Background: Mitochondrial superoxide flashes measured with mt-cpYFP were suggested to reflect mitochondrial matrix alkalinization.
Results: Simultaneous recordings of mt-cpYFP flashes with SNARF-1 and MitoSOX revealed that flashes are concurrent with an increase in superoxide and <0.1 pH unit alkalinization.
Conclusion: mt-cpYFP flashes are transient bursts of superoxide production coincident with modest matrix alkalinization.
Significance: This study validates mt-cpYFP as a novel superoxide sensor.
Keywords: Cardiac Muscle, Mitochondria, pH Regulation, Skeletal Muscle, Superoxide Ion, Mitochondrial Matrix Alkalinization, mt-cpYFP, Superoxide Flashes
Abstract
Superoxide flashes are transient bursts of superoxide production within the mitochondrial matrix that are detected using the superoxide-sensitive biosensor, mitochondria-targeted circularly permuted YFP (mt-cpYFP). However, due to the pH sensitivity of mt-cpYFP, flashes were suggested to reflect transient events of mitochondrial alkalinization. Here, we simultaneously monitored flashes with mt-cpYFP and mitochondrial pH with carboxy-SNARF-1. In intact cardiac myocytes and purified skeletal muscle mitochondria, robust mt-cpYFP flashes were accompanied by only a modest increase in SNARF-1 ratio (corresponding to a pH increase of <0.1), indicating that matrix alkalinization is minimal during an mt-cpYFP flash. Individual flashes were also accompanied by stepwise increases of MitoSOX signal and decreases of NADH autofluorescence, supporting the superoxide origin of mt-cpYFP flashes. Transient matrix alkalinization induced by NH4Cl only minimally influenced flash frequency and failed to alter flash amplitude. However, matrix acidification modulated superoxide flash frequency in a bimodal manner. Low concentrations of nigericin (< 100 nm) that resulted in a mild dissipation of the mitochondrial pH gradient increased flash frequency, whereas a maximal concentration of nigericin (5 μm) collapsed the pH gradient and abolished flash activity. These results indicate that mt-cpYFP flash events reflect a burst in electron transport chain-dependent superoxide production that is coincident with a modest increase in matrix pH. Furthermore, flash activity depends strongly on a combination of mitochondrial oxidation and pH gradient.
Introduction
Mitochondrial superoxide flashes (mSOFs),6 recently discovered events present in a wide range of cell types, are stochastic and quantal bursts of superoxide produced within the mitochondrial matrix that are detected using mitochondria-targeted cpYFP (mt-cpYFP) (1). Superoxide flashes occur concomitantly with a depolarization of the mitochondrial membrane potential and the opening of a large pore channel in the mitochondria inner membrane. mSOF activity is tightly coupled to mitochondrial respiration and is exquisitely dependent on both electron transport chain (ETC) and adenine nucleotide translocase functionality. mSOF activity is a highly sensitive biomarker of normal cellular metabolism (1–4) that is increased in several oxidative stress-related disorders including ischemia reperfusion (1), malignant hyperthermia (4), and ROS-induced apoptosis (5). However, the mechanism for mSOF generation remains incompletely defined.
The interpretation of mt-cpYFP flashes as superoxide-dependent events is based on considerable evidence from a wide variety of both in vitro and intact cell studies. Fluorescence emission of purified cpYFP is increased by superoxide anions, but not by other ROS/reactive nitrogen species or changes in redox potential, Ca2+, ATP, ADP, NAD(P)+, or NAD(P)H (1). In intact cells, mSOF frequency is strongly altered by multiple superoxide-modifying interventions including manganese-superoxide dismutase knockdown/knock-out (6, 7), ROS scavengers (Tiron), superoxide dismutase mimetics (MnTMPyP) (1), and mitochondria-targeted antioxidants (mito-TEMPO) (6). Moreover, an irreversible increase in MitoSOX fluorescence during each mSOF event further supports the role for a burst of superoxide production during a flash (2). Finally, flash activity is reduced during severe hypoxia, abolished under conditions of complete anoxia, and increased immediately upon reperfusion with oxygenated medium (1, 6).
However, like other GFP-based biosensors, cpYFP fluorescence is also pH-sensitive. A significant increase in purified cpYFP fluorescence at 488 nm excitation is observed upon alkalinization, with a pKa ∼8.5 (1). Recent studies using Arabidopsis mitochondria observed stochastic bursts in mt-cpYFP fluorescence with amplitude and spatiotemporal properties similar to those reported previously for mSOF activity (8, 9). However, the authors concluded that these events reflected a transient alkalinization of the mitochondrial matrix rather than a change in superoxide (8). This study also reported that global mt-cpYFP fluorescence in purified Arabidopsis mitochondria increased upon application of mitochondrial substrates and was not altered by conditions expected to either enhance (e.g. menadione, manganese-superoxide dismutase knockdown) or reduce (Tiron, TEMPOL) superoxide production, consistent with the events reflecting ETC-dependent proton efflux rather than superoxide production. However, effects of these interventions on spontaneous flash activity in Arabidopsis mitochondria were not reported. Mitochondria are highly dynamic organelles that constantly undergo oxidative phosphorylation to produce ATP needed to support an array of different cellular energy requirements. Because the ETC pumps protons out of the matrix and the resulting proton gradient is used to drive ATP production by the F1F0-ATPase, fluctuations in matrix pH in actively respiring mitochondria are not unexpected. Indeed, mitochondrial alkalinization transients in quiescent cells are observed using mito-SypHer, a mitochondria-targeted ratiometric pH-sensitive probe (10–13). Because mt-cpYFP is sensitive to both superoxide anions and pH, and the ETC is responsible for both proton efflux (a potential mechanism for pH spikes) and superoxide production (via electron slippage), it is possible that these two events are connected. Thus, mt-cpYFP flashes could reflect a concurrent ETC-dependent increase in both superoxide and pH within the mitochondrial matrix (10).
To directly address the issue of whether mt-cpYFP flashes reflect bursts in superoxide production and/or mitochondrial matrix alkalinization, we used a red-shifted pH-sensitive dye, carboxy-SNARF-1, to simultaneously monitor changes in matrix pH during mSOF events in mitochondria from both cardiac and skeletal muscle. The response of flash activity to both transient matrix alkalinization (NH4Cl) and acidification (nigericin) was also examined. In addition, mitochondria were purified from adult skeletal muscle to directly determine the influence of ETC blockade, superoxide scavenging, and matrix acidification on flash activity. The results demonstrate that mitochondrial mt-cpYFP flash activity reflects ETC- and pH-dependent superoxide production, with only a minor component due to a concurrent alkalinization of the mitochondrial matrix during each event.
EXPERIMENTAL PROCEDURES
Adult Rat Cardiac Myocyte Isolation, Culture, and Gene Transfer
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington. Cardiac myocytes were enzymatically isolated from the hearts of adult Sprague-Dawley rats (200–250 g) using previously reported approaches (1). Freshly isolated cardiac myocytes were plated on 25-mm coverslips coated with laminin (20 μg/ml) for 2 h to allow for cell attachment. Attached myocytes were infected with adenovirus carrying mt-cpYFP at an multiplicity of infection of 100. Myocytes were cultured in M199 medium (Sigma) supplemented with 0.02% BSA, 5 mm creatine, 2 mm l-carnitine, 5 mm taurine, 5 mm HEPES, and 0.1% insulin-transferrin-selenium-X. Myocytes were kept in culture for 48–72 h to allow adequate indicator expression prior to imaging.
pH Calibration in Intact Cardiac Myocytes
Intracellular SNARF-1 calibration was conducted using the same loading condition in myocytes and followed the procedure reported previously with modifications (14). Briefly, intact myocytes were incubated in the calibration solution containing: 140 mm KCl (KCl adjusted to keep K+ constant), 1.0 mm MgCl2, 11.0 mm dextrose, 2 mm EGTA, 15 mm 2,3-butanedione monoxime, 12 mm HEPES, and 10 μm nigericin. Solution pH varied from 7.0 to 9.0 by titration with 1 m KOH. The emission spectra were collected using the Lambda scan mode of the Zeiss LSM 510 Meta confocal microscope. The spectra were collected at three excitation wavelengths: 488, 514, and 543 nm, all of which have previously been tested by the manufacturer (Invitrogen) for pH measurement (31). Bandwidth was set at 10 nm. For pH calibration of mt-cpYFP and SNARF-1 simultaneously, we used the same imaging condition for flash and SNARF-1 ratio S2/S1 measurement. Dual excitation at 488 nm/405 nm or single excitation at 488 nm was used to determine the pH calibration curve for mt-cpYFP.
Purification of Isolated Mitochondria from Skeletal Muscle
Skeletal muscle-specific mt-cpYFP-expressing transgenic mice were housed at the University of Rochester School of Medicine and Dentistry, and all animal protocols were approved by the local University Committee on Animal Resources. Hindlimb muscle tissue from mt-cpYFP transgenic mice was dissected on ice in Chappell-Perry buffer (50 mm Tris, pH 7.4, 100 mm KCl, 5 mm MgCl2, and 1 mm EDTA) and manually minced using scissors. Minced muscle tissue was washed twice in Chappell-Perry buffer by gentle sedimentation using a bench-top centrifuge and digested on ice for 6 min in a buffer containing: 50 mm Tris pH 7.4, 180 mm KCl, 100 mm sucrose, and 1 mm EDTA, with 1 mg/ml proteases (from Streptomyces griseus Type XIV, Sigma). Digested tissue was then homogenized in a potter homogenizer equipped with a motor-driven pestle (four strokes at 500 rpm) and centrifuged at 3500 × g for 4 min at 4 °C. The supernatant was collected after being filtered through cheese cloth. The pellet was resuspended, rehomogenized, and centrifuged once more, and supernatant collected as above. The pellet was then digested again in the same protease solution at room temperature for 8 min and homogenized and centrifuged as above. The supernatants from each centrifugation were pooled and subjected to a higher speed centrifugation at 11,000 × g for 9 min at 4 °C. The resultant pellet was collected, resuspended in a buffer containing 50 mm Tris, pH 7.4, 100 mm KCl, and 1 mm EDTA, and recentrifuged at 11,000 × g for 9 min. The final pellet was resuspended in skeletal muscle respiration buffer (10 mm HEPES, pH 7.4, 125 mm KCl, 5 mm MgCl2, 2 mm K2HPO4, and 1 mg/ml BSA) with 10 mm glutamate, 5 mm malate, and 100 μm malonate (for Complex I) or 5 mm succinate (Complex II) as mitochondrial substrate. Mitochondria were stored on ice and used for experiments within 3 h of preparation.
Transfection of mt-cpYFP in Flexor Digitorum Brevis (FDB) Muscles by in Vivo Electroporation and Isolation of Transfected Fibers
For some experiments (see Fig. 3D), mt-cpYFP was transiently expressed in FDB muscle of 5–8-week-old male OF1 mice (Charles River Laboratories, L'Arbresle, France) by in vivo electroporation as described previously (2). All experiments and procedures were in accordance with the guidelines of the French Ministry of Agriculture (87/848) and of the European Community (86/609/EEC). They were approved by the local animal ethic committee of Rhone-Alpes.
FIGURE 3.
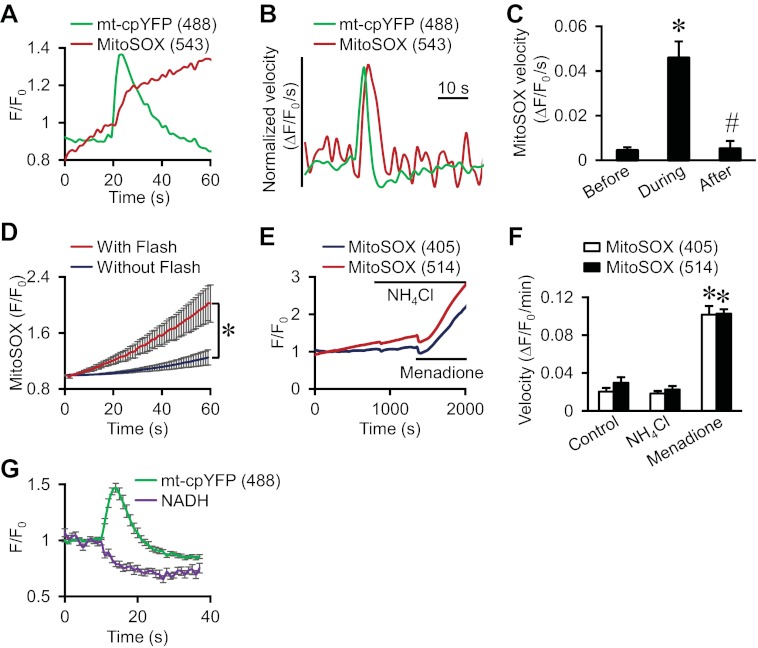
A stepwise increase in MitoSOX fluorescence occurs during mt-cpYFP flash activity. A, averaged traces showing increased rate of MitoSOX fluorescence (increased slope) during superoxide flash activity in cardiac myocytes. Traces were averaged from 11 flashes. Similar results were obtained from 3–4 cells from three hearts. B, averaged traces showing the rate of signal change (dF/dt) in MitoSOX and mt-cpYFP fluorescence during flash events. C, summarized data showing the rate of signal change in MitoSOX fluorescence before, during, and after flash events. Data are calculated from the trace in B. *, p < 0.001 versus before. #, p < 0.001 versus during. D, traces showing time dependent changes of MitoSOX fluorescence (F/F0) during 60 s of continuous recording in skeletal muscle fibers exhibiting flashes (With Flash) or devoid of flashes (Without Flash). n = 5–6. *, p < 0.05 versus with flash. E, MitoSOX fluorescence in cardiac myocytes increased in response to mitochondrial oxidation (200 μm menadione), but not matrix alkalinization (30 mm NH4Cl). Similar results were obtained from 18 cells in three separate experiments. F, summarized data showing the rate of change in MitoSOX fluorescence calculated from E. Data are mean ± S.E. n = 18 cells from three experiments. *, p < 0.05 versus control. G, mt-cpYFP flashes in cardiac myocytes were accompanied by a significant decrease in mitochondrial NADH autofluorescence, consistent with mitochondrial oxidation. Traces were averaged from 31 flashes in the same myocyte. Similar results were obtained from flashes in 15 cells from three rat hearts.
Single fibers were isolated from muscles 5 days after electroporation using a previously described procedure (15). In brief, muscles were placed in a Tyrode's solution containing 0.2% collagenase (Sigma, type I) for 50 min at 37 °C. After this treatment, muscles were kept at 4 °C in a Tyrode's solution containing 136 mm NaCl, 5 mm KCl, 2.6 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 10 mm glucose (pH 7.2), and used within 10 h. Single fibers were obtained by triturating the muscles within the experimental chamber. For experimental measurements, fibers were bathed in Tyrode's solution at room temperature.
Isolation of FDB Fibers
FDB fibers were isolated from mt-cpYFP transgenic mice using enzymatic digestion as described previously (4) (see Figs. 4–7). Acutely isolated single fibers were stored in medium containing DMEM/F12 (1:1); 2% FBS and 1% penicillin/streptomycin at 37 °C and transferred to regular rodent Ringer's solution (146 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, pH 7.4) prior to imaging.
FIGURE 4.
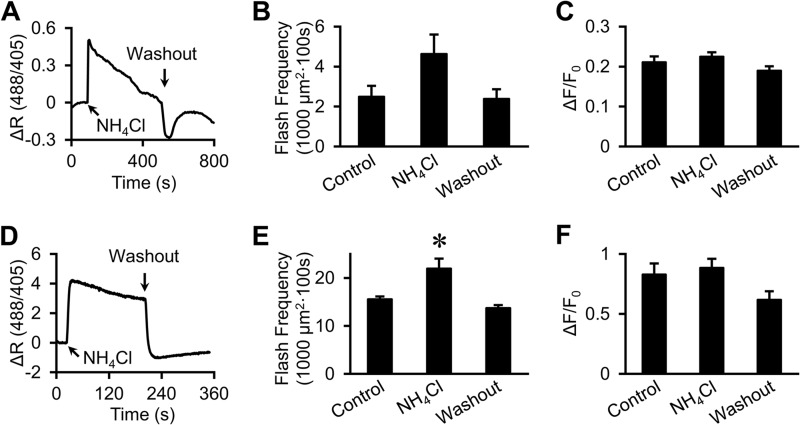
Mitochondrial alkalinization does not significantly alter mt-cpYFP flash amplitude. A, representative trace showing the response of mt-cpYFP base-line fluorescence in cardiac myocytes to transient mitochondrial alkalinization (10 mm NH4Cl). Similar results were obtained from 10 cells in four experiments. ΔR (488/405): the changes of 488/405 ratio over base line. B and C, averaged flash frequency and amplitude before and during NH4Cl application and washout in cardiac myocytes. Mitochondrial alkalinization slightly increased superoxide flash frequency (B), but did not alter flash amplitude (C). n = 43–100 flashes from 9–15 cells. D, same as A except from FDB fibers. E and F, averaged flash frequency and amplitude in FDB fibers before and during NH4Cl application and after washout. Matrix alkalinization resulted in a moderate increase in flash frequency (E) without altering flash amplitude (F). Data are mean ± S.E. n = 14 fibers. *, p < 0.05 versus control.
FIGURE 5.
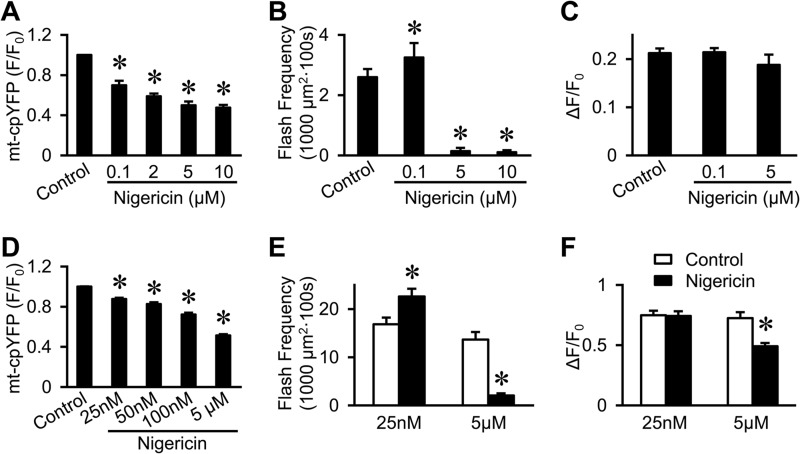
Bimodal regulation of flash frequency by nigericin-induced mitochondrial acidosis. A, dose-dependent decrease in base-line mt-cpYFP fluorescence by nigericin in intact cardiac myocytes. Data are mean ± S.E. n = 5–14 cells. *, p < 0.05 versus no nigericin. B and C, superoxide flash frequency in cardiac myocytes was significantly increased by mild acidosis (100 nm nigericin) and abolished by severe acidosis (5–10 μm nigericin), whereas flash amplitude is unchanged. Data are mean ± S.E. n = 35–273 flashes in 20–68 cells. *, p < 0.05 versus control. D–F, same as A–C except from intact FDB fibers. n = 12–25 cells. *, p < 0.05 versus control.
FIGURE 6.
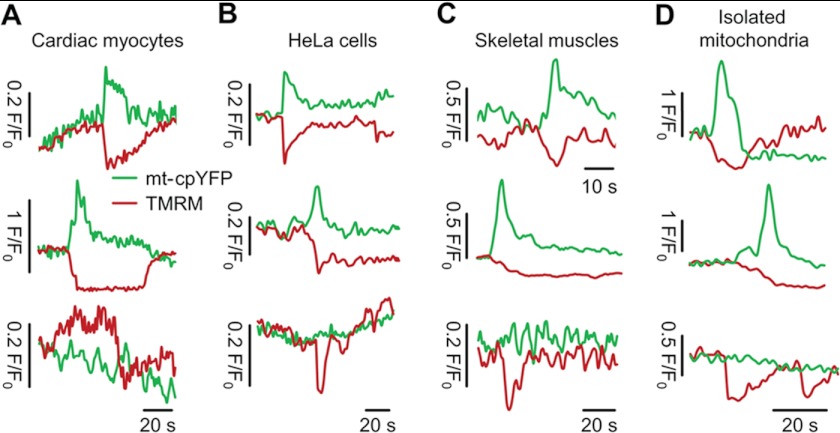
Dissociation between respective time courses of superoxide flash activity and mitochondrial membrane potential fluctuations. Three similar classes of mitochondrial membrane potential fluctuations were observed in cardiac myocytes (A), HeLa cells (B), skeletal muscle fibers (C), and mitochondria purified from skeletal muscle (D): i) a parallel decrease and recovery of the mitochondrial membrane potential during a flash (upper panel), ii) the decrease followed by a slow recovery of the mitochondrial membrane potential during a flash (middle panel), and iii) fluctuations in the mitochondrial membrane potential in the absence of flash activity (lower panel). Traces are representative of at least three experiments.
FIGURE 7.
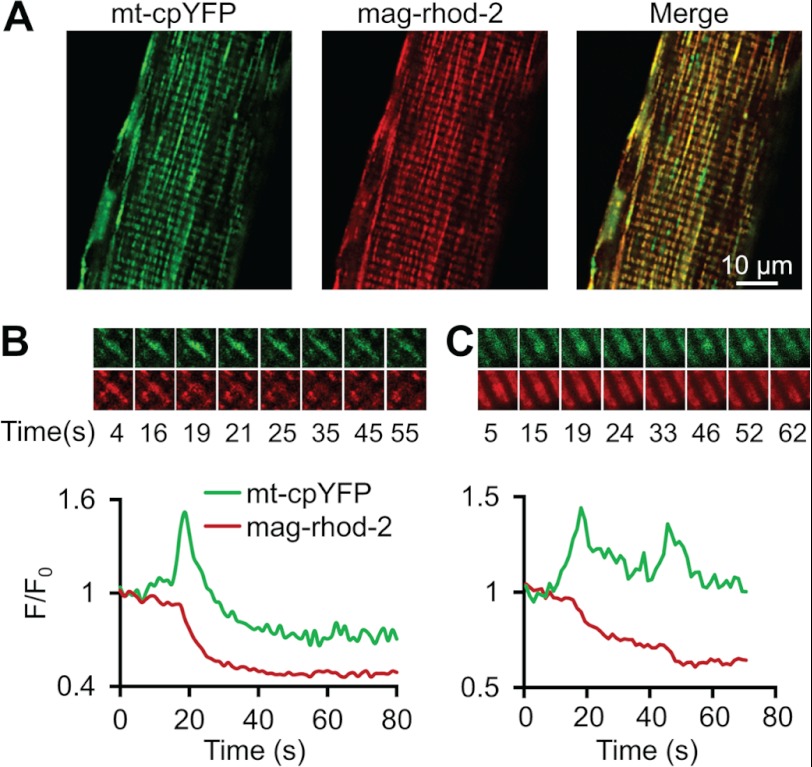
Release of mag-rhod-2 from mitochondria during mt-cpYFP flash events. A, confocal images of a representative FDB fiber obtained from an mt-cpYFP transgenic mouse after loading with mag-rhod-2. Images obtained during 488 nm excitation (mt-cpYFP, left), 543 nm excitation (mag-rhod-2, middle), and after merging the two images (Merge, right) demonstrate co-localization of mt-cpYFP and mitochondria-loaded mag-rhod-2. B and C, two examples of loss of mag-rhod-2 fluorescence during individual mt-cpYFP flash events. A series of raw images of the event (top) and time course (bottom) is shown for each region. A single large sustained decrease of mag-rhod-2 fluorescence was observed after a large amplitude flash (B), and two smaller stepwise decreases in mag-rhod-2 fluorescence were observed during two successive smaller amplitude flash events (C).
Imaging Mitochondrial Superoxide Flashes, pH, ROS, Membrane Potential, and NADH
Confocal imaging for cardiac myocytes was conducted using a Zeiss LSM 510 Meta confocal microscope equipped with a 40×, 1.3 NA oil immersion objective. Intact myocytes were incubated in Krebs-Henseleit buffer solution with 1 mm CaCl2 at room temperature. For flash measurements, dual-excitation imaging of mt-cpYFP was achieved by alternating excitation at 405 and 488 nm and collecting emission at >505 nm. Time-lapse x,y images were acquired at 1024 × 1024 resolution for 100 frames, 1.0 s/frame.
To monitor mitochondrial pH during flash events, carboxy-SNARF-1 (5 μm, Invitrogen) was loaded into cells at 4 °C for 30 min following a 2-h washout at room temperature to maximize mitochondrial localization. To simultaneously image mt-cpYFP and SNARF-1, we used dual-wavelength excitation at 405 nm (for mt-cpYFP) and 488 nm (for mt-cpYFP and SNARF-1) or 488 nm (for mt-cpYFP) and 543 nm (for SNARF-1). SNARF-1 fluorescence was collected at 545–595 nm (S1) and >615 nm (S2) for both 488 nm and 543 nm excitations. mt-cpYFP fluorescence was collected at 505–545 nm. SNARF-1 fluorescence was first normalized to base line (ΔF/F0), and then the S2/S1 ratio was calculated.
To monitor mitochondrial superoxide during flashes, MitoSOX Red (5 μm, Invitrogen) was loaded into mt-cpYFP-expressing cardiac myocytes at 37 °C for 10 min followed by washing two times. Tri-wavelength imaging of MitoSOX and mt-cpYFP was performed by tandem excitation at 405, 488, and 543 nm, and emission was collected at >560, 505–545, and >560 nm, respectively. In a subset of experiments, when only MitoSOX was loaded, dual-wavelength imaging was done by tandem excitation at 405 and 514 nm, and emission was collected at >560 nm. To avoid saturation by excessive oxidation, all MitoSOX experiments were completed within 30 min of loading.
To monitor mitochondrial membrane potential during flashes, TMRM (20 nm) was loaded into cells at room temperature for 20 min. Tri-wavelength excitation imaging of mt-cpYFP and TMRM was done by tandem excitation at 405, 488, and 543 nm, and emission was collected at 505–545, 505–545, and 560–657 nm, respectively.
A Zeiss LSM 510 META confocal/2-photon microscope with a 40×, 1.3 NA oil immersion objective was used to monitor intrinsic mitochondrial NADH autofluorescence during flash activity. Dual-excitation imaging was achieved by alternating excitation at 488 nm (confocal) and 720 nm (2-photon), and emission was collected at 500–550 nm and 390–465 nm, respectively.
Superoxide flash detection in skeletal muscle fibers isolated from transgenic mice was performed using a Nikon Eclipse C1 Plus confocal microscope (Nikon Instruments, Melville, NY) equipped with a Super Fluor 40× (1.3 NA) oil immersion objective. mt-cpYFP-expressing fibers were co-loaded with either TMRE (20 nm for 10 min at room temperature) to monitor mitochondrial membrane potential or Mag-Rhod-2 (4 μm for 45 min at 4 °C followed by a 2-h washout at 37 °C) to evaluate the opening of a large pore channel during flash activity. mt-cpYFP and TMRE/Mag-Rhod-2 were excited using 488 and 543 nm lasers and detected at 515/30 nm and 605/75 nm emission, respectively. Time-lapse x,y images were acquired at 512 × 512 resolution for 100 frames, 1.24 s/frame.
Muscle fibers isolated from transfected FDB (see Fig. 3D) were visualized using a Leica TCS SP5 acousto-optical beam splitter laser scanning confocal microscope (PLATIM of IFR128 BioSciences Gerland – Lyon Sud, France) equipped with a 40× oil immersion objective (1.4 NA). The excitation was provided by a diode laser (405 nm) and an argon laser (491 nm), and emission was collected at 500–550 nm.
Isolated mitochondria from adult skeletal muscle (see above) were immobilized on Cell-Tak-coated coverslips and imaged in skeletal muscle respiration buffer solution supplemented with the appropriate mitochondrial substrate (see Fig. 2). Flash activity was monitored using a Nikon Eclipse C1 Plus confocal microscope equipped with a Super Fluor 40× (1.3 NA) oil immersion objective. To simultaneously measure mitochondrial pH during flash activity, isolated mitochondria were loaded with carboxy-SNARF-1 (5 μm in Pluronic F-127 for 30 min at room temperature). mt-cpYFP and SNARF-1 were excited by 488 and 543 nm laser, with emission detected at 530 nm (for mt-cpYFP) and 670/20 nm (for SNARF-1), respectively. Time-lapse x,y image stacks were obtained using the same confocal settings as those for FDB fibers.
FIGURE 2.
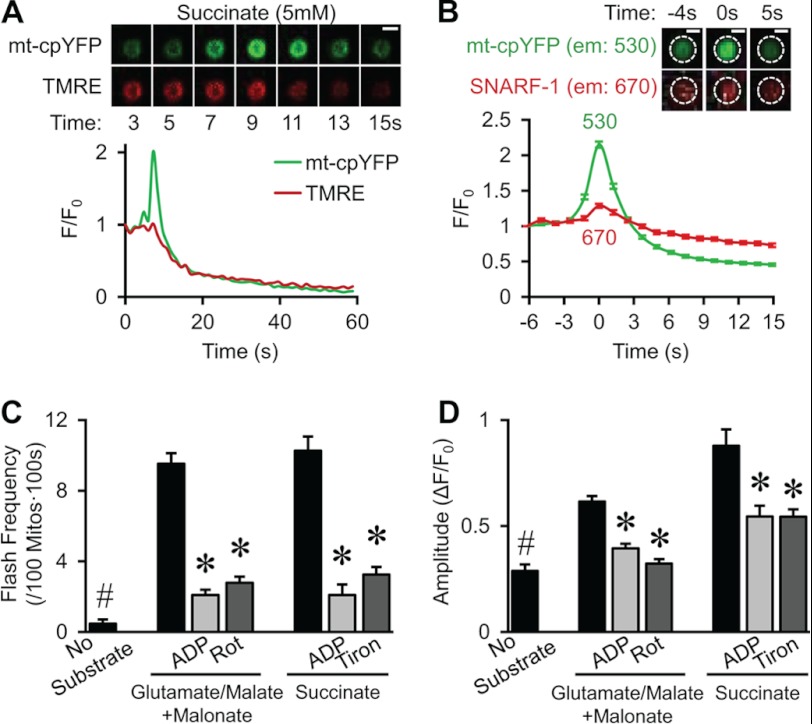
Modest matrix alkalinization during mt-cpYFP flash events in single mitochondria purified from skeletal muscle. A, top, time-lapse confocal images from a representative isolated mitochondrion showing the kinetics of an mt-cpYFP flash and simultaneous membrane potential depolarization (TMRE fluorescence) in the presence of 5 mm succinate. Bottom, corresponding flash time course for the event shown in the top panel. B, average (± S.E.) amplitude of mt-cpYFP (585/30 nm) and SNARF-1 (670/20 nm) fluorescence during flash events in purified mitochondria isolated from mt-cpYFP transgenic mice and loaded with SNARF-1. The two traces represent the average of 360 individual flashes in which the time of the peak of each flash was arbitrarily set as time 0. Only a modest increase in SNARF-1 fluorescence was observed during the peak of mt-cpYFP flash activity. Inset, mt-cpYFP (530 nm, top) and SNARF-1 (670 nm, bottom) fluorescence images obtained at the indicated times are shown for a representative mitochondrion. Dotted circles indicate mitochondrial area. Scale bars = 1 μm. C and D, averaged (± S.E.) frequency (C) and amplitude (D) of superoxide flashes in the presence of Complex I substrates (10 mm glutamate and 5 mm malate plus 100 μm malonate) or Complex II substrate (5 mm succinate), in the absence and presence of 2 mm ADP, 10 μm rotenone (Rot), or 1 mm Tiron. A marked reduction of flash frequency was observed during state 3 respiration and in the presence of either ETC blockade or superoxide scavenging. n = 14–18 experiments. *, p < 0.05 versus control with each substrate. #, p < 0.01 versus control in the presence of substrates. 100 Mitos·100s, 100 mitochondria × 100 s.
Image Processing, Data Analysis, and Statistics
mt-cpYFP flash properties (frequency, amplitude, duration, spatial width, etc.) were extracted from time-lapse image stacks using customized automated detection software programs (1, 4). Statistical analyses were performed using Microsoft Excel and SigmaPlot software suites. Student's t tests were used for single comparison, and one-way analysis of variance analysis followed by post hoc test were used for multiple comparisons. p < 0.05 was considered as statistically significant.
RESULTS
mt-cpYFP Flash Activity Is Coincident with Only Modest Matrix Alkalinization
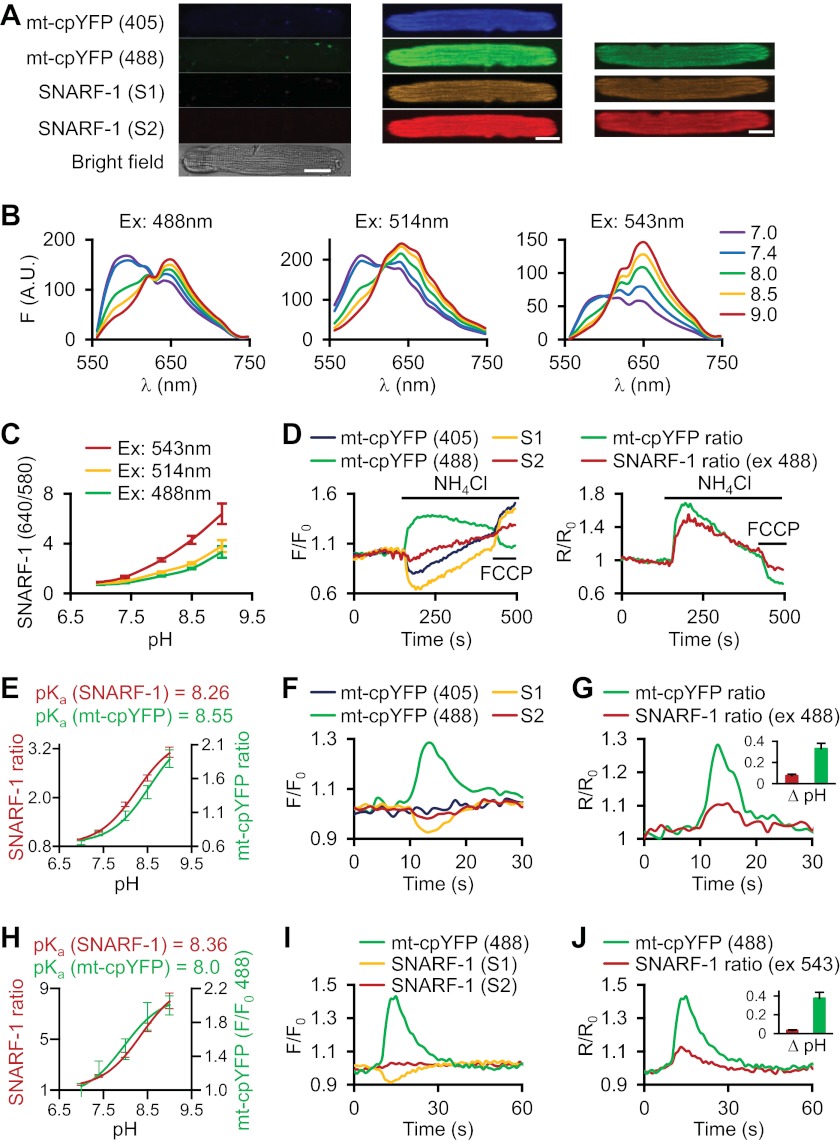
Previous observations in cardiac myocytes (1), skeletal muscle fibers (2), and Arabidopsis mitochondria (8) showed that during mt-cpYFP flashes, the fluorescence emission at a pH-sensitive excitation wavelength remain unchanged, suggesting that any pH change during an mt-cpYFP flash is minimal. To more precisely quantify the potential pH component of mt-cpYFP flashes, we simultaneously measured mitochondrial pH changes during mt-cpYFP flashes using carboxy-SNARF-1, a ratiometric pH indicator, preferentially loaded into mitochondria of mt-cpYFP-expressing cardiac myocytes. Although minimal background fluorescence was observed in control cardiac myocytes (no mt-cpYFP expression, no SNARF-1 loading, Fig. 1A, left panel), mt-cpYFP-expressing and SNARF-1-loaded myocytes showed a discrete mitochondrial staining at both 405/488 nm (Fig. 1A, middle panel) and 488/543 nm dual excitation (Fig. 1A, right panel). Detailed in-cell pH calibrations and spectral analyses of SNARF-1 using different excitation wavelengths are shown in Fig. 1, B and C, which revealed that all three excitation wavelengths are suitable for monitoring intracellular pH changes. In-cell calibration for mt-cpYFP and SNARF-1 revealed similar pKa values for these two probes (Fig. 1, E and H), validating the suitability of SNARF-1 for simultaneous detection of pH changes during mt-cpYFP flashes. Indeed, a transient matrix alkalinization evoked by 10 mm NH4Cl followed by acidification with 300 nm carbonyl cyanide p-trifluoromethoxyphenylhydrazone induced similar responses in mt-cpYFP (increase at 488 nm excitation, decrease at 405 nm excitation, and increase in 488/405 ratio) and SNARF-1 (decrease at S1 and increase in S2/S1 ratio) (Fig. 1D). In contrast, during the time course of robust mt-cpYFP flash events (peak ΔF/F0 = 0.26 ± 0.004) (Fig. 1, F and G), only a slight increase in SNARF-1 ratio (peak S2/S1 = 0.08 ± 0.001) was observed (Fig. 1G). According to the pH calibration curve (Fig. 1E), this increase in SNARF-1 signal represents a pH increase of ∼0.08 unit (assuming a resting mitochondrial matrix pH = 8.0) (Fig. 1G, inset, red bar). In contrast, if the large increase in mt-cpYFP signal during a flash was solely attributed to a change in pH, the flash would represent a pH increase of 0.33 unit (Fig. 1G, inset, green bar), which is >4 times larger than that quantified by SNARF-1. These results indicate that only a modest alkalinization of the mitochondrial matrix occurs during large amplitude mt-cpYFP flash events. Similar results were observed when SNARF-1 was excited using a 543 nm laser (Fig. 1, H–J).
FIGURE 1.
Single mitochondrial superoxide flash is accompanied by modest matrix alkalinization in adult cardiac myocytes. A, representative confocal images showing quad-wavelength scanning of cardiac myocytes showing low background fluorescence in the absence of any indicators (left), as well as simultaneous imaging of mt-cpYFP and SNARF-1 at 488 nm excitation (middle) and 543 nm excitation (right) in cardiac myocyte expressing mt-cpYFP and with mitochondrial loading of SNARF-1. Note that when using 543 nm for SNARF-1 excitation, 405 nm was not used for mt-cpYFP excitation because the filter setting for tri-wavelength excitation (405, 488, and 543) caused significant signal loss of the S2 channel. S1 and S2: SNARF-1 emissions collected at 545–595 nm (S1) and >615 nm (S2), respectively. B, emission spectra of SNARF-1 at 488 nm (left), 514 nm (middle), or 543 nm (right) excitation for pH calibration in intact myocytes. Traces were averaged from 9–12 cells from four experiments. A.U., arbitrary units. C, pH calibration of SNARF-1 fluorescence at the different excitation wavelengths used in intact adult cardiac myocytes. D, mitochondrial matrix alkalization (10 mm NH4Cl) or acidosis (300 nm carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP)) resulted in similar magnitude changes in SNARF-1 and mt-cpYFP fluorescence. Traces are representative of three experiments. E, pH calibration of mt-cpYFP and SNARF-1 fluorescence in adult cardiac myocytes using 488 nm excitation. n = 25–29 cells from six experiments. F and G, representative traces of superoxide flashes recorded from cardiac myocytes co-stained with SNARF-1 using 488 nm excitation revealed a slight increase in SNARF-1 ratio (G, alkalinization) and decrease in S1 wavelengths (F) during the flash. Traces were averaged from 11 flashes in the same myocyte. G, inset, estimated pH changes using the calibration curve in E at the peak of the flash in G, assuming that the SNARF-1 and mt-cpYFP signals were exclusively due to a change in matrix pH. Note the large difference in estimated pH changes, consistent with the majority of mt-cpYFP fluorescence signal not being due to matrix alkalinization. Similar results were obtained from flashes in 12 cells from five rat hearts. H–J, Same as E–G except using 543 nm excitation for SNARF-1 and 488 nm excitation for mt-cpYFP. Calibration (H) was performed on 19–23 cells from five experiments. Traces were averaged from 23 flashes. Error bars in C, E, G, H, and J indicate ± S.E.
To eliminate potential cytosolic contamination of the SNARF-1 signal, simultaneous mt-cpYFP and SNARF-1 measurements were also performed in purified mitochondria from skeletal muscle of mt-cpYFP transgenic mice (Fig. 2). Similar to flashes observed in intact skeletal muscle cells (2–4), isolated mitochondria exhibited robust spontaneous mt-cpYFP flash activity, in which each event occurs coincident with a depolarization in membrane potential (Fig. 2A). Moreover, flash activity in isolated mitochondria exhibited remarkably similar amplitude and spatiotemporal properties (Fig. 2A, bottom panel) as those observed in intact cells (1–4). Most importantly, only a modest increase in matrix pH (e.g. increase in SNARF-1 far red fluorescence) was observed during the peak of mt-cpYFP flash activity in isolated skeletal muscle mitochondria (Fig. 2B), similar to that observed in intact cardiac myocytes (Fig. 1, G and J). Furthermore, in isolated skeletal muscle mitochondria, average basal flash frequency was very low in the absence of substrate (0.5 ± 0.2 flashes/100 mitochondria × 100 s). The addition of either Complex I substrates glutamate and malate (in the presence of Complex II inhibitor malonate) or Complex II substrate succinate increased flash frequency ∼20 times (Fig. 2C). Subsequent addition of ADP to promote state 3 respiration greatly decreased flash frequency, suggesting that enhanced forward electron flux through the ETC decreased electron slippage for superoxide production. Both flash frequency (Fig. 2C) and amplitude (Fig. 2D) in isolated mitochondria were significantly decreased by either 10 μm rotenone or 1 mm Tiron, whereas flash duration remained unchanged (data not shown). These results are consistent with both the ETC dependence and the superoxide origin of mt-cpYFP flashes.
mt-cpYFP Flash Activity Is Coincident with a Stepwise Increase of MitoSOX Signal and Matrix Oxidation
To confirm a dominant superoxide-dependent component during mt-cpYFP flashes, cardiac myocytes were co-labeled with MitoSOX Red, a mitochondria-targeted superoxide-specific dye. The MitoSOX fluorescence increased significantly along the rising phase of each mt-cpYFP flash (Fig. 3A), and the rate of increase in MitoSOX fluorescence (dF/dt) closely tracked the time course of the corresponding mt-cpYFP flash (Fig. 3, B and C). A similar increase in MitoSOX fluorescence during flash activity was demonstrated previously in skeletal muscle fibers (2). Moreover, the time-dependent increase in MitoSOX fluorescence was significantly larger in skeletal muscle fibers where flashes were abundant (Fig. 3D), consistent with a burst in superoxide production during flash activity. Control experiments verified that MitoSOX fluorescence under these conditions was insensitive to NH4Cl-induced matrix alkalinization as the induced pH changes were outside the MitoSOX pH sensitivity (16), but still sensitive to superoxide produced by menadione (Fig. 3, E and F). Consistent with increased ETC activity and substrate utilization driving mitochondrial superoxide production during a flash, NADH (the primary substrate of the respiratory chain) autofluorescence decreased markedly during flash activity (Fig. 3G). Overall, the results in Figs. 1–3 indicate that although mt-cpYFP is sensitive to changes in matrix pH, the mt-cpYFP flashes primarily reflect a burst in mitochondrial superoxide production, with a small component due to a <0.1 pH unit alkalinization during each event.
Effects of Mitochondrial Matrix Alkalinization on Flash Frequency and Amplitude
Because the driving force for net proton efflux is decreased by matrix alkalinization, we determined the impact of NH4Cl application on mt-cpYFP flash properties. Specifically, if flash activity primarily reflects an increase in mitochondrial pH, then NH4Cl-induced matrix alkalinization should reduce the driving force for proton efflux, and thus, flash amplitude. Application of 10 mm NH4Cl indeed induced a rapid increase in mitochondrial pH (as evidenced by an increase in basal mt-cpYFP 488/405 ratio) in cardiac myocytes (Fig. 4A) and skeletal muscle FDB fibers (Fig. 4D). However, NH4Cl-induced matrix alkalinization had only minimal effects on mt-cpYFP flash properties in cardiac myocytes and FDB fibers. Specifically, NH4Cl-induced matrix alkalinization produced a moderate increase in flash frequency in both cardiac myocytes (Fig. 4B) and skeletal FDB fibers (Fig. 4E), in the absence of a change in flash amplitude (Fig. 4, C and F). The absence of an effect of NH4Cl-induced matrix alkalinization on flash amplitude indicates that net proton efflux is not a major determinant of flash amplitude in these cells.
Bimodal Dependence of Flash Frequency on Inner Mitochondrial Membrane Proton Gradient
Nigericin, a K+/H+ antiporter, can either mildly (nanomolar concentrations) or completely (micromolar concentrations) dissipate the proton gradient (ΔpHm) across the mitochondrial inner membrane (17, 18). Indeed, the addition of nigericin caused a concentration-dependent acidification of the mitochondrial matrix in both cardiac myocytes and FDB fibers (Fig. 5, A and D). In both cell types, low nanomolar concentrations of nigericin (25–100 nm) caused both a mild reduction in ΔpHm (as evidenced by an ∼15–25% decline in basal mt-cpYFP fluorescence; Fig. 5, A and D) and an increase in flash frequency (Fig. 5, B and E). Despite the mild dissipation of ΔpHm by low concentrations of nigericin, flash amplitude was unaltered in both cardiac myocytes and FDB fibers (Fig. 5, C and F). In contrast, high concentrations of nigericin (5–10 μm), which markedly dissipate ΔpHm (>50% decline in basal mt-cpYFP fluorescence, Fig. 5, A and D), almost completely abolished flash activity (Fig. 5, B and E). Apart from a moderate reduction in FDB fibers (Fig. 5F), flash amplitude was unchanged for the few events that remained in the presence of 5 μm nigericin (Fig. 5, C and F). Similar results were obtained in isolated skeletal muscle mitochondria, where flash frequency was also significantly increased by 1 nm nigericin (from 9.2 ± 1.3 to 14.9 ± 1.4 flashes/100 mitochondria × 100 s) and nearly abolished by 5 μm nigericin (0.8 ± 0.2 flashes/100 mitochondria × 100 s).
Diverse Membrane Potential Recovery Pattern during Flash Activity
The occurrence of mitochondrial pH spikes was proposed to represent a mechanism for restoring the mitochondrial proton motive force during a spontaneous fluctuation/flickering of the mitochondrial membrane potential (12). In this case, the time course of a pH spike should precisely mirror the kinetics of the mitochondrial membrane potential (TMRM/TMRE fluorescence). However, although all mt-cpYFP flashes occur coincident with a depolarization of the mitochondrial membrane potential, the time course of the two events are temporally correlated for only a subset of all flashes (Fig. 6, top row). In many other cases, mitochondrial membrane potential depolarization remained sustained during (and even after) flash decay (Fig. 6, middle row). In addition, many similar magnitude mitochondrial depolarizations occur in the complete absence of a flash, indicating that depolarization alone is not sufficient to drive these events (Fig. 6, bottom row). Moreover, the frequency of spontaneous membrane potential fluctuation events is ∼5 times higher than that for superoxide flashes in cardiac myocyte (data not shown) and ∼1.5 times higher in skeletal muscle fibers (2). Lack of a strict temporal correlation between flash and membrane potential events was observed across a wide diversity of preparations including cardiac myocytes (Fig. 6A), HeLa cells (Fig. 6B), skeletal muscle FDB fibers (Fig. 6C), and isolated mitochondria (Fig. 6D).
We previously demonstrated that mt-cpYFP flash events are coincident with a sustained loss of the fluorescence of rhod-2 or a Ca2+-insensitive rhod-2 analog in cardiac myocytes (1, 19). Similar results are also observed during flash activity in FDB fibers in which mitochondria were loaded with mag-rhod-2 (Fig. 7), which exhibits a substantially lower Ca2+ affinity (Kd = 70 μm) than rhod-2. Specifically, mag-rhod-2 was readily loaded into mitochondria as evidenced by co-localization with mt-cpYFP expression (Fig. 7A). A sustained loss in mag-rhod-2 fluorescence was observed during each mt-cpYFP event (Fig. 7, B and C), consistent with the opening of a large pore/channel (see “Discussion”) within the mitochondrial inner membrane that results in a sustained leak of the dye from the mitochondrial matrix rather than being due to a change in mitochondrial Ca2+.
DISCUSSION
In this study, we set out to determine the relative contribution of mitochondrial superoxide and pH to mt-cpYFP flash activity in cardiac myocytes and skeletal muscle FDB fibers. The results are consistent with mt-cpYFP flashes primarily reflecting a burst in production of superoxide anions within the matrix of actively respiring and energized mitochondria, although a minor contribution of matrix alkalinization during each event cannot be ruled out. This conclusion is also supported by the finding that flash activity was accompanied by mitochondrial oxidation, only modestly affected by strong matrix alkalinization, and showed a bimodal dependence on the proton gradient across the inner membrane.
The Superoxide Origin of mt-cpYFP Flashes
As discussed above, evidence that mt-cpYFP flashes reflect a burst in mitochondrial superoxide production is based on both in vitro and in vivo experiments across different cell types (1, 2, 6). Here, we demonstrate that superoxide flashes coincide with a decrease in mitochondrial NADH content (Fig. 3G) and an increase in MitoSOX signal (Fig. 3, A–C), further supporting the notion that mt-cpYFP flash activity reflects superoxide produced as a byproduct of mitochondrial bioenergetics. The proposal that mt-cpYFP flashes in isolated Arabidopsis mitochondria reflect transient events of matrix alkalinization was based largely on the observation that global mt-cpYFP fluorescence increased upon the addition of mitochondrial substrates in the absence of ADP (state 2) and that this increase was not modified by conditions expected to increase (menadione) or inhibit (Tiron, TEMPOL, and MnTMPyP) mitochondrial superoxide production (8). Although these results are consistent with a pH-mediated increase in mt-cpYFP fluorescence during mitochondrial energization, this does not necessarily indicate that transient bursts in mt-cpYFP fluorescence (flashes) reflect an increase in matrix pH, not only because mt-cpYFP is responsive to superoxide, but also because of ample evidence demonstrating strong oxidative and antioxidant regulation of flash frequency (1, 2, 4, 6). In addition, an increase in global mt-cpYFP fluorescence may be mechanistically different from the stochastic and transient bursts in mt-cpYFP fluorescence (flashes) observed in energized mitochondria. Here, we demonstrate that isolated mitochondria from skeletal muscle exhibit flashes when energized with glutamate and malate or succinate. Moreover, the frequency of these events is sensitive to ADP, mitochondrial ETC inhibition, and superoxide scavenging (Fig. 2C). Thus, mt-cpYFP flash activity in purified mitochondria also reflects ETC-dependent superoxide generation, consistent with previous observations in intact cardiac myocytes and skeletal muscle fibers (1, 2, 4, 6).
Matrix Alkalinization Is Not a Major Component of an mt-cpYFP Flash
Using the mitochondria-targeted pH-sensitive probe, mito-SypHer, Santo-Domingo and Demaurex (11, 12) and Azarias and Chatton (10) reported spontaneous oscillations in mitochondrial pH (termed pH spikes) in intact cells, with properties similar, but not identical, to those reported for mitochondrial superoxide flashes. A recent study from Schwarzländer et al. (20) used mt-cpYFP as a mitochondrial pH probe. The authors detected reciprocal time courses for mt-cpYFP spikes and mitochondrial membrane potential changes in isolated Arabidopsis mitochondria. The authors concluded that mitochondrial pH spikes reflected the same phenomenon as membrane potential pulsing. However, results in Fig. 3 demonstrate that a burst in superoxide occurs during an mt-cpYFP flash such that the majority of the mt-cpYFP signal during a flash is not the result of a change in matrix pH. Further, several lines of evidence from this study indicate that alkalinization cannot fully account for the fluorescence increase during mt-cpYFP flashes.
First of all, even during large amplitude flash events, mt-cpYFP probe fluorescence at the pH-sensitive 405-nm excitation wavelength (Fig. 1D) does not change (Fig. 1F) (1). The absence of a detectable change in mt-cpYFP fluorescence at 405 nm excitation suggests that any change in probe fluorescence during a flash due to matrix alkalinization must be very modest.
Second, measurement and calibration of changes in mitochondrial matrix pH with carboxy-SNARF-1 during a flash provided direct evidence for a modest pH alkalinization to the increase in mt-cpYFP fluorescence observed during a flash. Specifically, despite a similar pKa (Fig. 1, E and H) and pH-dependent response magnitudes for mt-cpYFP and carboxy-SNARF-1 (Fig. 1D, right panel), only a modest increase in carboxy-SNARF-1 ratio was observed during robust mt-cpYFP flashes in both cardiac myocytes (Fig. 1, G and J) and purified skeletal muscle mitochondria (Fig. 2B). Taken together, these findings indicate that the majority of the increase in mt-cpYFP signal during a flash results from an increase in superoxide production, with only a minor contribution due to modest (<0.1 pH unit) matrix alkalinization.
Third, strong NH4Cl-induced matrix alkalinization did not dramatically alter superoxide flash frequency, or more importantly, flash amplitude in cardiac myocytes and FDB fibers. If mt-cpYFP flashes primarily reflect pH fluctuations, then an increase in matrix pH should reduce the driving force for proton efflux, and thus, also reduce flash amplitude. Indeed, Santa-Domingo and Demaurex (11, 12) reported that NH4Cl-induced matrix alkalinization reduced pH alkalinization transient amplitude monitored with mito-SypHer by 70%. In contrast, flash amplitude remained unchanged in cardiac myocytes and FDB fibers following strong matrix alkalinization (Fig. 4, C and F). Currently, it is not clear whether mito-SypHer is sensitive to superoxide (21).
Fourth, the time course of pH spikes reported by Santa-Domingo and Demaurex (11, 12) and Azarias and Chatton (10) was precisely reciprocal to the time course of “dips” in mitochondrial membrane potential. Therefore, the authors proposed that pH spikes are produced in response to a spontaneous membrane potential flicker (22–24), and thus, are designed to restore the proton motive force by pumping protons to the intermembrane space (12). However, the recovery of the mitochondrial membrane potential observed during mt-cpYFP flashes does not always mirror the time course of flash decay (Fig. 6). These findings are in marked contrast to the property of pH spikes and argue against mt-cpYFP flash activity strictly reflecting a corrective response to spontaneous mitochondrial membrane potential flickers.
Fifth, the frequency of multiple, repetitive membrane potential pulses and mt-cpYFP transients in isolated mitochondria reported by Schwarzländer et al. (20) is much higher than that observed for flashes in cardiac myocytes and FDB fibers, where the incidence of two events originating from the same mitochondrion during the time course monitored (∼2 min) is extremely low (1–2% in cardiac myocytes, 3.6 ± 0.4% in skeletal muscle fibers, and 8.1 ± 0.9% in isolated skeletal muscle mitochondria). This discrepancy may be due to mt-cpYFP events in Arabidopsis mitochondria being fundamentally different from what is observed in mammalian mitochondria. Alternatively, the events observed in Arabidopsis mitochondria, as well as the pH spikes observed by Santa-Domingo and Demaurex (11, 12) and Azarias and Chatton (10), may reflect the subset of flash activity exhibiting precisely reciprocal flash and TMRE time courses observed in mitochondria in intact mammalian cells (Fig. 6, top row).
Finally, mt-cpYFP flash events are coincident with the opening of a large pore/channel within the inner mitochondrial membrane, which is not observed during pH spikes that do not exhibit a sustained loss of mitochondrial rhod-2 fluorescence (10). Our previous studies suggested that this large pore/channel is most likely the mitochondrial permeability transition pore (mPTP) because flash activity coincides with both mitochondrial depolarization and the irreversible loss of large organic molecules (Fig. 7) (1, 2, 19). As brief openings of the mPTP may both stimulate ETC activity by depolarizing mitochondria and also alter the structure and efficacy of the ETC components due to mitochondria swelling (1, 5), the ETC may become less efficient (e.g. dislocation of cytochrome c), albeit with increased activity, thus allowing for a greater degree of electron slippage to transiently increase superoxide production. This suggests that the opening of a large pore/channel is specifically related to a burst in superoxide production rather than more general pH fluctuations. Our recent study showed that basal ROS production serves as a critical trigger for superoxide flashes (25). A small increase in basal ROS may open the mPTP, causing solute exchange and mitochondria swelling that further enhance ETC-dependent superoxide production. We describe this process as spontaneous “mitochondrion-contained ROS-induced ROS release,” as opposed to evoked cell-wide ROS-induced ROS release induced by photo-stimulation (26, 27). Over time, depolarization of mitochondrial membrane will dramatically reduce electron slippage from Complex I (28), and thus, reduce the superoxide production, providing an intrinsic mechanism for flash termination (29).
It is also worth noting that given the negative mitochondrial membrane potential and high matrix pH, the opening of a nonspecific large pore/channel like the mPTP should permit significant proton influx, and thus acidification, rather than mild alkalinization, of the mitochondrial matrix. This paradox could be resolved by very brief openings of the pore sufficient to depolarize the mitochondrial membrane potential but cause limited proton influx, while also stimulating the ETC to pump out an even greater amount of protons, resulting in a net proton efflux reflected as a modest matrix alkalinization.
Azarias and Chatton (10) recently reported mitochondrial alkalinization transients coincident with mitochondrial Na+ spikes (increase in CoroNa Red fluorescence), as well as both mitochondrial depolarizations (decrease in TMRE fluorescence) and bursts in superoxide production (increase in MitoSOX fluorescence) (10). These results are consistent with the hypothesis that an increase in Na+ influx down its electrochemical gradient depolarizes the mitochondrial membrane potential, which stimulates a burst in ETC activity that increases both proton efflux across the inner membrane to alkalinize the matrix and electron leak to produce superoxide. Further investigations of the precise relationship between pH spikes and superoxide generation will help to clarify the role of the multiple processes that occur during a flash.
Superoxide Flash Activity Depends on a Mitochondrial Proton Gradient
A key result used to suggest that mt-cpYFP flashes reflect a pH spike was the observation that mt-cpYFP flash activity is abolished by selective collapse of the mitochondrial pH gradient with nigericin (8). However, mt-cpYFP flashes in that study (8) were obtained in the presence of 20 μm rotenone, a potent Complex I inhibitor that markedly reduces flash activity in both intact cells (1, 4) and energized mitochondria (Fig. 2C). Thus, the relevance of these events to those reported with intact Complex I functionality is unclear. Nevertheless, we confirmed that 5 μm nigericin nearly abolished flash activity in intact cardiac myocytes, FDB fibers, and isolated mitochondria even in the absence of rotenone. However, we also uncovered a bimodal regulation of mt-YFP flash by nigericin, activation at low concentration, and inhibition at high concentration, which cannot be readily reconciled with the pH spike hypothesis. Our interpretation for the nigericin results is that superoxide flash production requires a significant proton gradient. Indeed, ROS generation from Complex I depends strongly on the presence of the proton gradient across the mitochondrial inner membrane (18, 30). Thus, a small reduction in pH gradient could promote activity of the respiratory chain to enhance proton pumping across the inner membrane needed to restore the pH gradient and proton motive force, which at the same time leads to hyperpolarization of mitochondrial membrane potential. Both the enhanced proton pumping and membrane hyperpolarization can promote ETC-dependent superoxide production and trigger flash activity. Together, these results demonstrate that flash activity depends strongly on the pH gradient across the mitochondrial inner membrane, and thus, is consistent with superoxide production during a flash originating primarily from Complex I.
In summary, this study confirms that mt-cpYFP flashes are tightly linked to superoxide production in cardiac myocytes, skeletal muscle fibers, and isolated mitochondria. The results demonstrate that the mt-cpYFP flash signal primarily reflects a burst of superoxide production, with a potential minor component due to a modest concurrent alkalinization of the mitochondrial matrix. Although the precise mechanism responsible for the burst in superoxide production remains to be fully elucidated, this study directly addresses an ongoing controversy regarding the relationship between mitochondrial superoxide flash activity and pH spikes and provides evidence differentiating the respective contributions of these two events during a flash. Further studies are needed regarding the integrative regulation of pH transients and bursts in superoxide production within single mitochondria. In addition, the precise relationship between superoxide flash activity and spontaneous fluctuations in mitochondrial membrane potential and large pore opening also remain to be fully clarified.
This work was supported, in whole or in part, by National Institutes of Health Grants AR44657 (to R. T. D.), HL114760 (to W. W.), and HL093671 (to S. S. S.). This work was also supported by a Scientist Development Grant from the American Heart Association (to W. W.) and grants from the Université Lyon 1 and the CNRS (to S. P.), the Academia Dei Lincea Fund (to L. W. L.), and National Basic Research Program of China 2013CB531201 and 2011CB809102 and the National Science Foundation of China 31221002, 31130067 (to H. C).
- mSOF
- mitochondrial superoxide flashes
- mt-cpYFP
- mitochondria-targeted circularly permutated yellow fluorescent protein
- ETC
- electron transport chain
- FDB
- flexor digitorum brevis
- ROS
- reactive oxygen species
- TMRM
- tetramethylrhodamine, methyl ester
- TMRE
- tetramethylrhodamine, ethyl ester
- mPTP
- mitochondrial permeability transition pore
- Tiron
- Sodium 4,5-dihydroxy-1,3-benzenedisulfonate.
REFERENCES
- 1. Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Wang W., Mattson M. P., Kao J. P., Lakatta E. G., Sheu S. S., Ouyang K., Chen J., Dirksen R. T., Cheng H. (2008) Superoxide flashes in single mitochondria. Cell 134, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pouvreau S. (2010) Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PLoS One 5, e13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang H., Chen M., Ding Y., Shang W., Xu J., Zhang X., Zhang W., Li K., Xiao Y., Gao F., Shang S., Li J.-C., Tian X.-L., Wang S.-Q., Zhou J., Weisleder N., Ma J., Ouyang K., Chen J., Wang X., Zheng M., Wang W., Zhang X., Cheng H. (2011) Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 21, 1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei L., Salahura G., Boncompagni S., Kasischke K. A., Protasi F., Sheu S. S., Dirksen R. T. (2011) Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J. 25, 3068–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Q., Fang H., Shang W., Liu L., Xu Z., Ye T., Wang X., Zheng M., Chen Q., Cheng H. (2011) Superoxide flashes, early mitochondrial signals for oxidative stress-induced apoptosis. J. Biol. Chem. 286, 27573–27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang Z., Zhang W., Fang H., Zheng M., Wang X., Xu J., Cheng H., Gong G., Wang W., Dirksen R. T., Sheu S. S. (2011) Response to “A critical evaluation of cpYFP as a probe for superoxide”. Free Radic. Biol. Med. 51, 1937–1940 [DOI] [PubMed] [Google Scholar]
- 7. Hou Y., Ouyang X., Wan R., Cheng H., Mattson M. P., Cheng A. (2012) Mitochondrial superoxide production negatively regulates neural progenitor proliferation and cerebral cortical development. Stem Cells 30, 2535–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarzländer M., Logan D. C., Fricker M. D., Sweetlove L. (2011) The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide 'flashes'. Biochem. J. 437, 381–387 [DOI] [PubMed] [Google Scholar]
- 9. Schwarzländer M., Murphy M. P., Duchen M. R., Logan D. C., Fricker M. D., Halestrap A. P., Müller F. L., Rizzuto R., Dick T. P., Meyer A. J., Sweetlove L. J. (2012) Mitochondrial 'flashes': a radical concept repHined. Trends Cell Biol. 22, 503–508 [DOI] [PubMed] [Google Scholar]
- 10. Azarias G., Chatton J. Y. (2011) Selective ion changes during spontaneous mitochondrial transients in intact astrocytes. PLoS One 6, e28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santo-Domingo J., Demaurex N. (2011) Alkalinization transients in individual mitochondria. J. Gen. Physiol. 138, 16a [Google Scholar]
- 12. Santo-Domingo J., Demaurex N. (2012) Perspectives on: SGP symposium on mitochondrial physiology and medicine: the renaissance of mitochondrial pH. J. Gen. Physiol. 139, 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poburko D., Santo-Domingo J., Demaurex N. (2011) Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 286, 11672–11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skolnick R. L., Litwin S. E., Barry W. H., Spitzer K. W. (1998) Effect of ANG II on pHi, [Ca2+]i, and contraction in rabbit ventricular myocytes from infarcted hearts. Am. J. Physiol. 275, H1788–H1797 [DOI] [PubMed] [Google Scholar]
- 15. Jacquemond V. (1997) Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophys. J. 73, 920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robinson K. M., Janes M. S., Pehar M., Monette J. S., Ross M. F., Hagen T. M., Murphy M. P., Beckman J. S. (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 103, 15038–15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selivanov V. A., Zeak J. A., Roca J., Cascante M., Trucco M., Votyakova T. V. (2008) The role of external and matrix pH in mitochondrial reactive oxygen species generation. J. Biol. Chem. 283, 29292–29300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambert A. J., Buckingham J. A., Boysen H. M., Brand M. D. (2010) Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia. Aging Cell 9, 78–91 [DOI] [PubMed] [Google Scholar]
- 19. Wang X., Jian C., Zhang X., Huang Z., Xu J., Hou T., Shang W., Ding Y., Zhang W., Ouyang M., Wang Y., Yang Z., Zheng M., Cheng H. (2012) Superoxide flashes: Elemental events of mitochondrial ROS signaling in the heart. J. Mol. Cell Cardiol. 52, 940–948 [DOI] [PubMed] [Google Scholar]
- 20. Schwarzländer M., Logan D. C., Johnston I. G., Jones N. S., Meyer A. J., Fricker M. D., Sweetlove L. J. (2012) Pulsing of membrane potential in individual mitochondria: a stress-induced mechanism to regulate respiratory bioenergetics in Arabidopsis. Plant Cell 24, 1188–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quatresous E., Legrand C., Pouvreau S. (2012) Mitochondria-targeted cpYFP: pH or superoxide sensor? J. Gen. Physiol. 140, 567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buckman J. F., Reynolds I. J. (2001) Spontaneous changes in mitochondrial membrane potential in cultured neurons. J. Neurosci. 21, 5054–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duchen M. R., Leyssens A., Crompton M. (1998) Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J. Cell Biol. 142, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hüser J., Rechenmacher C. E., Blatter L. A. (1998) Imaging the permeability pore transition in single mitochondria. Biophys. J. 74, 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou T., Zhang X., Xu J., Jian C., Huang Z., Ye T., Hu K., Zheng M., Gao F., Wang X., Cheng H. (2013) Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal ROS elevation. J. Biol. Chem. 288, 4602–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zorov D. B., Filburn C. R., Klotz L. O., Zweier J. L., Sollott S. J. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aon M. A., Cortassa S., Marbán E., O'Rourke B. (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278, 44735–44744 [DOI] [PubMed] [Google Scholar]
- 28. Miwa S., Brand M. D. (2003) Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 31, 1300–1301 [DOI] [PubMed] [Google Scholar]
- 29. Wei L., Dirksen R. T. (2012) Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J. Gen. Physiol. 139, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lambert A. J., Brand M. D. (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382, 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Life Technologies Corp (2013) The Molecular Probes Handbook, Chapter 20, Section 20.2, Grand Island, NY [Google Scholar]