Background: The biological function of a former orphan receptor, GPR84, has not been clarified yet.
Results: GPR84 activation results in chemotaxis and cytokine production by the stimulation of potential ligands and the surrogate agonist.
Conclusion: GPR84 works as a proinflammatory receptor in myeloid cells.
Significance: This study provides new insights into the function of GPR84.
Keywords: Fatty acid, G protein coupled receptors (GPCR), Inflammation, Leukocyte, Pharmacology
Abstract
G protein-coupled receptor 84 (GPR84) is a putative receptor for medium-chain fatty acids (MCFAs), whose pathophysiological roles have not yet been clarified. Here, we show that GPR84 was activated by MCFAs with the hydroxyl group at the 2- or 3-position more effectively than nonhydroxylated MCFAs. We also identified a surrogate agonist, 6-n-octylaminouracil (6-OAU), for GPR84. These potential ligands and the surrogate agonist, 6-OAU, stimulated [35S]GTP binding and accumulated phosphoinositides in a GPR84-dependent manner. The surrogate agonist, 6-OAU, internalized GPR84-EGFP from the cell surface. Both the potential ligands and 6-OAU elicited chemotaxis of human polymorphonuclear leukocytes (PMNs) and macrophages and amplified LPS-stimulated production of the proinflammatory cytokine IL-8 from PMNs and TNFα from macrophages. Furthermore, the intravenous injection of 6-OAU raised the blood CXCL1 level in rats, and the inoculation of 6-OAU into the rat air pouch accumulated PMNs and macrophages in the site. Our results indicate a proinflammatory role of GPR84, suggesting that the receptor may be a novel target to treat chronic low grade inflammation associated-disease.
Introduction
G protein-coupled receptors (GPCRs)2 consist of a family of cell surface receptors that sense various extracellular stimuli, including light, odorants, peptides, nucleotides, neurotransmitters, and hormones (1). In the past decade, several GPCRs have been reported to be activated by free fatty acids (FFAs). These FFA-sensing GPCRs are proposed to play important roles in a variety of physiological situations (2). For example, GPR40 is a receptor for medium-chain fatty acids (MCFAs) and long-chain fatty acids (LCFAs) and enhances glucose-stimulated insulin secretion from pancreatic β-cells (3, 4). GPR119 is activated by LCFAs, leading to enhanced glucose-dependent insulin secretion from pancreatic β-cells increasing the release of a gut peptide, glucagon-like peptide 1 from intestinal neuroendocrine L-cells (5). GPR120, a receptor for MCFAs and LCFAs, is responsible for FFA-induced glucagon-like peptide 1 secretion from L-cells (6) and also works in macrophages and adipocytes, mediating potent anti-inflammatory and insulin-sensitizing effects (7). It is reported that the dysfunction of GPR120 leads to obesity in both mice and humans (8). GPR41 is primarily expressed in adipose tissue (9). Stimulation of short-chain fatty acids (SCFAs) results in leptin production in adipocytes (10). GPR43 is also activated by SCFAs, and adipocytes treated with the natural ligands exhibit a reduction in lipolytic activity (11, 12). Both GPR41 and GPR43 are expressed in polymorphonuclear leukocytes (PMNs) as well (9, 13), to which SCFAs elicit chemotaxis (13).
GPR84 was originally discovered by expressed sequence tag data mining (14) and cloned from a cDNA library prepared from human peripheral blood neutrophils (15). No close homologs of GPR84 could be identified. Its expression was mainly observed in bone marrow, lung, and peripheral blood leukocytes (15). GPR84 is now considered to be a member of FFA-sensing GPCRs. MCFAs with carbon chain lengths of 9–14 activate GPR84, coupling primarily to a pertussis toxin (PTX)-sensitive Gi/o pathway (16). To date, there are only a few published studies with respect to the function of this receptor. GPR84 activation in RAW264.7 cells, a murine macrophage-like cell line, amplifies LPS-stimulated IL-12 p40 production (16). GPR84 KO mice increased Th2 cytokine production (17). Nevertheless, physiological roles of GPR84 are still largely unknown.
We have screened an in-house bioactive molecule library and chemical library to test the abilities of these molecules to activate GPR84. In this study, we have identified 2- or 3-hydoxy MCFAs as potential ligands as well as conventional MCFAs and also discovered a surrogate agonist compound for GPR84. Furthermore, we have shown that GPR84 functions as a proinflammatory receptor both in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Materials
F12 Nutrient Mixture, RPMI 1640, DMEM, pcDNA3.1, and Bac-to-Bac baculovirus expression system were purchased from Invitrogen. DMEM without l-glutamine and inositol was purchased from ICN Biomedicals. All FFAs, phorbol 12-myristate 13-acetate (PMA), BSA, LPS, siRNAs for human GPR84, and a negative control siRNA were purchased from Sigma-Aldrich. PTX was purchased from Calbiochem. Casein sodium from milk was purchased from Tokyo Kasei (Tokyo, Japan). Ficoll-Paque PLUS was purchased from Amersham Biosciences. Human TNFα Quantikine ELISA kit, human IL-8 Quantikine ELISA kit, rat TNF-α Quantikine ELISA kit, and rat CXCL1/CINC-1 Quantikine ELISA kit were purchased from R&D Systems. LEADseeker Beads were purchased from GE Healthcare. pEGFP-N1 was purchased from Clontech. cAMP dynamic 2 was purchased from Cisbio. [35S]GTPγS and myo-[3H]inositol were purchased from GE Healthcare. pCEP-Gqi5-HA was purchased from Molecular Devices. Flow-count Fluorospheres were purchased from Beckman Coulter. Amaxa cell line Nucleofector kit C was purchased from Lonza (Cologne, Germany). GPR84 agonist, 6-n-octylaminouracil (6-OAU), was synthesized at Okayama University or in-house at Daiichi Sankyo Co. Ltd. (Tokyo, Japan).
Cloning and Cell Culture
The human GPR84 gene was cloned by PCR from human-derived cDNA. A sequence-confirmed cDNA was inserted into the mammalian expression vector pcDNA3.1(+). CHO-K1 cells were maintained in F12 nutrient mixture containing 10% FBS. CHO-GPR84 stable cell lines were generated by transfecting CHO-K1 cells with human GPR84 and subsequently selected in the presence of 400 μg/ml Geneticin. HEK293 cells were maintained in DMEM medium containing 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin. HEK293-GPR84-EGFP stable cell lines were generated by transfecting HEK293 cells with C-terminal EGFP-tagged human GPR84 in pEGFP-N1 and were subsequently cloned in 400 μg/ml Geneticin. Human leukemic monocyte lymphoma cell lines, U937 cells, were cultured in RPMI 1640 medium containing 10% FBS (R10F). All cells were cultured at 37 °C with 5% CO2.
GTPγS Binding Assay for High Throughput Screening (HTS)
Human GPR84 and bovine Gαi1 genes were fused in tandem in pFastBac1, and the fusion protein was expressed in Sf9 insect cells using the Bac-to-Bac baculovirus expression system. The membrane expressing GPR84-Gαi1 fusion protein was used for the agonist screening in 384 plates. For the agonist screening assay, Sf9 membrane fraction, [35S]GTPγS, and cold 5 μm GDP were incubated with test compounds at room temperature for 1 h. The incorporated [35S]GTPγS was measured by LEADseeker (GE Healthcare).
GTPγS Binding Assay
To confirm the results of HTS assay, a 96-well scale [35S]GTPγS binding assay was conducted at the same condition as HTS. The reactions were terminated by vacuum filtration through UniFilters-96 GF/B (PerkinElmer Life Sciences), and the filter plates were dried overnight. The retained radioactivity was quantified on a liquid scintillation counter, TopCount (Packard).
Phosphoinositide Accumulation Assay (PI Assay)
Human GPR84 and human GPR40 expression vectors constructed in pcDNA3.1(+) were transfected into HEK293 cells with (for GPR84) or without (for GPR40) pCEP-Gqi5-HA. The transfected cells were labeled overnight with myo-[3H]inositol in DMEM without l-glutamine and inositol. The labeling medium was then replaced to assay medium (DMEM without l-glutamine and inositol, plus 20 mm LiCl) including various concentrations of test compounds for 2 h. The radioactivity was measured with TopCount (Packard).
Internalization Assay
HEK293-GPR84-EGFP cell lines were seeded in 96-well plates, and the cells were treated with different concentrations of 6-OAU at 37 °C for 30 min. Fluorescence images were taken on a Nikon Eclipse TE300 microscope with an IN Cell Analyzer 1000 (GE Healthcare).
Preparation of PMNs
Human peripheral PMNs were obtained from venous blood of healthy volunteers in accordance with the ethical approval guidelines of the Daiichi Sankyo Co. Ltd. Ethics Board. Blood was prediluted in 1.2% (w/v) dextran T-250 in saline and sedimented at room temperature for 90 min. Then, PMNs were separated by density gradient centrifugation using a Ficoll-Paque Plus. Cells were suspended at 2 × 106 cells/ml in RPMI medium containing 0.2% FBS. The purity was >90% checked by flow cytometer analysis and >95% by nucleus morphology with Diff-Quick staining. Cell viability was >95% as judged by trypan blue exclusion.
PMNs Chemotaxis Assay
PMNs were plated onto 5-μm Transwell permeable supports (Corning; 2 × 105 cells/Transwell) in the absence or presence of the indicated concentration of FFAs or the surrogate agonist, 6-OAU in the lower chamber. Transwell permeable supports were incubated for 1 h at 37 °C in a humidified CO2 incubator; the number of cells that migrated through the Transwell was determined by manual counting or using flow cytometer (Cytomics FC500, Beckman Coulter)-based cell counting with flow-count Fluorospheres.
Chemotaxis Assay for CHO-GPR84 and U937 Cells
U937 cells were differentiated into macrophage-like cells after culturing for 2 days in the presence of PMA (10 ng/ml) to up-regulate GPR84 gene expression before the experiments. To inactivate Gαi protein, U937 cells were preincubated with PTX (5 ng/ml) for one night before the chemotaxis assay. Chemotaxis was examined by measuring the migration of CHO-GPR84 or U937 cells through a porous membrane (8.0-μm pore size, NeuroProbe Inc.), in response to various FFAs and 6-OAU, in a 96-well chemotaxis chamber (AB96, Ieda Trading). In the lower chamber (volume of 30.5 μl), we placed F12 medium with 0.1% BSA supplemented with the indicated concentration of FFAs or 6-OAU. Controls without any ligands were prepared in the same manner. Then, 200 μl of CHO-GPR84 (4 × 105 cells/ml) or U937 cells (1 × 106 cells/ml) suspended in F12 medium containing 0.1% BSA were added on the top of the filter for each well. The chambers were incubated for 4 h at 37 °C in a humidified incubator. The cells on the upper layer of the membrane were removed with a cotton swab and washed with PBS, and those that migrated, adhering to the lower side of the filter, were fixed with methanol and stained with Diff-Quick; optical density was measured using a microplate reader (Rainbow Thermo, TECAN Japan). The chemotaxis activities were expressed as a migration index: (A595 of experimental well − A595 of background well)/(A595 of medium control well − A595 of background well).
Cytokine Production Assay
PMA-differentiated U937 cells were suspended in R10F at a density of 2 × 104 cells/well in a 96-well plate and incubated for 1 h before stimulation. U937 cells were stimulated with 100 ng/ml LPS in the presence of various concentrations of 3-hydroxy lauric acid (3-OH-C12) or 6-OAU. For the control experiment, 0.025% DMSO/methanol (7:3) mixture in R10F was used. After the stimulation, cells were cultured for 16 h. TNFα in conditioned medium was quantified using sandwich ELISA kits according to the protocol provided by the manufacturer. In knockdown experiments, U937 cells were transfected using Amaxa cell line Nucleofector kit C with 30 nm predesigned siRNA duplexes against GPR84 and a negative control siRNA before PMA treatment. Three different siRNA duplexes against GPR84 were used in combination for nucleofection. The sequences for GPR84 were as follows: for duplex 1, 5′-GCCAAUUCCGCCAAGCAUA-3′ (sense) and 5′-UAUGCUUGGCGGAAUUGGC-3′ (antisense); for duplex 2, 5′-CCAAUUCCGCCAAGCAUAU-3′ (sense) and 5′-AUAUGCUUGGCGGAAUUGG-3′ (antisense); for duplex 3, 5′-CAGAAGAGCUCCGGAUUCU-3′ (sense) and 5′-AGAAUCCGGAGCUCUUCUG-3′ (antisense).
Human peripheral PMNs were suspended in R10F, plated at a density of 3.5 × 105 cells/well in a 96-well plate, and incubated for 1 h before stimulation. PMNs were stimulated with 100 ng/ml LPS in the presence of various concentrations of 3-OH-C12 or 6-OAU. For the control experiment, 0.025% DMSO/methanol (7:3) mixture in R10F was used. Cells were cultured for 4 h. IL-8 in conditioned medium was quantified using sandwich ELISA kits according to the protocol provided by the manufacturer.
Animal Experiments
Female Lewis rats were purchased from Charles River Laboratories (Tokyo, Japan). Four-week-old rats were used for the experiments. A GPR84 agonist, 6-OAU (1 mg/ml), was dissolved in sterile PBS containing 1% rat serum and injected into the jugular vein of rats. Control groups received sterile PBS containing 1% rat serum into the jugular vein instead of injections of the stimulus. Blood was collected 3 h after the 6-OAU injection. Serum cytokine concentrations were quantified using sandwich ELISA kits according to the protocol provided by the manufacturer.
Induction of rat skin air pouches was performed according to the method described by Rodrigues et al. (18). Seven ml of sterile air (using 0.22-μm filters) were insufflated into the subcutaneous tissue of the back trunk of rats under anesthesia 1 day before the experiments. Negative controls received 1 ml of vehicle (sterile saline containing 0.3% BSA). Positive controls received 1 ml of 6-OAU in vehicle (1 mg/ml) through the same route. Four hours after the inoculation of the stimulus, the animals were euthanized by carbon dioxide asphyxiation, and exudate in the air pouch was collected by washing the cavity with 1 ml of sterile PBS. The suspension was centrifuged at 400 × g for 5 min at 4 °C. Cells were counted by the Sysmex XT-2000i automated hematology analyzer. All animal experimental procedures were performed in accordance with the in-house guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
Statistical Analysis
Results are expressed as means ± S.D. Statistical analysis was conducted by Student's t test or parametric Dunnett's test for in vitro experiments and by Wilcoxon test or nonparametric Dunnett's test for in vivo experiments.
RESULTS
Potential Ligands for GPR84
Using a [35S]GTPγS binding assay that can detect Gαi pathway activation, we found that 2-hydroxy capric acid (2-OH-C10), 3-hydroxy capric acid (3-OH-C10), 2-hydroxy lauric acid (2-OH-C12), and 3-hydroxy lauric acid (3-OH-C12) activated GPR84 in a dose-dependent manner with a medium effective concentration (EC50) of 31, 230, 9.9, and 13 μm, respectively (Fig. 1). Lauric acid (C12) did not activate GPR84 in our [35S]GTPγS binding assay. To confirm the receptor activation by hydroxy-MCFAs, we tested the activity of various MCFAs with PI assay co-transfected with Gqi5 to detect Gαi signaling as intracellular PI accumulation, resulting in the same results (Fig. 2). These data proved that MCFAs with carbon chains, especially from 10 to 12, and their 2-OH or 3-OH forms activate GPR84 effectively as well as conventional MCFAs.
FIGURE 1.
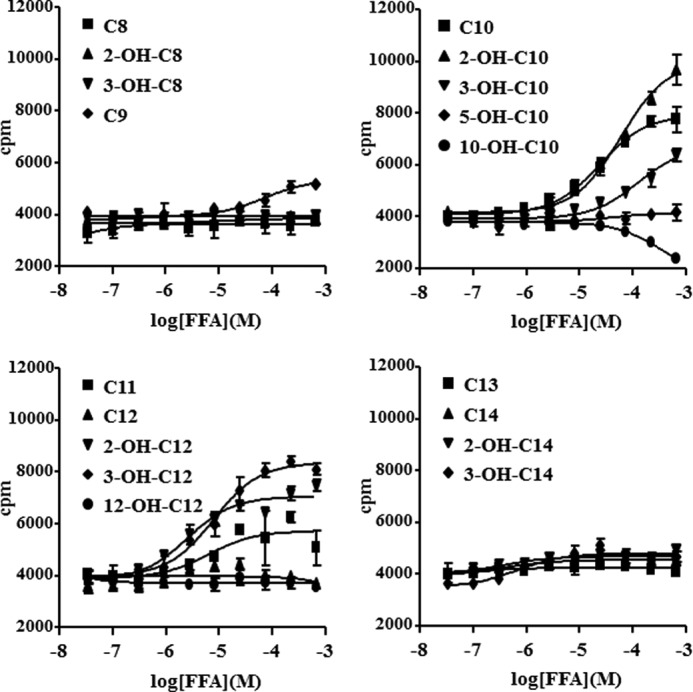
Potential ligands for GPR84. Membranes from Sf9 cells expressing human GPR84-fused with bovine Gαi protein were incubated with various FFAs for 1 h at room temperature. The data represent the means ± S.D. for quadruple determinations.
FIGURE 2.
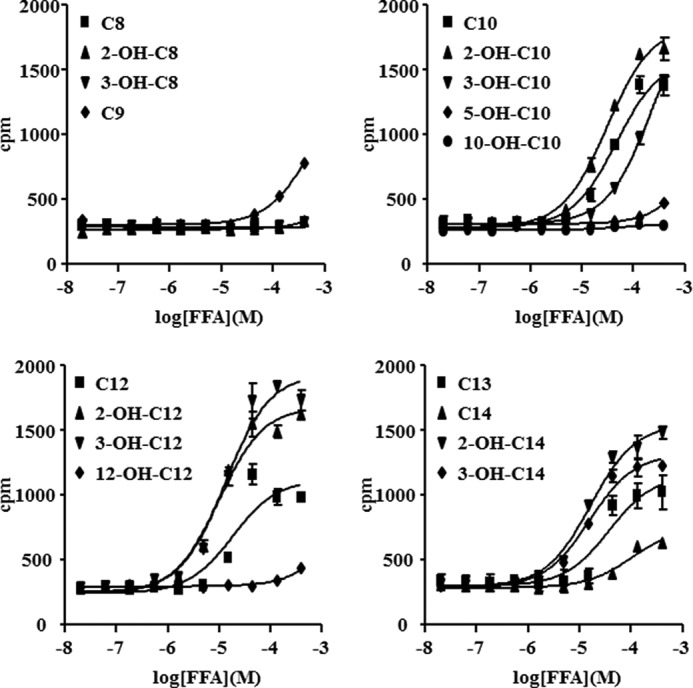
Effect of FFAs in PI assay. HEK293 cells were transfected with human GPR84 or control vector, pcDNA3.1, in the presence of Gqi5 plasmid. Cells were incubated with FFAs for 2 h at 37 °C. The data represent the means ± S.D. for triplicate determinations.
Surrogate Agonist for GPR84
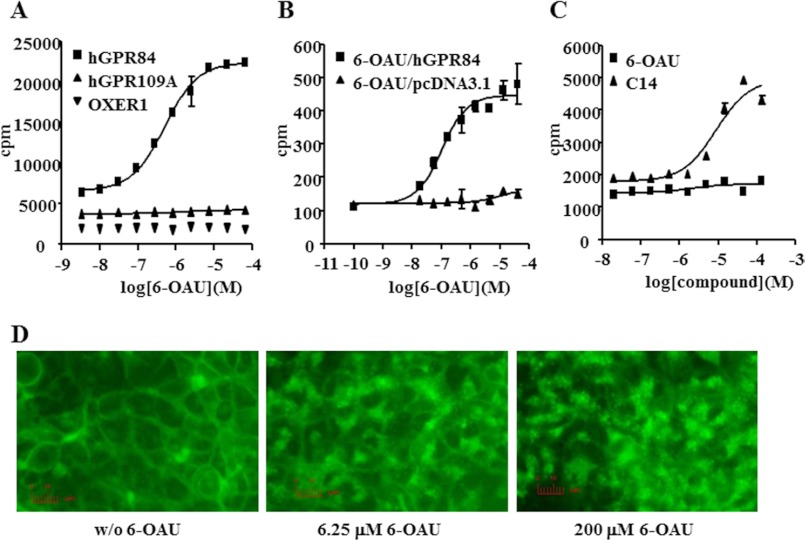
To determine a surrogate agonist for GPR84, we conducted a 384-well scale HTS for the in-house chemical library using a [35S]GTPγS binding assay and obtained some agonistic compounds. Among them, 6-OAU was confirmed to have the highest agonistic activity in the secondary 96-well scale [35S]GTPγS binding assay. 6-OAU increased [35S]GTPγS incorporated in Sf9 cell membranes expressing human GPR84-Gαi fusion protein with an EC50 of 512 nm (Fig. 3A). Other Gαi-coupled GPCR-Gαi fusions, human GPR109A and OXER1, did not respond to 6-OAU at all (Fig. 3A). 6-OAU activated human GPR84 in the presence of Gqi5 chimera in HEK293 cells with an EC50 of 105 nm in the PI assay (Fig. 3B). Although myristic acid (C14) activated GPR40, 6-OAU did not activate human GPR40 in HEK293 cells (Fig. 3C) in the PI assay. HEK293 cells stably expressing C-terminally EGFP-tagged human GPR84 (HEK293-GPR84-EGFP) were generated. Fluorescence of HEK293-GPR84-EGFP cells revealed prominent plasma membrane localization of GPR84 protein, and the internalization of GPR84 was observed from 6.25 μm 6-OAU stimulation (Fig. 3D). Stimulation with 6.25 μm 6-OAU began to induce GPR84-EGFP internalization, and the extensive internalization was observed at 200 μm 6-OAU stimulation (Fig. 3D). We conducted radio-ligand binding assays against 69 receptors, channels, or transporters to determine whether 6-OAU inhibits the specific binding or activity to their ligand or substrate. No more than 50% inhibition was observed for any receptors, channels, or transporters by the addition of 6-OAU at 10 μm (data not shown).
FIGURE 3.
Identification and characterization of a surrogate agonist for GPR84. A, effect of 6-OAU on the [35S]GTPγS binding assay. Membranes from Sf9 cells expressing human GPR84 (hGPR84), human GPR109A (hGPR109A), or OXER1 fused with bovine Gαi protein were incubated with 6-OAU for 1 h at room temperature. The data represent the means ± S.D. for quadruple determinations. B, effect of 6-OAU on GPR84 in the PI assay. HEK293 cells were transfected with human GPR84 or control vector, pcDNA3.1, in the presence of Gqi5 plasmid. Cells were incubated with various concentrations of 6-OAU or 3-OH-C12 for 2 h at 37 °C. The data represent the means ± S.D. for triplicate determinations. C, effect of 6-OAU on GPR40 in the PI assay. HEK293 cells were transfected with human GPR40 or control vector, pcDNA3.1. Cells were incubated with various concentrations of 6-OAU or C14 for 2 h at 37 °C. The data represent the means ± S.D. for triplicate determinations. D, receptor internalization assay. Internalization of stably expressing human GPR84-EGFP fusion protein in HEK293 cells was observed by fluorescence microscopy. Cells were treated without or with 0, 6.25, or 200 μm 6-OAU for 30 min.
Cellular Functions of GPR84
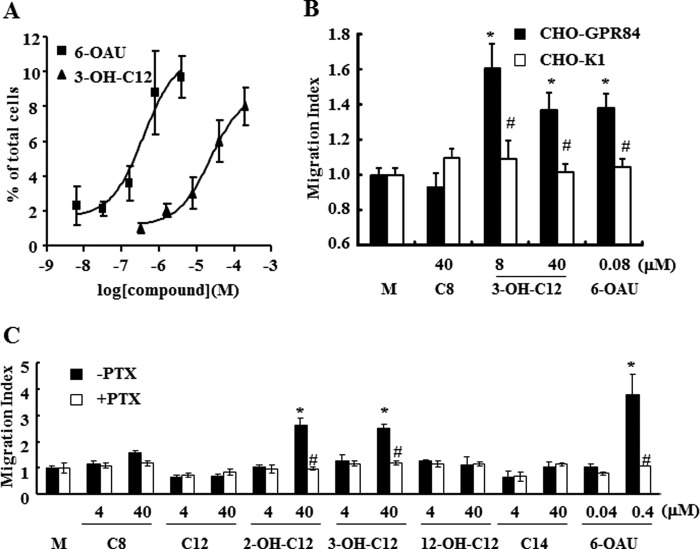
Expression analysis by quantitative PCR revealed that high levels of GPR84 gene expression were detected in human PMNs and macrophages. Additionally, GPR84 expression was increased in macrophages upon LPS stimulation (data not shown). These data suggest that GPR84 may play a role in PMNs and macrophages and may be involved in inflammatory conditions. We used agonistic MCFAs or a surrogate agonist, 6-OAU, to investigate the effect of GPR84 activation on chemotaxis and cytokine secretion for these GPR84-expressing cells. In a Transwell assay, 3-OH-C12 and the surrogate agonist, 6-OAU, provoked chemotaxis of PMNs prepared from human peripheral blood in a concentration-dependent manner with an EC50 of 24.2 μm and 318 nm, respectively (Fig. 4A). In a modified Boyden chamber assay, 3-OH-C12 and 6-OAU but not medium alone and caprylic acid (C8) gave rise to chemotaxis on CHO-GPR84 in contrast to the parental CHO-K1, which did not respond to these agonists, meaning that the chemotactic action is mediated by GPR84 activation (Fig. 4B). Also, 6-OAU (400 nm), 2-OH-C12 (40 μm), and 3-OH-C12 (40 μm) brought about chemotaxis on PMA-treated macrophage-like U937 cells. The inactive MCFAs (C8, C12, 12-hydroxy lauric acid (12-OH-C12), and myristic acid (C14)) in the [35S]GTPγS binding assay exhibited no chemotactic activity (Fig. 4C). The agonist-induced chemotaxis was blocked completely by preincubating cells with PTX, a specific inhibitor of Gi/o proteins (Fig. 4C). In addition, 3-OH-C12 and 6-OAU increased the secretion of IL-8 from LPS-stimulated PMNs (Fig. 5A), suggesting that those effects are mediated through GPR84 activation. In the absence of LPS, IL-8 production was less than one-tenth when compared with the result in the presence of LPS, although the minimal increased IL-8 production was observed (Fig. 5A). TNFα production from U937 macrophages was also amplified by 6-OAU and 3-OH-C12 stimulation in the presence of LPS (Fig. 5B). No effect on TNFα production was observed in the absence of LPS. The introduction of GPR84 siRNAs in U937 resulted in abrogation of the TNFα amplification effects caused by 3-OH-C12 and 6-OAU stimulation (Fig. 5B). Taken together, our results indicate that GPR84 could be involved in making an inflammatory condition through inducing chemotaxis and amplifying LPS-stimulated inflammatory cytokine production in PMNs and macrophages.
FIGURE 4.
Cellular functions of human GPR84. A, chemotaxis of human peripheral PMNs. PMNs isolated from human peripheral blood were applied onto the upper chamber of a Transwell permeable support. Various concentrations of 6-OAU or 3-OH-C12 were added in the lower chambers of the Transwell. Transwell permeable supports were incubated at 37 °C for 1 h. The chemotaxis activities were assessed as a percentage of migrated cells to a lower chamber across the membrane from an upper chamber. The data were expressed as the means ± S.D. of quadruple determinations. B, chemotaxis of human GPR84 stable transfectant (CHO-GPR84). CHO-GPR84 was generated as described under “Experimental Procedures.” CHO-GPR84 cells were applied onto the upper chambers of the 96-well chemotaxis chamber. Various concentrations of 6-OAU or FFAs were added into the lower chambers. The chemotaxis activities were expressed as a migration index: (A595 of experimental well − A595 of background well)/(A595 of medium control well − A595 of background well). The data were expressed as means ± S.D.; n = 5. C, chemotaxis of U937 cell lines. U937 cells were used for the experiment after differentiation into macrophage-like cells as described under “Experimental Procedures.” Differentiated U937 cells were applied onto the upper chambers of the 96-well chemotaxis chamber. Various concentrations of 6-OAU or FFAs were added into the lower chambers. The chemotaxis activities were expressed as the same as B. The data were expressed as the means ± S.D. of triplicate determinations. *, p < 0.001 when compared with the medium control group (M). #p < 0.001 when compared with the PTX-untreated group.
FIGURE 5.
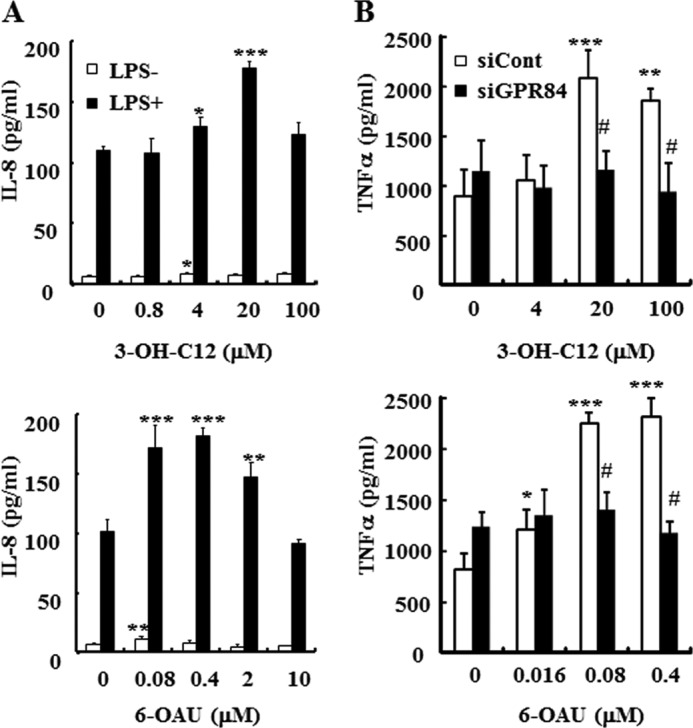
Effects of 6-OAU or 3-OH-C12 on cytokine production. A, cytokine production from human peripheral PMNs. PMNs isolated from human peripheral blood were stimulated by LPS in the presence or absence of 6-OAU or 3-OH-C12. Culture supernatant was collected 4 h after stimulation. IL-8 concentration in the supernatant was measured with a commercial ELISA kit. The data were expressed as the means ± S.D. of triplicate determinations. *, p < 0.05, **, p < 0.01, and ***, p < 0.001 when compared with the agonist untreated group. B, negative control siRNA or GPR84 siRNA-transfected U937 macrophages were stimulated by 100 ng/ml LPS in the presence or absence of 6-OAU or 3-OH-C12. Culture supernatant was collected 16 h after stimulation. TNFα concentration in the supernatant was measured with a commercial ELISA kit. The data were expressed as the means ± S.D. of triplicate determinations. *, p < 0.05, **, p < 0.01, and ***, p < 0.001 when compared with the agonist untreated group. #, p < 0.01 when compared with the negative control siRNA-transfected group.
Pharmacological Effects of 6-OAU on Rats in Vivo
As some of the functions of GPR84 emerged from the in vitro experiments, we studied whether 6-OAU exerts any inflammatory effects in vivo in rats. Injection of 6-OAU suspension in 1% rat serum into the rat jugular vein (10 mg/kg) leads to the elevation of a chemokine, CXCL1 concentration in the serum peaking at 3 h after the injection (Fig. 6A). Other cytokines tested (IL-1β, IL-2, IL-4, IL-6, IL-10, IL-22, TNFα, and IFNγ) and soluble ICAM-1 were not detected in this experiment (data not shown). In the rat air pouch model, 6-OAU at 1 mg/ml dissolved in 0.3% BSA attracted both PMNs and macrophages into the air pouch, peaking at 4 h after 6-OAU inoculation (Fig. 6B). These results strongly indicate that GPR84 could also be involved in making an inflammatory condition through chemokine production and chemotaxis in vivo.
FIGURE 6.
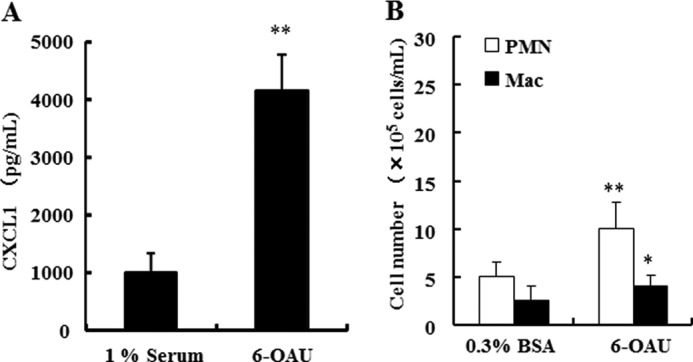
Proinflammatory effects of 6-OAU in vivo. A, 6-OAU (10 mg/kg) suspended in 1% rat serum or 1% rat serum as control was injected into the jugular vein in rats. Blood was collected from the jugular vein 3 h after 6-OAU injection. CXCL1 concentration in the serum was measured with a commercial ELISA kit. The data were expressed as the means ± S.D.; n = 5. **, p < 0.01 when compared with the 1% rat serum-injection group. B, 6-OAU suspended in 0.3% BSA or 0.3% BSA alone as control was injected into the dorsal air pouch prepared on rats. Infiltrated cells into the air pouch were collected with PBS 4 h after 6-OAU inoculation. The cell number was counted with the XT-2000i. The data were expressed as the means ± S.D.; n = 6. **, p < 0.01 and *, p < 0.05 when compared with the 0.3% BSA injection group.
DISCUSSION
We have shown that hydroxy-MCFAs, 2-OH-C10, 3-OH-C10, 2-OH-C12, and 3-OH-C12, work as potential ligands for GPR84 as well as the ligands previously reported: capric acid (C10), undecanoic acid (C11), and lauric acid (C12) (16). Although C12 activated GPR84 in the PI assay, this had no reaction with the [35S]GTPγS binding assay. C12 may need to be converted to 2-OH-C12 or 3-OH-C12 in the presence of cells. 2-Hydroxy-FAs are present in several tissues, especially in the brain, and are formed by fatty acid 2-hydroxylase and degraded by α-oxidation (19). 3-Hydroxy-FAs are formed during β-oxidation in peroxisomes. Its plasma concentration was reported to reach micromolar order in mitochondrial fatty acid β-oxidation disorders (20, 21). 3-Hydroxy-FAs are also found as a major component of the lipid A moiety of LPS (22). Considering the existence of hydroxy-carboxylic acid receptors, GPR81 (23), GPR109A (24), and GPR109B (25), which are the receptors for 2-hydroxy-propanoate, 3-hydroxy-butyrate, and 3-hydroxy-octanoate respectively, it is likely that GPR84 works as a sensor to both MCFAs and hydroxy-MCFAs on macrophages and PMNs.
A potent surrogate agonist can be instrumental in characterizing functionally unknown or less known GPCRs. Therefore, we subsequently screened the in-house chemical library with the [35S]GTPγS binding assay and obtained an agonistic compound, 6-OAU (26), which was originally synthesized for a completely different purpose than this study. 6-OAU also activated human GPR84 in the presence of Gqi5 chimeric G proteins in the PI assay. Furthermore, EGFP-labeled human GPR84 internalization was observed in a GPR84-dependent manner. All these data confirm that 6-OAU activates GPR84. Takeda et al. (27) reported diindolylmethane as a surrogate agonist for GPR84. Although diindolylmethane was also a hit in our HTS, 6-OAU activated GPR84 more potently than diindolylmethane (data not shown).
The specific and abundant expression of GPR84 in activated macrophages and PMNs in our study and others (16, 28) suggests the role of its ligands in modulating leukocyte functions, providing a potential involvement of the MCFA-GPR84 system to the proinflammatory state. As to the function of GPR84, Venkataraman et al. (17) reported that GPR84-deficient T cells exhibited increased production of Th2 cytokines, although it remains to be determined how GPR84 deficiency on macrophages and PMNs alters the cytokine production from T cells in a GPR84-deficient mice. In our preliminary experiments using GPR84-knock-out mice, we observed no difference between knock-out mice and WT mice with respect to Th2 cytokine production from T cells (data not shown). On the other hand, hydroxy-MCFAs and 6-OAU exert biological effects on human PMNs and macrophages including chemotaxis induction and cytokine production in this study. The chemotaxis of U937 macrophages suggests that GPR84 activation by its ligands is coupled to a PTX-sensitive Gi/o pathway as reported previously (16). Several groups have reported that saturated FAs directly activate either Toll-like receptor (TLR) 2-dependent or TLR4-dependent signaling in cultured macrophages and transfected cells (29, 30). As both 6-OAU and 3-OH-C12 produced a very small amount of IL-8 from PMNs and TNFα from U937 macrophages in the absence of LPS, the amplification effects on the cytokine production are not assumed to be mediated by TLR4 directly. Indeed, 6-OAU did not activate TLR2- or TLR4-dependent NF-κB-luciferase reporter (data not shown). Rather, as LPS stimulation to macrophages up-regulates GPR84 expression, this may allow the cells to easily respond to the ligands. Up-regulation of GPR84 expression by LPS stimulation in microglia and macrophages was also reported by others (28, 31). Then, to examine whether 6-OAU exerts any biological effects in vivo, we performed animal experiments. Unfortunately, because either 3-OH-C12 or 6-OAU had no or little effect on peritoneal PMNs prepared from mice, we used rats for the animal experiments. 6-OAU injection into the rat jugular vein led to the elevation of CXCL1, which was reported to have strong chemotactic potency on the migration of macrophages and neutrophils (32) and to be a major chemokine produced by LPS-stimulated rat macrophages (33). Taking these studies into consideration, they suggest that 6-OAU has the ability to stimulate macrophage and to induce CXCL1 production in vivo. Moreover, 6-OAU injection into the rat dorsal air pouch led to the accumulation of PMNs and macrophages in the site. Based on all of the above data, we suggest that the agonist for GPR84 works as a proinflammatory mediator in vivo.
Soydan et al. (34) found that 3-OH-C12 increased the amount of IL-6, one of proinflammatory cytokines, released by human blood cells in vitro. However, they were not able to detect 3-OH-C12 in the plasma of patients with metabolic syndrome, cancer, and rheumatoid arthritis (34). It remains to be determined whether hydroxy-MCFAs can be detected in tissues with inflammation. In the past, it has been difficult to relate the concentration of MCFAs required to activate the receptor to concentrations found under physiological or pathophysiological conditions. Therefore, MCFAs and hydroxy-MCFAs are still just potential ligands but not the bona fide “endogenous ligands.” However, it might be possible that MCFAs including the hydroxylated forms are liberated from tissues that are especially rich in fat because FFAs are released from adipocytes by TNFα-induced lipolysis (35). Considering that all other FFA-sensing receptors and hydroxy-carboxylic acid receptors work to regulate metabolic and/or inflammatory conditions (36–38), it is likely that the MCFAs-GPR84 system can be a new player for chronic low grade inflammation in adipose tissue, affecting this metabolic disorder. An increased number of M1 macrophages in adipose tissue has appeared to be a key factor in metabolic inflammation (39). Neutrophils are also observed to be infiltrated in adipose tissue and may have a role in the initiating event(s) of the inflammatory cascade (40). Nagasaki et al. (41) recently reported that high fat diet-fed mice up-regulated GPR84 expression in adipose tissue. TNFα-treated 3T3-L1 adipocytes remarkably enhanced GPR84 expression, and MCFAs down-regulated adiponectin mRNA expression. Altogether, a certain extent of communication between leukocytes and adipocytes via MCFA in adipose tissue is supposed to exist and may contribute to the state of chronic low grade inflammation.
In summary, we have identified hydroxy-MCFAs as potential ligands and have identified a potent surrogate agonist, 6-OAU. We have shown that the activation of GPR84 amplifies LPS-stimulated IL-8 production in PMNs and TNFα production in macrophages, and GPR84 agonists work as chemoattractants to both PMNs and macrophages in both in vitro and in vivo experiments. GPR84 should be a proinflammatory receptor and may be a novel, attractive target for treating chronic low grade inflammation associated-disease.
Acknowledgment
We thank Dr. Tomohisa Nagamatsu at Okayama University for providing 6-OAU.
Footnotes
- GPCR
- G protein-coupled receptor
- FA
- fatty acid
- MCFA
- medium-chain fatty acid
- LCFA
- long-chain fatty acid
- SCFA
- short-chain fatty acid
- PTX
- pertussis toxin
- PMA
- phorbol 12-myristate 13-acetate
- 6-OAU
- 6-n-octylaminouracil
- HTS
- high throughput screening
- PI
- phosphoinositide
- PMN
- polymorphonuclear leukocyte
- R10F
- RPMI 1640 medium containing 10% FBS
- 2-OH-C10
- 2-hydroxy capric acid
- 3-OH-C10
- 3-hydroxy capric acid
- 2-OH-C12
- 2-hydroxy lauric acid
- 3-OH-C12
- 3-hydroxy lauric acid
- TLR
- Toll-like receptor
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- DMSO
- dimethyl sulfoxide
- EGFP
- enhanced green fluorescent protein.
REFERENCES
- 1. Wellendorph P., Johansen L. D., Bräuner-Osborne H. (2009) Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol. Pharmacol. 76, 453–465 [DOI] [PubMed] [Google Scholar]
- 2. Blad C. C., Tang C., Offermanns S. (2012) G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 11, 603–619 [DOI] [PubMed] [Google Scholar]
- 3. Briscoe C. P., Tadayyon M., Andrews J. L., Benson W. G., Chambers J. K., Eilert M. M., Ellis C., Elshourbagy N. A., Goetz A. S., Minnick D. T., Murdock P. R., Sauls H. R., Jr., Shabon U., Spinage L. D., Strum J. C., Szekeres P. G., Tan K. B., Way J. M., Ignar D. M., Wilson S., Muir A. I. (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 [DOI] [PubMed] [Google Scholar]
- 4. Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., Tanaka H., Maruyama M., Satoh R., Okubo S., Kizawa H., Komatsu H., Matsumura F., Noguchi Y., Shinohara T., Hinuma S., Fujisawa Y., Fujino M. (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422, 173–176 [DOI] [PubMed] [Google Scholar]
- 5. Overton H. A., Babbs A. J., Doel S. M., Fyfe M. C., Gardner L. S., Griffin G., Jackson H. C., Procter M. J., Rasamison C. M., Tang-Christensen M., Widdowson P. S., Williams G. M., Reynet C. (2006) Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3, 167–175 [DOI] [PubMed] [Google Scholar]
- 6. Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11, 90–94 [DOI] [PubMed] [Google Scholar]
- 7. Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., Choquet H., Besnard P., Lecoeur C., Vivequin S., Ayukawa K., Takeuchi M., Ozawa K., Tauber M., Maffeis C., Morandi A., Buzzetti R., Elliott P., Pouta A., Jarvelin M. R., Körner A., Kiess W., Pigeyre M., Caiazzo R., Van Hul W., Van Gaal L., Horber F., Balkau B., Lévy-Marchal C., Rouskas K., Kouvatsi A., Hebebrand J., Hinney A., Scherag A., Pattou F., Meyre D., Koshimizu T. A., Wolowczuk I., Tsujimoto G., Froguel P. (2012) Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354 [DOI] [PubMed] [Google Scholar]
- 9. Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., Dowell S. J. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 10. Xiong Y., Miyamoto N., Shibata K., Valasek M. A., Motoike T., Kedzierski R. M., Yanagisawa M. (2004) Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. U.S.A. 101, 1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J. L., Tian H., Li Y. (2008) Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 [DOI] [PubMed] [Google Scholar]
- 12. Hong Y. H., Nishimura Y., Hishikawa D., Tsuzuki H., Miyahara H., Gotoh C., Choi K. C., Feng D. D., Chen C., Lee H. G., Katoh K., Roh S. G., Sasaki S. (2005) Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 [DOI] [PubMed] [Google Scholar]
- 13. Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 [DOI] [PubMed] [Google Scholar]
- 14. Wittenberger T., Schaller H. C., Hellebrand S. (2001) An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J. Mol. Biol. 307, 799–813 [DOI] [PubMed] [Google Scholar]
- 15. Yousefi S., Cooper P. R., Potter S. L., Mueck B., Jarai G. (2001) Cloning and expression analysis of a novel G-protein-coupled receptor selectively expressed on granulocytes. J. Leukoc. Biol. 69, 1045–1052 [PubMed] [Google Scholar]
- 16. Wang J., Wu X., Simonavicius N., Tian H., Ling L. (2006) Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. 281, 34457–34464 [DOI] [PubMed] [Google Scholar]
- 17. Venkataraman C., Kuo F. (2005) The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immunol. Lett. 101, 144–153 [DOI] [PubMed] [Google Scholar]
- 18. Rodrigues H. G., Vinolo M. A., Magdalon J., Fujiwara H., Cavalcanti D. M., Farsky S. H., Calder P. C., Hatanaka E., Curi R. (2010) Dietary free oleic and linoleic acid enhances neutrophil function and modulates the inflammatory response in rats. Lipids 45, 809–819 [DOI] [PubMed] [Google Scholar]
- 19. Guo L., Zhang X., Zhou D., Okunade A. L., Su X. (2012) Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J. Lipid Res. 53, 1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costa C. G., Dorland L., Holwerda U., de Almeida I. T., Poll-The B. T., Jakobs C., Duran M. (1998) Simultaneous analysis of plasma free fatty acids and their 3-hydroxy analogs in fatty acid β-oxidation disorders. Clin. Chem. 44, 463–471 [PubMed] [Google Scholar]
- 21. Jones P. M., Bennett M. J. (2011) Clinical applications of 3-hydroxy fatty acid analysis by gas chromatography-mass spectrometry. Biochim. Biophys. Acta 1811, 657–662 [DOI] [PubMed] [Google Scholar]
- 22. Parlesak A., Bode C. (1995) Lipopolysaccharide determination by reversed-phase high-performance liquid chromatography after fluorescence labeling. J. Chromatogr. A 711, 277–288 [DOI] [PubMed] [Google Scholar]
- 23. Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J., Sutton S. W., Li X., Yun S. J., Mirzadegan T., Mazur C., Kamme F., Lovenberg T. W. (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 284, 2811–2822 [DOI] [PubMed] [Google Scholar]
- 24. Taggart A. K., Kero J., Gan X., Cai T. Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T. J., Jin L., Liaw C., Chen R., Richman J., Connolly D., Offermanns S., Wright S. D., Waters M. G. (2005) d-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 280, 26649–26652 [DOI] [PubMed] [Google Scholar]
- 25. Ahmed K., Tunaru S., Langhans C. D., Hanson J., Michalski C. W., Kölker S., Jones P. M., Okun J. G., Offermanns S. (2009) Deorphanization of GPR109B as a receptor for the β-oxidation intermediate 3-OH-octanoic acid and its role in the regulation of lipolysis. J. Biol. Chem. 284, 21928–21933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimachi T., Tanaka K., Yoneda F. (1991) Synthesis of a proposed isomer of F420 having α-glutamyl bonding. J. Heterocyc. Chem. 28, 439–443 [Google Scholar]
- 27. Takeda S., Yamamoto A., Okada T., Matsumura E., Nose E., Kogure K., Kojima S., Haga T. (2003) Identification of surrogate ligands for orphan G protein-coupled receptors. Life Sci. 74, 367–377 [DOI] [PubMed] [Google Scholar]
- 28. Lattin J. E., Schroder K., Su A. I., Walker J. R., Zhang J., Wiltshire T., Saijo K., Glass C. K., Hume D. A., Kellie S., Sweet M. J. (2008) Expression analysis of G protein-coupled receptors in mouse macrophages. Immunome Res. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee J. Y., Zhao L., Youn H. S., Weatherill A. R., Tapping R., Feng L., Lee W. H., Fitzgerald K. A., Hwang D. H. (2004) Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279, 16971–16979 [DOI] [PubMed] [Google Scholar]
- 30. Suganami T., Tanimoto-Koyama K., Nishida J., Itoh M., Yuan X., Mizuarai S., Kotani H., Yamaoka S., Miyake K., Aoe S., Kamei Y., Ogawa Y. (2007) Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 27, 84–91 [DOI] [PubMed] [Google Scholar]
- 31. Bouchard C., Pagé J., Bédard A., Tremblay P., Vallières L. (2007) G protein-coupled receptor 84, a microglia-associated protein expressed in neuroinflammatory conditions. Glia 55, 790–800 [DOI] [PubMed] [Google Scholar]
- 32. Shibata F., Konishi K., Kato H., Komorita N., al-Mokdad M., Fujioka M., Nakagawa H. (1995) Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2α, CINC-2β, and CINC-3. Eur. J. Biochem. 231, 306–311 [DOI] [PubMed] [Google Scholar]
- 33. Nakagawa H., Shiota S., Takano K., Shibata F., Kato H. (1996) Cytokine-induced neutrophil chemoattractant (CINC)-2 α, a novel member of rat GRO/CINCs, is a predominant chemokine produced by lipopolysaccharide-stimulated rat macrophages in culture. Biochem. Biophys. Res. Commun. 220, 945–948 [DOI] [PubMed] [Google Scholar]
- 34. Soydan A. S., Dokmetas H. S., Cetin M., Koyuncu A., Kaptanoglu E., Elden H. (2006) The evaluation of the role of β-hydroxy fatty acids on chronic inflammation and insulin resistance. Mediators Inflamm. 2006, 64980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang X., Zhang X., Heckmann B. L., Lu X., Liu J. (2011) Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-α (TNF-α)-induced lipolysis in adipocytes. J. Biol. Chem. 286, 40477–40485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Talukdar S., Olefsky J. M., Osborn O. (2011) Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 32, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh D. Y., Lagakos W. S. (2011) The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 14, 322–327 [DOI] [PubMed] [Google Scholar]
- 38. Offermanns S., Colletti S. L., Lovenberg T. W., Semple G., Wise A., IJzerman A. P. (2011) International Union of Basic and Clinical Pharmacology. LXXXII: Nomenclature and Classification of Hydroxy-carboxylic Acid Receptors (GPR81, GPR109A, and GPR109B). Pharmacol. Rev. 63, 269–290 [DOI] [PubMed] [Google Scholar]
- 39. Romeo G. R., Lee J., Shoelson S. E. (2012) Metabolic syndrome, insulin resistance, and roles of inflammation - mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 32, 1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elgazar-Carmon V., Rudich A., Hadad N., Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 41. Nagasaki H., Kondo T., Fuchigami M., Hashimoto H., Sugimura Y., Ozaki N., Arima H., Ota A., Oiso Y., Hamada Y. (2012) Inflammatory changes in adipose tissue enhance expression of GPR84, a medium-chain fatty acid receptor: TNFα enhances GPR84 expression in adipocytes. FEBS Lett. 586, 368–372 [DOI] [PubMed] [Google Scholar]