Background: HLA-B27 is strongly associated with ankylosing spondylitis (AS).
Results: A change in the position (P) 2 anchor motif was detected in the 3% of HLA-B27 ligands identified, which showed significant homology to pathogenic bacterial sequences.
Conclusion: Unusual HLA-B*2705 ligands bind with different conformations to both AS-associated and non-AS-associated HLA-B27 subtypes.
Significance: This could be sufficient to initiate autoimmune damage in patients with AS-associated subtypes.
Keywords: Antigen Presentation, Autoimmune Diseases, Intracellular Processing, Major Histocompatibility Complex (MHC), Proteomics, Ankylosing Spondylitis, Arthritogenic Bacteria, HLA-B27 Alleles
Abstract
The presentation of short viral peptide antigens by human leukocyte antigen (HLA) class I molecules on cell surfaces is a key step in the activation of cytotoxic T lymphocytes, which mediate the killing of pathogen-infected cells or initiate autoimmune tissue damage. HLA-B27 is a well known class I molecule that is used to study both facets of the cellular immune response. Using mass spectrometry analysis of complex HLA-bound peptide pools isolated from large amounts of HLA-B*2705+ cells, we identified 200 naturally processed HLA-B*2705 ligands. Our analyses revealed that a change in the position (P) 2 anchor motif was detected in the 3% of HLA-B*2705 ligands identified. B*2705 class I molecules were able to bind these six GlnP2 peptides, which showed significant homology to pathogenic bacterial sequences, with a broad range of affinities. One of these ligands was able to bind with distinct conformations to HLA-B27 subtypes differentially associated with ankylosing spondylitis. These conformational differences could be sufficient to initiate autoimmune damage in patients with ankylosing spondylitis-associated subtypes. Therefore, these kinds of peptides (short, with GlnP2, and similar low affinity to all HLA-B27 subtypes tested but with unlike conformations in differentially ankylosing spondylitis-associated subtypes) must not be excluded from future researches involving potential arthritogenic peptides.
Introduction
Proteolytic degradation of self-proteins and pathogenic proteins in the cytosol by the combined actions of the proteasomes and degradative peptidases generates peptides, mostly of 8–10 residues, that are translocated to the endoplasmic reticulum lumen by the transporter associated with antigen processing (TAP)2 molecules. These short peptides assemble with human leukocyte antigen (HLA) class I heavy chain and β2-microglobulin molecules (1). Typically, this interaction is made possible by two major anchor residues at position 2 (P2) and the C terminus (CΩ) of the antigenic peptide (2, 3) that are deeply inserted into specific pockets of the antigen recognition site of the HLA class I molecule (4, 5). Finally, the stable trimolecular peptide-HLA-β2-microglobulin complexes are transported to the cell membrane and presented for cytotoxic T lymphocyte recognition (6). This recognition of pathogenic or self-peptide ligands can lead to the beneficial killing of pathogen-infected cells or initiate a pathologic autoimmune damage respectively.
One of the most interesting class I HLA alleles is the HLA-B27, which is strongly associated with ankylosing spondylitis (AS), a chronic inflammatory spondyloarthropathy (7). Furthermore, although most of the HLA-B27 subtypes are strongly associated with AS, HLA-B*2706 and -B*2709 are either not associated or perhaps only weakly associated with this disease (reviewed in Ref. 8), suggesting that this polymorphism subtype modulates disease susceptibility. Previous studies show an unambiguous functional distinction between the closely related AS-associated B*2704 and non-AS-associated B*2706 subtypes (9). However, after four decades of research, the basis for this association remains an enigma. Several hypotheses, each based on a particular feature of the HLA-B27 gene, have been proposed to elucidate this intriguing association, but none have yet satisfactorily explained the mechanism and the differential association of HLA-B27 subtypes with disease. The arthritogenic peptide hypothesis (10) assumes that HLA-B27 can present a microbial epitope, eliciting a normal cytotoxic T lymphocyte response against the pathogen. Unfortunately, some of these cytotoxic T lymphocytes would cross-react with an autologous self-ligand also presented by this molecule and show molecular mimicry with the primary pathogenic epitope. This cross-reaction promotes the autoimmune tissue injury and inflammation. In this hypothesis, the differential binding or immune recognition of either microbial or mimetic self-peptide(s) between the HLA subtypes explains the disparity in association with AS. The interest in this hypothesis has been highly renewed by recent studies on the role of the interaction between HLA-B27 and the endoplasmic reticulum aminopeptidase involved in the trimming of peptides for HLA class I antigen presentation, ERAP1 (11, 12).
The major outstanding feature of HLA-B*2705 specificity is its almost mandatory requirement for Arg at P2 (SYFPEITHI database (3)). In contrast, two ligands with GlnP2 were identified (13, 14). To broaden this study, by means of an immunoproteomics analysis of peptide pools isolated from HLA-B27+ cells the current study identifies, in addition to several hundred HLA-B27 ArgP2 self-ligands, a small fraction of peptides contained Gln at the P2 anchor motif. These peptides bind to HLA-B27 subtypes with differential association with AS and show significant homology to arthritogenic bacterial sequences.
EXPERIMENTAL PROCEDURES
Cell Lines and Antibodies
B27-C1R is an HLA-B*2705 transfectant (15) of the human lymphoid cell line HMy2.C1R (C1R) that expresses endogenous HLA class I antigens at low levels (16). RMA-S is a TAP-deficient murine cell line that expresses the mouse H-2b haplotype (17). The RMA-S transfectant cells expressing HLA-B*2705 (18), -B*2704 (19), or -B*2706 (19) have been previously described. All cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 5 μm β-mercaptoethanol. The monoclonal antibodies (mAbs) used in this study were W6/32 (specific for a monomorphic HLA-A, -B, and -C determinant) (20) and ME1 (which is specific for HLA-B27, -B7, and -Bw22) (21).
Synthesis of Peptides
Peptides were synthesized in a peptide synthesizer (model 433A; Applied Biosystems, Foster City, CA) and purified by reverse-phase HPLC. The molecular mass of peptides was established with MALDI-TOF MS, and composition was determined by μLC-MS/MS.
Isolation of HLA-bound Peptides
HLA-bound peptides were isolated from 4 × 1010 B27-C1R transfectant cells as described previously (22). Cells were lysed in 1% IGEPAL CA-630 (Sigma), 20 mm Tris/HCl buffer, and 150 mm NaCl, pH 7.5, in the presence of a protease inhibitor mixture. HLA-peptide complexes were isolated by affinity chromatography of the soluble fraction with the W6/32 mAb. HLA-bound peptides were eluted at room temperature with 0.1% aqueous trifluoroacetic acid (TFA) and concentrated with a Centricon 3 column (Amicon, Beverly, MA), as described previously (22).
Electrospray-Ion Trap Mass Spectrometry Analysis
Peptide mixtures recovered after the ultrafiltration step were concentrated with Micro-Tip reverse-phase columns (C18, 200 μl, Harvard Apparatus, Holliston, MA). Each C18 tip was equilibrated with 80% acetonitrile in 0.1% TFA, washed with 0.1% TFA, and then loaded with the peptide mixture. The tip was then washed with an additional volume of 0.1% TFA, and the peptides were eluted with 80% acetonitrile in 0.1% TFA. Peptide samples were then concentrated to ∼18 μl using vacuum centrifugation.
Recovered HLA class I peptides were analyzed in three HPLC procedures by μLC-MS/MS using an Orbitrap XL mass spectrometer (Thermo Electron) fitted with a capillary HPLC column (Eksigent, Dublin, CA). The peptides were resolved on a homemade ReproSil C18 capillary column (75-μm inner diameter) with a 7–40% acetonitrile gradient for 2 h in the presence of 0.1% formic acid, as in Ref. 22. The seven most intense masses that exhibited single-, double-, and triple-charge states were selected for fragmentation by collision-induced dissociation from each full mass spectrum.
Database Searches
Pep-Miner (23) was used for peak list generation of the μLC-MS/MS data. The HLA peptides were identified using the following search engines: Pep-Miner (23); Proteome Discoverer 1.0 SP1 (Thermo Electron), combining the results of Sequest 3.31 and Bioworks Browser 3.3.1 SP1 (Thermo Electron) (24); and Mascot (server 2.2, Matrix Science) (25), using the human and the viral sections of the National Center for Biotechnology Information (NCBI) database, which includes 448,769 proteins. The search was not limited by enzymatic specificity, the peptide tolerance was set to 0.005 Da, and the fragment ion tolerance was set to 0.5 Da. Identified peptides were selected if the following criteria were met: Pep-Miner score above 80; Mascot score above 20; Sequest Xcorr >1.7 for singly charged, >2.2 for doubly charged, and >2.9 for triply charged peptides; Ppep less than 1 × 10−4 with Bioworks Browser; Proteome Discovered score higher than 20; and mass accuracy of 0.005 Da (22). When the MS/MS spectra fitted more than one peptide, only the highest scoring peptide was analyzed. The false positive rate for peptide identification was set to 2% based on the search of a reversed database. In addition, the corresponding synthetic peptides were made, and their manually identified MS/MS spectra were used to confirm the assigned sequence of HLA-B27 ligands.
MHC/Peptide Stability Assays
The following synthetic peptides were used as controls in complex stability assays: Flu NP (SRYWAIRTR, HLA-B27-restricted) (26) and C4CON (QYDDAVYLK, HLA-Cw4-restricted) (27). RMA-S B*2705 transfectant cells, a cell line deficient in TAP that expresses low levels of cell surface MHC class I, were incubated at 26 °C for 16 h in RPMI 1640 medium supplemented with 10% heat-inactivated FBS. This allows the expression of empty MHC class I molecules (without antigenic peptide) at the cellular membrane that are stable at 26 °C but not at 37 °C. The cells were washed and incubated for 2 h at 26 °C with various concentrations of peptide in the same medium. The cells were maintained at 37 °C for an additional 4 h and then collected for flow cytometry. This method allows empty MHC class I molecules to become internalized and can thus discriminate between bound and unbound peptides. MHC expression was measured using 100 μl of hybridoma culture supernatant containing ME1 (anti-HLA-B27) mAb as described previously (28). Data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using CellQuest Pro 2.0 software (BD Biosciences). Cells incubated without peptides exhibited peak fluorescence intensities close to the background staining observed with secondary antibody alone. The fluorescence index was calculated for each time point as the ratio of peak channel fluorescence of the sample to that of the control incubated without peptide. Binding of peptides was also expressed as EC50, which is the molar concentration of the peptide at 50% of the maximum fluorescence obtained in a concentration range of 100–0.001 μm.
Statistical Analysis
To analyze statistical significance, an unpaired Student's t test was used. p values < 0.001 were considered to be significant.
RESULTS
HLA-B*2705 Peptidome
HLA-bound peptide pools were isolated from large amounts of B27-C1R cells. This peptide mixture was subsequently separated by reverse-phase HPLC and analyzed by mass spectrometry. Using several software technologies (see “Experimental Procedures”), 198 fragmentation spectra were resolved with high confidence parameters as peptidic sequences of different human cellular proteins (data not shown). As a control, a reverse database search of the same HLA-B27-bound peptide pool showed a 2% false positive rate.
Arg or Gln as the HLA-B27 P2 Anchor Motif
The classical anchor motif for HLA-B*2705 binding, Arg at the P2 residue (SYFPEITHI database (3)), was present in 193 (97%) of the detected ligands, and no differences with the HLA-B27 ligands previously described were found (data not shown). In contrast, this motif was absent in the other six ligands (Table 1). Although virtually all significant fragments of all MS/MS spectra were assigned as daughter ions of the tentative peptidic sequence (supplemental Figs. 1–6, upper panels), the bioinformatics assignment of each sequence was confirmed by identity with the MS/MS spectrum of each corresponding synthetic peptide (supplemental Figs. 1–6, lower panels). These confirmations of synthetic peptides indicate the correct database assignment of the HLA-B27 peptidome.
TABLE 1.
Summary of endogenous ligands with Gln2 detected by MS/MS analysis
CTCL, cutaneous T-cell lymphoma.
Two of the ligands with GlnP2 had been previously detected in an earlier study of the HLA-B*2705 peptidome (14) (Table 1). One peptide was derived from the cartilage-related protein ADAM10, two nested peptides were derived from the monomorphic β2-microglobulin of the HLA class I molecule, and the last three were derived from a tumor antigen, a hypothetical protein, and the SF3B2 protein, respectively (Table 1).
In summary, these results indicate that a total of six ligands with absent ArgP2 anchor motifs were endogenously processed and presented in the HLA-B*2705+ cell line. In addition, these data confirm that GlnP2 is an anchor motif derived from peptides bound to the HLA-B*2705 class I molecule.
GlnP2 Ligands Bind to the B*2705 Molecule
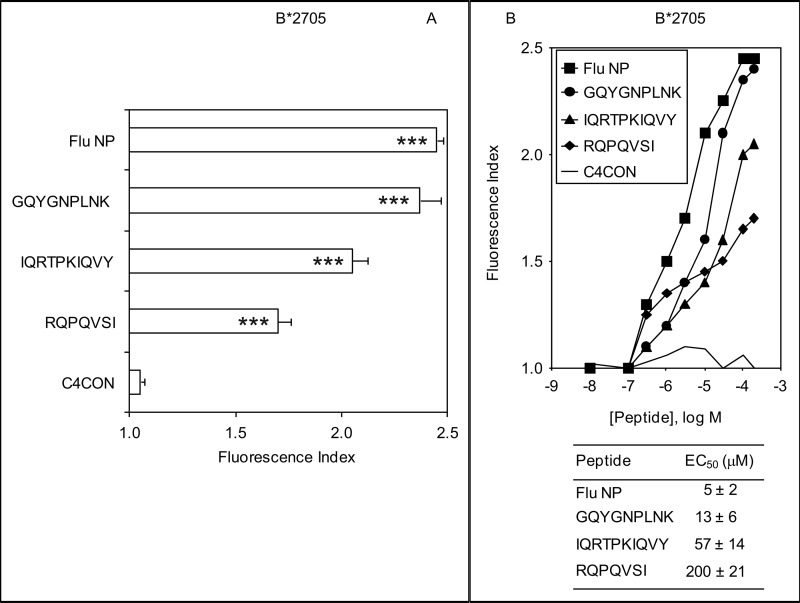
Following a similar strategy to the one used in this study, recent studies have identified several hundred HLA-B27 ligands by immunoprecipitation with the W6/32 mAb of HLA-B27-peptide complexes using the B27-C1R cell line (summarized in the SYFPEITHI database (3)). As these six ligands with GlnP2 do not possess the major HLA-B27 anchor motif ArgP2, one possibility is that they could have a defective interaction with the HLA-B27 class I molecule. To test this hypothesis, MHC/peptide complex stability assays were carried out using TAP-deficient RMA-S cells transfected with HLA-B*2705 and three of the six noncanonical ligands. These included the larger (IQRTPKIQVY) and shorter (RQPQVSI) ligands as well as a nonamer (GQYGNPLNK). The GQYGNPLNK synthetic peptide induced similar numbers of HLA-peptide surface complexes to a well known HLA-B*2705 epitope from the influenza virus, whereas the other two peptides induced fewer HLA-B*2705-peptide complexes (Fig. 1A). In addition, the relative MHC class I affinity was determined for all peptides. The GQYGNPLNK peptide bound to HLA-B*2705 class I molecules with EC50 values in the range commonly observed among natural ligands (Fig. 1B). In contrast, the HLA affinity was substantially less for the IQRTPKIQVY (EC50, 57 ± 14) and RQPQVSI (EC50, 200 ± 21) peptides, and thus, these peptides must be considered as medium and low affinity ligands, respectively (Fig. 1B). These data indicate that all ligands detected in the B27-C1R cell line were endogenously presented in association with the B*2705 molecule and that this molecule could bind peptides with a broad range of affinities.
FIGURE 1.
HLA-B*2705 stabilization assay of synthetic ligands. A, stability of HLA-B*2705/peptide complexes on the surface of RMA-S transfectant cells was measured by flow cytometry. The indicated peptides were used at 200 μm. Significant p values: ***, p < 0.001. B, the titration curves of synthetic GQYGNPLNK (circles), IQRTPKIQVY (triangles), and RQPQVSI (diamonds) peptides with HLA-B*2705 are depicted. The C4CON (solid line) (27) and Flu NP (squares) (26) peptides were used as negative and positive controls, respectively. The results, calculated as the fluorescence index (panel A) or EC50 values ± S.D. (panel B), are the mean of three or four independent experiments.
Binding of the RQPQVSI Peptide to HLA-B*2705 Molecules
Peptides without an N-terminal interaction with the HLA molecule have been identified as endogenously bound to HLA-B39 (29) and the murine MHC class I molecule H-2Dd (30), indicating that canonical MHC-peptide interactions in the P1 pocket are not always necessary for endogenous peptide presentation. Usually, the HLA-B*2705 molecule binds peptides of 8–13 residues (3, 31), but in this study, a 7-mer was found bound to this HLA class I molecule. One possibility for this is that the short RQPQVSI peptide binds to HLA-B27 molecules in the same manner that other peptides lacking the N-terminal binding residue do. Thus, new HLA-B*2705/peptide complex stability assays were carried out exchanging either the ArgP1 or the GlnP2 residues of the RQPQVSI peptide for alanine. Fig. 2 shows similar binding affinities for both the HLA-B*2705 natural ligands and the single Ala-substituted peptides. The exchange of both ArgP1 and GlnP2 residues by Ala abrogated binding to HLA-B*2705 molecules. Collectively, these data indicate that ArgP1 as well as GlnP2 residues of the RQPQVSI peptide are sufficient to support interactions with class I molecules.
FIGURE 2.
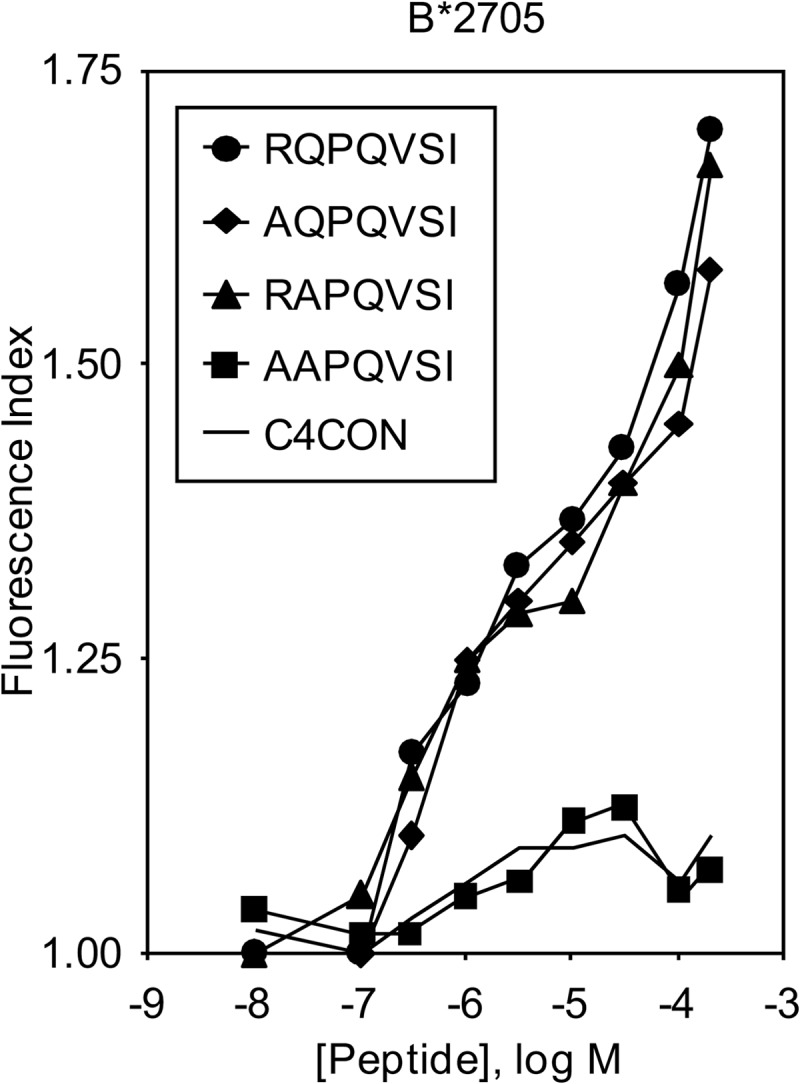
HLA-B*2705 stabilization assay of synthetic peptide RQPQVSI with Ala substitutions in anchor motifs. The stability of HLA-B*2705/peptide complexes on the surface of RMA-S transfectant cells was measured by flow cytometry. The titration curves of synthetic RQPQVSI (circles), AQPQVSI (diamonds), RAPQVSI (triangles), and AAPQVSI (squares) peptides with HLA-B*2705 are depicted. The results, which are the means of three independent experiments, are as shown in Fig. 1B.
GlnP2 Ligands Bind to HLA-B27 Subtypes with Differential Association with Ankylosing Spondylitis
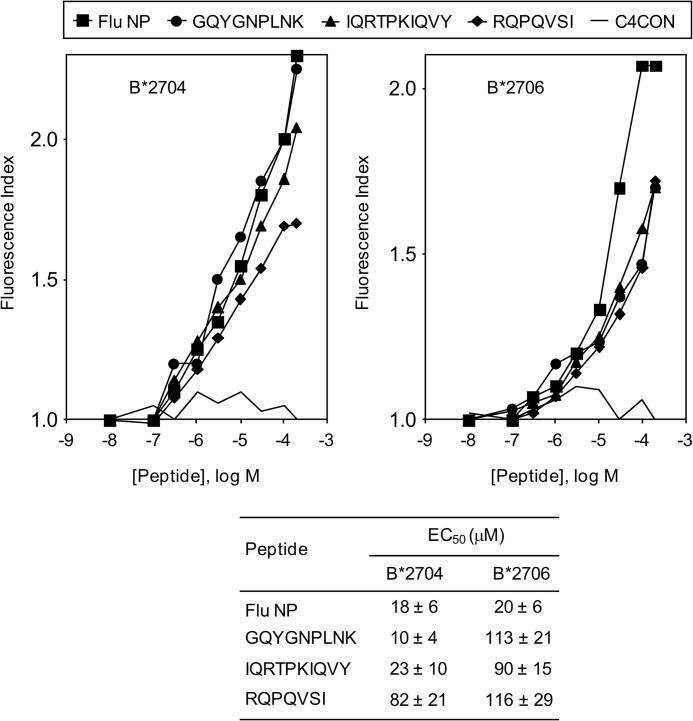
In contrast to HLA-B*2705, some HLA-B27 subtypes are not associated with AS (reviewed in Ref. 8). Thus, we selected a pair of HLA-B27 subtypes with differential association with this autoimmune disease. Although HLA-B*2706 is not associated with AS, its most related HLA-B27 subtype, B*2704, is an AS-associated subtype. Thus, a comparison of the specific binding of these HLA class I molecules to peptides with noncanonical GlnP2 residues was carried out. The binding to HLA-B*2706 was significantly less than that of B*2704 (and the B*2705) with both the GQYGNPLNK and the IQRTPKIQVY peptides; thus, these peptides must be considered low affinity ligands in the subtype associated with AS (Fig. 3). No differences in binding were found with the RQPQVSI peptide in both subtypes (Fig. 3). In summary, the three GlnP2 peptides studied bound to HLA-B*2706 with relatively low affinity. In addition, the differential binding between the B*2704 and B*2706 subtypes detected with the GQYGNPLNK and IQRTPKIQVY peptides, but not with RQPQVSI peptide, correlated with the previously reported repertoire of ArgP2 peptide specificity of these subtypes, where B*2704 but not B*2706 favors the binding of Tyr or Arg at residue carboxy-terminal of a peptide (PΩ) (32).
FIGURE 3.
HLA stabilization assay of subtypes with differential association with ankylosing spondylitis of synthetic ligands. The stability of HLA-B*2704 (left panel) or HLA-B*2706 (right panel)/peptide complexes on the surface of RMA-S transfectant cells was measured by flow cytometry. The code used and the results, which represent the means of three independent experiments, are as shown in Fig. 1B. Also, the calculated EC50 values ± S.D. are shown in the lower panel.
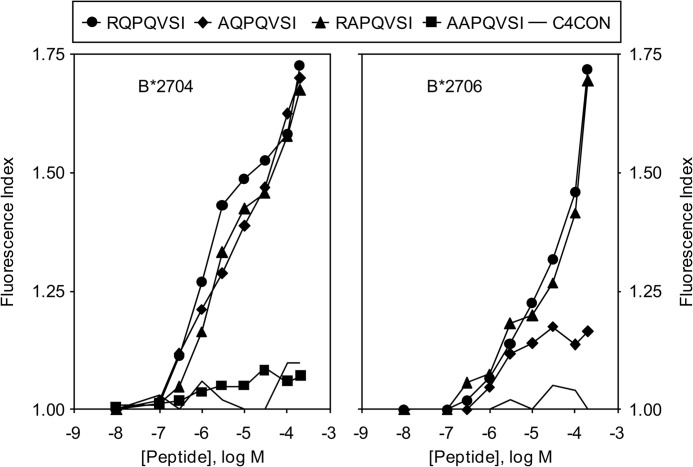
Differential Binding of the RQPQVSI Peptide to HLA-B27 Subtypes with Disparate Association with Ankylosing Spondylitis
The RQPQVSI peptide bound to the HLA-B*2704 and -B*2706 subtypes with similarly low affinities (Fig. 3). To study the requirements of the binding of these peptides to the related HLA-B27 subtypes, the exchange of alanine for either the ArgP1 or the GlnP2 residues of the RQPQVSI peptide was tested in complex stability assays. The relative binding to the B*2704 subtype of both natural ligands and Ala-substituted peptides was identical (Fig. 4, left panel). Thus, the RQPQVSI peptide bound in similar ways to two different AS-associated subtypes, B*2705 and B*2704. On the other hand, the ArgP1 residue of the RQPQVSI peptide is critical for binding to the B*2706 subtype (Fig. 4, right panel) but not to the B*2704 subtype (Fig. 4, left panel). Thus, in the interactions of this subtype with the RQPQVSI peptide, the ArgP1 residue is the anchor motif and mediates binding to this subtype, as do other peptides lacking the N-terminal binding residue (29, 30). In summary, the RQPQVSI peptide bound with a comparably low affinity, but with different conformation, to HLA-B27 subtypes associated (B*2705 and B*2704) or not associated (B*2706) with ankylosing spondylitis (Table 2).
FIGURE 4.
HLA stabilization assay of subtypes with differential association with ankylosing spondylitis of synthetic peptide RQPQVSI with Ala substitutions in anchor motifs. The stability of HLA-B*27/peptide complexes on the surface of RMA-S transfectant cells was measured by flow cytometry. The titration curves of synthetic RQPQVSI (circles), AQPQVSI (diamonds), RAPQVSI (triangles), and AAPQVSI (squares) peptides with HLA-B*2704 (left panel) or -B*2706 (right panel) are depicted. The results, which represent the means of three independent experiments, are as shown in Fig. 1B.
TABLE 2.
Binding to HLA-B27 subtypes with differential association to ankylosing spondylitis to synthetic peptide RQPQVSI with Ala substitutions in anchor motifs
| Subtype | Association with AS | RQPQVSIa | AQPQVSIa,b | RAPQVSIa,b | AAPQVSIb,c |
|---|---|---|---|---|---|
| B*2705 | Yes | + | + | + | − |
| B*2704 | Yes | + | + | + | − |
| B*2706 | No | + | − | + | NDc |
a Binding to HLA-B27 subtypes.
b The substitution for Ala is underlined.
c ND, not done.
Homology of GlnP2 Ligands with Pathogenic Bacterial Sequences
A comparison of the sequences of six natural HLA-B27 GlnP2 ligands identified in the current study with 12 species of bacteria associated with HLA-B27-dependent reactive arthritis (33) was carried out. Table 3 shows diverse bacterial sequences that differ from endogenous HLA-B27 peptides by 2–3 residues. Most of these changes are in surface-exposed regions of proteins; thus, they could modulate differential interactions with the T-cell receptor as compared with endogenous cellular HLA-B27 peptides as shown in the HLA-B*2705 crystal structure (34) (PDB: 1HSA). In addition, four proteins with HLA-B27 GlnP2 ligands, ADAM10, β2-microglobulin, cutaneous T-cell lymphoma antigen, and SF3B2, were included in a network of connective tissue disorders and inflammatory disease following analysis with the Ingenuity Pathway Analysis software.
TABLE 3.
Alignment of natural HLA-B27 peptides with sequences of bacteria associated with B27-dependent reactive arthritis
CTCL, cutaneous T-cell lymphoma.
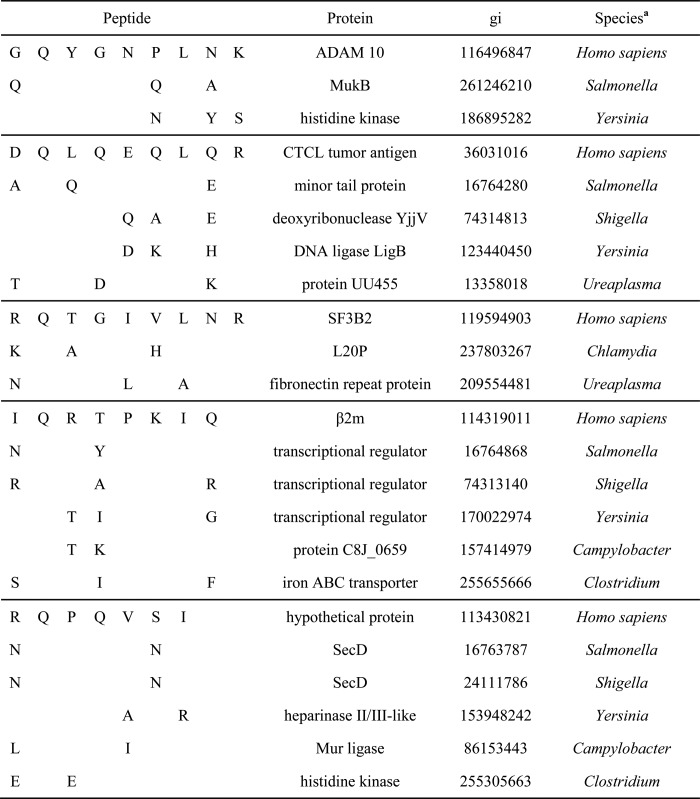
a Bacterial sequences analyzed: Salmonella typhimurium, Salmonella enteritidis, Salmonella paratyphi, Shigella flexneri, Shigella sonnei, Shigella dyssenteriae, Chlamydia trachomatis, Yersinia enterocolitica, Yersinia pseudotuberculosis, Campylobacter jejuni, Ureaplasma urealyticum, Clostridium difficile.
DISCUSSION
In this study, we have investigated several issues concerning the processing of natural HLA-B*2705 ligands, including the analysis of a refined binding motif and the binding of GlnP2 ligands to HLA-B27 subtypes differentially associated with AS. Previously, some HLA-B*2705 ligands with GlnP2 had been described (13, 14). In the present study, this change in the P2 anchor motif was detected in the 3% of HLA-B*2705 ligands identified. Thus, these data confirm the refined HLA-B*2705 binding motif. In addition, five of six ligands identified by mass spectrometry analysis show the preferential residue usage both P1 and/or PΩ positions previously described from canonical HLA-B*2705 ligands (14, 35). Thus, the GlnP2 ligands could interact with the pockets of the HLA class I-presenting molecule in the same way those ArgP2 canonical ligands.
Five of the six ligands with GlnP2 were derived from four proteins included in a network of connective tissue disorders. ADAM10 is a chondrocyte-derived metalloproteinase involved in joint pathology (36, 37). The β2-microglobulin is associated with HLA class I heavy chains and reduces HLA-B27 misfolding promoting arthritis and spondylitis in HLA-B27-transgenic rats (38). HD-CL-01 is an antigen of cutaneous T-cell lymphoma, and polyarthritis in the presence of this lymphoma is a phenomenon previously described (39–41). Lastly, SF3B2 is a component of the spliceosome complex (42) that has been linked to rheumatoid arthritis and several connective tissue diseases (43, 44).
All these human HLA-B27 GlnP2 ligands have homology with distinct arthritogenic bacterial sequences that differ from these endogenous HLA-B27 peptides by 2–3 residues. Remarkably, several arthritogenic bacterial sequences possess changes in the ArgP1 of the RQPQVSI sequence that could alter the binding conformations between the different subtypes of HLA-B27 associated or not associated with AS.
The observation that HLA-B*2704 and -B*2706 subtypes share a very broad fraction of ∼90% of their peptide repertoires (32) suggests that putative arthritogenic peptides could be confined in a first approximation to a relatively small portion of ligands shared by AS-associated subtypes such as B*2705 and B*2704 that are missing from the B*2706 peptide repertoire. The self-derived ligands with GlnP2, analyzed in the current study, bind to AS-associated class I subtypes (HLA-B*2705 and -B*2704) with a broad range of affinities, whereas they have shown a low affinity in their interaction with the non-AS-associated HLA-B*2706 allele. Thus, the differences of affinity in self-derived ligands could trigger autoimmune tissue injury in AS-associated versus non-AS-associated HLA-B27 class I positive individuals. In addition, our study indicates that some unusual HLA-B*2705 ligands, such as the RQPQVSI peptide, bind with similar affinity but different conformations to both AS-associated and non-AS-associated HLA-B27 subtypes. The HLA-B27 subtype-dependent conformation of canonical ArgP2 self-peptides was previously described (45, 46). Thus, these conformational differences, which could be detected by the fine specificity of autoreactive cytotoxic T lymphocytes, could be sufficient to initiate autoimmune damage in patients with AS-associated subtypes. Therefore, these kinds of peptides (short, with GlnP2, and with similar low affinity to all HLA-B27 subtypes tested but with dissimilar conformations in differentially AS-associated subtypes) must not be excluded from future research involving potential arthritogenic peptides.
In summary, these results inform us about the interaction between unusual GlnP2 self-derived ligands and different HLA-B27 subtypes in the pathogenic role of these class I molecules in the triggering of AS autoimmune disease.
Acknowledgment
We thank Dr. J. A. López de Castro (Centro de Biología Molecular Severo Ochoa, Madrid, Spain) for the cell lines.
This work was supported by grants from the Programa Ramón y Cajal and the Ministerio de Ciencia e Innovación (to D. L.) and from the Israel Science Foundation (ISF 916/05) (to A. A.).

This article contains supplemental Figs. 1–6.
- TAP
- transporter associated with antigen processing
- AS
- ankylosing spondylitis
- B27-C1R
- HMy2.C1R transfected with HLA-B*2705
- P2
- position 2
- μLC-MS/MS
- micro-tandem liquid chromatography/mass spectrometry.
REFERENCES
- 1. Shastri N., Schwab S., Serwold T. (2002) Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 20, 463–493 [DOI] [PubMed] [Google Scholar]
- 2. Parker K. C., Bednarek M. A., Coligan J. E. (1994) Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152, 163–175 [PubMed] [Google Scholar]
- 3. Rammensee H.-G., Bachmann J., Emmerich N. P. N., Bachor O. A., Stevanović S. (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50, 213–219 [DOI] [PubMed] [Google Scholar]
- 4. Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. (1987) Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329, 506–512 [DOI] [PubMed] [Google Scholar]
- 5. Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. (1987) The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 329, 512–518 [DOI] [PubMed] [Google Scholar]
- 6. York I. A., Goldberg A. L., Mo X. Y., Rock K. L. (1999) Proteolysis and class I major histocompatibility complex antigen presentation. Immunol. Rev. 172, 49–66 [DOI] [PubMed] [Google Scholar]
- 7. Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. (1973) Ankylosing spondylitis and HL-A 27. Lancet 1, 904–907 [DOI] [PubMed] [Google Scholar]
- 8. Marcilla M., López de Castro J. A. (2008) Peptides: the cornerstone of HLA-B27 biology and pathogenetic role in spondyloarthritis. Tissue Antigens 71, 495–506 [DOI] [PubMed] [Google Scholar]
- 9. López D., Rojo S., Calvo V., López de Castro J. A. (1992) Peptide-presenting similarities among functionally distant HLA-B27 subtypes revealed by alloreactive T lymphocytes of unusual specificity. J. Immunol. 148, 996–1002 [PubMed] [Google Scholar]
- 10. Benjamin R., Parham P. (1990) Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol. Today 11, 137–142 [DOI] [PubMed] [Google Scholar]
- 11. Evans D. M., Spencer C. C., Pointon J. J., Su Z., Harvey D., Kochan G., Oppermann U., Opperman U., Dilthey A., Pirinen M., Stone M.A, Appleton L., Moutsianas L., Moutsianis L., Leslie S., Wordsworth T., Kenna T. J., Karaderi T., Thomas G. P., Ward M. M., Weisman M. H., Farrar C., Bradbury L. A., Danoy P., Inman R. D., Maksymowych W., Gladman D., Rahman P., Spondyloarthritis Research Consortium of Canada (SPARCC), Morgan A., Marzo-Ortega H., Bowness P., Gaffney K., Gaston J. S., Smith M., Bruges-Armas J., Couto A. R., Sorrentino R., Paladini F., Ferreira M. A., Xu H., Liu Y., Jiang L., Lopez-Larrea C., Díaz-Peña R., López-Vázquez A., Zayats T., Band G., Bellenguez C., Blackburn H., Blackwell J. M., Bramon E., Bumpstead S. J., Casas J. P., Corvin A., Craddock N., Deloukas P., Dronov S., Duncanson A., Edkins S., Freeman C., Gillman M., Gray E., Gwilliam R., Hammond N., Hunt S. E., Jankowski J., Jayakumar A., Langford C., Liddle J., Markus H. S., Mathew C. G., McCann O. T., McCarthy M. I., Palmer C. N., Peltonen L., Plomin R., Potter S. C., Rautanen A., Ravindrarajah R., Ricketts M., Samani N., Sawcer S. J., Strange A., Trembath R. C., Viswanathan A. C., Waller M., Weston P., Whittaker P., Widaa S., Wood N. W., McVean G., Reveille J. D., Wordsworth B. P., Brown M. A., Donnelly P., Australo-Anglo-American Spondyloarthritis Consortium (TASC), and Wellcome Trust Case Control Consortium 2 (WTCCC2) (2011) Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haroon N., Tsui F. W., Uchanska-Ziegler B., Ziegler A., Inman R. D. (2012) Endoplasmic reticulum aminopeptidase 1 (ERAP1) exhibits functionally significant interaction with HLA-B27 and relates to subtype specificity in ankylosing spondylitis. Ann. Rheum. Dis. 71, 589–595 [DOI] [PubMed] [Google Scholar]
- 13. Alvarez I., Sesma L., Marcilla M., Ramos M., Marti M., Camafeita E., de Castro J. A. (2001) Identification of novel HLA-B27 ligands derived from polymorphic regions of its own or other class I molecules based on direct generation by 20 S proteasome. J. Biol. Chem. 276, 32729–32737 [DOI] [PubMed] [Google Scholar]
- 14. Ben Dror L., Barnea E., Beer I., Mann M., Admon A. (2010) The HLA-B*2705 peptidome. Arthritis Rheum. 62, 420–429 [DOI] [PubMed] [Google Scholar]
- 15. Calvo V., Rojo S., López D., Galocha B., López de Castro J. A. (1990) Structure and diversity of HLA-B27-specific T cell epitopes. Analysis with site-directed mutants mimicking HLA-B27 subtype polymorphism. J. Immunol. 144, 4038–4045 [PubMed] [Google Scholar]
- 16. Storkus W. J., Howell D. N., Salter R. D., Dawson J. R., Cresswell P. (1987) NK susceptibility varies inversely with target cell class I HLA antigen expression. J. Immunol. 138, 1657–1659 [PubMed] [Google Scholar]
- 17. Ljunggren H. G., Kärre K. (1985) Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J. Exp. Med. 162, 1745–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villadangos J. A., Galocha B., López de Castro J. A. (1994) Unusual topology of an HLA-B27 allospecific T cell epitope lacking peptide specificity. J. Immunol. 152, 2317–2323 [PubMed] [Google Scholar]
- 19. Galocha B., Lamas J. R., Villadangos J. A., Albar J. P., López de Castro J. A. (1996) Binding of peptides naturally presented by HLA-B27 to the differentially disease-associated B*2704 and B*2706 subtypes, and to mutants mimicking their polymorphism. Tissue Antigens 48, 509–518 [DOI] [PubMed] [Google Scholar]
- 20. Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. (1978) Production of monoclonal antibodies to group A erythrocytes, HLA, and other human cell surface antigens-new tools for genetic analysis. Cell 14, 9–20 [DOI] [PubMed] [Google Scholar]
- 21. Ellis S. A., Taylor C., McMichael A. (1982) Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum. Immunol. 5, 49–59 [DOI] [PubMed] [Google Scholar]
- 22. Infantes S., Lorente E., Barnea E., Beer I., Cragnolini J. J., García R., Lasala F., Jiménez M., Admon A., López D. (2010) Multiple, non-conserved, internal viral ligands naturally presented by HLA-B27 in human respiratory syncytial virus-infected cells. Mol. Cell Proteomics 9, 1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beer I., Barnea E., Ziv T., Admon A. (2004) Improving large-scale proteomics by clustering of mass spectrometry data. Proteomics 4, 950–960 [DOI] [PubMed] [Google Scholar]
- 24. Eng J. K., McCormack A. L., Yates J. R. (2009) An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 25. Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 26. Wang M., Lamberth K., Harndahl M., Røder G., Stryhn A., Larsen M. V., Nielsen M., Lundegaard C., Tang S. T., Dziegiel M. H., Rosenkvist J., Pedersen A. E., Buus S., Claesson M. H., Lund O. (2007) CTL epitopes for influenza A including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine 25, 2823–2831 [DOI] [PubMed] [Google Scholar]
- 27. Fan Q. R., Garboczi D. N., Winter C. C., Wagtmann N., Long E. O., Wiley D. C. (1996) Direct binding of a soluble natural killer cell inhibitory receptor to a soluble human leukocyte antigen-Cw4 class I major histocompatibility complex molecule. Proc. Natl. Acad. Sci. U.S.A. 93, 7178–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samino Y., López D., Guil S., Saveanu L., van Endert P. M., Del Val M. (2006) A long N-terminal-extended nested set of abundant and antigenic major histocompatibility complex class I natural ligands from HIV envelope protein. J. Biol. Chem. 281, 6358–6365 [DOI] [PubMed] [Google Scholar]
- 29. Yagüe J., Marina A., Vázquez J., López de Castro J. A. (2001) Major histocompatibility complex class I molecules bind natural peptide ligands lacking the amino-terminal binding residue in vivo. J. Biol. Chem. 276, 43699–43707 [DOI] [PubMed] [Google Scholar]
- 30. Samino Y., López D., Guil S., de León P., Del Val M. (2004) An endogenous HIV envelope-derived peptide without the terminal NH3+ group anchor is physiologically presented by major histocompatibility complex class I molecules. J. Biol. Chem. 279, 1151–1160 [DOI] [PubMed] [Google Scholar]
- 31. Sesma L., Galocha B., Vázquez M., Purcell A. W., Marcilla M., McCluskey J., López de Castro J. A. (2005) Qualitative and quantitative differences in peptides bound to HLA-B27 in the presence of mouse versus human tapasin define a role for tapasin as a size-dependent peptide editor. J. Immunol. 174, 7833–7844 [DOI] [PubMed] [Google Scholar]
- 32. Sesma L., Montserrat V., Lamas J. R., Marina A., Vázquez J., López de Castro J. A. (2002) The peptide repertoires of HLA-B27 subtypes differentially associated to spondyloarthropathy (B*2704 and B*2706) differ by specific changes at three anchor positions. J. Biol. Chem. 277, 16744–16749 [DOI] [PubMed] [Google Scholar]
- 33. Colmegna I., Cuchacovich R., Espinoza L. R. (2004) HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin. Microbiol. Rev. 17, 348–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. (1992) The three-dimensional structure of HLA-B27 at 2.1 Ä resolution suggests a general mechanism for tight peptide binding to MHC. Cell 70, 1035–1048 [DOI] [PubMed] [Google Scholar]
- 35. Lopez de Castro J. A., Alvarez I., Marcilla M., Paradela A., Ramos M., Sesma L., Vázquez M. (2004) HLA-B27: a registry of constitutive peptide ligands. Tissue Antigens 63, 424–445 [DOI] [PubMed] [Google Scholar]
- 36. McKie N., Edwards T., Dallas D. J., Houghton A., Stringer B., Graham R., Russell G., Croucher P. I. (1997) Expression of members of a novel membrane linked metalloproteinase family (ADAM) in human articular chondrocytes. Biochem. Biophys. Res. Commun. 230, 335–339 [DOI] [PubMed] [Google Scholar]
- 37. Chubinskaya S., Mikhail R., Deutsch A., Tindal M. H. (2001) ADAM-10 protein is present in human articular cartilage primarily in the membrane-bound form and is upregulated in osteoarthritis and in response to IL-1α in bovine nasal cartilage. J. Histochem. Cytochem. 49, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 38. Tran T. M., Dorris M. L., Satumtira N., Richardson J. A., Hammer R. E., Shang J., Taurog J. D. (2006) Additional human β2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 54, 1317–1327 [DOI] [PubMed] [Google Scholar]
- 39. Seleznick M. J., Aguilar J. L., Rayhack J., Fenske N., Espinoza L. R. (1989) Polyarthritis associated with cutaneous T cell lymphoma. J. Rheumatol. 16, 1379–1382 [PubMed] [Google Scholar]
- 40. Levy Y., George J., Abraham A., Afek A., Livneh A., Shoenfeld Y. (1997) Subcutaneous T-cell lymphoma in a patient with rheumatoid arthritis not treated with cytotoxic agents. Clin. Rheumatol. 16, 606–608 [DOI] [PubMed] [Google Scholar]
- 41. Sandberg Y., El Abdouni M., Lam K. H., Langerak A. W., Lugtenburg P. J., Dolhain R. J., Heule F. (2004) Clonal identity between skin and synovial tissue in a case of mycosis fungoides with polyarthritis. J. Am. Acad. Dermatol. 51, 111–117 [DOI] [PubMed] [Google Scholar]
- 42. Kaida D., Motoyoshi H., Tashiro E., Nojima T., Hagiwara M., Ishigami K., Watanabe H., Kitahara T., Yoshida T., Nakajima H., Tani T., Horinouchi S., Yoshida M. (2007) Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 3, 576–583 [DOI] [PubMed] [Google Scholar]
- 43. Hassfeld W., Steiner G., Studnicka-Benke A., Skriner K., Graninger W., Fischer I., Smolen J. S. (1995) Autoimmune response to the spliceosome. An immunologic link between rheumatoid arthritis, mixed connective tissue disease, and systemic lupus erythematosus. Arthritis Rheum. 38, 777–785 [DOI] [PubMed] [Google Scholar]
- 44. Hoffman R. W., Maldonado M. E. (2008) Immune pathogenesis of mixed connective tissue disease: a short analytical review. Clin. Immunol. 128, 8–17 [DOI] [PubMed] [Google Scholar]
- 45. Hülsmeyer M., Fiorillo M. T., Bettosini F., Sorrentino R., Saenger W., Ziegler A., Uchanska-Ziegler B. (2004) Dual, HLA-B27 subtype-dependent conformation of a self-peptide. J. Exp. Med. 199, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fabian H., Huser H., Loll B., Ziegler A., Naumann D., Uchanska-Ziegler B. (2010) HLA-B27 heavy chains distinguished by a micropolymorphism exhibit differential flexibility. Arthritis Rheum. 62, 978–987 [DOI] [PubMed] [Google Scholar]