Abstract
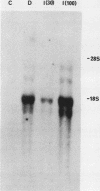
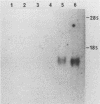
A 27-fold increase in 2',5'-oligoadenylate synthetase activity, an enzyme associated with the antiproliferative actions of interferon (IFN), was observed after treatment of HL-60 human leukemia cells with dimethyl sulfoxide (DMSO), an inducer of granulocytic differentiation of the cells. Enzyme activity was elevated after 24 h of exposure to DMSO, was maximal at 48 hours, and declined thereafter. A comparable increase was observed after treatment with 1 U of alpha interferon (IFN-alpha) per ml or 8 U of beta interferon (IFN-beta) per ml. Elevated levels of expression of other IFN-inducible genes, including type I histocompatibility antigen (HLA-B) mRNA and 2',5'-oligoadenylate phosphodiesterase activity, were also observed with DMSO treatment. DMSO-treated HL-60 cells had an increased amount of a 1.8-kilobase mRNA for oligoadenylate [oligo(A)] synthetase when compared with that of control cells; both DMSO- and IFN-treated HL-60 cells also expressed 1.6-, 3.4-, and 4.3-kilobase mRNA. The increase in both oligo(A) synthetase activity and mRNA levels was inhibited by polyclonal antiserum to human IFN-alpha; however, no IFN-alpha mRNA could be detected in the cells. Antiserum to IFN-beta or gamma interferon (IFN-gamma) had no effect on oligo(A) synthetase expression or activity nor was there any detectable IFN-beta 1 or IFN-beta 2 mRNA in the cells. The anti-IFN-alpha serum did not block the elevation of HLA-B mRNA in DMSO-treated cells. These observations suggest that the increased expression of oligo(A) synthetase in DMSO-treated cells may be mediated by the release of an IFN-alpha-like factor; however, the levels of any IFN-alpha mRNA produced in the cells were extremely low.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. E., Cayley P. J., Kerr I. M. Analysis of (2'-5')-oligo(A) and related oligonucleotides by high-performance liquid chromatography. Methods Enzymol. 1981;79(Pt B):208–216. doi: 10.1016/s0076-6879(81)79031-1. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., De Wit L., Poupart P., Opdenakker G., Van Damme J., Billiau A. Induction of a 26-kDa-protein mRNA in human cells treated with an interleukin-1-related, leukocyte-derived factor. Eur J Biochem. 1985 Oct 15;152(2):253–257. doi: 10.1111/j.1432-1033.1985.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Eppstein D. A., Marsh Y. V., Khan Z., Merigan T. C. Growth regulation of melanoma cells by interferon and (2'-5')oligoadenylate synthetase. Mol Cell Biol. 1983 May;3(5):780–786. doi: 10.1128/mcb.3.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Modjtahedi N., Brison O., Tovey M. G. Interferon modulation of c-myc expression in cloned Daudi cells: relationship to the phenotype of interferon resistance. Mol Cell Biol. 1986 May;6(5):1374–1378. doi: 10.1128/mcb.6.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Peled T., Rachmilewitz E. A. Self-renewal and commitment to differentiation of human leukemic promyelocytic cells (HL-60). J Cell Physiol. 1982 Oct;113(1):152–158. doi: 10.1002/jcp.1041130124. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Kaufman S. E., Weisbart R. H., Tomonaga M., Golde D. W. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):669–673. doi: 10.1073/pnas.83.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., White C. P., Siebenlist R. E., Sedmak J. J., Grossberg S. E. Cytotoxicity of human beta-interferon for differentiating leukemic HL-60 cells. Cancer Res. 1985 Nov;45(11 Pt 1):5369–5373. [PubMed] [Google Scholar]
- Henco K., Brosius J., Fujisawa A., Fujisawa J. I., Haynes J. R., Hochstadt J., Kovacic T., Pasek M., Schamböck A., Schmid J. Structural relationship of human interferon alpha genes and pseudogenes. J Mol Biol. 1985 Sep 20;185(2):227–260. doi: 10.1016/0022-2836(85)90401-2. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Krause D., Friedman R. M., Silverman R. H. Induction of ppp(A2'p)nA-dependent RNase in murine JLS-V9R cells during growth inhibition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4954–4958. doi: 10.1073/pnas.80.16.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Gilbert C. S., Stark G. R., Kerr I. M. Differential regulation of interferon-induced mRNAs and c-myc mRNA by alpha- and gamma-interferons. Eur J Biochem. 1985 Dec 2;153(2):367–371. doi: 10.1111/j.1432-1033.1985.tb09312.x. [DOI] [PubMed] [Google Scholar]
- Kimchi A. Increased levels of interferon-induced (2'--5') oligo-isoadenylate synthetase in mature T-lymphocytes and in differentiated Friend-erythroleukemic cells. J Interferon Res. 1981;1(4):559–569. doi: 10.1089/jir.1981.1.559. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Anti-mitogenic function of interferon-induced (2'-5')oligo(adenylate) and growth-related variations in enzymes that synthesize and degrade this oligonucleotide. Eur J Biochem. 1981;114(1):5–10. doi: 10.1111/j.1432-1033.1981.tb06163.x. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Enzymology of interferon action--a short survey. Methods Enzymol. 1981;79(Pt B):135–148. [PubMed] [Google Scholar]
- Mantei N., Weissmann C. Controlled transcription of a human alpha-interferon gene introduced into mouse L cells. Nature. 1982 May 13;297(5862):128–132. doi: 10.1038/297128a0. [DOI] [PubMed] [Google Scholar]
- May L. T., Sehgal P. B., LaForge K. S., Inouye M. Expression of the native alpha and beta interferon genes in human cells. Virology. 1983 Aug;129(1):116–126. doi: 10.1016/0042-6822(83)90400-2. [DOI] [PubMed] [Google Scholar]
- Merlin G., Chebath J., Benech P., Metz R., Revel M. Molecular cloning and sequence of partial cDNA for interferon-induced (2'-5')oligo(A) synthetase mRNA from human cells. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4904–4908. doi: 10.1073/pnas.80.16.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. N., Larsen H. S., Horohov D. W., Rouse B. T. Endogenous regulation of macrophage proliferative expansion by colony-stimulating factor-induced interferon. Science. 1984 Jan 13;223(4632):178–181. doi: 10.1126/science.6606850. [DOI] [PubMed] [Google Scholar]
- Ragg H., Weissmann C. Not more than 117 base pairs of 5'-flanking sequence are required for inducible expression of a human IFN-alpha gene. Nature. 1983 Jun 2;303(5916):439–442. doi: 10.1038/303439a0. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Yarden A., Zipori D., Kimchi A. Autocrine beta-related interferon controls c-myc suppression and growth arrest during hematopoietic cell differentiation. Cell. 1986 Jul 4;46(1):31–40. doi: 10.1016/0092-8674(86)90857-3. [DOI] [PubMed] [Google Scholar]
- Revel M. F., Kimchi A. Initial characterization of a spontaneous interferon secreted during growth and differentiation of Friend erythroleukemia cells. Mol Cell Biol. 1982 Dec;2(12):1472–1480. doi: 10.1128/mcb.2.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Wallach D., Merlin G., Schattner A., Schmidt A., Wolf D., Shulman L., Kimchi A. Interferon-induced enzymes: microassays and their applications: purification and assay of (2'-5')-oligoadenylate synthetase and assay of 2'-phosphodiesterase. Methods Enzymol. 1981;79(Pt B):149–161. doi: 10.1016/s0076-6879(81)79024-4. [DOI] [PubMed] [Google Scholar]
- Samanta H., Engel D. A., Chao H. M., Thakur A., García-Blanco M. A., Lengyel P. Interferons as gene activators. Cloning of the 5' terminus and the control segment of an interferon activated gene. J Biol Chem. 1986 Sep 5;261(25):11849–11858. [PubMed] [Google Scholar]
- Schwartz E. L., Maher A. M. Enhanced mitogenic responsiveness to granulocyte-macrophage colony-stimulating factor in HL-60 promyelocytic leukemia cells upon induction of differentiation. Cancer Res. 1988 May 15;48(10):2683–2687. [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa U., Ferbus D., Gabbianelli M., Pascucci B., Boccoli G., Louache F., Thang M. N. Effect of endogenous and exogenous interferons on the differentiation of human monocyte cell line U937. Cancer Res. 1988 Jan 1;48(1):82–88. [PubMed] [Google Scholar]
- Tovey M. G., Streuli M., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Vignaux F., Gigou M. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5038–5042. doi: 10.1073/pnas.84.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Wong W., Hyman R., Minden M., Robinson S. H. Analysis of commitment of human leukemia HL-60 cells to terminal granulocytic maturation. Cancer Res. 1985 May;45(5):2334–2339. [PubMed] [Google Scholar]
- Yarden A., Shure-Gottlieb H., Chebath J., Revel M., Kimchi A. Autogenous production of interferon-beta switches on HLA genes during differentiation of histiocytic lymphoma U937 cells. EMBO J. 1984 May;3(5):969–973. doi: 10.1002/j.1460-2075.1984.tb01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Melchner H., Höffken K. Commitment to differentiation of human promyelocytic leukemia cells (HL60): an all-or-none event preceded by reversible losses of self-renewal potential. J Cell Physiol. 1985 Dec;125(3):573–581. doi: 10.1002/jcp.1041250329. [DOI] [PubMed] [Google Scholar]