A). Expression of DC-SIGNR also allowed infection of Raji cells by SFTSV Gn/Gc pseudotypes but did not confer susceptibility to infection by pseudotypes bearing LACV- and RVFV Gn/Gc, indicating that bunyavirus glycoproteins can differ in lectin specificity. Inhibition studies with the DC-SIGN/DC-SIGNR dual-specific antibody 526 (28) and mannan, a mannose polymer known to block ligand binding to DC-SIGN (54), demonstrated that infection of Raji DC-SIGN cells was dependent on lectin expression (Fig. 7B). Dendritic cells express high levels of endogenous DC-SIGN (54) and are susceptible to SFTSV Gn/Gc-mediated infection (Fig. 2B). We therefore assessed if entry into monocyte-derived dendritic cells depends on the availability of DC-SIGN. Indeed, inhibition of DC-SIGN by antibody 526 and mannan had no effect on VSV G-driven entry but markedly reduced infection mediated by SFTSV Gn/Gc. Thus, SFTSV Gn/Gc can use DC-SIGN as a receptor for entry into cell lines and primary cells.
Fig 7.
DC-SIGN can serve as a host cell receptor for entry mediated by the glycoproteins of SFTSV. (A) Raji B cells engineered to express DC-SIGN or DC-SIGNR were infected with pseudotypes bearing the glycoproteins of VSV, SFTSV, LACV, or RVFV in triplicate. Parental Raji B cells were used as a control. The pseudotypes had been normalized for comparable infectivity on Vero cells. At 24 h postinfection, cell lysates were prepared and analyzed for luciferase activity. The results are representative of three independent experiments; the error bars indicate SD. (B) Parental Raji B cells or DC-SIGN-expressing Raji cells were preincubated with mannan (200 μg/ml), a mouse isotype control antibody (10 μg/ml), or the DC-SIGN/R-specific monoclonal antibody 526 (10 μg/ml) as indicated. The cells were then infected with pseudotypes bearing VSV G or SFTSV Gn/Gc, as described for panel A. Infection was quantified by luciferase activity at 24 h postinfection. Infection of Raji DC-SIGN cells in the absence of inhibitor was set as 100%. The results are representative of three independent experiments; the error bars indicate SD. (C) Primary human dendritic cells were preincubated with mannan (200 μg/ml) or the indicated antibodies (10 μg/ml) as described for panel B, followed by pseudotype infection. Cells were analyzed for luciferase activity at 24 h postinfection. Infection of dendritic cells in the absence of inhibitor (medium) was set as 100%. The error bars indicate SD. The results are representative of three independent experiments using two different donors and three independent pseudotype preparations.
DISCUSSION
Emerging bunyaviruses endanger animal and human health. Infection with Schmallenberg virus (SBV), a novel orthobunyavirus that recently emerged in Europe, is associated with congenital malformations in ruminants (55–57). The virus poses an economic threat to animal farmers but might not be an immediate danger to human health, since transmission of SBV and closely related bunyaviruses to humans has not been observed so far (58). In contrast, SFTSV is infectious for humans, and about 10 to 30% of the afflicted individuals succumb to the disease (6). The factors that determine whether bunyaviruses can cross the species barrier and cause disease in humans are incompletely understood, and the analysis of bunyavirus interactions with host cells will yield important insights. Here, we established a pseudotyping system that allows safe and convenient analysis of the interactions of the SFTSV glycoproteins (Gn/Gc) with host cell factors during viral entry into target cells. We show that SFTSV Gn/Gc mediates entry into a broad panel of animal and human cells in a pH-dependent fashion. Inhibitor experiments revealed that both pH and the activity of a serine protease in target cells are required for SFTSV Gn/Gc-driven infection and that infection can be blocked by sera from convalescent SFTS patients. Finally, evidence was obtained that SFTSV Gn/Gc can use DC-SIGN as a receptor or entry factor for host cell infection.
We employed a VSV-based pseudotyping system to analyze host cell entry driven by the Gn/Gc proteins of SFTSV, with the exception of the neutralization analysis, for which lentiviral vectors were employed. Preference was given to the VSV system, because supernatants from cells producing VSV-based pseudotypes generally exhibited higher infectivity than supernatants obtained from cells generating lentiviral pseudotypes. The reasons for these differences in infectivity are at present unknown but might involve slightly higher incorporation of bunyavirus glycoproteins into rhabdoviral than into lentiviral particles (see below). In the VSV system, the coding sequence for VSV G has been replaced by that of enhanced GFP (EGFP), and an extra transcriptional unit driving expression of luciferase has been inserted (32). The lack of VSV G can be complemented in trans by providing a heterologous glycoprotein (33, 35), including bunyavirus glycoproteins (59). Western blot analysis of cells transfected with SFTSV Gn/Gc with a C-terminal V5 tag detected a protein with a molecular mass of approximately 58 kDa. This signal is consistent with the processing of the precursor glycoprotein into the subunits Gn and Gc, with the latter being detected by the anti-V5 antibody. The same signal was also detected in lentiviral and rhabdoviral pseudotype preparations, indicating that the Gc protein is incorporated into heterologous viral particles, although potentially with different efficiencies. Particle incorporation of Gn was not formally demonstrated in the present study or compared to incorporation of the other bunyavirus glycoproteins tested. However, it is known that Gn and Gc associate and that the presence of both proteins is required for efficient bunyavirus entry, implying that the infectivity of our pseudoparticle preparations was due to the actions of both proteins.
Determining the range of cell types permissive to viral infection can provide important insights into viral pathogenesis. We found that cell lines derived from different human organs are susceptible to SFTSV Gn/Gc-driven infection, including cell lines from human lung, liver, and kidney. These results suggest that the SFTSV receptor(s) is broadly expressed and that the virus might be capable of multiorgan spread. Indeed, virus was detected in the spleens, livers, and kidneys of experimentally infected mice, with the spleen being the major site of viral replication, and pathology was observed in all of the aforementioned organs (60). Diverse animal cell lines were also susceptible to SFTSV Gn/Gc-dependent entry, in agreement with the finding by Yu and colleagues that DH82 cells (canine macrophage-like cells), Vero cells (African green monkey kidney cells), and L929 cells (murine fibroblasts) are permissive to infection with authentic SFTSV (6). Thus, SFTSV might be capable of infecting diverse animal species. However, the nature of the natural reservoir of the virus, from which it is transmitted via ticks to humans, remains to be determined. Notably, pseudotypes bearing LACV, RVFV, and SFTSV Gn/Gc showed overlapping but not identical tropisms for human and animal cells, suggesting that the respective viruses have different receptor requirements for host cell entry. THP-1, QT6, and Raji cells were refractory to entry by all bunyavirus Gn/Gc proteins analyzed, indicating that these cells are receptor negative and thus might be a useful tool for studies aiming at receptor identification.
The membrane fusion activity of viral envelope proteins can be stimulated by receptor engagement (39), low pH (61), or a combination thereof (62). Cellular entry driven by glycoproteins, which are triggered by acidic pH, can be inhibited by lysosomotropic agents, which neutralize the acidic milieu in host cell endosomes. These agents blocked cellular entry driven by SFTSV Gn/Gc in a dose-dependent manner, demonstrating that protons are required for SFTSV Gn/Gc-dependent host cell entry. In the case of EBOV and SARS-CoV infection, inhibition of viral infection by lysosomotropic agents stems from the blockade of the pH-dependent lysosomal cysteine proteases cathepsins B and L, which prime the viral glycoproteins for fusion (42, 43). Incubation of target cells with cathepsin B and L inhibitors did not interfere with SFTSV Gn/Gc-driven entry, indicating that SFTSV Gn/Gc do not rely on cathepsin B and L activity for host cell entry. However, entry mediated by both SFTSV and LACV Gn/Gc proteins was markedly inhibited by the serine protease inhibitor AEBSF. This finding suggests that SFTSV, LACV, and possibly other bunyaviruses might depend on proteolytic processing of their surface proteins in target cells for acquisition of infectivity, in addition to the well-established processing of the precursor Gn/Gc protein in productively infected cells (12). The nature of the responsible serine protease and its potential dependence on low pH remain to be determined. Our preliminary results suggest that cleavage of the SFTSV glycoprotein in SFTSV Gn/Gc-expressing cells is not sensitive to inhibition by AEBSF (data not shown), indicating that the processing of bunyavirus glycoproteins in the secretory pathway of infected cells and in the endocytic pathway of uninfected target cells is mediated by different proteases. While the processing of SFTSV Gn/Gc by an AEBSF-sensitive protease during entry into host cells remains to be demonstrated biochemically, the potential “double processing” of viral glycoproteins in infected and target cells is not without precedent. The glycoproteins of most Ebola virus species are efficiently cleaved by furin in infected or transfected cells (63) (with unknown functional consequences), but glycoprotein activation still depends on the activities of cathepsins B and L in target cells (42), although this model has recently been challenged by a study examining activation of authentic Ebola viruses (64). In addition, our unpublished results suggest that the spike protein of the emerging betacoronavirus Erasmus Medical Center (EMC) is cleaved in transfected cells but still requires the activity of cathepsins B and L in target cells to mediate infectious entry (data not shown).
Convalescent SFTS patients develop neutralizing antibodies (6), and the success of approaches to SFTSV vaccine development might depend on the induction of such antibodies. However, the quantification of the neutralizing antibody response currently depends on time-consuming experiments with authentic SFTSV, for which a high level of biosecurity is required. Our results indicate that pseudotypes bearing SFTSV Gn/Gc allow convenient and fast detection and quantification of neutralizing antibodies in sera from SFTS patients. The system might thus prove to be a useful tool in SFTSV diagnostics. In addition, the pseudotyping system will be instrumental in identifying epitopes in Gn and Gc that are targeted by neutralizing antibodies.
The receptor usage of bunyaviruses is incompletely understood. Hantaviruses were found to use integrins and DAF as receptor and coreceptor for host cell entry, and a correlation between integrin usage and pathogenicity has been proposed (19–21, 65). Recent studies show that phleboviruses can use the C-type lectin DC-SIGN for uptake into host cell endosomes, where the low pH triggers the membrane fusion activity of the viral glycoprotein (17, 18). Indeed, expression of DC-SIGN allowed SFTSV, RVFV, and LACV Gn/Gc-driven infection of DC-SIGN-expressing Raji B cells, which were otherwise nonsusceptible to infection. Dendritic cells and macrophages, both professional antigen-presenting cells, were susceptible to SFTSV G-mediated infection, and dendritic cells in mucosa and lymphoid organs express DC-SIGN (28, 54). Inhibition studies demonstrated that DC-SIGN is required for SFTSV Gn/Gc-dependent infection of monocyte-derived dendritic cells, indicating that the use of DC-SIGN as a receptor for host cell entry is conserved between bunyavirus genera. However, it needs to be pointed out that most, if not all, cell lines identified as susceptible in the present study do not express endogenous DC-SIGN (data not shown), indicating that the receptor requirement of SFTSV might be cell type dependent.
In sum, our results provide important insights into SFTSV host cell entry and might aid in the diagnosis and prevention of SFTSV infection. Recently, a novel bunyavirus, most closely related to SFTSV, was discovered in patients in Missouri who suffered from fever and thrombocytopenia (10). The tools and findings reported in the present study might therefore also be instrumental in the analysis of this novel virus. Finally, it should be noted that the results reported here were generated with vector systems and await confirmation with authentic viruses.
ACKNOWLEDGMENTS
We thank G. Herrler and G. Zimmer for BHK-G43 cells, the VSVΔG-GFP/luc virus, and the polyclonal antiserum directed against VSV G. We thank Sascha Knauf for the THP-1 monocytic cell line, Christina Karsten for advice concerning dendritic cell preparation, Astrid Krueger for expert technical assistance, and Michael Winkler for the gift of some cell lines, assistance with generation of HFF, and discussions.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Walter CT, Barr JN. 2011. Recent advances in the molecular and cellular biology of bunyaviruses. J. Gen. Virol. 92:2467–2484 [DOI] [PubMed] [Google Scholar]
- 2. Jonsson CB, Figueiredo LT, Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23:412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boshra H, Lorenzo G, Busquets N, Brun A. 2011. Rift Valley fever: recent insights into pathogenesis and prevention. J. Virol. 85:6098–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollidge BS, Gonzalez-Scarano F, Soldan SS. 2010. Arboviral encephalitides: transmission, emergence, and pathogenesis. J. Neuroimmune Pharmacol. 5:428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikegami T, Makino S. 2009. Rift valley fever vaccines. Vaccine 27(Suppl. 4):D69–D72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bao CJ, Guo XL, Qi X, Hu JL, Zhou MH, Varma JK, Cui LB, Yang HT, Jiao YJ, Klena JD, Li LX, Tao WY, Li X, Chen Y, Zhu Z, Xu K, Shen AH, Wu T, Peng HY, Li ZF, Shan J, Shi ZY, Wang H. 2011. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin. Infect. Dis. 53:1208–1214 [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 12:156–160 [DOI] [PubMed] [Google Scholar]
- 9. Gai Z, Liang M, Zhang Y, Zhang S, Jin C, Wang SW, Sun L, Zhou N, Zhang Q, Sun Y, Ding SJ, Li C, Gu W, Zhang F, Wang Y, Bian P, Li X, Wang Z, Song X, Wang X, Xu A, Bi Z, Chen S, Li D. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin. Infect. Dis. 54:249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 367:834–841 [DOI] [PubMed] [Google Scholar]
- 11. Marklewitz M, Handrick S, Grasse W, Kurth A, Lukashev A, Drosten C, Ellerbrok H, Leendertz FH, Pauli G, Junglen S. 2011. Gouleako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J. Virol. 85:9227–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmaljohn CS, Nichol ST. 2007. Bunyaviridae, p 1741–1789 In Knipe DM, Howley PM, Fields virology, 5th ed Lippincott Williams & Wilson, Philadelphia, PA [Google Scholar]
- 13. Nakitare GW, Elliott RM. 1993. Expression of the Bunyamwera virus M genome segment and intracellular localization of NSm. Virology 195:511–520 [DOI] [PubMed] [Google Scholar]
- 14. Salanueva IJ, Novoa RR, Cabezas P, Lopez-Iglesias C, Carrascosa JL, Elliott RM, Risco C. 2003. Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments. J. Virol. 77:1368–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lappin DF, Nakitare GW, Palfreyman JW, Elliott RM. 1994. Localization of Bunyamwera bunyavirus G1 glycoprotein to the Golgi requires association with G2 but not with NSm. J. Gen. Virol. 75:3441–3451 [DOI] [PubMed] [Google Scholar]
- 16. Wasmoen TL, Kakach LT, Collett MS. 1988. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology 166:275–280 [DOI] [PubMed] [Google Scholar]
- 17. Lozach PY, Mancini R, Bitto D, Meier R, Oestereich L, Overby AK, Pettersson RF, Helenius A. 2010. Entry of bunyaviruses into mammalian cells. Cell Host. Microbe 7:488–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lozach PY, Kuhbacher A, Meier R, Mancini R, Bitto D, Bouloy M, Helenius A. 2011. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10:75–88 [DOI] [PubMed] [Google Scholar]
- 19. Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. 1998. Beta3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 73:3951–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krautkramer E, Zeier M. 2008. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 82:4257–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Martin TD, Carrington M, KewalRamani VN. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17–23 [DOI] [PubMed] [Google Scholar]
- 23. Gramberg T, Hofmann H, Moller P, Lalor PF, Marzi A, Geier M, Krumbiegel M, Winkler T, Kirchhoff F, Adams DH, Becker S, Munch J, Pöhlmann S. 2005. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 340:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Habjan M, Penski N, Spiegel M, Weber F. 2008. T7 RNA polymerase-dependent and -independent systems for cDNA-based rescue of Rift Valley fever virus. J. Gen. Virol. 89:2157–2166 [DOI] [PubMed] [Google Scholar]
- 25. Plassmeyer ML, Soldan SS, Stachelek KM, Roth SM, Martin-Garcia J, Gonzalez-Scarano F. 2007. Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066-1087) as the fusion peptide. Virology 358:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, Doms RW, Bates P, Pöhlmann S. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115–123 [DOI] [PubMed] [Google Scholar]
- 27. Gao F, Li Y, Decker JM, Peyerl FW, Bibollet-Ruche F, Rodenburg CM, Chen Y, Shaw DR, Allen S, Musonda R, Shaw GM, Zajac AJ, Letvin N, Hahn BH. 2003. Codon usage optimization of HIV type 1 subtype C gag, pol, env, and nef genes: in vitro expression and immune responses in DNA-vaccinated mice. AIDS Res. Hum. Retroviruses 19:817–823 [DOI] [PubMed] [Google Scholar]
- 28. Jameson B, Baribaud F, Pöhlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944 [DOI] [PubMed] [Google Scholar]
- 30. Chong H, Hong K, Zhang C, Nie J, Song A, Kong W, Wang Y. 2008. Genetic and neutralization properties of HIV-1 env clones from subtype B/BC/AE infections in China. J. Acquir. Immune Defic. Syndr. 47:535–543 [DOI] [PubMed] [Google Scholar]
- 31. Kuhl A, Hoffmann M, Muller MA, Munster VJ, Gnirss K, Kiene M, Tsegaye TS, Behrens G, Herrler G, Feldmann H, Drosten C, Pöhlmann S. 2011. Comparative analysis of Ebola virus glycoprotein interactions with human and bat cells. J. Infect. Dis. 204(Suppl. 3):S840–S849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger Rentsch M, Zimmer G. 2011. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS One 6:e25858 doi:10.1371/journal.pone.0025858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanika A, Larisch B, Steinmann E, Schwegmann-Wessels C, Herrler G, Zimmer G. 2005. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J. Gen. Virol. 86:1455–1465 [DOI] [PubMed] [Google Scholar]
- 34. Niwa H, Yamamura K, Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 [DOI] [PubMed] [Google Scholar]
- 35. Takada A, Robison C, Goto H, Sanchez A, Murti KG, Whitt MA, Kawaoka Y. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 94:14764–14769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Doherty U, Swiggard WJ, Malim MH. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Superti F, Seganti L, Ruggeri FM, Tinari A, Donelli G, Orsi N. 1987. Entry pathway of vesicular stomatitis virus into different host cells. J. Gen. Virol. 68:387–399 [DOI] [PubMed] [Google Scholar]
- 38. McClure MO, Marsh M, Weiss RA. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7:513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClure MO, Sommerfelt MA, Marsh M, Weiss RA. 1990. The pH independence of mammalian retrovirus infection. J. Gen. Virol. 71:767–773 [DOI] [PubMed] [Google Scholar]
- 40. Hofmann H, Hattermann K, Marzi A, Gramberg T, Geier M, Krumbiegel M, Kuate S, Uberla K, Niedrig M, Pöhlmann S. 2004. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 78:6134–6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wool-Lewis RJ, Bates P. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 102:11876–11881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gnirss K, Kuhl A, Karsten C, Glowacka I, Bertram S, Kaup F, Hofmann H, Pöhlmann S. 2012. Cathepsins B and L activate Ebola but not Marburg virus glycoproteins for efficient entry into cell lines and macrophages independent of TMPRSS2 expression. Virology 424:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80:4174–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hollidge BS, Nedelsky NB, Salzano MV, Fraser JW, Gonzalez-Scarano F, Soldan SS. 2012. Orthobunyavirus entry into neurons and other mammalian cells occurs via clathrin-mediated endocytosis and requires trafficking into early endosomes. J. Virol. 86:7988–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santos RI, Rodrigues AH, Silva ML, Mortara RA, Rossi MA, Jamur MC, Oliver C, Arruda E. 2008. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 138:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- 49. Mulherkar N, Raaben M, de la Torre JC, Whelan SP, Chandran K. 2011. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology 419:72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hunt CL, Kolokoltsov AA, Davey RA, Maury W. 2011. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire Ebola virus. J. Virol. 85:334–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Araki N, Johnson MT, Swanson JA. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aleksandrowicz P, Marzi A, Biedenkopf N, Beimforde N, Becker S, Hoenen T, Feldmann H, Schnittler HJ. 2011. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J. Infect. Dis. 204(Suppl. 3):S957–S967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsegaye TS, Pöhlmann S. 2010. The multiple facets of HIV attachment to dendritic cell lectins. Cell Microbiol. 12:1553–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 55. Garigliany MM, Hoffmann B, Dive M, Sartelet A, Bayrou C, Cassart D, Beer M, Desmecht D. 2012. Schmallenberg virus in calf born at term with porencephaly, Belgium. Emerg. Infect. Dis. 18:1005–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muskens J, Smolenaars AJ, van der Poel WH, Mars MH, vanWuijckhuise L, Holzhauer M, van Weering H, Kock P. 2012. Diarrhea and loss of production on Dutch dairy farms caused by the Schmallenberg virus. Tijdschr. Diergeneeskd. 137:112–115 [PubMed] [Google Scholar]
- 57. van den Brom R, Luttikholt SJ, Lievaart-Peterson K, Peperkamp NH, Mars MH, van der Poel WH, Vellema P. 2012. Epizootic of ovine congenital malformations associated with Schmallenberg virus infection. Tijdschr. Diergeneeskd. 137:106–111 [PubMed] [Google Scholar]
- 58. Ducomble T, Wilking H, Stark K, Takla A, Askar M, Schaade L, Nitsche A, Kurth A. 2012. Lack of evidence for Schmallenberg virus infection in highly exposed persons, Germany, 2012. Emerg. Infect. Dis. 18:1333–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ogino M, Ebihara H, Lee BH, Araki K, Lundkvist A, Kawaoka Y, Yoshimatsu K, Arikawa J. 2003. Use of vesicular stomatitis virus pseudotypes bearing hantaan or Seoul virus envelope proteins in a rapid and safe neutralization test. Clin. Diagn. Lab. Immunol. 10:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin C, Liang M, Ning J, Gu W, Jiang H, Wu W, Zhang F, Li C, Zhang Q, Zhu H, Chen T, Han Y, Zhang W, Zhang S, Wang Q, Sun L, Liu Q, Li J, Wang T, Wei Q, Wang S, Deng Y, Qin C, Li D. 2012. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc. Natl. Acad. Sci. U. S. A. 109:10053–10058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Helenius A, Kartenbeck J, Simons K, Fries E. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mothes W, Boerger AL, Narayan S, Cunningham JM, Young JA. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679–689 [DOI] [PubMed] [Google Scholar]
- 63. Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. 1998. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc. Natl. Acad. Sci. U. S. A. 95:5762–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marzi A, Reinheckel T, Feldmann H. 2012. Cathepsin B and L are not required for Ebola virus replication. PLoS Negl. Trop. Dis. 6:e1923 doi:10.1371/journal.pntd.0001923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Popugaeva E, Witkowski PT, Schlegel M, Ulrich RG, Auste B, Rang A, Kruger DH, Klempa B. 2012. Dobrava-Belgrade hantavirus from Germany shows receptor usage and innate immunity induction consistent with the pathogenicity of the virus in humans. PLoS One 7:e35587 doi:10.1371/journal.pone.0035587 [DOI] [PMC free article] [PubMed] [Google Scholar]