Background: NK produce IFN-γ and GM-CSF in response to CpG-ODN in the presence of accessory cytokines.
Results: This production involves NF-κB, STAT3, UNC93b1 and IL-12. IFN-γ is MyD88- and TLR9-dependent, whereas GM-CSF depends on the adaptor STING.
Conclusion: NK present an alternative mechanism of sensing of CpG-ODN.
Significance: These results open new horizons in the understanding of NK cells activation by microbial DNA.
Keywords: Cytokine, Interferon, Natural Killer (NK) cell, Signal Transduction, Toll-like Receptors (TLR), GM-CSF, IFN-gamma, STING, TLR9
Abstract
Natural killer (NK) cells are important for innate immunity in particular through the production of IFN-γ and GM-CSF. Both cytokines are important in restoration of immune function of tolerized leukocytes under inflammatory events. The expression of TLRs in NK cells has been widely studied by analyzing the mRNA of these receptors, rarely seeking their protein expression. We previously showed that murine spleen NK cells express TLR9 intracellularly and respond to CpG oligodeoxynucleotide (CpG-ODN) by producing IFN-γ and GM-CSF. However, to get such production the presence of accessory cytokines (such as IL-15 and IL-18) was required, whereas CpG-ODN or accessory cytokines alone did not induce IFN-γ or GM-CSF. We show here that TLR9 overlaps with the Golgi apparatus in NK cells. Furthermore, CpG-ODN stimulation in the presence of accessory cytokines induces the phosphorylation of c-Jun, STAT3, and IκBα. IFN-γ and GM-CSF production requires NF-κB and STAT3 activation as well as Erk-dependent mechanisms for IFN-γ and p38 signaling for GM-CSF. Using knock-out-mice, we show that UNC93b1 and IL-12 (produced by NK cells themselves) are also necessary for IFN-γ and GM-CSF production. IFN-γ production was found to be MyD88- and TLR9-dependent, whereas GM-CSF was TLR9-independent but dependent on STING (stimulator of interferon genes), a cytosolic adaptor recently described for DNA sensing. Our study thereby allows us to gain insight into the mechanisms of synergy between accessory cytokines and CpG-ODN in NK cells. It also identifies a new and alternative signaling pathway for CpG-ODN in murine NK cells.
Introduction
Natural killer (NK)3 cells are important actors of innate immunity with a predominant role during viral infections. It has now been clearly established that NK cells also play a key role during bacterial infection (for review see, Ref. 1). NK cells contribute to the inflammatory reaction by the production of many cytokines, particularly two potent immune-stimulatory cytokines IFN-γ and GM-CSF, with each playing a key role in innate immunity (2, 3). IFN-γ is a well known proinflammatory cytokine and has been recognized as a factor involved in endotoxin-induced lethality (4, 5). GM-CSF has similarly been shown to contribute to many inflammatory disorders (6).
NK cells have been shown to express Toll-like receptors (TLRs), primarily determined by PCR measurement of TLR transcript, whereas few have demonstrated expression at the protein level. Various detection methods have yielded conflicting results (mRNA or surface expression) (7–10). We recently showed that murine NK cells do indeed express TLR2, TLR4, and TLR9 at the protein level. In contrast to other leukocytes such as macrophages in which TLR2 and TLR4 are present on the surface, NK cells express these receptors intracellularly, similarly to TLR9 (11). Additionally, we found that purified splenic naive NK cells were highly responsive to CpG-ODN, a synthetic oligodeoxynucleotide mimicking bacterial DNA recognized as an agonist of TLR9. CpG-ODN is a potent inducer of IFN-γ and GM-CSF, but only in the presence of accessory cytokines such as IL-2, IL-15, and/or IL-18 (11), which alone are unable to induce cytokine production. Thus, NK cells require two signals for IFN-γ and GM-CSF production: from accessory cytokines and from the receptors sensing CpG-ODN.
The aim of the present study was to decipher the mechanisms involved in IFN-γ and GM-CSF induction and to identify the intracellular actors during production. We are first to report that TLR9 expression overlaps with the Golgi apparatus in NK cells. We found that CpG-ODN stimulation in the presence of accessory cytokines induces the phosphorylation of c-Jun, STAT3, and IκBα. IFN-γ and GM-CSF production requires NF-κB and STAT3 activation as well as Erk-dependent mechanisms for IFN-γ and p38 signaling for GM-CSF. Using knock-out mice GM-CSF production was surprisingly MyD88- and TLR9-independent in contrast to IFN-γ, which used the classical CpG-ODN sensing via TLR9. Therefore, we investigated the possible role of the STING (stimulator of interferon genes), a cytosolic adaptor recently described and associated with TLR-independent sensing of bacterial DNA (12, 13). Our results indicate that NK cell cytokine production may occur independently of TLR9, yet require STING and UNC93b1 as well as NK-produced IL-12.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies against the following antigens were used: anti-IL-12p40/p70-PE, anti-NKp46-V450, and anti-NKp46-A647 were from BD Biosciences, and anti-TLR9/CD289-A488 was from Imgenex (San Diego, CA). The corresponding isotype and fluorophore controls were obtained from the same companies. For immunofluorescence, the antibodies against the following antigens were used: anti-TMEM173-STING from Acris Antibodies GmbH (Herford, Germany) and anti-p65 from Cellular Signaling Technology (Beverly, MA). The corresponding secondary antibody, conjugated with Texas Red and A488 respectively, were obtained from the same companies. The FcReceptor-blocking reagent and the Inside stain kit were obtained from Miltenyi Biotec (Bergisch-Gladbach, Germany). The murine recombinant cytokines (IL-2, IL-15) were from Peprotech, and IL-18 was from Medical and Biological Laboratories. ELISA kits to detect GM-CSF, IFN-γ, IL-1β, IL-6, and TNF-α were obtained from R&D Systems and used according to the recommendations of the manufacturer. The multiplex combination used to detect phosphorylated AKT, ERK1–2, Iκβα, c-Jun, JNK, p38, STAT2, and STAT3 was provided by Bio-Rad Laboratories. The murine CpG-ODN sequence was as follows: 5′-TCCATGACGTTCCTGATGCT-3′ and was synthesized by Sigma-Genosys (The Woodlands, TX). The corresponding CpG-ODN sequence was customized to be coupled to Alexa Fluor 488 (A488) by Integrated DNA Technologies (Coralville, IA). The inhibitors were as follows: Pepinh-MYD (MyD88, 20 μm) was obtained from InvivoGen (San Diego, CA); Stattic was used at 1 μm (STAT3), SP600125 at 10 μm (c-Jun), Bay 11-7085 at 1 μm (NF-κB), and SB203580 at 10 μm (p38) were from TOCRIS Bioscience (Bristol, UK); 10 μm LY294002 (PI3K) and 20 μm PD-98059 (ERK1,2) were from Calbiochem. The nucleus and Golgi apparatus probes Hoechst 33342 and BODIPY-TR ceramide, respectively, were obtained from Invitrogen. radioimmune precipitation assay buffer was obtained from Sigma Aldrich.
Mice
Studies were performed with 8- to 12-week-old male mice. The wild-type C57BL/6(J) was obtained from Janvier (Le Genest-St.-Isle, France). The transgenic mice in a C57BL/6 background were obtained as follows: il-12r2β−/− mice from the Transgenose Institute animal breeding facility (Orléans, France); the ifn-α/βR, ifn-γ, myd88, and tlr9−/− mice from the animal facility of the Institut Pasteur (Paris, France); the trif−/− mice from the animal facility of the A*STAR Singapore Immunology Network (Singapore); the sting-deficient mice and unc93b1−/− from the animal facility of the University of Massachusetts Medical School (Worcester, MA). Animals were housed in the animal facilities under specific pathogen-free conditions. Protocols, performed in compliance with the NIH Animal Welfare Insurance no. A5476-01 issued on 02/07/2007, were approved by the veterinary team of the Institut Pasteur animal facility.
Preparation of Murine NK Cells and in Vitro Culture Conditions
NK cells were enriched from spleen homogenates by negative selection using the NK cell isolation kit on untouched mouse NK cells (Miltenyi Biotec) as described previously (11). NK cell purity was assessed by flow cytometry after CD3−NKp46+ staining and was >95%. After purification, cells were plated at 1 × 105 cells/well in 96-well plates in RPMI 1640 (LONZA, Rockland, ME) supplemented with 10% FCS (PAA Laboratories, Pasching, Austria), streptomycin at 0.1 mg/ml, and penicillin at 100u/ml (Sigma-Aldrich).
For GM-CSF and IFN-γ measurements, cells were incubated for 48 h at 37 °C and 5% CO2 in the presence or absence of IL-15 and IL-18 (10 ng/ml) and CpG-ODN (1 μm). In some experiments, peritoneal cells were prepared and cultured as described previously and were used as controls (14). For the in vitro Golgi/TLR9 co-localization assay, purified NK cells were first incubated 30 min at 4 °C in the presence of the BODIPY-TR probe at 5 μm, incubated for 30 min at 37 °C and 5% CO2, and then fixed in 2% paraformaldehyde, permeabilized, and stained for intracellular TLR9. The same intracellular stain protocol was used to stain STING in naive cells, and p65 as described by Gouin et al. (15) (after IL-15, IL-18, and CpG-ODN stimulation). For the STING co-localization assay, a CpG-DNA coupled to A647 was used (1 μm). The staining was analyzed using a Leica SP5 confocal microscope. To perform the bioplex to detect the phosphorylated signaling proteins, 3 × 106 NK cells per test were cultured in culture tubes for 30 min or 1 h in the presence or absence of the IL-15, IL-18, IL-15/IL-18 (10 ng/ml), and/or CpG-ODN (1 μm). Cells were then resuspended and homogenized in 50 μl of radioimmune precipitation assay buffer. Cell lysates were kept at −80 °C until analysis.
NK cell surface antigens were labeled in staining buffer (PBS, 2 mm EDTA, and 0.5% FCS) with antibodies at the concentration suggested by the manufacturers. Intracellular staining was performed after fixation and permeabilization of the cells using the Inside Stain kit (Myltenyi Biotec), according to the manufacturer's instructions. All flow cytometry data were acquired and analyzed on a MACSQuant flow cytometer (Myltenyi Biotec).
Statistical Analysis
All experiments were performed at least three times, using at least n = 3 mice per assay. One-way ANOVA and Fisher least significance difference test were used for statistical analysis. A p value < 0.05 was considered to be significant.
RESULTS
TLR9 Overlaps with the Golgi Apparatus in NK Cells and IFN-γ and GM-CSF Production Requires NF-κB and STAT3
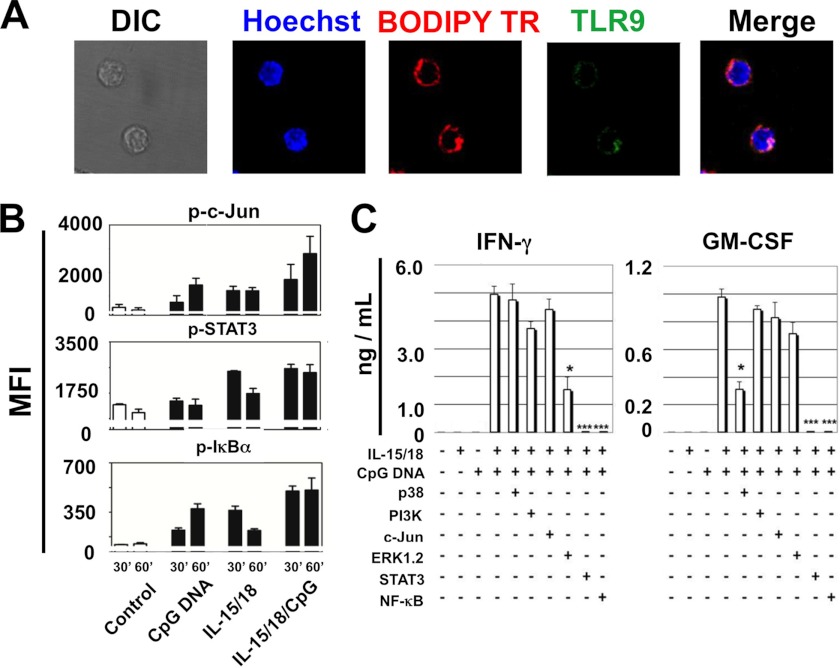
We previously showed by flow cytometry that similar to myeloid cells, TLR9 was intracellular in murine NK cells (11). In the present report we confirmed this finding and in addition showed by confocal microscopy that TLR9 overlaps with the Golgi apparatus (Fig. 1A). A more precise localization is however difficult in primary NK cells because of the small cytosol in this cell type. To better understand the signaling of CpG-ODN in NK cells, we analyzed the phosphorylation of signaling molecules described in the literature to be induced by accessory cytokines (IL-15/IL-18) or CpG-ODN. Whole cell extracts were prepared after 30 or 60 min of stimulation and analyzed by the Bioplex technology. Within these molecules, we observed that IL-15 and IL-18 alone and/or together induced the phosphorylation of c-Jun (Fig. 1B). In the presence of CpG-ODN and IL-15/IL-18 c-Jun phosphorylation was consistent at 30 min but appeared more sustained at 1 h as compared with IL-15/IL-18 alone. CpG and IL-18 also induced STAT3 phosphorylation and the level was again higher when stimulatory cytokines and CpG-ODN were added simultaneously. Finally, CpG-ODN and stimulatory cytokines added alone induced some phosphorylation on IκBα with higher p-IκBα observed when both CpG-ODN and IL-15/IL-18 were present simultaneously. p38 and ERK were phosphorylated after stimulation with CpG, IL-15/18, or their combination, but the level of activation was comparable for all conditions. No synergistic effect was observed (data not shown). No difference in the phosphorylation of the signaling molecules AKT, JNK, or STAT2 was observed (data not shown). The contribution of various signaling molecules on IFN-γ and GM-CSF production was also analyzed using specific inhibitors (Fig. 1C). The optimal concentration for each inhibitory drug was chosen according to the literature (16–19) and our own experience (20). CpG-ODN or IL-15/IL-18 alone did not induce any IFN-γ or GM-CSF secretion. In contrast, when co-signaling they synergized inducing thousands of pg/ml of both cytokines. This induction was completely abolished by specific inhibitors of STAT3 or NF-κB and partially diminished by the ERK1/2 inhibitor for IFN-γ and by p38 inhibitor for GM-CSF.
FIGURE 1.
A, intracellular localization of TLR9 by confocal microscopy showing overlapping with the Golgi apparatus (BODIPY cytochemistry). Nucleus is visualized with Hoechst. Scale bar, 5 μm. B, phosphorylated signaling molecules profile at 30 and 60 min of stimulation. DIC, differential interference contrast. The phosphorylation is measured by bioplex technology and expressed as mean fluorescence intensity (MFI). Data are mean ± S.E. of three experiments. C, pharmacological inhibition of p38, PI3K, c-Jun, ERK1/2, STAT3, and NF-κB. Purified spleen NK cells were cultured in the presence of IL-15/IL-18 (10 ng/ml each) and/or CpG-ODN (1 μm) with or without the inhibitors. After 48 h of in vitro stimulation, IFN-γ and GM-CSF were measured by ELISA in the supernantants. Data are mean ± S.E. of five experiments. *, p < 0.05; ***, p < 0.001 (one-way ANOVA and Fisher least significance difference test). 30', 30 min; 60', 60 min.
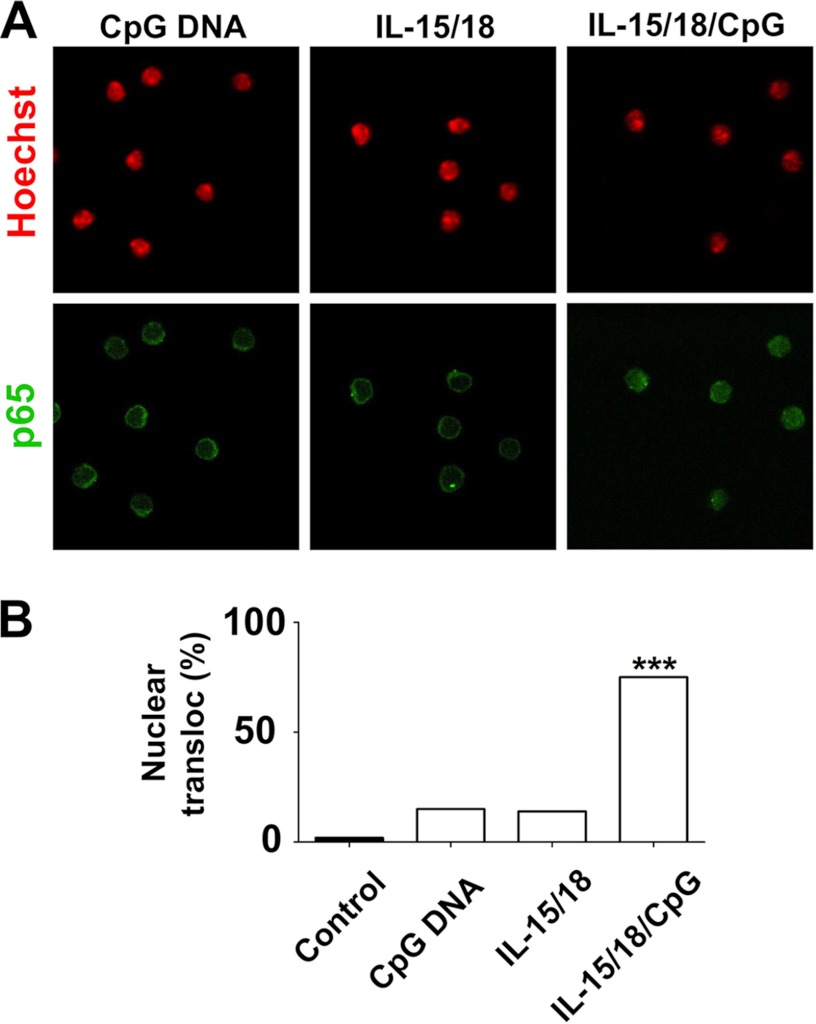
Both Accessory Cytokines IL-15/IL-18 and CpG-ODN Are Necessary for NF-κB p65 Nuclear Translocation
Purified NK cells were primed overnight with IL-15 and IL-18 and then challenged with CpG-ODN. Nuclear translocation of p65 was measured by immunofluorescence after 60-min stimulation. As shown in Fig. 2A, no or low nuclear localization of NF-κB was present in cells stimulated with CpG-ODN or IL-15/IL-18 alone. Similarly, no nuclear localization was seen in unstimulated cells (control, image not shown). In contrast, immunofluorescence staining of the p65 subunit revealed a high nuclear localization of this transcription factor after CpG-ODN + IL-15/IL-18 challenge. Cells were counted in order to estimate the percentage of nucleus positive for p65 (Fig. 2B). The percentage of positive cells for nuclear p65 was ∼20% in cells treated with CpG-ODN or IL-15/IL-18 alone but reached 75% when both stimuli were present.
FIGURE 2.
A, both accessory cytokines IL-15/IL-18 and CpG-ODN are necessary for NF-κB p65 nuclear translocation. Purified NK cells were primed overnight with IL-15 and IL-18 (10 ng/ml each) and then challenged with CpG-ODN (1 μm). Nuclear translocation of p65 was measured by immunofluorescence after 60-min stimulation. Hoeschst was used to stain the nucleus. B, to quantify the results shown in A, the percentage of nuclear translocation was determined by counting 100 cells. The figure is representative of three independent experiments. ***, p < 0.001 versus unstimulated cells (control) using one-way ANOVA and Fisher least significance difference test.
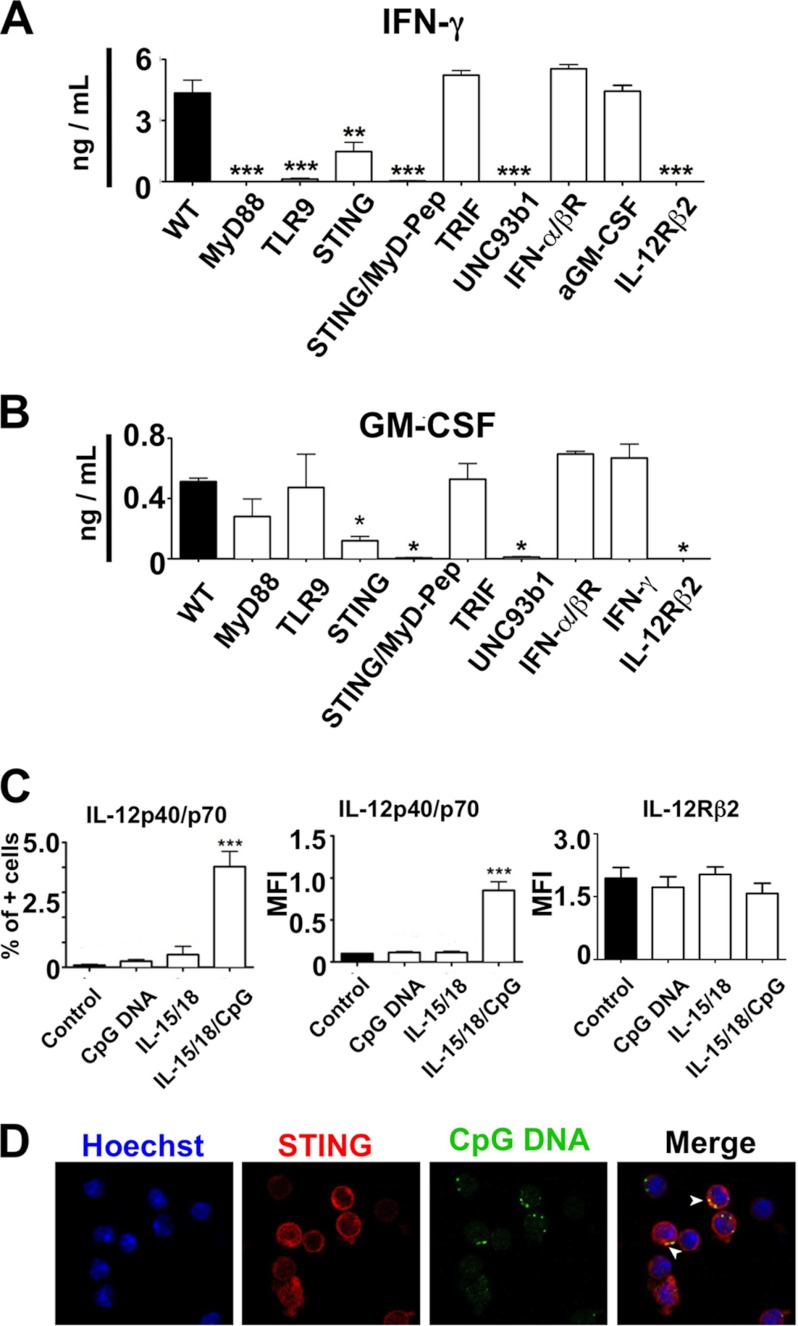
TLR9-independent GM-CSF Induction by CpG-ODN and the Role of STING and UNC93b1 for Cytokine Production by NK Cells
Using deficient mice, we evaluated whether IFN-γ and GM-CSF productions were fully dependent of the TLR9 signaling pathway for NK cells. In the case of IFN-γ, we demonstrated an absence of production in myd88 and tlr9−/− NK cells (Fig. 3A). Interestingly, IFN-γ production was also abolished in the absence of UNC93b1, a molecule important for TLR9 migration from the endoplasmic reticulum to the Golgi apparatus (21). In addition, we found a partial effect of STING, a newly described adaptor involved in cytoplasmic sensing double-stranded DNA (22), on IFN-γ production by NK cells. Indeed, IFN-γ levels were significantly decreased for STING deficient NK cells identifying a new actor involved in CpG-ODN sensing. As shown in Fig. 3A, the absence of STING did not totally abolish IFN-γ production, but its presence was necessary for optimal production. The remaining IFN-γ production was completely abolished when sting−/− NK cells were incubated with a MyD88 inhibitor (MyD88 peptide), suggesting the existence of two pathways for CpG-ODN sensing: one involving STING and one needing the adaptor protein MyD88 for downstream signaling. For GM-CSF, the results were more striking and surprising, considering no significant alteration of GM-CSF production in myd88 or tlr9−/− NK cells was found. In contrast, GM-CSF production was profoundly and significantly altered in the absence of STING. Similar to IFN-γ, GM-CSF production was totally abolished if, in addition to STING, the MyD88-dependent pathway was blocked.
FIGURE 3.
Purified spleen NK cells from WT and mice deficient for MyD88, TLR9, STING, TRIF, UNC93b1, IFN-α/βR, and IL-12RB2 were stimulated with accessory cytokines (IL-15 and IL-18 at 10 ng/ml each) and CpG-ODN (1 μm) for 48 h, and IFN-γ (A) and GM-CSF (B) were measured in the supernatants by ELISA. The MyD88 inhibitory peptide (MyD-Pep) was also applied on the sting−/− cells at 25 μm. Data are mean ± S.E. of five experiments. *, p < 0.05; *, p < 0.01; ***, p < 0.001 versus WT using one-way ANOVA and Fisher least significance difference test. C, purified NK cells were primed overnight with IL-15 and IL-18 (10 ng/ml each) and challenged with CpG-ODN (1 μm) in presence of GolgiStop. IL-12p40/70 was then stained intracellularly using specific antibodies and detected on the gated NKp46+ cells. The left histogram shows the IL-12p40/70 stain as percentage of positive cells, the middle graph shows the mean fluorescence intensity (MFI), and the right histograms show the expression of IL-12Rβ2 on the NK cells cultured in the same conditions. Data are the mean ± S.E. of five experiments. ***, p < 0.001 versus cells without stimulation (control) using one-way ANOVA and Fisher least significance difference test. D, purified NK cells were stimulated with IL-15/IL-18 and CpG-ODN labeled with A488. After 60 min, the cells were fixed, stained with an anti-STING antibody, and processed for confocal microscopy. The nucleus is visualized with Hoechst. Arrows indicate spots where an proximally overlapping was observed for cytosolic STING and speckled CpG-ODN stain.
As expected, the production of both cytokines was independent of TRIF (TIR domain-containing adaptor-inducing interferon-β), which was used as a negative control. We also investigated a possible role of IFN-α/β. Using ifn-α/βR−/− NK cells, we showed that indeed they do not participate in the induction mechanisms of IFN-γ or GM-CSF. Finally, we investigated whether IFN-γ or GM-CSF may participate in one another's induction. For this purpose, we utilized ifn-g−/− NK cells to study the influence on GM-CSF and anti-GM-CSF blocking antibodies to measure the effect of GM-CSF on IFN-γ production. Our results indicate that the lack of IFN-γ had no influence on GM-CSF, and the blockage of GM-CSF did not alter IFN-γ production.
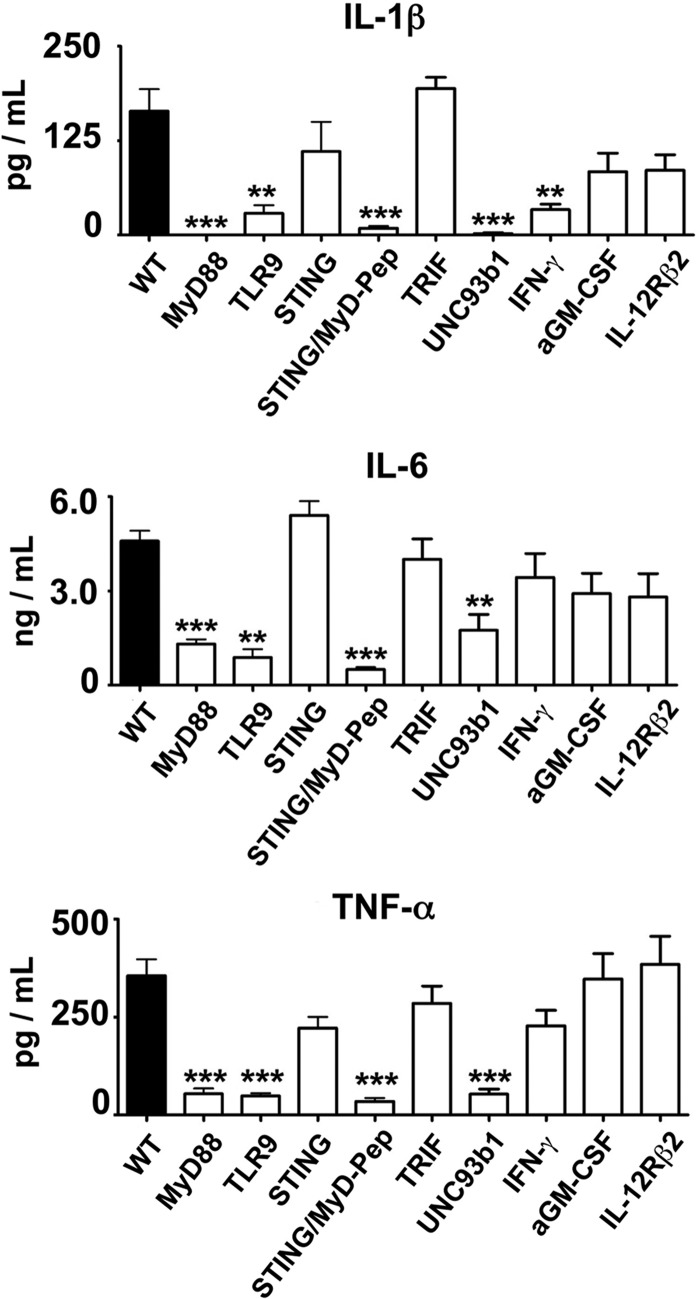
To determine whether the TLR9-independent and STING-dependent cytokine production was specific of NK cells, we measured cytokine production by macrophages in response to CpG-ODN. Because GM-CSF production was extremely low (below 30 pg/ml), we measured IL-1β, IL-6, and TNF (Fig. 4). We found that cytokine production by STING deficient macrophages was not reduced as compared with WT macrophages, whereas it was significantly diminished in the absence of MyD88, TLR9, or with the blockade of MyD88 (MyD peptide) as classically reported. In contrast to STING, the response to CpG-ODN in macrophages was dependent on UNC93b1, similar to NK cells.
FIGURE 4.
Purified peritoneal macrophages from WT and knock-out mice for MyD88, TLR9, STING, TRIF, UNC93b1, IFN-α/βR, and IL-12Rβ2 were stimulated with CpG-ODN (1 μm) for 24 h, and IL-1β (A), IL-6 (B), and TNF (C) were measured in the supernatants by ELISA. The MyD88 inhibitory peptide (MyD-Pep) was also applied on the sting−/− cells at 25 μm. Data are the mean ± S.E. of five experiments. *, p < 0.05; *, p < 0.01; ***, p < 0.001 versus WT using one-way ANOVA and Fisher least significance difference test.
Participation of IL-12 in IFN-γ and GM-CSF Induction in NK Cells
Interestingly, the production of IFN-γ and GM-CSF by NK cells was totally abolished in the absence of IL-12Rβ2, one of the two chains forming the IL-12 receptor (Fig. 3, A and B). IL-12 is usually derived from accessory cells but considering the high experimental purity (>95%), the capacity of NK cells to produce IL-12 and self-activate by an autocrine action was considered. We chose to analyze IL-12 production by flow cytometry and intracellular staining for two reasons. First, IL-12 production may be below the threshold of classical ELISA, and second, the cytokine may be consumed by NK cells themselves. A tangential example is type I IFNs, which are easily measured by RT-PCR but very hard to detect by ELISA in culture supernatants. As shown in Fig. 3C, IL-12-positive NK cells were barely detected when stimulated by CpG-ODN or IL-15/IL-18 alone, both in terms of percentage of positive cells and mean fluorescence intensity. In contrast, IL-15/IL-18 and CpG-ODN synergized and induced a significant increase in IL-12-positive NK cells. Although in these culture conditions the percentage of positive cells remained low, IL-12 was significantly increased in comparison to unstimulated cells or cells stimulated with CpG-ODN or IL-15/IL-18 alone. The different stimulations (CpG-ODN and/or accessory cytokines) had no effect on the expression of the IL-12Rβ2 chain.
STING and CpG-ODN Overlap in NK Cell Cytoplasm
Finally, we investigated whether STING and CpG-ODN overlapped in NK cells. As shown in Fig. 3D, STING was strongly expressed in the cytoplasm of NK cells. The figure shows NK cells stimulated with IL-15/IL-18 and CpG-ODN, but the result was similar without accessory cytokines. It can be seen, thanks to A488-labeled CpG-ODN, that there is an uptake of the oligodeoxynucleotide by the NK cells. Confocal microscopy shows that STING and CpG-ODN overlap in different locations within the NK cells (white arrows in the merge).
DISCUSSION
In a previous report, we showed by flow cytometry that similar to myeloid cells, TLR9 was intracellular in murine NK cells (11). TLR9 is a pattern recognition receptor sensing unmethylated bacterial DNA whose triggering is mimicked by CpG-ODN. CpG-ODN stimulation induced cytokine production by macrophages and dendritic cell maturation, a response that was absent for cells from tlr9−/− mice (23). Additionally, the TLR adaptor protein MyD88 is involved, co-localized with TLR9, and tagged CpG-ODN into the endosome compartment (24). In the present report, we confirmed the intracellular localization of TLR9 in NK cells and in addition showed that it overlaps with the Golgi apparatus.
In a previous study, we also found that purified splenic naive NK cells were highly responsive to CpG-ODN, in terms of IFN-γ and GM-CSF production, but only in the presence of accessory cytokines such as IL-2, IL-15, and/or IL-18 (11), which alone are unable to induce cytokine production.
To better understand the synergy between accessory cytokines (IL-15/IL-18) and CpG-ODN and their signaling in NK cells, we analyzed the phosphorylation of signaling molecules described in the literature to be induced by them. We found that IL-15 and IL-18 alone and/or together induced the phosphorylation of c-Jun and IκBα, which is consistent with the literature, where IL-15 has been shown to activate the JNK and NF-κB pathways in myeloid cells (25). Nevertheless, in the presence of CpG-ODN and IL-15/IL-18, their phosphorylation appeared more sustained at 1 h as compared with IL-15/IL-18 alone. CpG-ODN and IL-18 also induced STAT3 phosphorylation, and the level was again higher when stimulatory cytokines and CpG-ODN were added simultaneously. The contribution of NF-κB and STAT3 on IFN-γ and GM-CSF production by NK cells was confirmed using specific inhibitors. In addition, using microscopy and immunofluorescence, we showed that no or low nuclear localization of NF-κB was present in cells stimulated with CpG-ODN or IL-15/IL-18 alone. In contrast, the p65 subunit of NF-κB revealed a high nuclear localization after challenge with both CpG-ODN and IL-15/IL-18. Finally, using specific inhibitors, we observed that IFN-γ and GM-CSF secretion was independent of PI3K but partially dependent on ERK1/2 for IFN-γ and p38 for GM-CSF.
Using deficient mice, we evaluated whether IFN-γ and GM-CSF productions were fully dependent of the TLR9 signaling pathway for NK cells. In the case of IFN-γ, we demonstrated an absence of production in myd88 and tlr9−/− NK cells, which was consistent with the literature. Interestingly, IFN-γ production was also absent in unc93b1−/− NK cells. UNC93b1 has been described to play an important role for TLR9 migration from the endoplasmic reticulum, where TLR9 is retained (26), to the Golgi apparatus, and then to the endosomal compartment where it encounter its agonist hypomethylated DNA after cellular uptake (27, 28). In addition, we found a partial effect of STING on IFN-γ production by NK cells. Indeed, IFN-γ levels were significantly decreased for STING-deficient NK cells identifying a new actor involved in CpG-ODN sensing. STING is a newly described cytoplasmic adaptor involved in the sensing double-stranded DNA (22), which localizes near the endoplasmic reticulum in the presence of intracellular DNA (29). We found that STING absence was necessary for optimal IFN-γ production by NK cells. In contrast to IFN-γ, which was mostly dependent on the classical TLR9 and MyD88 pathway, GM-CSF production was profoundly and significantly altered in the absence of STING and UNC93b1. Thus, we identified STING as a new actor in CpG-ODN sensing in NK cells, adding an intracytoplasmic detection of DNA in addition to the classical endosomal TLR9-dependent detection system. STING is involved in cytosolic DNA sensing, but it is an ER adaptor protein (29). Regarding UNC93b1, it has been predicted to be a multispanning transmembrane protein (30) and similarly to STING has been located in the ER (31). Thus, one can make the assumption that UNC93b1 may be also important for STING function and/or correct location, as already shown for TLR3, TLR7, and TLR9.
The TLR9-independent and STING-dependent cytokine production was specific of NK cells and was not found in macrophages. This negative result in macrophages does not exclude a role of STING in other cell types. For example, Landrigan et al. (32) recently identified a TLR9-, MyD88-, and endosomal-independent sensing of CpG-ODN in CD4+ T lymphocytes but did not identify the receptor responsible for CPG-ODN detection. It would be intriguing to verify whether similarly to NK cells, STING is the molecule involved in this sensing in T lymphocytes. In contrast to STING the response to CpG-ODN in macrophages was dependent on UNC93b1, similar to NK cells. The latter is most probably due to the fact that UNC93b1 allows the endosomal localization of TLR9, as reported previously (21). Finally, we found that STING and CpG-ODN overlapped in NK cells. This result is consistent with the observation by Hagele et al. (33) that DNA oligonucleotides present in endosomes may reach the cytoplasm. The existence of STING as an alternative cytosolic sensor for bacterial DNA may be an advantage for the detection of intracytoplasmic bacteria.
As expected, the production of both cytokines was independent of TRIF. This result is in accordance with the literature where TRIF has been shown to be involved in TLR3 and TLR4 signaling pathways (34, 35) but not TLR9. We also investigated a possible role of IFN-α/β, considering type I interferons have been described to prime NK cells for cytotoxicity or IFN-γ production (36, 37). Even if this effect appears to be related to antiviral response and sensing of poly-I:C (dsRNA), we wanted to ensure that type I interferons did not affect NK cell response to CpG-ODN. Using inf-α/βR −/− NK cells, we showed that indeed they do not participate in the induction mechanisms of IFN-γ or GM-CSF.
Our results indicate that the lack of IFN-γ had no influence on GM-CSF and the blockage of GM-CSF did not alter IFN-γ production. In contrast and interestingly, the production of IFN-γ and GM-CSF by NK cells was totally abolished in the absence of IL-12Rβ2, one of the two chains forming the IL-12 receptor. This suggests that IL-12 is necessary for the priming of NK cells, whereas IL-2, IL-15, and IL-18 alone or in combination are unable to induce cytokine production by murine NK cells. IL-12 is the only cytokine able to activate NK cells without the need of additional stimulus such as TLR agonists (3, 11). IL-12 is usually derived from accessory cells but considering the high experimental purity (>95%), the capacity of NK cells to produce IL-12 and self-activate by an autocrine action was considered. We show that IL-15/IL-18 and CpG-ODN synergized and induced a significant increase in IL-12-positive NK cells. The different stimulations (CpG-ODN and/or accessory cytokines) had no effect on the expression of the IL-12Rβ2 chain.
Taken together, the present data underlie the existence of an alternative mechanism of sensing and response to CpG-ODN, a typical TLR9 agonist. This signaling pathway in NK cells involves STING, a recently described cytosolic adaptor involved in the sensing of DNA. This pathway contributes fully to GM-CSF production by NK cells and partially to that of IFNγ. Furthermore, the production of both cytokines is dependent on IL-12, produced by NK cells themselves. Detection of bacterial DNA contributes to the immune response for several infections. The discovery of this alternative pathway brings insight into the understanding of bacterial DNA sensing and may be useful for the design of new vaccine adjuvant therapies.
Acknowledgments
We thank Dr. Bruce Beutler and Dr. Noelle Doyen for the kind gift of unc93b1 and tlr9 knock-out mice, respectively, and Brian Mozeleski for grammatical corrections.
Footnotes
- NK
- natural killer
- TLR
- Toll-like receptor
- ANOVA
- analysis of variance.
REFERENCES
- 1. Souza-Fonseca-Guimaraes F., Adib-Conquy M., Cavaillon J. M. (2012) Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol. Med. 18, 270–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hayakawa Y., Huntington N. D., Nutt S. L., Smyth M. J. (2006) Functional subsets of mouse natural killer cells. Immunol. Rev. 214, 47–55 [DOI] [PubMed] [Google Scholar]
- 3. Huntington N. D., Vosshenrich C. A., Di Santo J. P. (2007) Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7, 703–714 [DOI] [PubMed] [Google Scholar]
- 4. Doherty G. M., Lange J. R., Langstein H. N., Alexander H. R., Buresh C. M., Norton J. A. (1992) Evidence for IFN-γ as a mediator of the lethality of endotoxin and tumor necrosis factor-α. J. Immunol. 149, 1666–1670 [PubMed] [Google Scholar]
- 5. Car B. D., Eng V. M., Schnyder B., Ozmen L., Huang S., Gallay P., Heumann D., Aguet M., Ryffel B. (1994) Interferon-γ receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamilton J. A. (2002) GM-CSF in inflammation and autoimmunity. Trends Immunol. 23, 403–408 [DOI] [PubMed] [Google Scholar]
- 7. Chalifour A., Jeannin P., Gauchat J. F., Blaecke A., Malissard M., N'Guyen T., Thieblemont N., Delneste Y. (2004) Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 104, 1778–1783 [DOI] [PubMed] [Google Scholar]
- 8. Lauzon N. M., Mian F., MacKenzie R., Ashkar A. A. (2006) The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell. Immunol. 241, 102–112 [DOI] [PubMed] [Google Scholar]
- 9. Sawaki J., Tsutsui H., Hayashi N., Yasuda K., Akira S., Tanizawa T., Nakanishi K. (2007) Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int. Immunol. 19, 311–320 [DOI] [PubMed] [Google Scholar]
- 10. Martinez J., Huang X., Yang Y. (2010) Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 6, e1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Souza-Fonseca-Guimaraes F., Parlato M., Fitting C., Cavaillon J. M., Adib-Conquy M. (2012) NK cell tolerance to Toll-like receptor agonists mediated by regulatory T cells after polymicrobial sepsis. J. Immunol. 188, 5850–5858 [DOI] [PubMed] [Google Scholar]
- 12. Prantner D., Darville T., Nagarajan U. M. (2010) Stimulator of IFN gene is critical for induction of IFN-β during Chlamydia muridarum infection. J. Immunol. 184, 2551–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koppe U., Högner K., Doehn J. M., Müller H. C., Witzenrath M., Gutbier B., Bauer S., Pribyl T., Hammerschmidt S., Lohmeyer J., Suttorp N., Herold S., Opitz B. (2012) Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. J. Immunol. 188, 811–817 [DOI] [PubMed] [Google Scholar]
- 14. Fitting C., Dhawan S., Cavaillon J. M. (2004) Compartmentalization of tolerance to endotoxin. J. Infect. Dis. 189, 1295–1303 [DOI] [PubMed] [Google Scholar]
- 15. Gouin E., Adib-Conquy M., Balestrino D., Nahori M. A., Villiers V., Colland F., Dramsi S., Dussurget O., Cossart P. (2010) The Listeria monocytogenes InlC protein interferes with innate immune responses by targeting the IκB kinase subunit IKKα. Proc. Natl. Acad. Sci. U.S.A. 107, 17333–17338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandt B., Abou-Eladab E., Tiedge M., Walzel H. (2010) Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis. 1, e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanda N., Kamata M., Tada Y., Ishikawa T., Sato S., Watanabe S. (2011) Human β-defensin-2 enhances IFN-γ and IL-10 production and suppresses IL-17 production in T cells. J. Leukoc. Biol. 89, 935–944 [DOI] [PubMed] [Google Scholar]
- 18. Couture L. A., Piao W., Ru L. W., Vogel S. N., Toshchakov V. Y. (2012) Targeting Toll-like receptor (TLR) signaling by Toll/interleukin-1 receptor (TIR) domain-containing adapter protein/MyD88 adapter-like (TIRAP/Mal)-derived decoy peptides. J. Biol. Chem. 287, 24641–24648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogbomo H., Michaelis M., Kreuter J., Doerr H. W., Cinatl J., Jr. (2007) Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 581, 1317–1322 [DOI] [PubMed] [Google Scholar]
- 20. Kapetanovic R., Parlato M., Fitting C., Quesniaux V., Cavaillon J. M., Adib-Conquy M. (2011) Mechanisms of TNF induction by heat-killed Staphylococcus aureus differ upon the origin of mononuclear phagocytes. Am. J. Physiol. Cell Physiol. 300, C850–859 [DOI] [PubMed] [Google Scholar]
- 21. Kim Y. M., Brinkmann M. M., Paquet M. E., Ploegh H. L. (2008) UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 [DOI] [PubMed] [Google Scholar]
- 22. Ishikawa H., Ma Z., Barber G. N. (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788-U740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 [DOI] [PubMed] [Google Scholar]
- 24. Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., Lien E., Nilsen N. J., Espevik T., Golenbock D. T. (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 [DOI] [PubMed] [Google Scholar]
- 25. Chenoweth M. J., Mian M. F., Barra N. G., Alain T., Sonenberg N., Bramson J., Lichty B. D., Richards C. D., Ma A., Ashkar A. A. (2012) IL-15 Can Signal via IL-15Rα, JNK, and NF-κB To Drive RANTES Production by Myeloid Cells. J. Immunol. 188, 4149–4157 [DOI] [PubMed] [Google Scholar]
- 26. Tabeta K., Hoebe K., Janssen E. M., Du X., Georgel P., Crozat K., Mudd S., Mann N., Sovath S., Goode J., Shamel L., Herskovits A. A., Portnoy D. A., Cooke M., Tarantino L. M., Wiltshire T., Steinberg B. E., Grinstein S., Beutler B. (2006) The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7, 156–164 [DOI] [PubMed] [Google Scholar]
- 27. Leifer C. A., Kennedy M. N., Mazzoni A., Lee C., Kruhlak M. J., Segal D. M. (2004) TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173, 1179–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engel A., Barton G. M. (2010) Compartment-specific control of signaling from a DNA-sensing immune receptor. Sci. Signal 3, pe45. [DOI] [PubMed] [Google Scholar]
- 29. Ishikawa H., Barber G. N. (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature 455, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kashuba V. I., Protopopov A. I., Kvasha S. M., Gizatullin R. Z., Wahlestedt C., Kisselev L. L., Klein G., Zabarovsky E. R. (2002) hUNC93B1: a novel human gene representing a new gene family and encoding an unc-93-like protein. Gene 283, 209–217 [DOI] [PubMed] [Google Scholar]
- 31. Brinkmann M. M., Spooner E., Hoebe K., Beutler B., Ploegh H. L., Kim Y. M. (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landrigan A., Wong M. T., Utz P. J. (2011) CpG and non-CpG oligodeoxynucleotides directly costimulate mouse and human CD4+ T cells through a TLR9- and MyD88-independent mechanism. J. Immunol. 187, 3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hägele H., Allam R., Pawar R. D., Reichel C. A., Krombach F., Anders H. J. (2009) Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am. J. Pathol. 175, 1896–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672 [DOI] [PubMed] [Google Scholar]
- 35. Hennessy E. J., Parker A. E., O'Neill L. A. (2010) Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307 [DOI] [PubMed] [Google Scholar]
- 36. Beuneu H., Deguine J., Bouvier I., Di Santo J. P., Albert M. L., Bousso P. (2011) Cutting Edge: A dual role for type I IFNs during polyinosinic-polycytidylic acid-induced NK cell activation. J. Immunol. 187, 2084–2088 [DOI] [PubMed] [Google Scholar]
- 37. Boudreau J. E., Stephenson K. B., Wang F., Ashkar A. A., Mossman K. L., Lenz L. L., Rosenthal K. L., Bramson J. L., Lichty B. D., Wan Y. (2011) IL-15 and type I interferon are required for activation of tumoricidal NK cells by virus-infected dendritic cells. Cancer Res. 71, 2497–2506 [DOI] [PubMed] [Google Scholar]