Background: PAP-III is a secretory protein expressed in injured nerves.
Results: Extracellular PAP-III formed fibrillar structures upon proteolytic N-terminal processing, interacted with neuronal surfaces, and enhanced neurite extension.
Conclusion: Fibrillar PAP-III formed around injury sites may serve as a scaffold for the growth of regenerating axons.
Significance: Clarifying fibrillar PAP-III functions would provide a novel strategy for nerve regeneration.
Keywords: Neurite Outgrowth, Neurochemistry, Neurons, Protease, Regeneration, Pancreatitis-associated Protein, PAP, Reg, Regenerating Gene, Regenerating Islet-derived
Abstract
Pancreatitis-associated protein (PAP)-III, also known as regenerating gene/regenerating islet-derived (Reg)-IIIγ, is a small secretory protein whose expression is substantially induced in injured nerves. Here, we found that PAP-III protein underwent proteolytic N-terminal processing by trypsin-like protease(s) in injured sciatic nerves after axotomy. In vitro studies demonstrated that the N terminus-truncated PAP-III (ΔN-PAP-III) polymerized into a filament with a relatively uniform diameter of 10–20 nm, and the filaments formed higher order structures in a Na+ concentration-dependent manner. When the ΔN-PAP-III fibers were added to the culture media, the ΔN-PAP-III fibers were tightly attached to neurites and somata of primary cortical neurons in vitro. In contrast, little association with glial cells was observed. When dense matrices of ΔN-PAP-III fibers were sheeted on a culture dish, neurites preferentially adhered to the fibers, and neurite extension was enhanced. This neurite outgrowth activity was significantly suppressed by preincubation with antibodies against PAP-III. These results imply that the released PAP-III might be cleaved and forms ΔN-PAP-III fibers at the nerve injury sites. Consequently, these resulting fibers would provide regenerating axons with a platform for extension.
Introduction
Pancreatitis-associated protein (PAP)2 family members are small secretory proteins structurally classified as the calcium-dependent type (C-type) lectin. PAP-I was initially identified as a secretory molecule in the pancreas of rats with acute pancreatitis (1), and thereafter PAP-II and -III were cloned by the screening of cDNA libraries (2, 3). Because several family members have been identified in various animal species, the nomenclature of these molecules remains complicated (4, 5). The PAP family in rats consists of orthologs of the type III subclass of regenerating gene/regenerating islet-derived (Reg) family in mice; PAP-I (also called Reg-2) corresponds to Reg-IIIβ, PAP-II to Reg-IIIα, and PAP-III to Reg-IIIγ. PAP/Reg-III family members have been shown to play roles in the digestive system; for instance, PAP-I expression is enhanced by inflammation in intestine as well as in pancreas and acts as an anti-inflammatory molecule (6–8). PAP-III, whose expression is induced by an interaction of epithelial cells with bacteria in the gut, controls the innate immune system by killing bacteria (9–12).
In addition to the digestive system, several studies have revealed pivotal roles of PAP molecules in neuronal regeneration. Although the basal expression of PAP family members is almost nonexistent in the adult nervous system, they are markedly induced following neural injury. The expression of PAP-I is specifically induced in injured neurons both in the central nervous system and the peripheral nervous system (4, 13–18). It has been suggested that secreted PAP-I promotes neuronal regeneration by acting as a neuronal autocrine/paracrine survival factor as well as a mitogen for Schwann cells (13, 15, 18–20). In contrast to PAP-I, functional consequences of PAP-III expression have been less studied. The expression of PAP-III is also induced in injured neurons after central nervous system injury (16, 17). Unlike PAP-I, the induction of PAP-III expression does not occur in injured neurons in the peripheral nervous system; however, prominent expression has been observed in injured nerves in which Schwann cells serve as the putative sources of PAP-III (4). Our study further reveals that PAP-III secreted around injured peripheral nerves acts as a potent chemoattractant for macrophages, which is required for the removal of myelin and axonal debris in injured nerves (21–23). PAP-III overexpression by adenoviral injection into injured sciatic nerves not only promoted macrophage infiltration into injured nerve but also enhanced axonal regeneration in rats (23). Although these results demonstrated a crucial role of PAP-III in nerve regeneration, it is possible that PAP-III may possess additional functions. In this study we addressed this possibility and found that PAP-III formed fibrillar structures upon N-terminal proteolytic processing and associated specifically with neurons to promote neurite extension.
EXPERIMENTAL PROCEDURES
Animals and Surgery
All experimental procedures were conducted in accordance with the standard guidelines for animal experiments of the Osaka City University and Nagoya University Graduate Schools of Medicine. Adult male Wistar rats, weighing about 150 g (SLC, Hamamatsu, Japan), were anesthetized with pentobarbital (40 mg/kg), and their sciatic nerves were transected with scissors. Control nerves and distal segments of injured nerves 1, 3, and 7 days after surgery were harvested for RT-PCR and Western blot analyses.
RT-PCR
Total RNA was obtained by the acid guanidine isothiocyanate/phenol/chloroform extraction method. cDNA was converted from total RNA with SuperScript III (Invitrogen) and used for PCR amplification. Amplification was carried out in 23 cycles for GAPDH or 32 cycles for PAP-III at 94 °C for 30 s, at 60 °C for 30 s, and at 72 °C for 30 s using primers described in our previous paper (4). The amplified products were loaded onto a 1.2% agarose gel and stained with ethidium bromide.
Western Blotting
Sciatic nerves and ilea were lysed in a buffer containing 8 m urea and 2% CHAPS as described previously (24). The supernatants (16–22 μg) were loaded onto 5–20% gradient gels (Wako Chemicals, Osaka, Japan) together with 10 ng of recombinant PAP-III protein treated with or without trypsin as described below. Blots were prepared on polyvinylidene difluoride membranes (Millipore). The membrane was probed with a mouse monoclonal anti-GAPDH antibody (4300; Ambion, Huntingdon, UK) or a rabbit polyclonal anti-PAP-III antibody produced in our previous study and then with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) (4). Images were visualized by chemiluminescence (ECL Prime; GE Healthcare).
Precipitability of N Terminus-truncated PAP-III (ΔN-PAP-III) Processed by Trypsin
Recombinant PAP-III protein was purified from Pichia pastoris transformed with a rat PAP-III expression vector (4, 25). One microgram of PAP-III protein was treated with 0.1 μg of trypsin in a 20-μl solution of 25 mm Tris (pH 7.5) containing 0, 25, or 150 mm NaCl for 2, 5, or 12 h at room temperature. Then the reaction mixtures were centrifuged at 20,000 × g for 20 min at 4 °C, and supernatants and pellets were dissolved in 2 × and 1 × Laemmli sample buffer, respectively. After boiling, both samples were subjected to 15% SDS-PAGE, and proteins were visualized by staining with Coomassie Brilliant Blue R-250.
Microscopic Observation of ΔN-PAP-III Structure
PAP-III protein (2.5 μg) was treated with or without 0.25 μg of trypsin in a 25-μl solution of 25 mm Tris (pH 7.5) containing 0, 25, or 150 mm NaCl. For the control experiment, an equivalent amount (77 ng) of synthesized N-terminal 11 amino acids (EDAKEDVPTSR37) of PAP-III (PAP-III (N)) was diluted in a 25-μl solution of 25 mm Tris (pH 7.5) containing 150 mm NaCl. To examine the Ca2+ effect on the fiber shape, 25 mm Tris (pH 7.5), 150 mm NaCl solutions containing 2 mm EGTA or CaCl2 were used. The solutions were then diluted in 250 μl of the same buffers and incubated for 3 h at room temperature with poly-d-lysine (PDL)-coated 12-mm round glass coverslips placed on the bottom of 24-well culture dishes. We consistently used PDL in the present study because PDL cannot be hydrolyzed by trypsin (26). After a brief wash, proteins were fixed with 4% paraformaldehyde and then stained by a polyclonal anti-PAP-III antibody and anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen) for fluorescence observation. Images were acquired by confocal microscopes (TCS-SP5, Leica, Heidelberg, Germany; FV10i, Olympus, Tokyo, Japan). For scanning electron microscopy analyses, ΔN-PAP-III prepared on the coverslips was fixed with 2.5% glutaraldehyde and subsequently with 1% OsO4 in 0.1 m phosphate buffer. After dehydration with an ascending series of ethanol, the samples were immersed in isoamyl acetate and dried with a critical point dryer. Then the surfaces of the samples were coated with platinum using a sputter coater (E-1030; Hitachi, Tokyo, Japan). Images were acquired by S-4700 (Hitachi).
Generation of Monoclonal Anti-PAP-III Antibody
Rat monoclonal antibody was raised in accordance with the previous reports (27, 28). Briefly, a 10-week-old female WKY rat (SLC) was immunized with 100 μg of ΔN-PAP-III protein. Three weeks after immunization, lymph nodes obtained from the rat were dispersed, and lymphocytes were fused with mouse myeloma Sp2/0-Ag14 cells. The supernatants of hybridoma clones were screened by ELISA and subsequent immunostaining of ΔN-PAP-III fibers. A hybridoma clone was chosen, and the culture supernatant was loaded onto Hi-Trap SP column (GE Healthcare) to purify the antibody.
Primary Cultures
Cortical neurons were prepared from cerebra of embryonic day (E) 18 embryonic Wistar rats as described previously (29). Neurons were seeded on PDL-coated 12-mm round glass coverslips placed on the bottom of 24-well culture dishes (1.5 × 105 cells/well) and precultured in Neurobasal medium (Invitrogen) containing 0.05 mg/ml penicillin/streptomycin (Invitrogen), 0.5 mm glutamine, and B27 supplement (Invitrogen) for 12–18 h or 10 days for the interaction assay. Astrocytes were also prepared from E18 Wistar rats (30) and maintained in DMEM containing 10% FBS and 0.05 mg/ml penicillin/streptomycin (hereafter referred to as DMEM/FBS). Microglia were isolated from a mixed culture of brains of neonatal Wistar rats on day 14–21 (31). Schwann cells were prepared from sciatic nerves of neonatal Wistar rats (32) and maintained in DMEM/FBS containing 10 μm forskolin (Calbiochem). Before the interaction assay, astrocytes, microglia, and Schwann cells were seeded on PDL-coated glass coverslips placed on the bottom of 24-well culture dishes and pre-cultured in DMEM/FBS for 12–18 h.
Interaction of ΔN-PAP-III Fibers with Primary Cultured Cells
PAP-III protein (2.5 μg) was treated with or without 0.25 μg of trypsin in a 25-μl solution of 25 mm Tris (pH 7.5) containing 150 mm NaCl for 2 h at room temperature, and the reaction was stopped by the addition of 2.5 μg of trypsin inhibitor (Sigma). For the control experiment, an equivalent amount (77 ng) of PAP-III (N) was used. The reaction mixtures were added to culture media of each culture, and then cells were incubated for 24 h at 37 °C. After a brief wash with phosphate-buffered saline (PBS), samples were fixed with 4% paraformaldehyde and subjected to immunocytochemistry using polyclonal anti-PAP-III and mouse monoclonal anti-α-tubulin antibodies (Sigma) (24). Alexa Fluor 488- or 594-conjugated secondary antibodies (Invitrogen) and Alexa Fluor 594-conjugated phalloidin (Invitrogen) were used for visualization. Images were acquired by confocal microscopes (TCS-SP5 and FV10i). To examine the inhibition of interactions by anti-PAP-III antibodies, ΔN-PAP-III fibers were incubated with 2.5 μg of a normal rabbit IgG (R&D Systems), normal rat IgG (Sigma), polyclonal or monoclonal anti-PAP-III antibody for 2 h at room temperature before addition to the culture media.
Neurite Extension on ΔN-PAP-III Fibers
Trypsin digestion of PAP-III was performed on the top of coverslips to prepare dense fiber matrices of ΔN-PAP-III. PAP-III protein (1.0 μg) was diluted in a 10-μl solution of 25 mm Tris (pH 7.5) without NaCl. Immediately after the addition of 0.1 μg of trypsin, the solution was put on the center of PDL-coated 12-mm round glass coverslips placed on the bottom of 24-well culture dishes and incubated for 2 h at 37 °C. Coverslips were washed briefly with PBS, incubated for 30 min with 250 μl of PBS containing 1.0 μg of trypsin inhibitor, and then washed several times again. To examine antibody neutralization, coverslips were incubated with 250 μl of PBS containing anti-rabbit IgG (1.6 μg), rat IgG (0.8 μg), polyclonal (1.6 μg), or monoclonal anti-PAP-III antibody (0.8 μg) for 2 h at room temperature. After brief washes, cortical neurons were seeded at a density of 1.5 × 105 or 5.0 × 103 cells/well. Neurons were maintained in Neurobasal medium containing 0.05 mg/ml penicillin/streptomycin, 0.5 mm glutamine, and B27 supplement (Invitrogen) for 36 h, fixed with 4% paraformaldehyde, and subjected to immunocytochemistry using polyclonal anti-PAP-III and anti-α-tubulin antibodies.
Effects of Full-length PAP-III and N-terminal Peptide on Neurite Extension
Cortical neurons were seeded at a density of 5.0 × 103 cells/well on PDL-coated 12-mm round glass coverslips placed on the bottom of 24-well culture dishes in the culture media described above. Full-length PAP-III (2.5 μg) and the equivalent amount (77 ng) of PAP-III (N) were added to the culture media immediately after seeding. Cells were cultures for 36 h, fixed with 4% paraformaldehyde, and stained by anti-α-tubulin antibody.
Quantification of Neurite Length
Cortical neurons cultured at a density of 5.0 × 103 cells/well were prepared as described above. Low magnification images were acquired randomly using a fluorescent microscope BX41 (Olympus), and neurites of individual neurons were measured using the Neuron J plug-in in the ImageJ software (National Institutes of Health, Bethesda, MD). At least 88 neurons from three independent cultures were analyzed.
RESULTS
Proteolytic N-terminal Processing of PAP-III after Injury
We first examined the expression of PAP-III in injured sciatic nerves. PAP-III mRNA was substantially induced in the injured nerves in 1 day, and the expression gradually decreased (Fig. 1A). Western blotting using PAP-III antibody demonstrated a similar pattern of expressions of PAP-III protein (Fig. 1B). We detected a ∼14-kDa band in the injured nerves throughout the period, whereas the molecular mass of full-length protein was ∼16-kDa. Trypsin-like proteases were reported to act on PAP-III and remove the N-terminal 11 amino acids to generate a stable ∼14-kDa product (25, 33). We therefore digested recombinant PAP-III protein with trypsin in vitro to compare their sizes. The size of the bands detected in injured nerves was approximately equal to that of ΔN-PAP-III cleaved by trypsin (Fig. 1B). The size of the product was also almost identical to that observed in the small intestine, where removal of N-terminal 11 amino acids by trypsin-like protease(s) was reported previously (Fig. 1C) (34). These results suggest that N-terminal processing of PAP-III occurs at the nerve injury sites.
FIGURE 1.
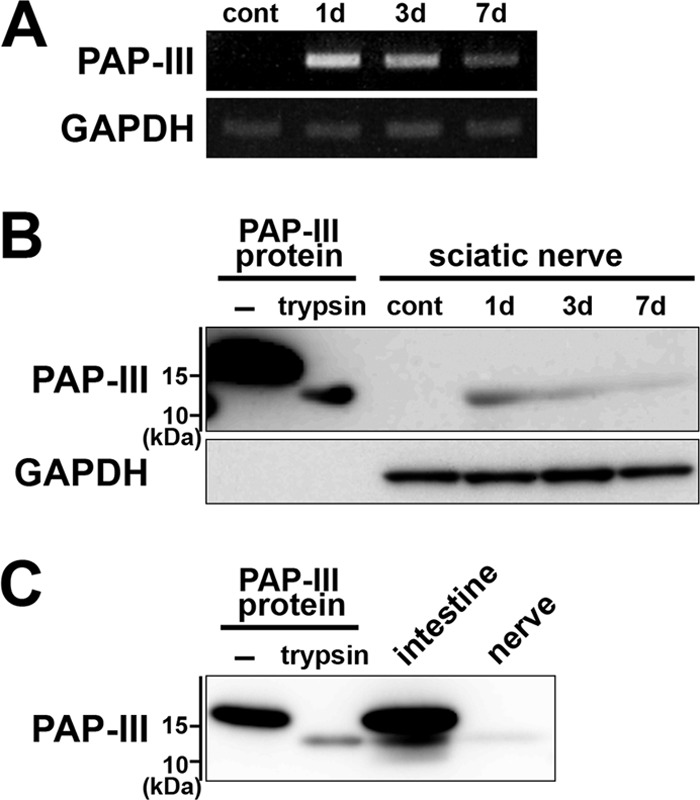
N-terminal proteolytic processing of PAP-III in injured sciatic nerves of rats. A, induction of PAP-III mRNA in injured sciatic nerves. Nerves were collected 0 (cont), 1, 3, and 7 days (d) after axotomy. Expression of PAP-III mRNA was examined by RT-PCR. B, processing of PAP-III protein in injured sciatic nerves. Extracts of sciatic nerves collected 0 (cont), 1, 3, and 7 days (d) after axotomy were analyzed by Western blotting using anti-PAP-III antibody. Recombinant PAP-III protein treated with or without (−) trypsin was used for the comparison. C, comparison of the product size between the injured nerves and small intestine. Extracts of the small intestine (intestine) and sciatic nerves (nerve) collected 1 day after axotomy were analyzed by Western blotting. Recombinant PAP-III protein treated with or without (−) trypsin was also loaded.
Na+ Concentration-dependent Higher Order Structures of ΔN-PAP-III
Previous studies reported that ΔN-PAP-III became insoluble upon trypsin processing and sedimented spontaneously (25, 33). Because these experiments were performed only under the Na+-free conditions, insolubility/precipitability of ΔN-PAP-III under the physiological concentration of Na+ was unknown. Thus, we examined precipitability of ΔN-PAP-III in the neutral buffer containing different concentrations of NaCl 2 h after the trypsin treatment (Fig. 2A). ΔN-PAP-III was entirely precipitated by centrifugation at 20,000 × g without NaCl, which is in line with previous reports (25, 33). Although ΔN-PAP-III remained in the pellet fraction even in the presence of 25 mm NaCl, the protein appeared in the supernatant fraction under 150 mm NaCl. When the incubation was prolonged up to 12 h under 150 mm NaCl, the precipitation of ΔN-PAP-III was increased and could not be detected in the supernatant (Fig. 2B). These results imply that ΔN-PAP-III gradually loses solubility, and the speed of this precipitation was dependent on the salt concentration.
FIGURE 2.
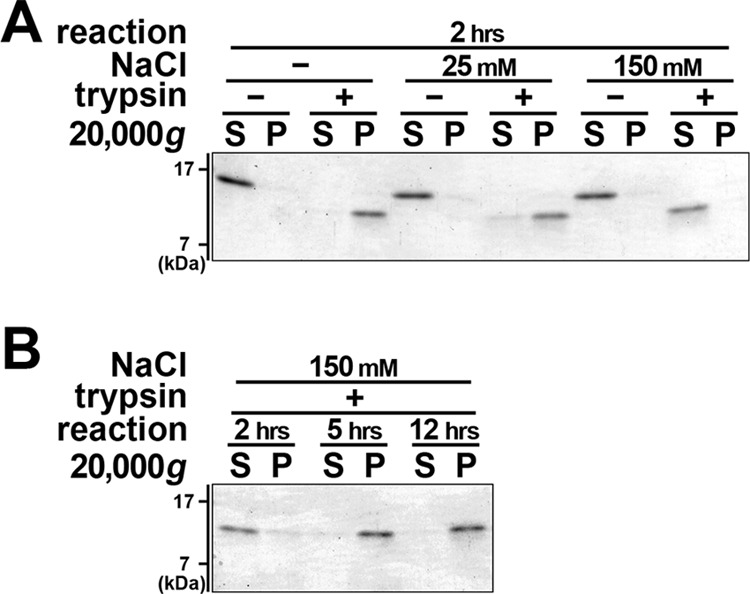
Precipitability of ΔN-PAP-III depends on Na+ concentrations. A, PAP-III protein was incubated with (+) or without (−) trypsin in 25 mm Tris (pH 7.5) containing 0 (−), 25, and 150 mm NaCl for 2 h (hrs). B, PAP-III protein was treated with trypsin (+) in 25 mm Tris (pH 7.5) containing 150 mm NaCl for 2, 5, and 12 h. Solutions were centrifuged at 20,000 × g, and proteins in supernatant (S) and pellet (P) fractions were resolved by SDS-PAGE followed by Coomassie Brilliant Blue staining. ΔN-PAP-III is precipitated under 0 and 25 mm but not under 150 mm NaCl after 2 h of incubation. ΔN-PAP-III becomes precipitated even in the presence of 150 mm NaCl after 5 h of incubation.
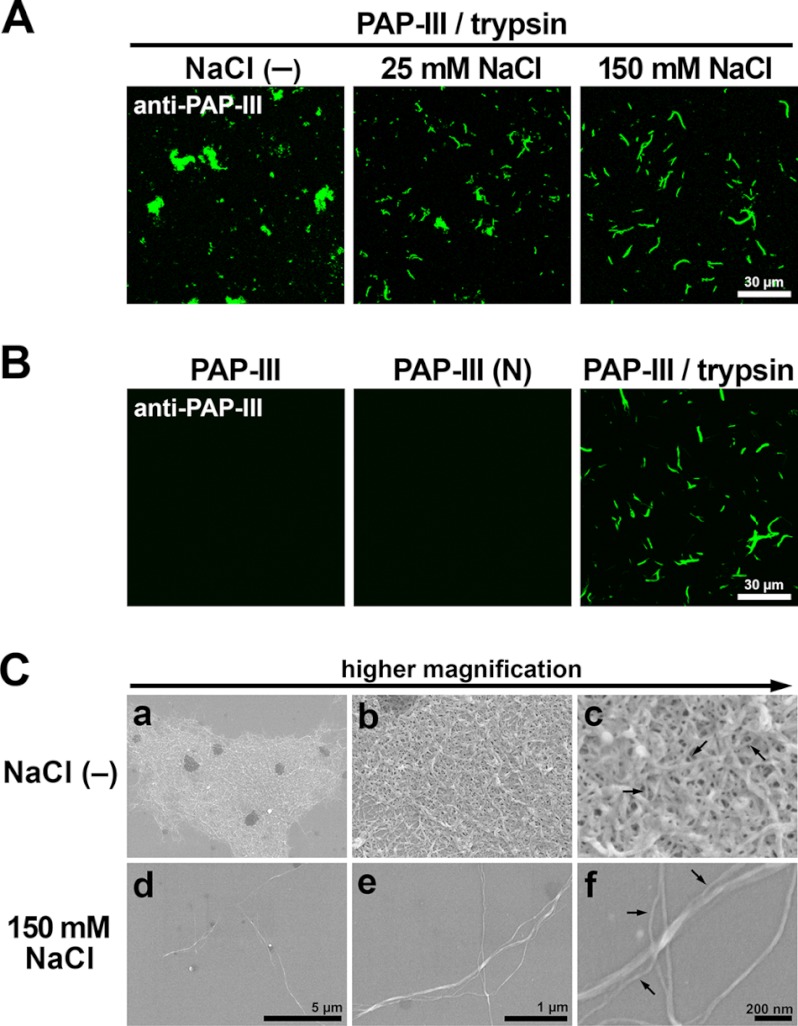
We next determined overall shapes of the insoluble ΔN-PAP-III by immunofluorescence and found apparent differences among different Na+ concentrations (Fig. 3A). The ΔN-PAP-III formed a sheet-like structure in a neutral buffer without NaCl; however, it formed fibrils under 150 mm NaCl. Intermediate NaCl concentrations resulted in intermediate structures. Signals could not be detected in case of full-length protein or N-terminal 11 amino acids of PAP-III (PAP-III (N)) (Fig. 3B), indicating that ΔN-PAP-III was the component of these structures. PAP-III is structurally classified as C-type lectin; however, Ca2+ did not affect the shapes (data not shown). To further characterize these insoluble structures, EM analyses were performed (Fig. 3C). Scanning electron microscopy demonstrated that a filament with a diameter of 10–20 nm was the minimal constituent in common. The sheet-like (meshwork) structure, under the NaCl-free condition, consisted of matrices of filaments branched and entangled tightly (Fig. 3C, a–c). In contrast, the filaments formed thicker bundles in the presence of 150 mm NaCl (Fig. 3C, d–f). These results indicate that the conformation patterns by ΔN-PAP-III filaments depend on environmental salt conditions, although ΔN-PAP-III polymerizes into a primary filament with a consistent diameter. We prepared either the fibrils or the meshwork matrices for the following assays.
FIGURE 3.
Fibrillar structure of ΔN-PAP-III. A, PAP-III treated with trypsin in 25 mm Tris (pH 7.5) containing 0 (−), 25, and 150 mm NaCl was attached to PDL-coated coverslips and visualized by immunofluorescence using anti-PAP-III antibody. Scale bar, 30 μm. B, full-length PAP-III, PAP-III (N), and PAP-III treated with trypsin in 25 mm Tris (pH 7.5) containing 150 mm NaCl were incubated with PDL-coated coverslips and stained by anti-PAP-III antibody. Scale bar, 30 μm. C, PAP-III treated with trypsin in 25 mm Tris (pH 7.5) containing 0 (−) (a–c) or 150 mm (d–f) NaCl was analyzed by scanning electron microscopy. Scale bars, 5 μm (a and d), 1 μm (b and e), and 200 nm (c and f). Note that filaments with a diameter of 10–20 nm are the minimum constituents in common in both conditions (arrows).
Association of ΔN-PAP-III Fibers with Neuronal Surfaces
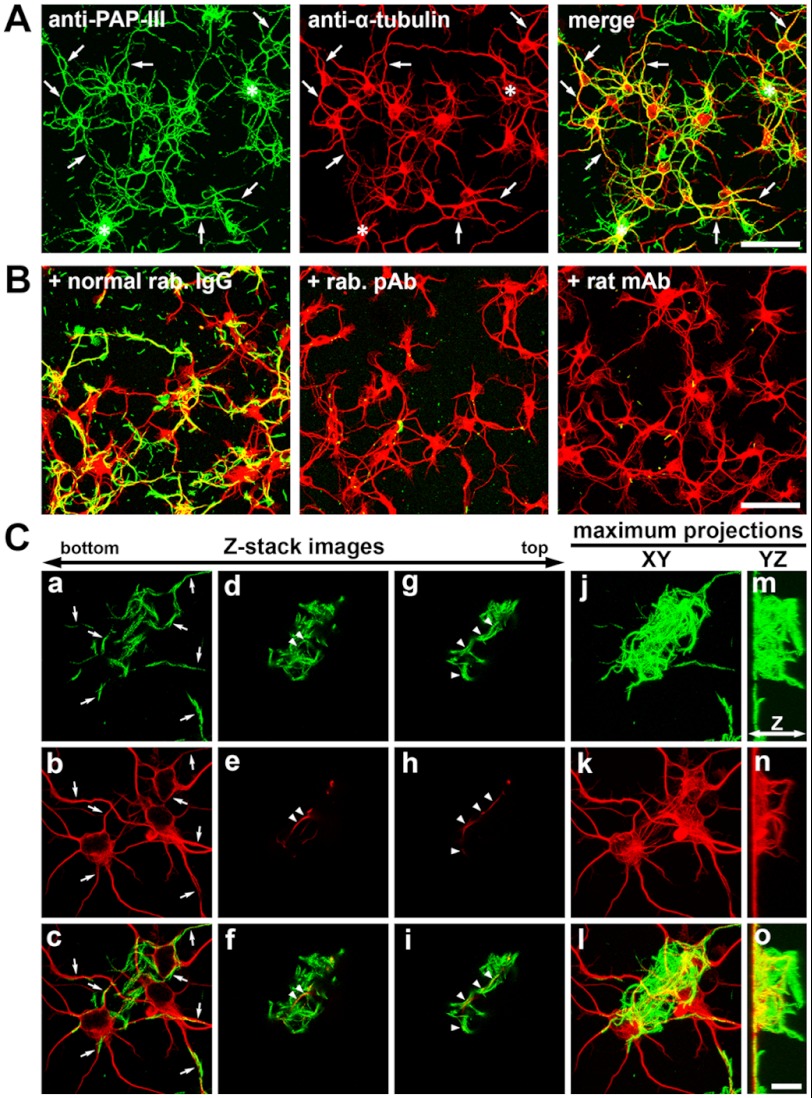
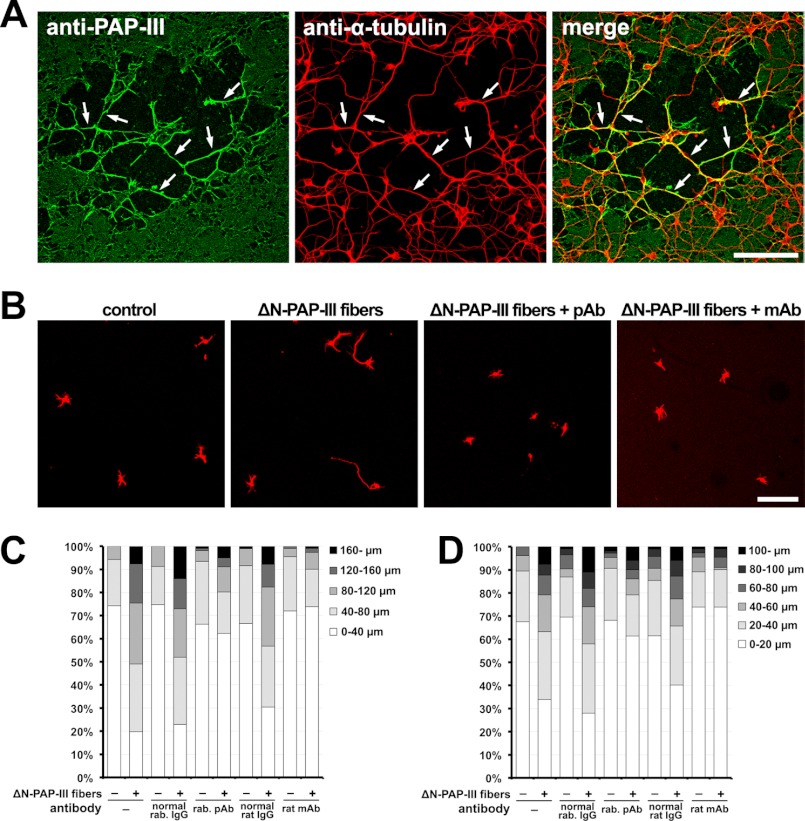
To address functions of extracellular ΔN-PAP-III fibers in the injured nervous system, we prepared primary cultures, added ΔN-PAP-III fibers to the culture media, and observed interactions of ΔN-PAP-III fibers with the cultured cells. The fibrillar ΔN-PAP-III produced in the presence of 150 mm NaCl was used for this experiment to observe the interactions in detail. Significant interactions with glial cells were not observed (Fig. 4). Exogenously added ΔN-PAP-III fibers adhered to the cell-free area of coverslips as well as on glial cells, although there were moderate tendencies for the ΔN-PAP-III fibers to attach preferentially to cellular surfaces. In contrast, significant interaction with neurons was observed (Fig. 5). When we added ΔN-PAP-III fibers to cultured cortical neurons, which were cultured for 12–18 h after seeding, ΔN-PAP-III fibers associated predominantly with growing neurites as well as neuronal somata (Fig. 5A). These associations were entirely blocked by preincubation of ΔN-PAP-III fibers with the rabbit polyclonal anti-PAP-III antibody before adding to the culture media (Fig. 5B). Moreover, the inhibition was confirmed by the use of rat monoclonal antibody, which we produced using ΔN-PAP-III fibers as an antigen. Control rabbit or rat IgG had no effect on the interactions (Fig. 5B and data not shown). In line with Fig. 3B, neither full-length PAP-III nor PAP-III (N) exhibited any signals on neuronal surfaces (data not shown). Such tight associations were also observed using matured cortical neurons, which were precultured for 10 days (data not shown).
FIGURE 4.
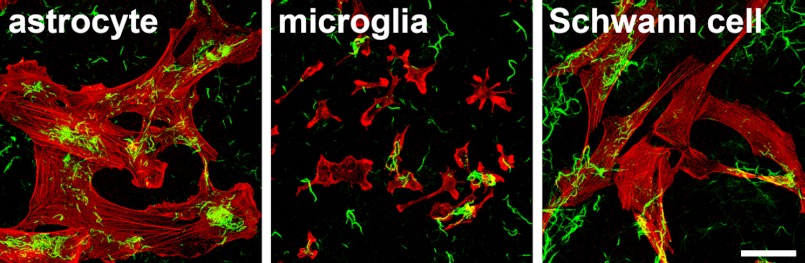
Weak interactions of exogenously added ΔN-PAP-III fibers with glial cells. ΔN-PAP-III fibers produced in 25 mm Tris (pH 7.5) containing 150 mm NaCl were added to the culture media of astrocytes, microglia, and Schwann cells, and incubated for 24 h. After fixation, ΔN-PAP-III fibers and cellular shapes were visualized by anti-PAP-III antibody (green) and phalloidin (red) staining, respectively. Scale bar, 50 μm.
FIGURE 5.
Association of exogenously added ΔN-PAP-III fibers with neurons. ΔN-PAP-III fibers produced in 25 mm Tris (pH 7.5) containing 150 mm NaCl were added to the culture media of primary cortical neurons precultured for 12–18 h. Twenty-four hours after incubation, the preparations were fixed and immunostained with anti-PAP-III (green) and anti-α-tubulin (red) antibodies. A, ΔN-PAP-III fibers associated with neurites (arrows) as well as somata. Complexes of accumulated fibrils were often formed and accumulated on somata (asterisks). B, the associations were blocked by preincubating ΔN-PAP-III fibers with rabbit polyclonal (+rab. pAb) or rat monoclonal anti-PAP-III (+rat mAb) antibody, but not with normal rabbit IgG (+normal rab. IgG). C, high magnification Z-stack images of regions where large complexes of ΔN-PAP-III fibers accumulates on somata (indicated by asterisks in A). Superficial images of the coverslips show a clear association of ΔN-PAP-III fibers with neurites (a–c, arrows). The top images show the upward growth of some neurites into accumulated fibrillar complex (d–f and g–i, arrowheads). Maximum projections of XY (j–l) and YZ (m–o) planes. Neurites grow upward only in the presence of fibrillar complexes (m–o). Scale bars, 50 μm (A and B) and 10 μm (C).
Neurite Outgrowth Activity of ΔN-PAP-III Fibers
As shown by asterisks in Fig. 5A, a large complex of the accumulated fibrils was often observed on neuronal somata. Precise observations of these regions by confocal microscopy revealed that some neurites grew upward into the accumulated fibrillar complex (Fig. 5C). This prompted us to examine the possibility that ΔN-PAP-III fibers might serve as a scaffold or substrate for neurite extension (Fig. 6).
FIGURE 6.
Neurite extension on ΔN-PAP-III fibers immobilized on glass coverslips. Dense matrices of ΔN-PAP-III fibers were prepared on PDL-coated glass coverslips by digesting ΔN-PAP-III without NaCl. Primary cortical neurons were seeded on them and cultured for 36 h. Neuronal shapes were defined by immunostaining with anti-α-tubulin antibody (red). A, image of regions where bundles of ΔN-PAP-III fibrils were sparsely formed (green). Neurites (arrows) as well as somata adhere predominantly to the thick fibrils. Scale bar, 100 μm. B, representative images of neurons extending neurites on PDL (control), PDL+ΔN-PAP-III fibers (ΔN-PAP-III fibers) in the presence of rabbit polyclonal (ΔN-PAP-III fibers+pAb) or rat monoclonal (ΔN-PAP-III fibers+mAb) antibody. Scale bar, 100 μm. C, quantification of the total neurite length. D, quantification of length of the longest neurite.
We next prepared patchy dense matrices of ΔN-PAP-III on PDL-coated glass coverslips and then seeded cortical neurons on them. The patchy dense matrices were successfully formed on the coverslips with some areas where the thick bundles were sparsely formed (Fig. 6A). In these areas, the neuronal somata adhered predominantly to the region where the bundled ΔN-PAP-III fibrils were formed. Moreover, neurites extended along the networks of fibrils, indicating that the neurites were guided by the ΔN-PAP-III fibers for their extension.
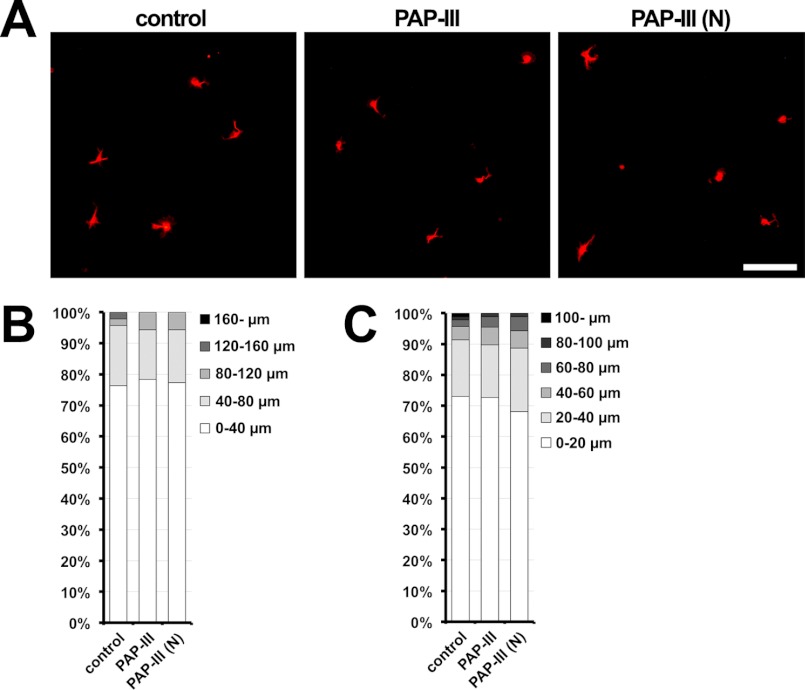
Finally, we examined whether the ΔN-PAP-III fibers promoted the neurite outgrowth (Fig. 6, B–D). Cortical neurons were cultured at low densities on dense matrices of ΔN-PAP-III for 36 h, and their morphologies were evaluated. Neurons grown on ΔN-PAP-III matrices had longer neurites than those grown on PDL. This activity was mostly neutralized by preincubation with anti-PAP-III antibodies before seeding neurons. To examine a possibility that the full-length PAP-III and the cleaved N-terminal fragment also had a similar effect, we carried out similar experiments using either PAP-III and PAP-III (N). However, the full-length PAP-III hardly adhered to any types of the coated coverslips (Fig. 3B), and the adhesion of cleaved N-terminal peptide (PAP-III (N)) was ambiguous due to the failures of the detections by the antibodies examined. In any event, the preincubation with PAP-III or PAP-III (N) did not show significant neurite elongation activity. To verify whether the soluble PAP-III and PAP-III (N) had neurite elongation activity, we added the proteins to the cultured media of cortical neurons and evaluated their morphologies. Neither the full-length PAP-III nor PAP-III (N) showed significant neurite extension activities (Fig. 7), indicating that those peptides do not function as a soluble neurite elongation factor. Taken together, these results suggest that extracellular ΔN-PAP-III fibers promote neurite extension by functioning as a scaffold or a substrate for neurites.
FIGURE 7.
No significant effects of exogenously added full-length PAP-III or PAP-III (N) on neurite extension. Primary cortical neurons were seeded on PDL-coated glass coverslips and cultured for 36 h in media containing no PAP-III (control), full-length PAP-III, or PAP-III (N). Neuronal shapes were defined by immunostaining with anti-α-tubulin antibody (red). A, representative images of neurons. Scale bar, 100 μm. B, quantification of the total neurite length. C, quantification of length of the longest neurite.
DISCUSSION
In this study we have demonstrated that the nerve injury-induced secretory protein PAP-III is cleaved and subsequently forms 10–20 nm filamentous structures. In vitro, the filaments provide neurites with a scaffold to facilitate neurite elongation. Previously, we demonstrated that PAP-III expression was substantially induced in injured peripheral nerves (4, 23). Although in those studies we did not address the cleavage form of PAP-III, we found, in the present study, a ∼14-kDa product of PAP-III in injured nerves throughout the period we examined (Fig. 1B). It is unlikely that this smaller product is derived from a splicing variant because no mRNA variants have been identified in our present and previous studies and in the NCBI database. A previous study showed that intestinal Reg-IIIγ, the mouse ortholog of PAP-III, actually underwent processing by trypsin-like protease(s) at the C terminus of conserved Arg37 in vivo (34). This cleavage site was identical to that where trypsin recognized the recombinant protein in vitro, and the N-terminal processing at this site could be essential for the bactericidal activity as well as conformation of the protein. Although the cleavage site of PAP-III remained uncertain in injured nerves, the size of the product was almost equal to the intestinal one (Fig. 1C), and it is likely that PAP-III is also cleaved at the C terminus of Arg37 in vivo. Processing enzyme(s) for PAP-III remain to be identified in vivo. Along with the context of the previous report in the intestine (34), we assume that PAP-III might be cleaved in the extracellular milieu immediately after secretion. An identification of potent protease(s) could be important for understanding how PAP-III activity is regulated by the nerve injury.
PAP family members and their orthologs are commonly cleaved at the C terminus of Arg37 (5, 25, 33–35). In addition to PAP-III, rat PAP-I and its putative human ortholog hepatocarcinoma-intestine-pancreas (HIP)/PAP were shown to become insoluble after the N-terminal processing at neutral pH (25, 33, 35). However, those studies were carried out in the absence of Na+, which was a nonphysiological condition. We therefore examined the correlation between Na+ concentration and the precipitability of ΔN-PAP-III, and we demonstrated that the precipitability and the precipitated fibrillar structures varied depending on the Na+ concentrations. The precipitability and precipitation speed of ΔN-PAP-III became lower and slower, respectively, as Na+ concentration increased (Fig. 2). However the present study demonstrated that the precipitation and the formation of filamentous structure could occur even under physiological conditions (Figs. 2 and 3). The present confocal and subsequent EM studies demonstrated that the precipitation, which was a polymerized ΔN-PAP-III, was composed of relatively uniform primary filament (10–20 nm in diameter) in the presence or absence of Na+ (Fig. 3). However, the conformational patterns by the primary filaments were different depending on the concentration of Na+. In the presence of 150 mm NaCl, several filaments made a thicker bundle. In contrast, in lower concentrations of Na+ the bundles composed of the primary filaments showed branched and entangled structures and eventually formed a meshwork, which was in accordance with the observation in a previous paper (25). Some members of the PAP family were biochemically shown to form oligomers (36, 37), and furthermore the degree of oligomerization of human HIP/PAP was shown to be dependent on Na+ concentration (38). The oligomerization state of the protein before processing may have an influence on the conformation such as bundling, branching, and meshwork composed of ΔN-PAP-III filaments.
To address significances of the fibrillar structure, we prepared dense mesh-like matrices of ΔN-PAP-III on coverslips and demonstrated that the neurites were preferentially adhered to and elongate along the fibrillar ΔN-PAP-III (Fig. 6). Neurite elongation was significantly promoted on dense matrices of ΔN-PAP-III, and the neurite elongation activity of the fibrillar ΔN-PAP-III was decreased to almost control levels by preincubation of the matrices with antibodies against PAP-III (Fig. 6, B–D). Recently, it was reported that full-length Reg-I, which belong to a distant family of PAP/Reg-III, stimulated neurite outgrowth (39). This was not the case with PAP-III. Neuronal morphologies were unchanged when the full-length protein was added to the culture media of cortical neurons (Fig. 7). In addition, the N-terminal peptide cleaved by trypsin, PAP-III (N), did not alter neurite length (Fig. 7). These results indicate that the cleaved N-terminal fragment and the full-length PAP-III, which are soluble, do not function as a neurite elongation factor and suggest that the N-terminal truncation of PAP-III or composition of the fibrillar structure would be necessary for promoting neurite outgrowth.
One of the intriguing findings in this study is that the association of the fibrillar ΔN-PAP-III is neuronal cell-specific (Fig. 5). Specific interactions between the fibrillar ΔN-PAP-III and glial cells or various nonneuronal cell lines were not observed (Fig. 4 and data not shown), suggesting that there may exist neuronal surface-specific molecule(s) having an affinity with ΔN-PAP-III. PAP family members are reported to bind to certain carbohydrates such as mannan, chitin, and bacterial peptidoglycan as a lectin (9, 35, 40). Thus, it is likely that ΔN-PAP-III recognizes and binds to sugar chains of membrane-bound proteins or lipids, which are preferentially expressed on neurons. Alternatively, it is also possible that ΔN-PAP-III may directly bind to the neuronal membrane via receptor-like molecules. Fibrillar proteins such as collagen and fibronectin promote neurite outgrowth by binding to their receptors, such as a combination of integrin heterodimers expressed on neuronal surfaces (41, 42). Further studies are needed to investigate molecular mechanisms underlying the neuron-specific adhesion and the neurite outgrowth activity of the fibrous ΔN-PAP-III.
In this study we demonstrated the neurite elongation activity of ΔN-PAP-III using the cortical neurons. This suggests that neurites of cortical neurons are capable of binding to ΔN-PAP-III and the adhesion could promote the neurite elongation. Indeed a traumatic injury of the cortex also demonstrated an induction of PAP-III expression (16). However, in the case of central nervous system injury, most neurons are highly susceptible to injury and eventually die without elongating neurites. In this respect, when injured neurons are successfully protected by some treatments, the ΔN-PAP-III may be advantageous for the protected neurons to promote their neurite elongation.
Regarding the actual structure of ΔN-PAP-III in vivo, we were not able to identify the fibrillar structure in the nerve injury site. Although our antibody clearly visualized PAP-III proteins in other brain lesion models (16, 17), immunohistochemical analyses did not show convincing results in injured nerve. One possibility may be that PAP-III conformation is significantly changed upon the processing. As our antibody was raised against full-length PAP-III (4), this antibody may not recognize ΔN-PAP-III in vivo. Alternatively, antigenic site(s) within PAP-III might be masked by some binding proteins such as proteoglycans in vivo. In any event, the present indirect evidences suggest that PAP-III undergoes processing and possibly interacts with regenerating axons. To further demonstrate the interaction between fibrillar PAP-III and regenerating axon in vivo, a good antibody that identifies ΔN-PAP-III in vivo would be necessary.
In conclusion, we have demonstrated a novel activity of PAP-III in vitro. The N-terminal cleaved PAP-III, ΔN-PAP-III, is capable of forming fibrils around injury sites and would serve as a scaffold or substrate for the growth of regenerating axons. However, the full-length PAP-III may attract macrophages/microglia to the injury site (23). The dual functions of PAP-III may be switched depending on its cleavage state. It has been demonstrated that the antibacterial activity of mouse Reg-IIIγ and human HIP/PAP is tightly controlled by an inhibitory N-terminal prosegment that is removed by trypsin or a trypsin-like protease in vivo (34, 40). They proposed that the N-terminal fragment of PAP molecules controls a two-state conformational switch between the biologically active and inactive states of the protein. In an analogous manner, the cleavage of the N-terminal region of PAP-III in injured nerves may also switch the function of the protein from a macrophage chemoattractant to an activator of neurite elongation. The proteolytic regulation of the dual PAP-III functions at the injured nerve site may be crucial for proper nerve regeneration.
Acknowledgments
We thank Dr. S. Kawahara, I. Noura, C. Kadono, and Y. Tabata for technical assistance and A. Asano for secretarial assistance. Chemiluminescence, scanning electron and confocal microscopy were performed at the Central Laboratory of Osaka City University Medical School.
This work was supported in part by Grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Agency.
- PAP
- pancreatitis-associated protein
- HIP
- hepatocarcinoma-intestine-pancreas
- ΔN-PAP-III
- N terminus-truncated PAP-III
- PAP-III (N)
- N-terminal 11 amino acids of PAP-III
- PDL
- poly-d-lysine
- Reg
- regenerating gene/regenerating islet-derived.
REFERENCES
- 1. Keim V., Iovanna J. L., Rohr G., Usadel K. H., Dagorn J. C. (1991) Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology 100, 775–782 [DOI] [PubMed] [Google Scholar]
- 2. Frigerio J. M., Dusetti N. J., Garrido P., Dagorn J. C., Iovanna J. L. (1993) The pancreatitis-associated protein III (PAP III), a new member of the PAP gene family. Biochim. Biophys. Acta 1216, 329–331 [DOI] [PubMed] [Google Scholar]
- 3. Frigerio J. M., Dusetti N. J., Keim V., Dagorn J. C., Iovanna J. L. (1993) Identification of a second rat pancreatitis-associated protein: messenger RNA cloning, gene structure, and expression during acute pancreatitis. Biochemistry 32, 9236–9241 [DOI] [PubMed] [Google Scholar]
- 4. Namikawa K., Fukushima M., Murakami K., Suzuki A., Takasawa S., Okamoto H., Kiyama H. (2005) Expression of Reg/PAP family members during motor nerve regeneration in rat. Biochem. Biophys. Res. Commun. 332, 126–134 [DOI] [PubMed] [Google Scholar]
- 5. Hunt S. P., Kiyama H., Smith A. J. H., C. L. (2007) Which gene Reg2 or Reg3β was targeted that affected liver regeneration? Reply. Hepatology 45, 1585–1586 [DOI] [PubMed] [Google Scholar]
- 6. Gironella M., Iovanna J. L., Sans M., Gil F., Peñalva M., Closa D., Miquel R., Piqué J. M., Panés J. (2005) Anti-inflammatory effects of pancreatitis-associated protein in inflammatory bowel disease. Gut 54, 1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Algül H., Treiber M., Lesina M., Nakhai H., Saur D., Geisler F., Pfeifer A., Paxian S., Schmid R. M. (2007) Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J. Clin. Invest. 117, 1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gironella M., Folch-Puy E., LeGoffic A., Garcia S., Christa L., Smith A., Tebar L., Hunt S. P., Bayne R., Smith A. J., Dagorn J. C., Closa D., Iovanna J. L. (2007) Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut 56, 1091–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cash H. L., Whitham C. V., Behrendt C. L., Hooper L. V. (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brandl K., Plitas G., Schnabl B., DeMatteo R. P., Pamer E. G. (2007) MyD88-mediated signals induce the bactericidal lectin Reg-IIIγ and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204, 1891–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandl K., Plitas G., Mihu C. N., Ubeda C., Jia T., Fleisher M., Schnabl B., DeMatteo R. P., Pamer E. G. (2008) Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaishnava S., Yamamoto M., Severson K. M., Ruhn K. A., Yu X., Koren O., Ley R., Wakeland E. K., Hooper L. V. (2011) The antibacterial lectin Reg-IIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Livesey F. J., O'Brien J. A., Li M., Smith A. G., Murphy L. J., Hunt S. P. (1997) A Schwann cell mitogen accompanying regeneration of motor neurons. Nature 390, 614–618 [DOI] [PubMed] [Google Scholar]
- 14. Averill S., Davis D. R., Shortland P. J., Priestley J. V., Hunt S. P. (2002) Dynamic pattern of reg-2 expression in rat sensory neurons after peripheral nerve injury. J. Neurosci. 22, 7493–7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tebar L. A., Géranton S. M., Parsons-Perez C., Fisher A. S., Bayne R., Smith A. J., Turmaine M., Perez-Luz S., Sheasby A., De Felipe C., Ruff C., Raivich G., Hunt S. P. (2008) Deletion of the mouse Reg-IIIβ (Reg2) gene disrupts ciliary neurotrophic factor signaling and delays myelination of mouse cranial motor neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ampo K., Suzuki A., Konishi H., Kiyama H. (2009) Induction of pancreatitis-associated protein (PAP) family members in neurons after traumatic brain injury. J Neurotrauma 26, 1683–1693 [DOI] [PubMed] [Google Scholar]
- 17. Kawahara S., Konishi H., Morino M., Ohata K., Kiyama H. (2011) Pancreatitis-associated protein-I and pancreatitis-associated protein-III expression in a rat model of kainic acid-induced seizure. Neuroscience 175, 273–280 [DOI] [PubMed] [Google Scholar]
- 18. März-Weiss P., Kunz D., Bimmler D., Berkemeier C., Özbek S., Dimitriades-Schmutz B., Haybaeck J., Otten U., Graf R. (2011) Expression of pancreatitis-associated protein after traumatic brain injury: a mechanism potentially contributing to neuroprotection in human brain. Cell. Mol. Neurobiol. 31, 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishimune H., Vasseur S., Wiese S., Birling M. C., Holtmann B., Sendtner M., Iovanna J. L., Henderson C. E. (2000) Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat. Cell Biol. 2, 906–914 [DOI] [PubMed] [Google Scholar]
- 20. Fang M., Huang J. Y., Ling S. C., Rudd J. A., Yew D. T., Han S. (2010) Effects of Reg-2 on survival of spinal cord neurons in vitro. Anat. Rec. 293, 464–476 [DOI] [PubMed] [Google Scholar]
- 21. Beuche W., Friede R. L. (1984) The role of nonresident cells in Wallerian degeneration. J. Neurocytol. 13, 767–796 [DOI] [PubMed] [Google Scholar]
- 22. Stoll G., Griffin J. W., Li C. Y., Trapp B. D. (1989) Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 18, 671–683 [DOI] [PubMed] [Google Scholar]
- 23. Namikawa K., Okamoto T., Suzuki A., Konishi H., Kiyama H. (2006) Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J. Neurosci. 26, 7460–7467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konishi H., Namikawa K., Kiyama H. (2006) Annexin III implicated in the microglial response to motor nerve injury. Glia 53, 723–732 [DOI] [PubMed] [Google Scholar]
- 25. Graf R., Schiesser M., Scheele G. A., Marquardt K., Frick T. W., Ammann R. W., Bimmler D. (2001) A family of 16-kDa pancreatic secretory stress proteins form highly organized fibrillar structures upon tryptic activation. J. Biol. Chem. 276, 21028–21038 [DOI] [PubMed] [Google Scholar]
- 26. Stahmann M. A., Tsuyuki E., Tsuyuki H. (1956) The synthesis and enzymatic hydrolysis of poly-d-lysine. J. Biol. Chem. 222, 479–485 [PubMed] [Google Scholar]
- 27. Kishiro Y., Kagawa M., Naito I., Sado Y. (1995) A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct. Funct. 20, 151–156 [DOI] [PubMed] [Google Scholar]
- 28. Ushijima R., Sakaguchi N., Kano A., Maruyama A., Miyamoto Y., Sekimoto T., Yoneda Y., Ogino K., Tachibana T. (2005) Extracellular signal-dependent nuclear import of STAT3 is mediated by various importin alphas. Biochem. Biophys. Res. Commun. 330, 880–886 [DOI] [PubMed] [Google Scholar]
- 29. Maeda M., Ampo K., Kiryu-Seo S., Konishi H., Ohba N., Kadono C., Kiyama H. (2005) The p53-independent nuclear translocation of cyclin G1 in degenerating neurons by ischemic and traumatic insults. Exp. Neurol. 193, 350–360 [DOI] [PubMed] [Google Scholar]
- 30. Nakanishi K., Okouchi Y., Ueki T., Asai K., Isobe I., Eksioglu Y. Z., Kato T., Hasegawa Y., Kuroda Y. (1994) Astrocytic contribution to functioning synapse formation estimated by spontaneous neuronal intracellular Ca2+ oscillations. Brain Res. 659, 169–178 [DOI] [PubMed] [Google Scholar]
- 31. Nakajima K., Shimojo M., Hamanoue M., Ishiura S., Sugita H., Kohsaka S. (1992) Identification of elastase as a secretory protease from cultured rat microglia. J. Neurochem 58, 1401–1408 [DOI] [PubMed] [Google Scholar]
- 32. Matsuoka I., Meyer M., Thoenen H. (1991) Cell type-specific regulation of nerve growth factor (NGF) synthesis in nonneuronal cells: comparison of Schwann cells with other cell types. J. Neurosci. 11, 3165–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiesser M., Bimmler D., Frick T. W., Graf R. (2001) Conformational changes of pancreatitis-associated protein (PAP) activated by trypsin lead to insoluble protein aggregates. Pancreas 22, 186–192 [DOI] [PubMed] [Google Scholar]
- 34. Mukherjee S., Partch C. L., Lehotzky R. E., Whitham C. V., Chu H., Bevins C. L., Gardner K. H., Hooper L. V. (2009) Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J. Biol. Chem. 284, 4881–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Medveczky P., Szmola R., Sahin-Tóth M. (2009) Proteolytic activation of human pancreatitis-associated protein is required for peptidoglycan binding and bacterial aggregation. Biochem. J. 420, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bödeker H., Keim V., Fiedler F., Dagorn J. C., Iovanna J. L. (1999) PAP I interacts with itself, PAP II, PAP III, and lithostathine/regIα. Mol. Cell. Biol. Res Commun. 2, 150–154 [DOI] [PubMed] [Google Scholar]
- 37. Hassanain E., Huan C., Mueller C. M., Stanek A., Quan W., Viterbo D., Bluth M. H., Zenilman M. E. (2011) Pancreatitis-associated proteins' regulation of inflammation is correlated with their ability to aggregate. Pancreas 40, 1151–1153 [DOI] [PubMed] [Google Scholar]
- 38. Ho M. R., Lou Y. C., Lin W. C., Lyu P. C., Huang W. N., Chen C. (2006) Human pancreatitis-associated protein forms fibrillar aggregates with a native-like conformation. J. Biol. Chem. 281, 33566–33576 [DOI] [PubMed] [Google Scholar]
- 39. Acquatella-Tran Van Ba I., Marchal S., François F., Silhol M., Lleres C., Michel B., Benyamin Y., Verdier J. M., Trousse F., Marcilhac A. (2012) Regenerating islet-derived 1alpha (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin tumor-like 3 (EXTL3) receptor. J. Biol. Chem. 287, 4726–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lehotzky R. E., Partch C. L., Mukherjee S., Cash H. L., Goldman W. E., Gardner K. H., Hooper L. V. (2010) Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc. Natl. Acad. Sci. U.S.A. 107, 7722–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Müller U., Bossy B., Venstrom K., Reichardt L. F. (1995) Integrin α8β1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol. Biol. Cell 6, 433–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bradshaw A. D., McNagny K. M., Gervin D. B., Cann G. M., Graf T., Clegg D. O. (1995) Integrin α2β1 mediates interactions between developing embryonic retinal cells and collagen. Development 121, 3593–3602 [DOI] [PubMed] [Google Scholar]