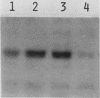
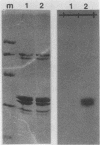
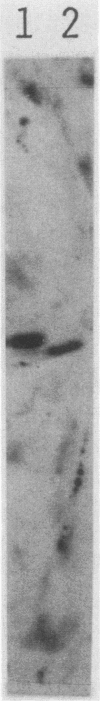
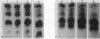Abstract
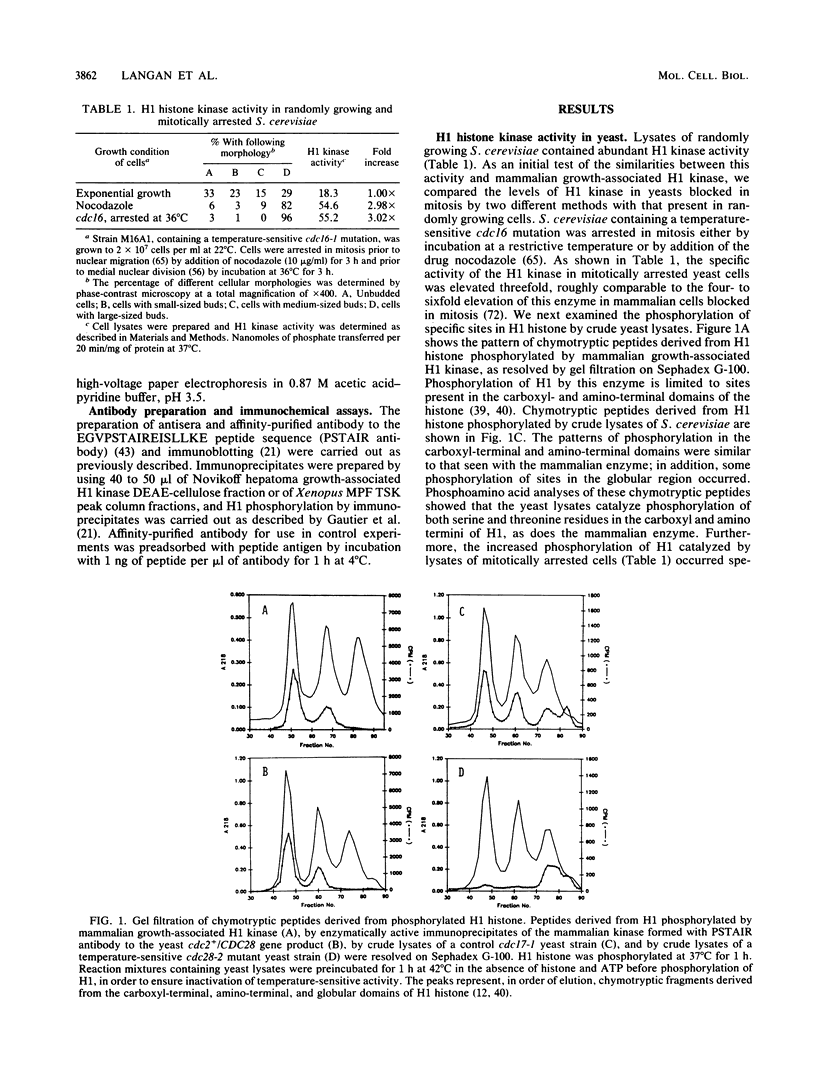
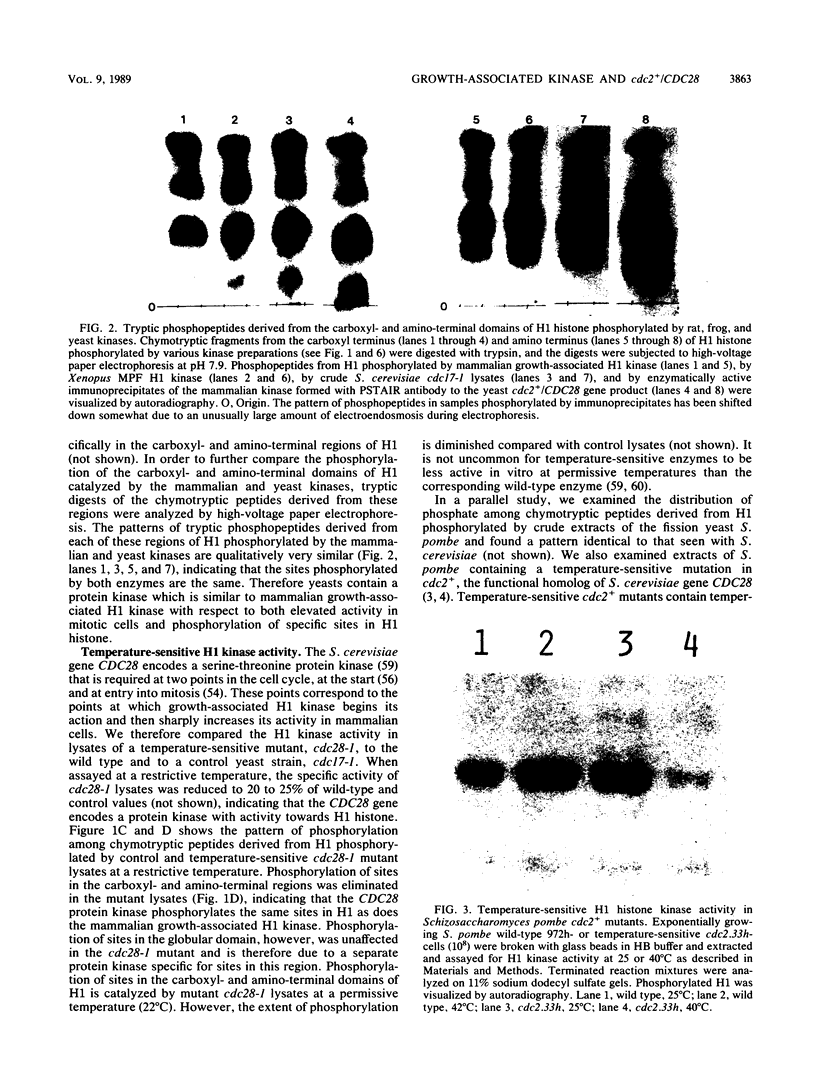
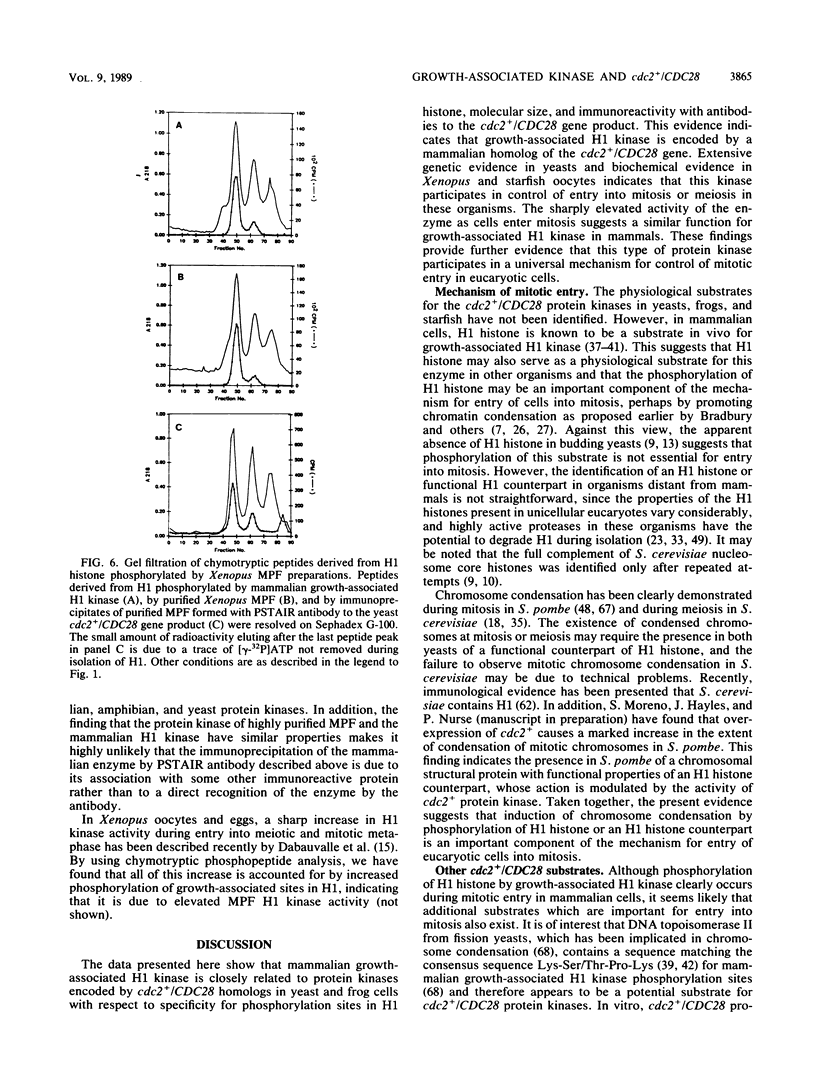
Mammalian growth-associated H1 histone kinase, an enzyme whose activity is sharply elevated at mitosis, is similar to cdc2+ protein kinase from Schizosaccharomyces pombe and CDC28 protein kinase from Saccharomyces cerevisiae with respect to immunoreactivity, molecular size, and specificity for phosphorylation sites in H1 histone. Phosphorylation of specific growth-associated sites in H1 histone is catalyzed by yeast cdc2+/CDC28 kinase, as shown by the in vitro thermal lability of this activity in extracts prepared from temperature-sensitive mutants. In addition, highly purified Xenopus maturation-promoting factor catalyzes phosphorylation of the same sites in H1 as do the mammalian and yeast kinases. The data indicate that growth-associated H1 kinase is encoded by a mammalian homolog of cdc2+/CDC28 protein kinase, which controls entry into mitosis in yeast and frog cells. Since H1 histone is known to be an in vivo substrate of the mammalian kinase, this suggests that phosphorylation of H1 histone or an H1 histone counterpart is an important component of the mechanism for entry of cells into mitosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Booher R., Beach D. Site-specific mutagenesis of cdc2+, a cell cycle control gene of the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1986 Oct;6(10):3523–3530. doi: 10.1128/mcb.6.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Langan T. A. Molecular basis of control of mitotic cell division in eukaryotes. Nature. 1974 Jun 7;249(457):553–556. doi: 10.1038/249553a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandt W. F., Patterson K., von Holt C. The histones of yeast. The isolation and partial structure of the core histones. Eur J Biochem. 1980 Sep;110(1):67–76. doi: 10.1111/j.1432-1033.1980.tb04841.x. [DOI] [PubMed] [Google Scholar]
- Brandt W. F., Von Holt C. The occurrence of histone H3 and H4 in yeast. FEBS Lett. 1976 Jun 15;65(3):386–390. doi: 10.1016/0014-5793(76)80153-6. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Cole R. D. Regions of high and low cationic charge in a lysine-rich histone. J Biol Chem. 1970 Mar 25;245(6):1458–1466. [PubMed] [Google Scholar]
- Certa U., Colavito-Shepanski M., Grunstein M. Yeast may not contain histone H1: the only known 'histone H1-like' protein in Saccharomyces cerevisiae is a mitochondrial protein. Nucleic Acids Res. 1984 Nov 12;12(21):7975–7985. doi: 10.1093/nar/12.21.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. C., Langan T. A., Matthews H. R., Bradbury E. M. H1 histone kinases from nuclei of Physarum polycephalum. Biochemistry. 1983 Jan 4;22(1):30–37. doi: 10.1021/bi00270a005. [DOI] [PubMed] [Google Scholar]
- Dabauvalle M. C., Doree M., Bravo R., Karsenti E. Role of nuclear material in the early cell cycle of Xenopus embryos. Cell. 1988 Feb 26;52(4):525–533. doi: 10.1016/0092-8674(88)90465-5. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Dresser M. E., Giroux C. N. Meiotic chromosome behavior in spread preparations of yeast. J Cell Biol. 1988 Mar;106(3):567–573. doi: 10.1083/jcb.106.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982 May;29(1):43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal Biochem. 1978 Jul 1;87(2):566–575. doi: 10.1016/0003-2697(78)90707-8. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Histones of Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):4131–4138. [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Sequential phsophorylation of histone subfractions in the Chinese hamster cell cycle. J Biol Chem. 1975 May 25;250(10):3936–3944. [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. The metabolism of histone fractions. VI. Differences in the phosphorylation of histone fractions during the cell cycle. Arch Biochem Biophys. 1973 Jan;154(1):212–218. doi: 10.1016/0003-9861(73)90051-9. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis R. J., Langan T. A., Matthews H. R., Hardie D. G., Bradbury E. M. Advance of mitosis by histone phosphokinase. Exp Cell Res. 1976 Feb;97(2):418–425. doi: 10.1016/0014-4827(76)90634-0. [DOI] [PubMed] [Google Scholar]
- Johmann C. A., Gorovsky M. A. Purification and characterization of the histones associated with the macronucleus of Tetrahymena. Biochemistry. 1976 Mar 23;15(6):1249–1256. doi: 10.1021/bi00651a012. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa T., Kojima H., Miyakawa I., Sando N. Meiotic karyotype of the yeast Saccharomyces cerevisiae. Exp Cell Res. 1984 Jul;153(1):259–265. doi: 10.1016/0014-4827(84)90469-5. [DOI] [PubMed] [Google Scholar]
- Labbe J. C., Lee M. G., Nurse P., Picard A., Doree M. Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature. 1988 Sep 15;335(6187):251–254. doi: 10.1038/335251a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S. Further characterization of the F1-histone phosphokinase of metaphase-arrested animal cells. J Cell Biol. 1973 Aug;58(2):317–331. doi: 10.1083/jcb.58.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Characterization of highly phosphorylated subcomponents of rat thymus H1 histone. J Biol Chem. 1982 Dec 25;257(24):14835–14846. [PubMed] [Google Scholar]
- Langan T. A. Methods for the assessment of site-specific histone phosphorylation. Methods Cell Biol. 1978;19:127–142. doi: 10.1016/s0091-679x(08)60018-7. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Hayes M. K., Maller J. L. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci U S A. 1988 May;85(9):3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. B., Paik W. K., Borun T. W. The relationship of histone phosphorylation to deoxyribonucleci acid replication and mitosis during the HeLa S-3 cell cycle. J Biol Chem. 1973 Aug 25;248(16):5660–5667. [PubMed] [Google Scholar]
- Masaracchia R. A., Maller J. L., Walsh D. A. Histone 1 phosphotransferase activities during the maturation of oocytes of Xenopus laevis. Arch Biochem Biophys. 1979 Apr 15;194(1):1–12. doi: 10.1016/0003-9861(79)90589-7. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Huebner V. D. Nuclear protein kinases. Mol Cell Biochem. 1984;59(1-2):81–99. doi: 10.1007/BF00231306. [DOI] [PubMed] [Google Scholar]
- McCully E. K., Robinow C. F. Mitosis in the fission yeast Schizosaccharomyces pombe: a comparative study with light and electron microscopy. J Cell Sci. 1971 Sep;9(2):475–507. doi: 10.1242/jcs.9.2.475. [DOI] [PubMed] [Google Scholar]
- Mende L. M., Waterborg J. H., Mueller R. D., Matthews H. R. Isolation, identification, and characterization of histones from plasmodia of the true slime mold Physarum polycephalum using extraction with guanidine hydrochloride. Biochemistry. 1983 Jan 4;22(1):38–51. doi: 10.1021/bi00270a006. [DOI] [PubMed] [Google Scholar]
- Mendenhall M. D., Jones C. A., Reed S. I. Dual regulation of the yeast CDC28-p40 protein kinase complex: cell cycle, pheromone, and nutrient limitation effects. Cell. 1987 Sep 11;50(6):927–935. doi: 10.1016/0092-8674(87)90519-8. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Piggott J. R., Rai R., Carter B. L. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982 Jul 22;298(5872):391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin-Stricker C. Histone H1 kinase from mouse plasmacytoma. Further characterization and molecular structure. Eur J Biochem. 1984 Jul 16;142(2):317–322. doi: 10.1111/j.1432-1033.1984.tb08288.x. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980 Jul;95(3):561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani R. A., Fangman W. L. Yeast gene CDC8 encodes thymidylate kinase and is complemented by herpes thymidine kinase gene TK. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5821–5825. doi: 10.1073/pnas.81.18.5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Srebreva L., Zlatanova J., Miloshev G., Tsanev R. Immunological evidence for the existence of H1-like histone in yeast. Eur J Biochem. 1987 Jun 1;165(2):449–454. doi: 10.1111/j.1432-1033.1987.tb11459.x. [DOI] [PubMed] [Google Scholar]
- Standart N., Minshull J., Pines J., Hunt T. Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev Biol. 1987 Nov;124(1):248–258. doi: 10.1016/0012-1606(87)90476-3. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Neff N. F., Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Furber V. Yeast chromatin structure. FEBS Lett. 1976 Jul 15;66(2):274–280. doi: 10.1016/0014-5793(76)80521-2. [DOI] [PubMed] [Google Scholar]
- Toda T., Yamamoto M., Yanagida M. Sequential alterations in the nuclear chromatin region during mitosis of the fission yeast Schizosaccharomyces pombe: video fluorescence microscopy of synchronously growing wild-type and cold-sensitive cdc mutants by using a DNA-binding fluorescent probe. J Cell Sci. 1981 Dec;52:271–287. doi: 10.1242/jcs.52.1.271. [DOI] [PubMed] [Google Scholar]
- Uemura T., Morikawa K., Yanagida M. The nucleotide sequence of the fission yeast DNA topoisomerase II gene: structural and functional relationships to other DNA topoisomerases. EMBO J. 1986 Sep;5(9):2355–2361. doi: 10.1002/j.1460-2075.1986.tb04504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg C., Reed S. I. Control of the yeast cell cycle is associated with assembly/disassembly of the Cdc28 protein kinase complex. Cell. 1988 Sep 23;54(7):1061–1072. doi: 10.1016/0092-8674(88)90121-3. [DOI] [PubMed] [Google Scholar]
- Woodford T. A., Pardee A. B. Histone H1 kinase in exponential and synchronous populations of Chinese hamster fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4669–4676. [PubMed] [Google Scholar]
- Zeilig C. E., Langan T. A. Studies on the mechanism of activation of mitotic histone H1 kinase. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1372–1379. doi: 10.1016/0006-291x(80)91625-3. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]