Abstract
Aeromonas hydrophila AH-3 lateral flagella are not assembled when bacteria grow in liquid media; however, lateral flagellar genes are transcribed. Our results indicate that A. hydrophila lateral flagellar genes are transcribed at three levels (class I to III genes) and share some similarities with, but have many important differences from, genes of Vibrio parahaemolyticus. A. hydrophila lateral flagellum class I gene transcription is σ70 dependent, which is consistent with the fact that lateral flagellum is constitutively transcribed, in contrast to the characteristics of V. parahaemolyticus. The fact that multiple genes are included in class I highlights that lateral flagellar genes are less hierarchically transcribed than polar flagellum genes. The A. hydrophila lafK-fliEJL gene cluster (where the subscript L distinguishes genes for lateral flagella from those for polar flagella) is exclusively from class I and is in V. parahaemolyticus class I and II. Furthermore, the A. hydrophila flgAMNL cluster is not transcribed from the σ54/LafK-dependent promoter and does not contain class II genes. Here, we propose a gene transcriptional hierarchy for the A. hydrophila lateral flagella.
INTRODUCTION
Swarming motility is defined as a rapid multicellular movement of bacteria across a surface that is powered by rotating flagella. Most bacteria that swarm have multiple constitutive flagella distributed randomly on the cell surface (peritrichous flagella) and increase the flagellum number per cell on contact with surfaces (1, 2). On the other hand, polar flagellated bacteria have developed two different strategies to swarm: some bacteria, such as Pseudomonas aeruginosa, synthesize an alternative polar flagellar motor that can propel bacteria on surfaces (3, 4), and others, such as Vibrio parahaemolyticus, Aeromonas spp., and Rhodospirillum centenum, have developed lateral flagella distributed randomly on the cell surface which are induced when grown on solid surfaces or in viscous environments (5, 6).
Phylogenetic analysis and organization of lateral flagellar genes suggest that this flagellar system originated in Betaproteobacteria and Gammaproteobacteria from a duplication of the entire flagellar gene complex in the nonenteric gammaproteobacterial lineage, which was then horizontally transferred to the Betaproteobacteria and the enteric bacteria (7). In contrast to polar or peritrichous flagella systems (primary systems), lateral flagellar systems lack fliO, and the fliEFGHIJKLMNPQR gene cluster is split into two gene clusters (fliEFGHIJL and fliMNPQRL [where the subscript L distinguishes genes for lateral flagella from those for polar flagella]). The fliKLL (lafEF) genes are arranged in the fliDL-motBL (lafB-lafU) cluster (5, 8).
The best-studied functional lateral flagellar systems are those of V. parahaemolyticus and A. hydrophila. Both are encoded by 38 genes distributed in six clusters, while V. parahaemolyticus genes are distributed in two discontinuous regions on chromosome II (9); A. hydrophila genes are distributed in a unique chromosomal region (10, 11). V. parahaemolyticus flgAMNL and motYL-lafK-fliEFGHIJL clusters are transcribed divergently from flgBCDEFGHIJKLL, fliMNPQRL-flhBAL, lafA, and fliDSTKLAL-motABL. A. hydrophila orthologous genes exhibit the same distribution, although only flgAMNL genes are transcribed divergently. Furthermore, A. hydrophila does not contain any gene orthologous to V. parahaemolyticus motYL (12), and a modification accessory factor gene, maf-5 (13), which is independently transcribed, is present between flgLL and lafA.
Synthesis and assembly of any flagellar system is regulated coordinately by a transcriptional cascade composed of three or four levels of hierarchy: class I to III or I to IV (14, 15). In polar flagellated Gammaproteobacteria, such as Vibrio, mesophilic Aeromonas, and Pseudomonas species, four levels of hierarchy have been described. Transcription of class II and III is σ54 dependent, and transcription of class IV is σ28 dependent (16, 17, 18). At the top of the hierarchy is a σ54-associated transcriptional activator (FlrA in A. hydrophila) which activates σ54-dependent promoters preceding the class II clusters. One of the class II clusters encodes a two-component signal-transducing system (FlrBC in A. hydrophila) whose regulator (FlrC) activates class III genes. The class III-transcribed A. hydrophila σ28 factor, which activates transcription of class IV genes, is class II transcribed in Vibrio spp., and flagellar hierarchy is independently transcribed in P. aeruginosa (16, 17, 18).
Inducible peritrichous flagella (lateral flagella) of V. parahaemolyticus and A. hydrophila do not posses an FlhDC master regulator and are σ54 dependent (9, 19) as polar flagella. In this work, we investigated the A. hydrophila lateral flagellar transcriptional hierarchy by two techniques: promoter-lacZ fusion assays (used to measure β-galactosidase activity in several mutant backgrounds) and reverse transcription-PCR (RT-PCR) assays. Until now, little was known about A. hydrophila's transcriptional hierarchy, translational and posttranslational regulatory mechanisms that ensure careful regulation, proper assembly of several different proteins, and whether or not there was a large enough quantity of individual lateral flagellar subunits.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown on Luria-Bertani (LB) Miller broth and LB Miller agar at 37°C, while Aeromonas strains were grown either in tryptic soy broth (TSB) or tryptic soy agar (TSA) at 30°C. When required, ampicillin (50 μg/ml), kanamycin (50 μg/ml), rifampin (100 μg/ml), spectinomycin (50 μg/ml), chloramphenicol (25 μg/ml), and tetracycline (20 μg/ml) were added to the media.
Table 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Genotype and/or phenotypea | Reference or source |
|---|---|---|
| Strains | ||
| A. hydrophila | ||
| AH-3 | A. hydrophila wild type, serogroup O:34 | 45 |
| AH-405 | AH-3, spontaneous Rifr | 39 |
| AH-4427 | AH-405ΔflaB; flaA::Kmr | 10 |
| AH-5502 | AH-405; rpoN::Kmr | 19 |
| AH-5503 | AH-405; lafK::Kmr | 19 |
| AH-3::lafS | AH-405; lafS::Kmr | This work |
| AH-3ΔlafA | AH-405ΔlafA | This work |
| E. coli | ||
| DH5α | F− endA hdsR17(rk− mk+) supE44 thi-1 recA1 gyr-A96 ϕ80lacZ | 25 |
| MC1061λpir | thi thr1 leu6 proA2 his4 argE2 lacY1 galK2 ara14 xyl5 supE44 λpir | 46 |
| Plasmids | ||
| pGEM-T easy | Cloning vector, Apr | Promega |
| pRK2073 | Helper plasmid, Spr | 46 |
| pUC4-KIXX | Source of Tn5-derived nptII gene (Kmr) | Pharmacia |
| pDM4 | Suicide plasmid, pir dependent with sacAB genes, oriR6K, Cmr | 27 |
| pDM-LafSKm | pDM4 with AH-3 lafS::Km, Cmr Kmr | This work |
| pDM-LAFA | pDM4 with AH-3ΔlafA, Cmr | This work |
| pBAD33-Gm | pBAD33 arabinose-induced expression vector with Gmr | 28 |
| pBAD33Gm-LAFK | pBAD33 with AH-3 lafK gene, Gmr | This work |
| pBAD33Gm-LAFS | pBAD33 with AH-3 lafS gene, Gmr | This work |
| pDN19 lacΩ | Promoterless lacZ fusion vector, Spr Smr Tcr | 29 |
| pDNlac-fliMLp | fliML promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-lafKp | lafK promoter-lacZ fusion in pDN19lacΩ, Tcr | 18 |
| pDNlac-flgMLp | flgML promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-flgALp | flgAL promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-flgBLp | flgBL promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-maf-5p1 | maf-5 promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-maf-5p2 | maf-5 promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-lafAp | lafA promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-lafBp | lafB promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
| pDNlac-lafTp | lafT promoter-lacZ fusion in pDN19lacΩ, Tcr | This work |
Tcr, tetracycline resistant; Kmr, kanamycin resistant; Apr, ampicillin resistant; Rifr, rifampin resistant; Cmr, chloramphenicol resistant; Spr, spectinomycin resistant; Smr, streptomycin resistant; Gmr, gentamicin resistant.
Motility assays (swarming and swimming).
Freshly grown bacterial colonies were transferred with a sterile toothpick into the center of swarm agar (1% tryptone, 0.5% NaCl, 0.5% agar) or swim agar (1% tryptone, 0.5% NaCl, 0.25% agar). The plates were incubated face up for 16 to 24 h at 25°C, and motility was assessed by examining the migration of bacteria through the agar from the center toward the periphery of the plate. Moreover, swimming motility was assessed by light microscopy observations in liquid media.
DNA techniques.
DNA manipulations were carried out essentially according to standard procedures (20). DNA restriction endonucleases and E. coli DNA polymerase Klenow fragment were obtained from Promega. T4 DNA ligase and alkaline phosphatase were obtained from Invitrogen and GE Healthcare, respectively. PCR was performed using BioTaq DNA polymerase (Ecogen) in a Gene Amplifier PCR system and a PerkinElmer 2400 thermal cycler.
Nucleotide sequencing and computer sequence analysis.
Plasmid DNA for sequencing was isolated by a Qiagen plasmid purification kit (Qiagen, Inc., Ltd.) as recommended by the suppliers. Double-stranded DNA sequencing was performed by using the Sanger dideoxy-chain termination method (21) with the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). Custom-designed primers used for DNA sequencing were purchased from Sigma-Aldrich. The DNA sequences were inspected in the GenBank and EMBL databases at the National Center for Biotechnology Information (NCBI) (22). The Terminator search program in the GCG Wisconsin package was used to search for factor-independent transcriptional terminators. Neural Network Promoter Prediction, PromScan (23), and PRODORIC (24) were used to search promoter sequences.
Total RNA extraction and RT-PCR.
Total RNA was isolated, by RNA Protect bacterial reagent (Qiagen) and an RNeasy Minikit (Qiagen), from A. hydrophila AH-3 and rpoN, lafK, and lafS mutant strains grown in liquid medium (TSB), viscous medium (TSB plus 18% [wt/vol] Ficoll), or solid agar (TSA). To ensure that RNA was devoid of contaminating DNA, the preparation was treated with RNase-free TurboDNase I (Ambion). First-strand cDNA synthesis was carried out with Moloney-murine leukemia virus (M-MuLV) reverse transcriptase (New England BioLabs) and random oligonucleotides (Promega) on 5 μg of total DNase-digested RNA. The reaction mixtures were incubated at 25°C for 10 min, 37°C for 120 min, and 75°C for 15 min. Control reaction mixtures lacking reverse transcriptase were used to confirm that RNA samples were not contaminated with genomic DNA (RT negative controls). PCR, second-strand synthesis, and subsequent DNA amplification were carried out using the Accuprime TaqDNA polymerase (Invitrogene), specific oligonucleotides, and 30 PCR cycles. Amplicons were analyzed by agarose gel electrophoresis with ethidium bromide staining. A. hydrophila ribosomal 16S primers were used as a control for the cDNA template. RT-PCR amplifications were performed at least twice with total RNA preparations obtained from a minimum of two independent extractions.
Mapping the A. hydrophila AH-3 fliML, lfgAL, lafK, lafB, and lafT transcription start sites by RACE PCR.
Amplifications of the A. hydrophila AH-3 fliML, lfgAL, lafK, lafB, and lafT cDNA 5′ ends were performed using the 5′ random amplification of cDNA ends (RACE) system, version 2.0 (Invitrogen). Total RNA extraction from A. hydrophila AH-3 was performed as mentioned above. First-strand cDNA was synthesized using the entire volume of DNase-digested total RNA (5 μg), the fliML, lfgAL, lafK, lafB, and lafT internal primers GSP1-FLIML (5′-ATCTTGCAAGGTGTG-3′), GSP1-LfgAL (5′-GAGCTTGGAACAAATC-3′), GSP1-LafK (5′-GATATAACGAGCCAGTC-3′), GSP1-LafB (5′-TTTCGACAAACTTCTTG-3′), and GSP1-LafT (5′-AATTATCGATGATGAAAC-3′), respectively, and the Thermoscript RT-PCR system (Invitrogen) at 45°C for 45 min. Reverse transcriptase was deactivated at 85°C for 5 min, and 1 μl of RNase H was then added and incubated at 37°C for 20 min. Purification of cDNA with S.N.A.P. columns, as well as tailing of purified cDNA using terminal deoxynucleotidyl transferase and dCTP, was done according to the 5′ RACE system, version 2.0, instructions. Confirmation of cDNA was performed after each step by PCR with nested primers. Tailed cDNA was amplified by primary PCR using 10 μM each primer, the 5′ RACE abridged anchor primer (AAP) that binds to the tailed cDNA sequence, and GSP2-FLIML (5′-AGATGTCGACCTGATATTGG-3′), GSP2-FLGAL (5′-ATCTCCGGTACGAATGGT-3′), GSP2-LafK (5′-TTCATTAACCAGGATGAC G-3′), GSP2-LafB (5′-GCATTCTCCAACCCACTAT-3′), and GSP2-LafT (5′-TTCAT CGAGTGCCTTCAT-3′), which bind to the respective internal gene sequences. The PCR program applied was 94°C for 1 min and then 35 cycles of 94°C for 45 s, 55°C for 30 s, and 72°C for 1 min, followed by an extension at 72°C for 5 min. PCR products were analyzed by agarose gel electrophoresis, and amplified bands were excised from the gel, purified, and sequenced with GSP2-FLIML, GSP2-FLGAL, GSP2 LafK, GSP2-LafB, or GSP2-LafT primer.
Construction of defined mutants.
To obtain the A. hydrophila AH-3::lafS mutant, lafS was amplified by PCR with 5′-CGCGGATCCAACCCAAGCCAGAGTTGAG-3′ and 5′-CGCGGATCCATGAAACACCAGGACACA-3′ (the BamHI site is underlined), ligated into vector pGEMTeasy (Promega), and transformed into E. coli DH5α (25). The Tn5-derived kanamycin resistance cartridge (nptll) from pUC4-KIXX was obtained by SmaI digestion, and the cassette was inserted into the XbaI blunt-ended restriction internal site of lafS. The cartridge contains an outward-reading promoter that ensures the expression of downstream genes when inserted in the correct orientation; however, such insertion will alter the regulation of those genes (26). The presence of a single BglII site in the SmaI-digested cassette allowed its orientation to be determined. Constructs containing the mutated genes were ligated to suicide vector pDM4 (27), electroporated into E. coli MC1061λpir, and plated on chloramphenicol-kanamycin plates at 30°C to obtain the pDM-LafSKm plasmid.
The chromosomal in-frame lafA deletion mutant A. hydrophila AH-3ΔlafA was constructed by allelic exchange as described by Milton et al. (27). Briefly, DNA regions flanking the lafA gene were amplified using the primers A (5′-CGCGGATCCTTTGGTGTCGACTTCTCCT-3′), B (5′-CCCATCCACTAAACTTAAACAAGAGTTCAGCTGGTTCTGG-3′), C (5′-TGTTTAAGTTTAGTGGATGGGAGCACCAATATGACCAAGAA-3′), and D (CGCGGATCCCAGCACCATGTTGACCTT-3′) in two sets of asymmetric PCRs to amplify DNA fragments of 779 (pair AB) and 733 (pair CD) bp, respectively. DNA fragments AB and CD were annealed at their overlapping regions (double-underlined letters in primers B and C) and amplified as a single fragment using primers A and D. The fusion product was purified, BamHI digested (the BamHI site is underlined in primers A and D), ligated into BglII-digested and phosphatase-treated pDM4 vector (27), electroporated into E. coli MC1061λpir, and plated on chloramphenicol plates at 30°C to obtain pDM-LAFA plasmid. Plasmid pDM-LafSKm or pDM-LAFA was transferred into an A. hydrophila AH-405 rifampin-resistant (Rifr) strain by triparental matings using E. coli MC1061λpir containing the insertion constructs and the mobilizing strain HB101/pRK2073. Transconjugants were selected on plates containing chloramphenicol, kanamycin, and rifampin or containing chloramphenicol and rifampin. PCR analysis confirmed that the vector had integrated correctly into the chromosomal DNA. After sucrose treatment, transconjugants that were Rifr, Kmr, and Cms or were Rifr and Cms were chosen and confirmed by PCR.
Plasmid constructions.
Plasmids pBAD33Gm-LAFK and pBAD33Gm-LAFS, containing the complete lafK and lafS genes of A. hydrophila AH-3, respectively, under the arabinose promoter (PBAD) on pBAD33-Gm (28) were obtained. Oligonucleotides 5′-GGATATCTAATGATAGCGGGGTTAC-3′ and 5′-CCCAAGCTTCATCAGCTTGTTTCGCACCT-3′ generate a band of 1,841 bp containing the lafK gene, and oligonucleotides 5′-TCCCCCGGGAACCCAAGCCAGAGTTGAG-3′ and 5′-CCCAAGCTTATGAAACACCAGCACACA-3 generate a band of 1,162 bp containing the lafS gene (the EcoRV site is in italics, the SmaI site is double underlined, and the HindIII site is underlined). The amplified bands were digested with EcoRV or SmaI and HindIII and ligated into SmaI- and HindIII-digested pBAD33-Gm vector (19) to construct the pBAD33Gm-LAFK and pBAD33Gm-LAFS recombinant plasmids. Plasmids were independently introduced into E. coli DH5α (25) and sequenced.
Construction of flagellar promoter-lacZ fusions.
Oligonucleotide primer pairs for the A. hydrophila AH-3 promoter regions of the fliML, flgML, flgAL, flgBL, maf-5, lafA, lafB, and lafT genes (19) are listed in Table 2. Primers were designed to amplify fragments of 493 to 1,560 bp that encompassed regions both upstream and downstream of the predicted start codon. Restriction sites were added to some primers for cloning purposes. Promoter fragments were PCR amplified from A. hydrophila AH-3 genomic DNA, ligated into pGEM-T Easy (Promega), and transformed into E. coli DH5α (25). DNA inserts containing fliML, flgML, flgAL, maf-5, and lafA promoters were recovered by EcoRI/BamHI restriction digestion, inserts containing lafB and flgBL promoters were recovered by EcoRI/BglII restriction digestion, and the insert containing lafT promoter was recovered by SmaI/BamHI restriction digestion. The BglII restriction site in the lafB insert is 158 bp downstream from the lafB start codon. The EcoRI restriction sites come from the pGEM-T Easy plasmid. Digested fragments were ligated into plasmid pDN19lacΩ EcoRI/BamHI-digested or EcoRI blunt-ended BamHI (29), transformed into E. coli DH5α (25), and selected for tetracycline resistance (Tcr). The final constructs were confirmed by DNA sequencing.
Table 2.
Primers used for lateral flagellar promoter-lacZ fusion construction
| Promoter | Primer sequencea | Siteb |
|---|---|---|
| fliMLp | 5′-TGCAACAGAGAGCAAACCG-3′ | −486 fliML |
| 5′-CGGGATCCTGAGTTGTTCTCGGTCTG-3′ | +114 fliML | |
| flgMLp | 5′-AGCAAGAGCAATCGGAATC-3′ | −325 flgML |
| 5′-CGGGATCCCATCCGATGTAGTTGCCAAT-3′ | +168 flgML | |
| flgALp | 5′-GGAAGATCTTCCCGGTGATTTTCATATTC-3′ | −494 flgAL |
| 5′-CGGGATCCCGTGCATTCAGCCAGATAG-3′ | +386 flgAL | |
| flgBLp | 5′-CGGGATCCCGTGCATTCAGCCAGATAG-3′ | −419 flgBL |
| 5′-GGAAGATCTTCCCGGTGATTTTCATATTC-3′ | +361 flgBL | |
| maf-5p1 | 5′-GAGCTCTGCGCAAAGAAA-3′ | −521 maf-5 |
| 5′-CGGGATCCTCAAGTGCAAGACCAGAGC-3′ | +339 maf-5 | |
| maf-5p2 | 5′-CGTTGACCCGAGAAGTCA-3′ | −1490 maf-5 |
| 5′-CGGGATCCTCGATCCAGCCTTGAAA-3′ | −523 maf-5 | |
| lafAp | 5′-TGTATGGCACTGGGTTGG-3′ | −1519 lafA |
| 5′-GCGGATCCGGTGGTCATGGAAGCAAA-3′ | +41 lafA | |
| llafBp | 5′-TCTGCTGAAAACCGGTGG-3′ | −553 lafB |
| 5′-TTCTGCGCCTGTAAATTG-3′ | +194 lafB | |
| lafTp) | 5′-TCCCCCGGGTGAGTTTGACACATCACCC-3′ | −732 lafT |
| 5′-CGCGGATCCATGAAACACCAGGACACA-3′ | +53 lafT |
Underlined letters show the BamHI restriction site. Double-underlined letters show the BglII restriction site. Italicized letters show the SmaI restriction site.
Numbers are numbers of nucleotides upstream (−) or downstream (+) from the start site for the indicated gene.
Transmission electron microscopy (TEM).
Three independent samples of bacterial suspensions grown in TSB or TSB medium with 18% (wt/vol) Ficoll at 25°C were placed on Formvar-coated grids and negatively stained with a 2% solution of uranyl acetate (pH 4.1). Preparations were observed on a Hitachi 600 transmission electron microscope.
Immunoblotting assays.
A. hydrophila grown on plates (TSA), in viscous medium (TSB plus 18% [wt/vol] Ficoll), or in liquid cultures (TSB) at 25°C was used to analyze lateral flagellins by Western blotting. For analysis of cytoplasmic fractions, cells grown on plates were collected with 20 mM MgCl2 in 100 mM Tris (pH 8.0) and harvested by centrifugation (5,000 × g). Cells grown in liquid or viscous medium were collected by centrifugation (5,000 × g). Both were suspended in 20 mM MgCl2 in 100 mM Tris (pH 8.0) and diluted to an optical density at 600 nm of 0.8. Flagella were removed from the cells by shearing in a vortex with a glass bar for 3 to 4 min and then passing repetitively (minimum of six times) through a syringe. Cells without flagella on their surface were collected by centrifugation at 8,000 × g for 30 min, resuspended in the same cold buffer, and subjected to French press cell lysis. After shearing, the supernatants were also collected for analysis. The lysates were centrifuged at 5,000 × g to remove unbroken cells. After centrifugation at 4°C for 1 h at 115,000 × g, the soluble fraction (cytoplasmic fraction) remained in the supernatant while the insoluble fraction (membrane-enriched fraction) was retained in the pellet. The cytoplasmic fraction was analyzed by SDS-PAGE and transferred to nitrocellulose membranes, and the membranes were blocked with bovine serum albumin (3 mg/ml) and probed with polyclonal rabbit anti-polar or anti-lateral flagellin antibodies (1:1,000) that were previously obtained (11). The unbound antibody was removed by three washes in phosphate-buffered saline (PBS), and a goat anti-rabbit alkaline phosphatase-conjugated secondary antibody (1:1000) was added. The unbound secondary antibody was removed by three washes in PBS. The bound conjugate was then detected by the addition of 5-bromo-4-chloroindolylphosphate disodium-nitroblue tetrazolium. Incubations were carried out for 1 h, and washing steps with 0.05% Tween 20 in phosphate-buffered saline were included after each incubation step.
For analysis of whole cells and bacterial supernatants before shearing, cells grown in liquid or viscous medium were collected at an optical density at 600 nm of 0.8, and cells grown in plates were collected with 20 mM MgCl2 in 100 mM Tris (pH 8.0) and diluted to an identical optical density at 600 nm. Both were harvested by centrifugation (5,000 × g), and cell suspensions as well as supernatants were analyzed by Western blotting with anti-lateral flagellin antibodies (1:1,000).
β-Galactosidase assays.
The promoter-lacZ fusion plasmids described above were introduced into several A. hydrophila strains (Table 1). The cultures were grown in TSB or TSB medium with 18% (wt/vol) Ficoll at 25°C to an optical density at 600 nm of 0.6 or 0.8. Bacterial cells were permeabilized with chloroform and sodium dodecyl sulfate (SDS) overnight and assayed for β-galactosidase activity as described by Miller (30). All experiments were performed at least 3 separate times.
Statistical analysis.
The data obtained from the β-galactosidase assays were analyzed by the t test using Microsoft Excel software.
RESULTS
Lateral flagellar genes of A. hydrophila AH-3 are transcribed, but lateral flagella are not expressed in liquid media.
A. hydrophila and V. parahaemolyticus both have dual flagellar systems (polar and lateral flagella) but do not share structural or regulatory genes, and both contribute to motility in semisolid plates (9, 18, 19). A. hydrophila polar flagellum is constitutive; however, lateral flagella are induced in highly viscous media or on surfaces. Both flagellar types have σ54-dependent response regulators, FlrA and LafK, which are essential for polar and lateral flagellum generation, respectively (10, 19). Furthermore, despite A. hydrophila FlrA and LafK showing 57% similarity to each other, their C-terminal domains might recognize different DNA binding regions, and LafK is unable to compensate for the FlrA mutation and vice versa (18, 19). In order to know whether viscosity conditions regulate transcription of lateral flagella, we measured the β-galactosidase activity of pDNlac-lafKp (lafKp-lacZ) after growth in liquid (TSB) and viscous media (TSB with 18% [wt/vol] Ficoll or 3% [wt/vol] gelatin) as well as on solid plates (TSA) (Fig. 1B). Data showed similar β-galactosidase values after growth in liquid and viscous media or on solid plates. Lateral flagellin (LafA) transcription was analyzed by measuring β-galactosidase activity of pDNlac-lafAp (lafAp-lacZ). Lateral flagellin transcription in liquid media shows a very slight reduction compared to transcription in viscous media or on solid plates, although lateral flagella are not produced in liquid media (Fig. 1A and B). However, the TEM assays showed lateral flagella in 85% of bacterial cells grown in TSB with Ficoll and in 80% of cells grown in TSA. Levels of β-galactosidase activity from other lateral flagella promoters, such as flgAL, flgBL, and lafB promoters, were also analyzed, with all of them being similar in liquid and viscous media (Fig. 1B). In addition, RT-PCR assays showed that lafK, lafA, flgAL, flgBL, and lafB transcription are viscosity/surface independent, since they are transcribed in liquid and viscous media as well as on solid plates (Fig. 1C). No RT-PCR product was obtained with primer pairs for flgAL and flgBL, which are divergently transcribed genes, eliminating the possibility of residual DNA in the RNA samples from liquid cultures.
Fig 1.
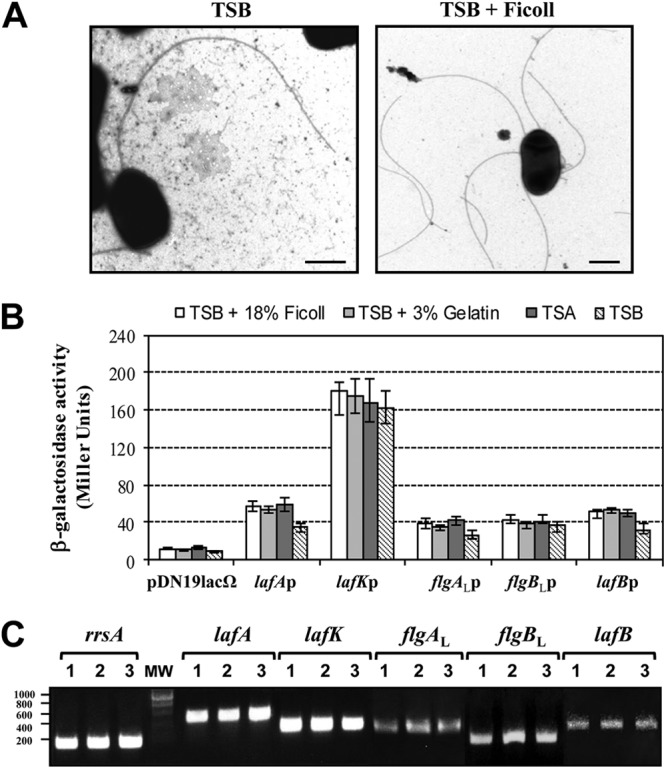
(A) Transmission electron microscopy of A. hydrophila AH-3 (wild type) grown at 25°C in TSB and TSB with 18% (wt/vol) Ficoll. Bacteria were gently placed onto Formvar-coated copper grids and negatively stained using a 2% solution of uranyl acetate. Bar, 0.5 μm. (B) Analysis of β-galactosidase activity of pDNlac-lafKp, pDNlac-lafAp, pDNlac-flgALp, pDNlac-flgBLp, and pDNlac-lafBp plasmids in A. hydrophila AH-405 after growth in liquid (TSB) medium, viscous medium (TSB plus 18% [wt/vol] Ficoll or 3% [wt/vol] gelatin), and solid agar (TSA) at 25°C. As a control, we also measured the pDN19lacΩ promoterless plasmid. The results shown are representative of three independent experiments. (C) RT-PCR amplification of lafK, lafA, flgAL, flgBL, and lafB from cDNA of AH-3 after growth in liquid medium (TSB), viscous medium (TSB plus 18% [wt/vol] Ficoll), or solid agar (TSA) (lanes 1, 2, and 3, respectively). A. hydrophila ribosomal 16S (rrsA) amplification was used as a control for cDNA template. RT-PCR amplifications were performed at least twice with total RNA preparations obtained from a minimum of two independent extractions. The Ecogen molecular weight marker (MW) was used.
The presence of lateral flagellin (LafA) in the cytoplasmic fraction of A. hydrophila AH-3 and the mutants AH-4427 (without polar flagellin), AH-3ΔlafA (without lateral flagellin), and AH-5502 (rpoN) grown in liquid or viscous medium or on solid plates was analyzed by Western blotting with anti-lateral flagellin (1:1,000) polyclonal antibodies (Fig. 2A). We analyzed the presence of polar flagellins by Western blotting with anti-polar flagellin (1:1,000) polyclonal antibodies in these strains (Fig. 2B). AH-3 and AH-4427 mutant cytoplasmic fractions obtained after growth in viscous media or on solid plates showed positive reactions with anti-lateral flagellin serum but were unable to react with similar fractions grown in liquid media. AH-3ΔlafA and AH-5502 (rpoN) cytoplasmic fractions were unable to react with anti-lateral flagellin serum. AH-3 and AH-3ΔlafA cytoplasmic fractions reacted positively with polar flagellin serum and were unable to react with AH-4427 and AH-5502 (rpoN) cytoplasmic fractions in all growth conditions tested. Furthermore, we analyzed the presence of lateral flagellin by Western blotting in whole cells of AH-3 before shearing, as well as in the supernatants before and after shearing, when grown in liquid or viscous medium or on solid plates. Nonlateral flagellin was detected in whole cells or supernatants from liquid cultures before and after shearing (Fig. 2C). These data suggest that lateral flagellin is not translated in liquid media, although it is transcribed.
Fig 2.

(A) Western blot analysis with anti-lateral flagellin (1:1,000) polyclonal antibodies of cytoplasmic fractions of A. hydrophila AH-3 (wild type), mutant AH-4427 (without polar flagellin), AH-3ΔlafA (without lateral flagellin), and AH-5502 (rpoN) (lanes 1, 2, 3, and 4, respectively) of bacteria grown in liquid (TSB) or viscous medium (TSB plus 18% [wt/vol] Ficoll) at 25°C in TSB. (B) Western blot analysis with anti-polar flagellin (1:1,000) polyclonal antibodies of cytoplasmic fractions of the same strains and grown conditions as those described for panel A. (C) Western blot analysis with anti-lateral flagellin (1:1,000) polyclonal antibodies of whole cells (WC) and supernatant before shearing (S) and after shearing (SS) of A. hydrophila AH-3 grown in liquid medium (TSB), viscous medium (TSB plus 18% [wt/vol] Ficoll), or an agar plate (TSA) (lanes 1, 2, and 3, respectively).
The A. hydrophila AH-3 σ54 factor (RpoN) is not involved in lafK transcription.
A. hydrophila AH-3 encodes an alternative σ54 sigma factor (RpoN) which is essential for both polar and lateral flagellum expression (10, 19). Lateral flagellar clusters contain only one gene that encodes a σ54-dependent response regulator, LafK (10, 38, 43). In silico analysis of the A. hydrophila upstream lafK sequence did not shown putative σ54 promoter sequences. In order to establish if there was any relationship between the σ54 factor (RpoN) and lafK transcription, we measured the β-galactosidase activity of A. hydrophila wild-type and rpoN mutant (AH-5502) strains carrying the lateral flagella gene promoter-lacZ fusion plasmid pDNlac-lafKp. Similar β-galactosidase activities were detected in both strains (Fig. 3B). In addition, RT-PCR assays showed lafK transcription in both the wild type and the rpoN mutant (Fig. 3C).
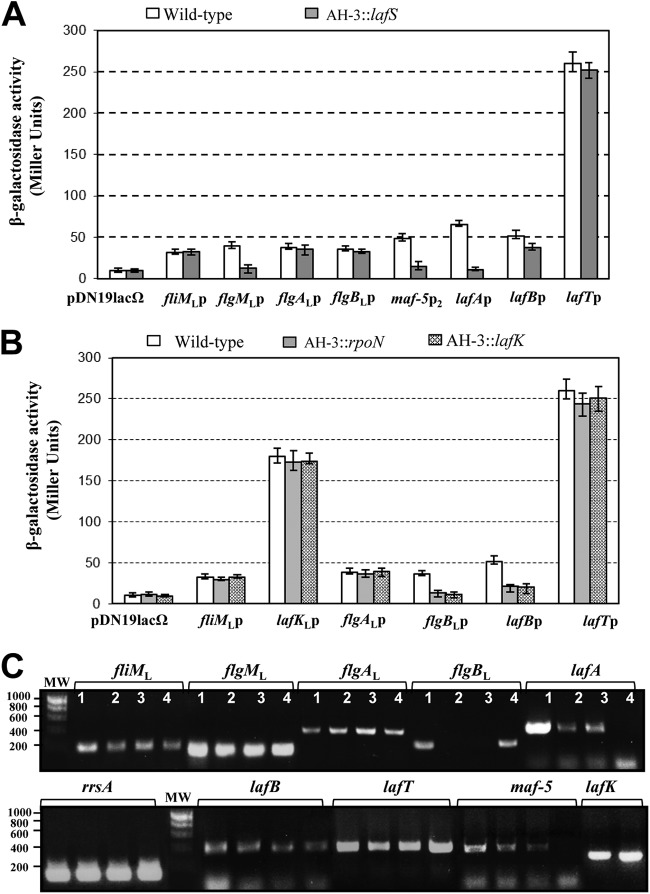
Fig 3.
Analysis of β-galactosidase activity after growth in TSB with 18% Ficoll at 25°C. (A) pDNlac-fliMLp, pDNlac-flgMLp, pDNlac-flgALp, pDNlac-flgBLp, pDNlac-maf-5p2, pDNlac-lafAp, pDNlac-lafBp, and pDNlac-lafTp plasmids in A. hydrophila wild-type (AH-405) and lafS mutant (AH-3::lafS) strains. (B) pDNlac-fliMLp, pDNlac-lafKp, pDNlac-flgALp, pDNlac-flgBLp, pDNlac-lafBp, and pDNlac-lafTp plasmids in the A. hydrophila wild type (AH-405) and rpoN (AH-5502) and lafK (AH-5503) mutant strains. pDNlac-lafKp was not analyzed in the lafK mutant. As a control, we measured the pDN19lacΩ promoterless plasmid. The results shown are representative of three independent experiments. Bars represent standard deviations. (C) RT-PCR amplification of fliML, lafK, flgML, flgAL, flgBL, maf-5, lafA, lafB, and lafT from cDNA of AH-3 (lane 1), AH-3::rpoN (2), AH-3::lafK (3), and AH-3::lafS (4) mutants. A. hydrophila ribosomal 16S (rrsA) amplification was used as a control for the cDNA template. RT-PCR amplifications were performed at least twice with total RNA preparations obtained from a minimum of two independent extractions. The Ecogen molecular weight marker (MW) was used.
Given that lafK transcription was σ54 independent, we performed 5′ RACE, as described in the Materials and Methods, to further analyze the lafK promoter region. Primary PCR of tailed cDNA using primers AAP and GSP2-LafK gave a unique DNA band of 620 bp (Fig. 4). Sequence of the amplified band indicates that it was tailed with G residues. The lafK transcription start was located 335 nucleotides (nt) upstream from the lafK translation start site, and DNA sequence upstream of the transcription start contains a σ70 promoter sequence (TTGAAT-N16-TATGAT) (Fig. 4). Furthermore, in silico analysis of the lateral flagellar region of A. caviae Sch3N (also showing dual flagellar systems) allowed us to identify a σ70 promoter sequence 331 bp upstream of the lafK start codon (5′-TTGAAT-N16-TATCAT-3′).
Fig 4.
(A) Amplification of the A. hydrophila AH-3 lafK, fliML, flgAL, lafB, and lafT cDNA 5′ end was performed using the 5′ RACE system, version 2.0 (Invitrogen). Amplicons were obtained by PCR using primers AAP and GSP2-LafK (lafKp), GSP2-FLIML (fliMLp), GSP2-FLGAL (flgALp), GSP2-LAFB (lafBp), and GSP2-LAFT (lafTp). Lanes: 1, PCR negative control; 2, primary PCR template; MW, molecular weight standard (Ecogen). Underlined sequences show start codons, asterisks show locations of the transcriptional start sites, and boldface nucleotides show potential consensus sequences. (B) Alignment in silico of σ28 and σ54 promoter elements in A. hydrophila lateral flagellar promoters. The consensus σ28 sequence is from Kutsukake (47). The consensus σ54 sequence is from Barrios (48).
Identification of A. hydrophila lateral flagellum σ28-dependent promoters.
Transcriptions of polar and peritrichous flagellar late genes are σ28 dependent (16, 17, 18, 31). The lafB-U cluster of the A. hydrophila lateral flagellar chromosomal region contains a gene, lafS, which encodes a sigma factor orthologous to the V. parahaemolyticus σ28 factor FliAL and homologous to the A. hydrophila σ28 factor FliA (38/54 and 34/54% identity/similarity, respectively) (10, 11, 23). In A. hydrophila, mutation of LafS abolishes lateral flagellum formation (11), and lateral flagella were restored by complementation with the pBAD33Gm-LAFS plasmid in the presence of 0.2% l-arabinose. In silico sequence analysis of A. hydrophila AH-3 lateral flagellum genes show a putative σ28 promoter sequence upstream of the anti-σ28 factor flgML (19), putative σ54 promoter sequences upstream of fliML, flgAL, flgBL, maf-5, lafB, and the lateral flagellin gene, lafA (11, 19), and several putative promoter sequences upstream of the motor gene lafT. In order to study which of these lateral flagella genes were σ28 dependent, we independently transferred the promoter-lacZ fusion plasmids pDNlac-fliMLp (fliMLp-lacZ), pDNlac-flgMLp (flgMLp-lacZ), pDNlac-flgALp (flgALp-lacZ), pDNlac-flgBLp (flgBLp-lacZ), pDNlac-maf-5p1 (maf-5p1-lacZ), pDNlac-lafAp (lafAp-lacZ), pDNlac-lafBp (lafBp-lacZ), and pDNlac-lafTp (lafTp-lacZ) into A. hydrophila AH-405 (AH-3 with rifampin resistance) and the lafS mutant (AH-3::lafS). Transconjugants were chosen and β-galactosidase activity measured. Transcription from the flgML and lafA promoters appeared to be highly affected by the lafS mutation, since 90 and 98% reduction of β-galactosidase activity, respectively, was found in the AH-3::lafS mutant compared to that of the wild-type AH-405. However, the activity of the lafB promoter was only slightly affected in the AH-3::lafS mutant (31% reduction), and activities from fliML, flgAL flgBL, lafB, and lafT promoters exhibited comparable values for both strains (Fig. 3A). The maf-5p1-lacZ cluster does not contain any promoter region, since β-galactosidase activity in the wild-type AH-405 was similar to that obtained with the pDN19lacΩ promoterless plasmid (data not shown). Sequence analysis of the maf-5 upstream region found a pseudogene which encodes an incomplete flagellin fragment homologous (77/79% identity/similarity) to the C-terminal region of ASA_0374 of A. salmonicida A449 (32). We amplified the region both upstream and downstream of the predicted pseudogene start codon and cloned it into the plasmid pDN19lacΩ to generate the promoter-lacZ fusion plasmid pDNlac-maf-5p2 (maf-5p2-lacZ). This plasmid was transferred by triparental conjugation into A. hydrophila AH-405 and the AH-3::lafS mutant. The measurement of β-galactosidase activity from the maf-5p2 promoter is significant only for wild-type AH-405 and was similar to the one obtained with the pDN19lacΩ promoterless plasmid in the lafS mutant (Fig. 3A).
Total RNAs from A. hydrophila AH-3 and the lafS mutant were used to amplify internal fragments of fliML, flgML, flgAL, flgBL, maf-5, lafA, lafB, and lafT transcripts, but no lafA and maf-5 amplicons were obtained from the lafS mutant (Fig. 3C). Furthermore, analysis of flgML-flgAL transcription in the wild type by RT-PCR showed that these two genes were cotranscribed. The results suggest that flgML, maf-5, and lafA transcription are σ28 dependent, and flgML is also transcribed from the flgAL promoter.
Identification of A. hydrophila LafK-dependent σ54 lateral flagellar promoters.
Promoters recognized by the σ54 holoenzyme require specialized enhancer-binding proteins, which bind specific sequences located in a relatively remote position from the transcription start site (33). Lateral flagellar clusters only contain one gene that encodes a σ54 enhancer-binding protein, LafK (34), which is required for V. parahaemolyticus lateral flagellum transcription (9). In A. hydrophila the LafK mutation abolishes lateral flagellum formation and swarming motility (19), as the wild-type phenotype is restored by complementation with the pBAD33Gm-LAFK plasmid in the presence of 0.2% l-arabinose. To investigate which of the A. hydrophila lateral flagellar clusters are σ54 and LafK dependent, β-galactosidase activities of the A. hydrophila wild type and the lafK (AH-5503) and rpoN (AH-5502) mutants carrying the promoter-lacZ fusion plasmids pDNlac-fliMLp (fliMLp-lacZ), pDNlac-lafKp (lafKp-lacZ), pDNlac-flgALp (flgALp-lacZ), pDNlac-flgBLp (flgBLp-lacZ), pDNlac-lafBp (lafBp-lacZ), and pDNlac-lafTp (lafTp-lacZ) were measured. Activities from flgBL and lafB promoters appeared to be affected in both LafK and RpoN mutant strains. The flgBL promoter showed a reduction of 93% in both mutants, and the lafB promoter show a reduction of 75 and 73% in the RpoN and LafK mutants, respectively (Fig. 3B). No significant variations were obtained from fliML, lafK, flgAL, and lafT promoters in any of these mutants. Furthermore, RT-PCRs to compare fliML, flgAL, flgBL, lafB, and lafT gene transcription in the wild type and the RpoN and LafK mutants showed fliML, flgAL, lafB, and lafT amplicons in the wild type and both mutants, whereas no flgBL amplicon was found in the RpoN and LafK mutants (Fig. 3C). These results suggest that the flgBL promoter is σ54 and LafK dependent.
To identify the fliML, flgAL, lafB, and lafT promoter regions, amplification of the A. hydrophila AH-3 fliML, flgAL, lafB, and lafT cDNA 5′ ends was performed using 5′ RACE as described in Materials and Methods. Primary PCR of tailed cDNA using primers AAP and GSP2-FLIML or GSP2-LafT give amplicons of 537 and 428 bp, respectively. However, primers AAP and GSP2-FLGAL or GSP2-LAFB render two amplicons of 650 and 827 bp and 700 and 588 bp, respectively. DNA sequence of the amplified bands indicates that amplicons were tailed with G residues. The fliML and lafT transcription starts were located 85 and 12 nt upstream from the fliML and lafT translation start sites, respectively. The flgAL transcription starts were located 158 and 332 nt upstream from the flgAL start site. The lafB transcription starts were located 39 and 158 nt upstream from the lafB start site. We were able to identify σ70 promoter sequences in DNA regions upstream of the transcription starts of fliML, flgAL, and lafT. Both σ28 and σ54 promoter sequences were found upstream of the lafB transcription start (Fig. 4A).
DISCUSSION
Functional lateral flagellar systems, whose flagella are randomly distributed over the cell surface in a manner similar to that of the peritrichous flagella of Enterobacteriaceae, were reported in polar flagellated bacteria with dual flagellar systems, such as A. hydrophila and V. parahaemolyticus (5). Lateral flagellar systems of these two species are encoded by 38 genes, 37 of which are orthologous and do not share either structural or regulatory genes with polar flagellar systems. Despite these similarities, A. hydrophila lateral flagellum genes are distributed in a unique chromosomal region, whereas V. parahaemolyticus lateral flagellar genes are distributed in two discontinuous chromosomal regions (9, 11, 19). The presence of two active flagellar systems implies a high energetic cost for a bacterium, therefore lateral flagellar synthesis should be carefully regulated in response to different environmental conditions. Different environmental conditions have been associated with lateral flagellar induction (35, 36), but the most extensive association is growth in viscous media or on a solid surface, which reduces polar flagellum motility. However, while V. parahaemolyticus and Azospirillium brasilense defects in polar flagellum formation or motility allow lateral flagellum expression (37, 38, 39), Aeromonas sp. polar flagellum defects do not induce constitutive lateral flagella (10, 40). This difference suggests that Aeromonas polar flagella do not act as mechanosensors and that lateral flagellar regulation is not linked to polar flagella.
The V. parahaemolyticus lateral flagellar system is the best studied at the regulatory level, and it has been demonstrated that its viscosity/surface-dependent expression is transcriptionally regulated (41). Transcription of V. parahaemolyticus lateral flagellar genes is organized into 3 levels (class I to III), where the first level contains the unique lateral flagellum σ54-associated transcriptional activator, lafK, and the third level contains the lateral flagellin gene, lafA. Lateral flagella of A. hydrophila are also expressed in viscous media or on solid surfaces (Fig. 1A), but their regulatory mechanisms have not been determined yet. Our data show that A. hydrophila lateral flagellar genes are transcribed in liquid and viscous media or on solid surfaces, although lateral flagellin is not transduced in liquid media. All of the data suggest that lateral flagella expression should be regulated by transductional mechanisms.
Two transcriptional hierarchy models in Gamma- and Alphaproteobacteria lateral flagella have been described (7, 9, 42). Until now, V. parahaemolyticus lateral flagellar transcriptional hierarchy has represented the Gammaproteobacteria model. In this model, the σ54-associated transcriptional activator, LafK, controls transcription of class II lateral flagellum genes which contain the σ28 factor (fliAL) involved in transcription of class III lateral flagellum genes (9). In order to establish the A. hydrophila lateral flagellar cluster transcription hierarchy, promoter-lacZ fusions with lateral flagellum promoters were analyzed in defined A. hydrophila lafK, lafS, and rpoN mutants. Furthermore, transcription analysis of genes in different lateral flagellar clusters in these A. hydrophila mutants were tested by RT-PCR assays.
Class III.
In V. parahaemolyticus the σ28 factor (FliAL) is involved in transcription of late genes, such as lateral flagellin, the anti-sigma factor flgML, and motor components (motABL), but also some middle genes, such as flgKLL and fliDSTKLAL (9). Our data show that A. hydrophila flgML, maf-5, and lafA are transcribed from σ28-dependent promoters, with the maf-5 promoter being upstream of the pseudoflagelin gene, and fliML, flgBL, flgAL, and lafT are transcribed from σ28-independent promoters (Fig. 3A and C). Furthermore, the slight reduction of β-galactosidase activity of lafB promoter fusion and the presence of the lafB transcript in the LafS mutant suggest that the lafBCXEFSTU cluster is transcribed from different promoters, with one of them being characterized as σ28 dependent. A similar situation has been reported in the V. parahaemolyticus lateral flagellar orthologous cluster fliDSTKLAL-motABL (9) (Fig. 4 and 5).
Fig 5.

Comparative proposed A. hydrophila and Vibrio parahaemolyticus (9) lateral flagellar gene transcription hierarchies. Diagrams show the three levels of lateral flagellar hierarchy, class I to III. Class I genes are at the top of the hierarchy, being σ70 dependent (dep.) in Aeromonas. One of the class I genes encodes a σ54-associated transcriptional activator (LafK) that activates σ54-dependent promoters preceding the class II clusters. One of the class II genes encodes the σ28 factor (LafS), which activates the transcription of class III genes. In Aeromonas, the σ28 factor might also be transcribed from another promoter.
Class II.
Transcription of the V. parahaemolyticus lateral flagellar genes included in the class II level are σ54 and LafK dependent (9). A. hydrophila LafK is essential for lateral flagella formation; however, in contrast to the case for V. parahaemolyticus, lafK, flgAL and lafT are transcribed from σ54/LafK-independent promoters and are not class II genes (Fig. 5). Our results also suggest that fliML is transcribed from σ54/LafK-independent promoters and flgB is transcribed from a σ54/LafK-dependent promoter, the sequence of which was predicted in silico. Furthermore, assays indicate that lafB promoter activity was only 73 to 75% reduced in the LafK and RpoN mutants (Fig. 3B), and the amplification of the lafB cDNA 5′ ends by 5′ RACE shows two promoter sequences, a σ28 promoter sequence (TAAGGG-N17-GTCGAAA) and a σ54 promoter sequence (TGGCAT-N5-TTCTG), with the latter being more active (Fig. 4). These results indicate that lafBCXEFSTU is transcribed from two promoters. A similar situation is described for V. parahaemolyticus, although it has not been studied at the transcriptional level. The σ28 factor lafS is contained in the lafBCXEFSTU cluster, but the lack of LafK does not prevent lafS transcription in Aeromonas. RT-PCR assays showed transcription of maf-5 and lafA in LafK and RpoN mutants (Fig. 3C). Data suggest that lafS is transcribed from a second promoter in a σ54/LafK-independent manner and is less active than the promoter upstream of lafB. Amplification of the lafS cDNA 5′ ends by 5′ RACE allowed us to obtain an amplicon (data not shown).
Class I.
As previously indicated, A. hydrophila lateral flagellum regulatory cascade class I seems to include more that one gene. Although previous in silico analysis showed putative σ54 promoter sequences upstream of lafK, fliML, and flgAL (19), the data obtained now show that upstream regions of fliML, flgAL, lafT, and lafK contain σ70 promoter sequences (Fig. 4). A. hydrophila lateral flagellum class I gene transcription is σ70 dependent, as has been reported for A. hydrophila polar flagellar class I genes. Data are consistent with the fact that A. hydrophila lateral flagellar genes are transcribed in liquid and viscous media and on solid medium, in contrast to V. parahaemolyticus (41). A. hydrophila lateral flagella are not induced by mutation of polar flagellum genes, as happens in V. parahaemolyticus. In V. parahaemolyticus, the lafK promoter is located upstream of motYL, which encodes a lateral motor protein that does not possess an orthologue in A. hydrophila, and genes of the motYL-lafK-fliEFGHIJL cluster are classified as class I and II. In addition, the V. parahaemolyticus fliMNPQRL-flhABL lateral flagellar cluster is classified as class I (43), as is the case in A. hydrophila. In A. hydrophila, lafS transcription feeds into class I, since LafK mutation does not abolish transcription of σ28-dependent promoters (Fig. 3C). The A. hydrophila lateral flagellar transcriptional hierarchy is complex, since many clusters of genes are transcribed independently of LafK. LafK is not strictly the master lateral flagellar regulator in A. hydrophila.
Our results indicate that despite A. hydrophila lateral flagella only being expressed in viscous media or on solid surfaces, their genes are transcribed in liquid, although lateral flagellin was not detected in liquid media. Recently, it has been described that the A. hydrophila AH-3 lateral flagellin is glycosylated (44), although it is nonglycosylated in V. parahaemolyticus, and this fact could contribute to the complexity of their lateral flagella transcription hierarchy and the important differences between these two bacteria. An A. hydrophila AH-3 in-frame deletion mutant of the pseudaminic acid biosynthetic gene pseB homologue resulted in the abolition of lateral flagella formation by posttranscriptional regulation of the flagellin, which was restored by complementation with the wild-type pseB homologue or Campylobacter pseB (data not shown).
ACKNOWLEDGMENTS
This work was supported by Plan Nacional de I + D (Ministerio de Educación, Ciencia y Deporte and Ministerio de Sanidad, Spain) and Generalitat de Catalunya (Centre de Referència en Biotecnologia).
We thank Maite Polo for her technical assistance and the Servicios Científico-Técnicos from the University of Barcelona.
Footnotes
Published ahead of print 18 January 2013
REFERENCES
- 1. Harshey RM, Matsuyama T. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. U. S. A. 91:8631–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearns DB. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8:634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doyle TB, Hawkins AC, McCarter LL. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J. Bacteriol. 186:6341–6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toutain CM, Zegans ME, O'Toole GA. 2005. Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J. Bacteriol. 187:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merino S, Shaw JG, Tomás JM. 2006. Bacterial lateral flagella: an inducible flagella system. FEMS Microbiol. Lett. 263:127–135 [DOI] [PubMed] [Google Scholar]
- 6. Shinoda S, Okamoto K. 1977. Formation and function of Vibrio parahaemolyticus lateral flagella. J. Bacteriol. 129:1266–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu R, Ochman H. 2007. Origins of flagellar gene operons and secondary flagellar systems. J. Bacteriol. 189:7098–7104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren CP, Beatson SA, Parkhill J, Pallen MJ. 2005. The Flag-2 locus, an ancestral gene cluster, is potentially associated with a novel flagellar system from Escherichia coli. J. Bacteriol. 187:1430–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stewart BJ, McCarter LL. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 185:4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canals R, Ramirez S, Vilches S, Horsburgh G, Shaw JG, Tomás JM, Merino S. 2006. Polar flagellum biogenesis in Aeromonas hydrophila. J. Bacteriol. 188:542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gavín R, Rabaan AA, Merino S, Tomás JM, Gryllos I, Shaw JG. 2002. Lateral flagella of Aeromonas species are essential for epithelial cell adherence and biofilm formation. Mol. Microbiol. 43:383–397 [DOI] [PubMed] [Google Scholar]
- 12. Okabe M, Yakushi T, Kojima M, Homma M. 2002. MotX and MotY, specific components of the sodium-driven flagellar motor, colocalize to the outer membrane in Vibrio alginolyticus. Mol. Microbiol. 46:125–134 [DOI] [PubMed] [Google Scholar]
- 13. Karlyshev AV, Linton D, Gregson NA, Wren BW. 2002. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148:473–480 [DOI] [PubMed] [Google Scholar]
- 14. Chilcott GS, Hughes KT. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCarter LL. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dasgupta N, Wolfgang MC, Goodman AL, Arora SK, Jyot J, Lory S, Ramphal R. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809–824 [DOI] [PubMed] [Google Scholar]
- 17. Prouty MG, Correa NE, Klose KE. 2001. The novel sigma54 and sigma28 dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 39:1595–1609 [DOI] [PubMed] [Google Scholar]
- 18. Wilhelms M, Molero R, Shaw JG, Tomas JM, Merino S. 2011. Transcriptional hierarchy of Aeromonas hydrophila polar-flagellum genes. J. Bacteriol. 193:5179–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canals R, Altarriba M, Vilches S, Horsburgh G, Shaw JG, Tomás JM, Merino S. 2006b. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 188:852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 21. Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U. S. A. 74:5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altschul FS, Madden TL, Schaffer AA, Zhang J, Zang Z, Miller W, Lipman J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Studholme DJ, Dixon R. 2003. Domain architectures of sigma54-dependent transcriptional activators. J. Bacteriol. 185:1757–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Münch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, Jahn D. 2005. Virtual footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187–4189 [DOI] [PubMed] [Google Scholar]
- 25. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 26. Bott M, Meyer M, Dimroth P. 1995. Regulation of anaerobic citrate metabolism in Klebsiella pneumoniae. Mol. Microbiol. 18:533–546 [DOI] [PubMed] [Google Scholar]
- 27. Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimenez N, Lacasta A, Vilches S, Reyes M, Vazquez J, Aquillini E, Merino S, Regué M, Tomás JN. 2009. Genetics and proteomics of Aeromonas salmonicida lipopolysaccharide core biosynthesis. J. Bacteriol. 191:2228–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Totten PAA, Lory S. 1990. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J. Bacteriol. 172:7188–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller JH. 1992. A short course in bacterial genetics, 2nd ed Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 31. Ohnishi K, Kutsukake K, Suzuki H, Iino T. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol. Gen. Genet. 221:139–147 [DOI] [PubMed] [Google Scholar]
- 32. Reith ME, Singh RK, Curtis B, Boyd JM, Bouevitch A, Kimball J, Munholland J, Murphy C, Sarty D, Williams J, Nash JH, Johnson SC, Brown LL. 2008. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics 18:9–427 doi:10.1186/1471-2164-9-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buck M, Gallegos MT, Studholme DJ, Guo Y, Gralla JD. 2000. The bacterial enhancer-dependent sigma 54 transcription factor. J. Bacteriol. 182:4129–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merino S, Tomás JM. 2009. Lateral flagella systems. In Jarrell K. (ed.), Pili and flagella: current research and future trends. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 35. Berleman JE, Bauer CE. 2005. A che-like signal transduction cascade involved in controlling flagella biosynthesis in Rhodospirillum centenum. Mol. Microbiol. 55:1390–1402 [DOI] [PubMed] [Google Scholar]
- 36. Madi L, Kessel M, Sadovnik E, Henis Y. 1988. Electron microscopic studies of aggregation and pellicle formation in Azospirillum spp. Plant Soil 109:115–121 [Google Scholar]
- 37. Alexandre G, Rohr R, Bally R. 1999. A phase variant of Azospirillum lipoferum lacks a polar flagellum and constitutively expresses mechanosensing lateral flagella. Appl. Environ. Microbiol. 65:4701–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawagishi I, Maekawa Y, Atsumi T, Homma M, Imae Y. 1995. Isolation of the polar and lateral flagellum-defective mutants in Vibrio alginolyticus and identification of their flagellar driving energy sources. J. Bacteriol. 177:5158–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCarter LL, Hilmen M, Silvermanm M. 1988. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 54:345–351 [DOI] [PubMed] [Google Scholar]
- 40. Altarriba A, Merino S, Gavín R, Canals R, Rabaan A, Shaw JG, Tomas JM. 2003. A polar flagella operon (flg) of Aeromonas hydrophila contains genes required for lateral flagella expression. Microb. Pathog. 34:249–259 [DOI] [PubMed] [Google Scholar]
- 41. Belas R, Simon M, Silverman M. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang Y, Tang T, Li J-L. 2007. Isolation of a flagellar operon in Azospirillum brasilense and functional analysis of FlbD. Res. Microbiol. 158:521–528 [DOI] [PubMed] [Google Scholar]
- 43. Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. 2011. Surface sensing in Vibrio parahaemolyticus triggers a program of gene expression that promotes colonization and virulence. Mol. Microbiol. 79:240–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilhelms M, Fulton KM, Twine SM, Tomás JM, Merino S. 2012. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J. Biol. Chem. 287:27851–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Merino S, Camprubi S, Tomas JM. 1991. The role of lipopolysaccharide in complement-killing of Aeromonas hydrophila strains of serotype O:34. J. Gen. Microbiol. 137:1583–1590 [DOI] [PubMed] [Google Scholar]
- 46. Rubires X, Saigi F, Piqué N, Climent N, Merino S, Albertí S, Tomás JM, Regué M. 1997. A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 179:7581–7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kutsukake K, Ohya Y, Iino T. 1990. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J. Bacteriol. 172:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barrios H, Valderrama B, Morett E. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acid Res. 27:4305–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]