Abstract
Hydrogenotrophic methanogenic Archaea are defined by an H2 requirement for growth. Despite this requirement, many hydrogenotrophs are also capable of growth with formate as an electron donor for methanogenesis. While certain responses of these organisms to hydrogen availability have been characterized, responses to formate starvation have not been reported. Here we report that during continuous culture of Methanococcus maripaludis under defined nutrient conditions, growth yields relative to methane production decreased markedly with either H2 excess or formate excess. Analysis of the growth yields of several mutants suggests that this phenomenon occurs independently of the storage of intracellular carbon or a transcriptional response to methanogenesis. Using microarray analysis, we found that the expression of genes encoding coenzyme F420-dependent steps of methanogenesis, including one of two formate dehydrogenases, increased with H2 starvation but with formate occurred at high levels regardless of limitation or excess. One gene, encoding H2-dependent methylene-tetrahydromethanopterin dehydrogenase, decreased in expression with either H2 limitation or formate limitation. Expression of genes for the second formate dehydrogenase, molybdenum-dependent formylmethanofuran dehydrogenase, and molybdenum transport increased specifically with formate limitation. Of the two formate dehydrogenases, only the first could support growth on formate in batch culture where formate was in excess.
INTRODUCTION
Methanogenic Archaea (methanogens) play a key role in the anaerobic breakdown of organic matter. Five of six known orders of methanogens, termed “hydrogenotrophic,” derive their energy through the use of H2 to reduce CO2 to methane. Hydrogenotrophic methanogens lack a cytochrome-containing electron transport chain and are proposed to conserve energy by the mechanism of electron bifurcation (1, 2). H2 availability has profound physiological and regulatory effects in hydrogenotrophic methanogens. For example, in Methanothermobacter thermautotrophicus, when H2 is present in excess, growth in relation to methane production (YCH4, grams [dry weight] of cells per mole of methane) decreases (3, 4), suggesting that energy spilling (otherwise known as growth uncoupling or overflow metabolism [5]) occurs. In M. thermautotrophicus, Methanococcus maripaludis, and other methanogens, H2 availability also alters the expression of key genes of methanogenesis (4, 6–10). The mechanisms for the effects on growth yield and gene expression are unknown.
In addition to H2, formate may also be a primary electron donor for methanogenesis in the environment (11, 12), and several hydrogenotrophic species can use formate without the addition of H2. For the catabolic process of methanogenesis, formate and H2 are interchangeable electron donors; all four reductive steps can proceed with electrons derived directly from either electron source (13–16). H2, however, is essential for the anabolic process of CO2 fixation (17, 18) and for an anaplerotic input to methanogenesis (16). During growth on formate, a formate:H2 lyase activity generates the necessary H2 (14–16).
Since formate acts independently of H2 as an electron donor for methanogenesis, we asked whether its availability similarly affects growth yield and gene expression. We identify four different response patterns with regard to four conditions, H2 limitation and excess and formate limitation and excess. Interestingly, a discrete set of genes responds specifically to formate limitation.
MATERIALS AND METHODS
Strains and batch culture conditions.
Strains, plasmids, and primers are listed in Table S1 in the supplemental material. Deletion mutants were either taken from previous studies or generated in this study as described previously (13, 19). Briefly, where possible, deletion constructs were digested out of the plasmid pCRprtneo and ligated directly to pCRuptneo (14); otherwise, PCR products containing the deletion of interest were generated and ligated into pCRuptneo. Strain MM901 was then transformed with the deletion constructs and subjected to a selection/counterselection system dependent on neomycin (1 to 5 mg ml−1) and 6-azauracil (250 μg ml−1) to generate mutants of interest (13). Strains were grown on McCas medium as described before (19). To test for auxotrophy of the cdh and ehb mutants, sodium acetate (normally 10 mM) or Casamino Acids (normally 0.2% [wt/vol]) were omitted, respectively. When formate instead of H2 was supplied as the electron donor, McCas medium was amended with 200 mM sodium formate (with an equivalent reduction in NaCl to maintain sodium osmolarity) and 200 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7); the headspace was 140 kPa N2-CO2 (80:20) (14). For growth curves, a 4% inoculum was used and optical density at 660 nm (OD660) was monitored. For all experiments, cultures were grown at 37°C.
Growth and microarray analysis of M. maripaludis grown in a chemostat under defined nutrient limitation.
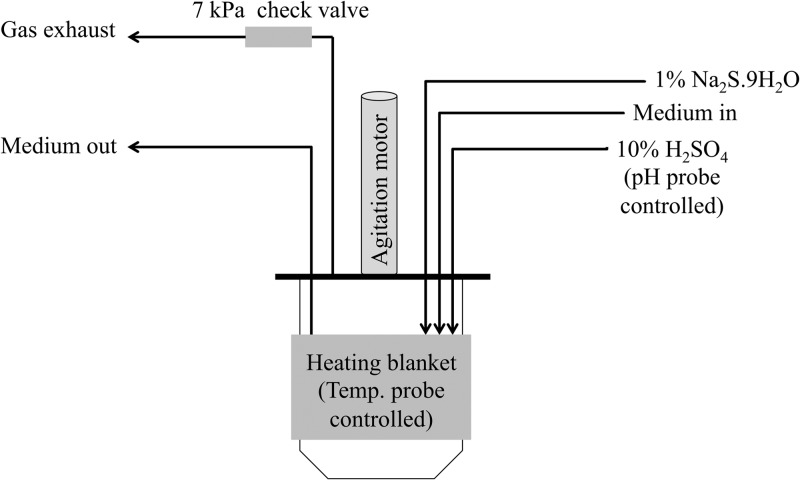
M. maripaludis strain MM901 was grown in a chemostat on H2 as described previously (8, 20, 21). For the ehb mutant, 0.1% (wt/vol) Casamino Acids was added to chemostat medium and 100 μg ml−1 of ampicillin was included to prevent bacterial contamination. For unknown reasons, when Casamino Acids (Fisher BioReagents catalog number BP1424-500) were used, limitation occurred at a lower phosphate level (0.02 mM versus 0.12 mM in minimal medium). For growth on formate, the chemostat setup is diagramed in Fig. 1. Conditions were modified as follows unless otherwise indicated. Medium contained 380 mM sodium formate in place of NaCl. Although Cl− is important for cellular function, replacing NaCl with sodium formate is standard practice and has no known deleterious effects on growth (22), suggesting that other Cl−-containing salts in our medium (e.g., MgCl2) are sufficient. MOPS buffer was not included during continuous culture on formate. Formate utilization results in a pH increase by the following equation: 4NaCOOH + 2H2O → CH4 + 3CO2 + 4NaOH. Therefore, a pH probe was used to monitor and maintain pH at 6.95 with the automated addition of 10% (vol/vol) H2SO4. The agitation rate was 250 rpm. The gas mixture initially supplied to the chemostat was H2-Ar-CO2-1% H2S (21, 125, 40, and 14 ml min−1) (8). After the OD increased above 0.6 (24 h), the medium flow was turned on at 0.083 liter h−1. Medium was either nonlimiting (described above), formate limiting (modified to contain 200 mM sodium formate and 180 mM NaCl), nitrogen limiting (2 mM NH4Cl), or phosphate limiting (0.08 mM K2HPO4). Also at this point, gas flow through the vessel was shut off, a 7-kPa check valve was installed on the exhaust line to maintain positive pressure within the vessel while also allowing for exhaust of gaseous metabolic by-products, and a 1% Na2S · 9H2O feed was started into the vessel at 0.83 ml h−1. Forty-eight hours later, samples were taken for physiological measurements or microarray analysis. If conditions were nutrient limited, nonlimiting medium was then reintroduced. A subsequent increase in OD confirmed that nutrient limitation had been imposed. If the chemostat was allowed to run longer, iron sulfides precipitated several days after the start of Na2S addition and attached to surfaces within the culture vessel (data not shown).
Fig 1.
Schematic of chemostat setup with formate as the electron donor. A more detailed diagram of the chemostat setup for H2-based growth can be found in reference 20.
Samples were collected for microarray analysis as follows. A total of 1.5 ml of chemostat culture (OD660, ∼0.65) was centrifuged at 13,000 × g for 30 s, and supernatant was discarded. The pellet was immediately placed in a dry ice-ethanol bath and stored at −80°C until RNA extraction and microarrays could be performed. Microarrays were run as described previously (23).
Growth yield calculations.
To calculate growth yield of M. maripaludis during H2 limitation or excess, culture OD660, gas flow rate, and percent methane in the gas outflow were determined. Methane concentrations were measured on a Buck Scientific model 910 GC equipped with a flame ionization detector. Carrier gases were air (110 kPa), H2 (180 kPa), and He (170 kPa). Growth rate based on the chemostat dilution was one doubling per 499 min. Finally, an OD660 of 1.0 was assumed to equal 0.34 g (dry cell mass) per liter (15). The following equation was used to determine dry cell mass produced per mole of CH4 (YCH4): (OD660/CH4 [ml/min]) × (0.34 g/OD660) × (1/499 min) × (22,400 ml/mol).
To determine the yield of grams (dry mass) per mole of formate consumed, the offset of pH in the chemostat vessel by 10% (vol/vol) H2SO4 addition was monitored (YH2SO4). H2SO4 consumption was monitored over ∼18 h, and YH2SO4 was calculated as follows: (OD660/[milliliters of acid/hour]) × (0.34 g/OD660) × [1/(499/60)] × (543.5 ml of acid/mol).
YH2SO4 was converted to YCH4 assuming that each mole of H+ from H2SO4 offsets the pH change from 1 mol of NaOH produced by sodium formate utilization. Therefore, 2 mol of H2SO4 corresponds to 4 mol of formic acid and 1 mol of CH4.
Microarray data accession number.
Microarray data have been deposited under GEO accession number GSE42111.
RESULTS
Chemostat system for growth of M. maripaludis with formate.
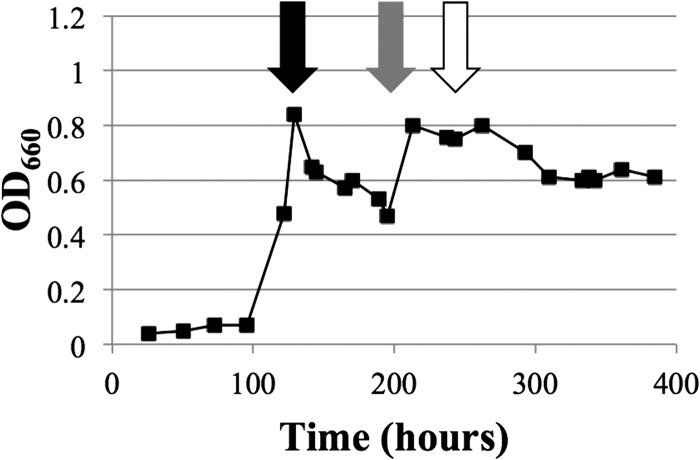
Continuous culture of M. maripaludis on formate required modification of our protocol for growth on H2. Even though the medium contained formate, it was necessary to first establish a culture in the chemostat vessel by sparging with gas containing H2. When H2 was subsequently excluded, culture OD steadily decreased (Fig. 2). M. maripaludis requires H2 for methanogenesis and anabolism; normally, formate:H2 lyase activity generates this H2 (14–18). Maintaining H2-free gas flow likely flushed all H2 from the vessel, inhibiting growth. We also tried changing the agitation rate of the vessel (Fig. 2), as this affects the rate of H2 removal from the system; however, this had no noticeable effect. We therefore completely stopped gas flow in order to maintain physiologically relevant levels of autochthonous H2 (Fig. 2). A 7-kPa check valve was installed to maintain positive pressure within the vessel while allowing escape of gaseous metabolic products. In addition, M. maripaludis requires sulfide as a reducing agent and a sulfur source for growth (24); therefore, a system for the addition of Na2S, instead of gaseous H2S, was devised. OD rebounded under these conditions, and reliable steady-state growth of M. maripaludis on formate was achieved with a constant OD of 0.8 (Fig. 2). Finally, phosphate limitation was introduced, and culture OD dropped to a steady-state value of 0.6, confirming our ability to impose nutrient limitation of M. maripaludis grown on formate (Fig. 2) similarly to nutrient limitation on H2 (20).
Fig 2.
Growth behavior of M. maripaludis in a chemostat with formate. Plot shows OD over time in response to various perturbations. Initially the vessel was sparged with gas containing H2 (H2-Ar-CO2-1% H2S; 21, 125, 40, and 14 ml min−1). At 121 h (black arrow), medium flow was started and H2 was turned off while flow of other gases was continued (Ar-CO2-1% H2S; 20, 20, and 6 ml min−1); these gassing rates were stepped down to 5, 20, and 2 ml min−1 at hour 170. At 195 h (gray arrow), all gas flow was terminated and 1% Na2S · 9H2O addition was started. Finally, at 243 h (white arrow), the medium flowing into the vessel was switched to a limiting level of phosphate. Medium flow was maintained at 0.083 liter/h throughout except between hours 165 and 195, when it was 0.062 liter/h. Agitation was 250 rpm throughout except after hour 121, when it was maintained between 50 and 100 rpm.
Changes in growth yield occur with both H2 and formate as electron donors.
Our chemostat system allowed us to determine whether H2 excess affects growth yield in M. maripaludis. We compared three continuous cultures: H2 limited, phosphate limited, and ammonia limited. Growth rate and cell density were held similar under all conditions. Since H2 was in excess when phosphate or ammonia limited growth, two comparisons yielded information on the effect of H2 limitation. YCH4 (grams [cell mass] per mole of CH4 produced) under H2 limitation was 2.86 ± 0.58 (mean ± standard deviation [SD]), compared to 0.77 ± 0.20 under phosphate or ammonia limitation (Fig. 3A). Hence, H2 excess results in a marked decrease in growth yield in M. maripaludis.
Fig 3.
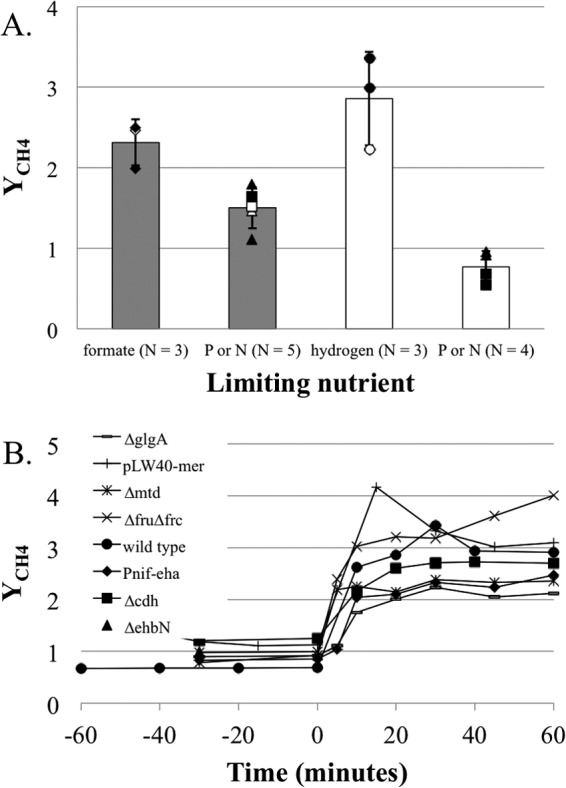
Growth yields for M. maripaludis under different nutrient limitations. (A) Cell yield (grams [dry weight]) per mole of CH4 (YCH4) in steady-state culture grown with H2 or formate excess or limitation. Error bars represent 1 SD around the mean. P and N limitation samples were pooled to represent H2 or formate excess growth. Gray bars, formate growth; white bars, H2 growth. Diamonds, formate-limited samples; circles, H2-limited samples; squares, N-limited samples; triangles, P-limited samples. Open symbols indicate samples used for microarray analysis. (B) Changes to YCH4 for various mutants upon transition from H2 excess (phosphate limitation) to H2 limitation at time zero.
We then asked whether formate limitation would have the same effect. A decrease in YCH4 due to H2 excess has previously been demonstrated in hydrogenotrophic methanogens, but whether it occurs with formate excess has not been determined. Again, we compared three continuous cultures: formate limited, phosphate limited, and ammonia limited. Since continuous culture on formate required the cessation of gas flow through the system, we could not accurately measure the rate of CH4 generation directly. Instead, we used the rate of acid addition, reflecting the rate of formic acid utilization, as an indirect measure for calculating YCH4. YCH4 under formate limitation was 2.31 ± 0.29, compared to 1.50 ± 0.26 under phosphate or ammonia limitation (Fig. 3A). Hence, the lowest YCH4 levels were observed with H2 excess, intermediate levels occurred with formate excess, and the highest levels occurred with formate limitation or H2 limitation (H2 excess versus formate excess, P = 0.002; H2 limitation versus formate limitation, P = 0.218; formate excess versus formate limitation, P = 0.006).
Changes in mRNA abundance or energy storage capacity cannot account for differences in YCH4.
A variety of hydrogenotrophic methanogens display altered YCH4 values in response to changes in H2 availability (4, 6, 7), suggesting a mechanism common to all organisms. In all methanogens tested, the coenzyme F420-dependent steps of methanogenesis are also transcriptionally regulated in response to H2 (4, 8–10). Therefore, we tested the importance of these H2-regulated genes in altering growth yields on M. maripaludis. Genes for both of the F420-reducing hydrogenases (fru and frc) or the F420-dependent methylene-tetrahydromethanopterin (H4MPT) dehydrogenase (mtd) were genetically eliminated. The gene for the F420-dependent methylene-H4MPT reductase (mer) is essential and, therefore, was overexpressed from the replicative vector pLW40 (25) rather than genetically eliminated. Each of these mutant strains displayed a marked change in YCH4 upon transition from H2 excess to H2 limitation similar to the wild type (Fig. 3B).
The membrane-bound, energy-converting hydrogenase Eha acts to anaplerotically sustain methanogenesis by reducing ferredoxin that can be used by formylmethanofuran dehydrogenase to reduce CO2 to formylmethanofuran (16). This reaction consumes membrane potential and could account for differences in growth yields between H2 excess and H2 limitation. As eha is required for methanogenesis in wild-type M. maripaludis, we elected to place it under the control of the nitrogenase promoter (Pnif) in an effort to control and repress its expression in the presence of ammonium. When grown in batch culture with formate as the electron donor (i.e., low H2 concentrations), this strain has a slow growth phenotype, suggesting that Eha levels are indeed repressed (see Fig. S1 in the supplemental material). The Pnif-eha construct also displayed a marked difference in growth yield in response to H2 availability (Fig. 3B).
Finally, alterations in growth yield in M. maripaludis could be due to changes in cellular composition such that energy storage occurs under H2 excess. As YCH4 decreases with H2 excess and phosphate limitation, energy storage as polyphosphates is unlikely despite the fact that this capability has been demonstrated for methanogens from the order Methanosarcinales (26). M. maripaludis was shown to synthesize glycogen (27); therefore, a deletion mutant for glycogen synthetase (glgA) was generated (28). This mutant still displayed a marked difference in YCH4 between H2 excess and H2 limitation, suggesting that the lowered growth yield under H2 excess is not due to an investment in glycogen synthesis. Additionally, deletion mutants for carbon monoxide dehydrogenase (cdh, essential for the first step of CO2 fixation) and the energy-converting hydrogenase ehb (important for generating reduced electron carriers for CO2 assimilation) were generated. As expected, the cdh and ehb mutants were auxotrophic for acetate and Casamino Acids, respectively (17, 18). With these mutants, too, trends in YCH4 values under the two conditions were similar to those with the wild type, suggesting that differential investment in CO2 assimilation cannot account for the differences in growth yield (Fig. 3B). However, we cannot discount the possibility that under H2 excess, resources are diverted toward higher energy content in some form, such as cellular lipid content or composition.
Transcriptional response of genes encoding F420-dependent steps of methanogenesis.
We compared formate-grown cultures to H2-grown cultures. In addition, we determined if the effect of formate limitation when formate is the electron donor is similar to the effect of H2 limitation when H2 is the electron donor. Microarray analysis was performed using samples from the six nutrient-limited cultures described above. In each case, nutrient supplementation after sampling resulted in an increase in OD, verifying that the nutrient in question had been limiting (Table 1). A seventh culture was grown on formate, and high levels of all nutrients were provided; in this case OD was continuously high. In addition, expression levels of key genes responded as expected, since transcript levels for genes for nitrogenase (nifH) and phosphate transporter (pstB) and mtd increased with ammonia, phosphate, and H2 limitation, respectively (8, 9, 29).
Table 1.
Nutrient-limited conditions for M. maripaludis grown in a chemostat
| Sample | Condition |
OD660 |
Log2 expressiona |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formate (mM) | H2 (ml/min) | Phosphate (mM) | Ammonia (mM) | Limited | Non-limited | mtd | fdhB2 | nifH | pstB | |
| Undefined limitation | 380 | 0 | 0.8 | 10 | 0.89 | 2.07 | 2.14 | −0.33 | −0.1 | |
| Formate limitation | 200 | 0 | 0.8 | 10 | 0.64 | 0.87 | 1.87 | 3.33 | −0.56 | −0.12 |
| Formate: P limitation | 380 | 0 | 0.08 | 10 | 0.69 | 0.86 | 0.82 | 0.23 | −0.47 | 4.71 |
| Formate: N limitation | 380 | 0 | 0.8 | 2 | 0.66 | 0.89 | 1.78 | 0.28 | 5.33 | −0.14 |
| H2 limitation | 0 | 21 | 0.8 | 10 | 0.68 | >1.0 | 3.08 | 0.41 | −0.34 | 0.05 |
| H2: P limitation | 0 | 110 | 0.12 | 10 | 0.67 | >1.0 | −3.84 | 0.29 | 0.06 | 4.06 |
| H2: N limitation | 0 | 110 | 0.8 | 2.8 | 0.59 | >1.0 | −2.89 | 0.02 | 5.89 | −0.1 |
Log2 expression ratios are relative to a single arbitrary standard (23). Boldface type indicates expression levels consistent with the imposed nutrient limitation.
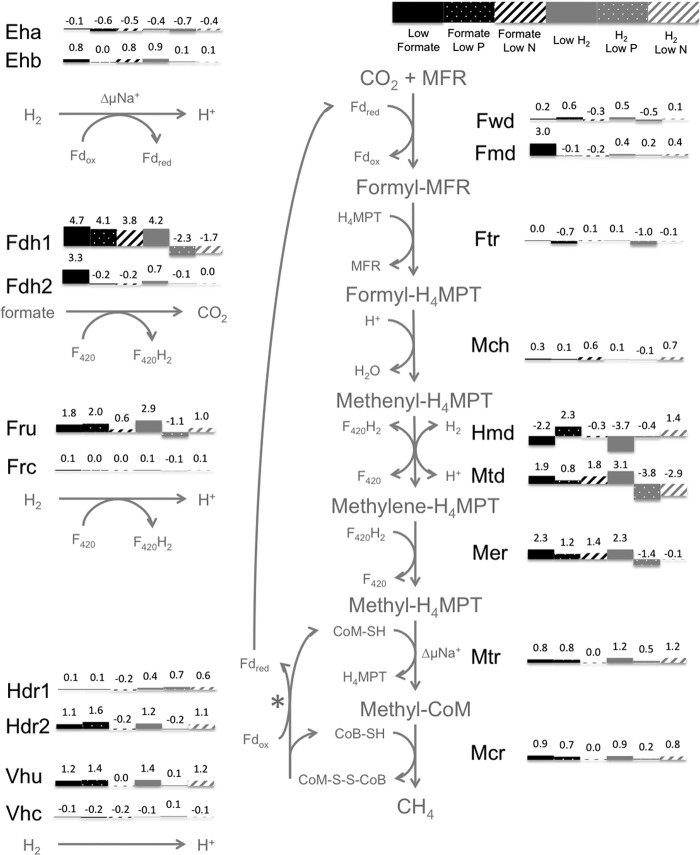
With H2 limitation, nearly all of the genes for the F420-dependent steps of methanogenesis showed a marked increase in mRNA abundance, in agreement with previous results (8). Thus, fdh1 (encoding formate dehydrogenase), mtd (encoding F420-dependent methylene-H4MPT dehydrogenase), mer (encoding methylene-H4MPT reductase), and fru (encoding F420-reducing hydrogenase) had higher mRNA abundance with H2 limitation than with either condition of H2 excess (phosphate limitation or ammonia limitation) (Fig. 4; see also Table S2 in the supplemental material). In contrast, the formate-grown cultures showed a different pattern: nearly all of the F420-dependent activities that showed increased mRNA abundance under H2 limitation also had high mRNA abundance during formate growth under all three conditions (Fig. 4) as well as in the seventh culture, in which all nutrients were present at high levels (see Table S2). fru displayed a single exception, showing increased mRNA abundance with formate except when nitrogen was limiting. Hence, growth on formate as the electron donor, whether formate was limiting or not, had an effect similar to that of growth on H2 limitation.
Fig 4.
Expression of methanogenesis genes in response to nutrient limitations. Bars and numbers show log2 expression ratios relative to an arbitrary standard (23). Values are for genes encoding active-site enzymes (except fdh; see below). ΔμNa+, change in Na+ across membrane; H4MPT, tetrahydromethanopterin; MFR, methanofuran; CoM-SH, coenzyme M; CoB-SH, coenzyme B; CoM-S-S-CoB, heterodisulfide of CoM and CoB; F420/F420H2, coenzyme F420; Fdox/Fdred, ferredoxin; Eha/Ehb, energy-converting hydrogenase A or B; Fdh, formate dehydrogenase; Ftr, formyltransferase; Fmd/Fwd, formylmethanofuran dehydrogenase; Fru/Frc, F420-reducing hydrogenase; Hdr, heterodisulfide reductase; Hmd, H2-dependent methylene-H4MPT dehydrogenase; Mch, methenyl-H4MPT cyclohydrolase; Mcr, methyl-CoM reductase; Mer, methylene-H4MPT reductase; Mtd, F420-dependent methylene-H4MPT dehydrogenase; Mtr, methyl-H4MPT:CoM methyltransferase; Vhu/Vhc, Hdr-associated hydrogenase. *, either H2 or formate acts as the electron donor for the reaction. (Note: fdhB transcript abundance [MMP0139 and MMP1297] was used to assess fdh expression. There is a known artifact where portions of fdhA2 [MMP0138] sometimes appear highly expressed under H2 limitation in microarrays, most likely due to probe cross-hybridization with fdhA1 [MMP1298] transcripts which share high [∼73%] nucleotide identity [23]. However, both gene expression and protein abundance analyses have demonstrated that fdhA2 is not differentially expressed between H2 limitation and excess [8, 9]. Due to this documented ambiguity in determining fdhA2 gene expression using microarray data, fdhA was ignored in our analysis in favor of fdhB.)
Formate limitation leads to increased mRNA levels of a formate dehydrogenase and other molybdenum-associated functions.
In contrast to most F420-dependent steps of methanogenesis, certain genes did respond specifically to formate limitation: a second formate dehydrogenase operon fdh2 (Fig. 4), the genes for the molybdenum-containing formylmethanofuran dehydrogenase (fmd), and several molybdenum transport genes (see Table S2 in the supplemental material). In contrast, neither fdh1 (which followed the pattern of other genes for F420-dependent steps of methanogenesis) nor the genes for tungsten-containing formylmethanofuran dehydrogenase (fwd) showed differences in mRNA abundance during formate-limited growth (Fig. 4). Since the two fdh operons were expressed differently, we checked the ability of each to support growth on formate. Deletion of fdhA1 eliminated growth on formate, while deletion of fdhA2 had no effect in batch culture (Fig. 5A). Deletion mutants for fdhA1 and fdhA2 should have no polar effects on formate transport (fdhC) or carbonic anhydrase (CA) (Fig. 5B).
Fig 5.
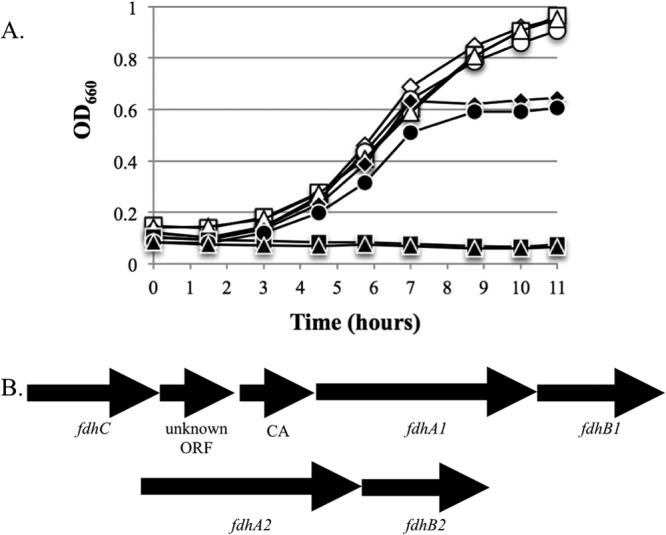
Growth of M. maripaludis fdh mutants on H2 or formate. (A) Open symbols, growth on H2; filled symbols, growth on formate; diamonds, wild type; squares, ΔfdhA1; circles, ΔfdhA2; triangles, ΔfdhA1-ΔfdhA2. Data are averages of duplicate growth experiments. Similar curves were observed in replicate experiments. (B) Structure of the operons for fdh1 and fdh2.
A distinct pattern occurred with hmd, which encodes the H2-dependent methylene-H4MPT dehydrogenase (Fig. 4). Although regulation appeared complex, H2 limitation clearly resulted in low mRNA abundance, in agreement with previous results (9, 30). Formate limitation had a similar effect.
DISCUSSION
We observed four different response patterns with regard to H2 and formate (Table 2). First, mRNA transcript levels for the F420-dependent steps of methanogenesis were high under three conditions, H2 limitation, formate limitation, and formate excess, and were low only with H2 excess. One possibility is that the presence of formate could act independently of H2 limitation. However, since the same set of genes is affected, we favor the hypothesis that the presence of formate or H2 limitation acts through the same set of regulatory signals (see below). The second response pattern, that of growth yield, was similar: YCH4 was high with H2 limitation or formate limitation, had an intermediate level with formate excess, and was lowest with H2 excess. The third response pattern was shown with only one gene: hmd had decreased expression with H2 limitation or formate limitation compared to formate excess or H2 excess. Fourth, one set of genes responded specifically to formate limitation: fdh2, fmd, and genes for molybdenum transport increased.
Table 2.
Summary of regulatory and physiological responses to H2 and formatea
| Gene(s) | H2 excess | Formate excess | H2 limitation | Formate limitation |
|---|---|---|---|---|
| F420-dependent genesb | — | ↑ | ↑ | ↑ |
| hmd | — or ↑ | — or ↑ | ↓ | ↓ |
| YCH4 | Low | Intermediate | High | High |
| fdh2, fmd, molybdenum transport genes | — | — | — | ↑ |
—, baseline expression; ↑, increased expression; ↓, decreased expression.
Genes for F420-dependent steps of methanogenesis (except fdh2).
During growth on formate, a low level of H2 is present due to its production and reutilization (14). It is tempting to speculate that H2 is a primary signal and that different response thresholds determine the different regulatory patterns. In this model, the highest H2 levels occur with H2 excess. The next step down in H2 occurs with growth on formate supplied in excess; at this point transcript levels for F420-dependent steps in methanogenesis increase and YCH4 increases to an intermediate level. The next lower H2 level occurs with H2 limitation, where YCH4 reaches a high level and hmd mRNA decreases in abundance. Hmd is an unusual [Fe] hydrogenase that has a low affinity for H2 (31), so when H2 levels are low, Hmd is of limited utility.
F420H2 concentrations are directly affected by H2 concentrations in the growth medium (32); therefore, it was previously suggested that when H2 limits growth, the genes for F420-dependent processes are upregulated to maintain a constant availability of F420H2 (8). When M. maripaludis is grown with formate as the electron donor, F420H2-dependent formate:H2 lyase activity is essential for growth, explaining the need to maintain high levels of F420-dependent enzymes under these conditions (14–16). A previous study that analyzed protein abundance of M. maripaludis cultures grown under different H2 gassing regimens demonstrated that changes in mRNA levels correspond to changes in protein abundance for these enzymes (9).
The remaining set of genes, fdh2, fmd, and molybdenum transport genes, could respond to the lowest extreme of H2 or could respond directly to a limiting level of formate. Fdh and Fmd both contain a molybdopterin cofactor (33). However, it is unlikely that these genes responded to molybdenum limitation as a primary signal, since a switch to medium containing higher formate and identical molybdate concentrations resulted in an increased OD, verifying that formate, not molybdenum, was the limiting nutrient (Table 1). We suggest that formate limitation leads to fdh2 expression and that molybdenum transport and fmd expression are secondary and tertiary responses, in which increased demand for molybdenum leads to increased molybdenum transport and increased intracellular molybdenum induces fmd transcription. Consistent with this, increased molybdate in cultures of M. thermautotrophicus resulted in increased fmd expression (34).
The different patterns of mRNA abundance for fdh1 and fdh2 suggest different roles for the two formate dehydrogenases. In a previous study by Wood et al., our lab reported that either Fdh was sufficient for growth on formate, whereas a later study by Lupa et al. reported that only Fdh1 supported growth on formate unless a Δfdh1 mutant was incubated for prolonged periods, ∼70 h (15, 35). This suggests that enrichment of a suppressor mutation may have accounted for the initial observations of Wood et al.; the data in our present study agree with this interpretation. We propose that Fdh1 functions for sustained growth on formate or for a transition from growth on H2 to growth on formate as H2 becomes limiting, while Fdh2 functions only at low formate concentrations. However, since a mutant containing only fdh2 (the fdh1 deletion mutant) could not be grown in the lab on formate without a probable suppressor mutation altering its expression pattern, we could not test the growth of this mutant under formate-limited conditions.
The differences in growth yield that occurred in M. maripaludis were striking, reflected in YCH4 values that varied as much as 4-fold. The fact that mutants for several genes in carbon metabolism (glgA, cdh, and ehb) display this phenotype suggests that energy storage does not account for differences in YCH4 between H2 excess and limitation or formate excess and limitation. Additionally, mutation of each of the F420 metabolizing enzymes of methanogenesis had no impact on the ability of M. maripaludis to alter growth yields in response to H2. Therefore, we tentatively propose that differences in YCH4 values in hydrogenotrophic methanogens are due to energy spilling, or the dissipation of energy when the metabolic electron donor is present in excess. The energy yield of hydrogenotrophic methanogens relies on electron bifurcation at the heterodisulfide reductase complex (1), where exergonic and endergonic electron flows are coupled via reduced ferredoxin (Fig. 4). One possibility is that this coupling partially breaks down. To make up for the endergonic requirement, electron flow through the energy-converting hydrogenase Eha, which normally functions anaplerotically (16), could increase with a concomitant consumption of membrane potential. However, if this is the case, we saw no evidence in the form of any regulation of Eha or any other steps of methanogenesis at the transcriptional level. Indeed, a promoter switch mutant for eha still displayed differences in cell yield in response to H2 availability. As an alternative, energy spilling in methanogenic Archaea may be more commonplace, perhaps involving a futile cycle that consumes ATP or membrane potential. Such cycling has been observed in Escherichia coli starved for potassium (36). Indeed, organisms from all three domains of life have documented examples of energy spilling metabolism (5). In methanogenic Archaea, energy spilling could be beneficial to maintain low concentrations of H2 or formate in order to prevent nutrient use by competitors or to maximize the energetics of metabolism during syntrophic growth: rapid nutrient consumption shifts the equilibrium of syntrophic metabolism, allowing for robust growth of both partners despite energy spilling on the part of the methanogen.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant DE-FG02-08ER64685 from the Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy. Experiments on energy spilling were supported by grant DE-FG02-05ER15709 from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy. K.C.C. was supported in part by Public Health Service, National Research Service Award T32 GM07270, from the National Institute of General Medical Sciences. Part of this work conducted by ENIGMA was supported by the Office of Science, Office of Biological and Environmental Research, of the U.S. Department of Energy under contract no. DE-AC02-05CH11231.
Footnotes
Published ahead of print 18 January 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02141-12.
REFERENCES
- 1. Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6: 579–591 [DOI] [PubMed] [Google Scholar]
- 2. Thauer RK. 2012. The Wolfe cycle comes full circle. Proc. Natl. Acad. Sci. U. S. A. 109: 15084–15085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Poorter LM, Geerts WJ, Keltjens JT. 2007. Coupling of Methanothermobacter thermautotrophicus methane formation and growth in fed-batch and continuous cultures under different H2 gassing regimens. Appl. Environ. Microbiol. 73: 740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morgan RM, Pihl TD, Nolling J, Reeve JN. 1997. Hydrogen regulation of growth, growth yields, and methane gene transcription in Methanobacterium thermoautotrophicum deltaH. J. Bacteriol. 179: 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell JB. 2007. The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 13: 1–11 [DOI] [PubMed] [Google Scholar]
- 6. Fardeau ML, Peillex JP, Belaich JP. 1987. Energetics of the growth of Methanobacterium thermoautotrophicum and Methanococcus thermolithotrophicus on ammonium chloride and dinitrogen. Arch. Microbiol. 144: 128–131 [Google Scholar]
- 7. Tsao JH, Kaneshiro SM, Yu SS, Clark DS. 1994. Continuous culture of Methanococcus jannaschii, an extremely thermophilic methanogen. Biotechnol. Bioeng. 43: 258–261 [DOI] [PubMed] [Google Scholar]
- 8. Hendrickson EL, Haydock AK, Moore BC, Whitman WB, Leigh JA. 2007. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. U. S. A. 104: 8930–8934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia Q, Wang T, Hendrickson EL, Lie TJ, Hackett M, Leigh JA. 2009. Quantitative proteomics of nutrient limitation in the hydrogenotrophic methanogen Methanococcus maripaludis. BMC Microbiol. 9: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukhopadhyay B, Johnson EF, Wolfe RS. 2000. A novel pH2 control on the expression of flagella in the hyperthermophilic strictly hydrogenotrophic methanarchaeaon Methanococcus jannaschii. Proc. Natl. Acad. Sci. U. S. A. 97: 11522–11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stams AJ, Plugge CM. 2009. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 7: 568–577 [DOI] [PubMed] [Google Scholar]
- 12. Dolfing J, Jiang B, Henstra AM, Stams AJ, Plugge CM. 2008. Syntrophic growth on formate: a new microbial niche in anoxic environments. Appl. Environ. Microbiol. 74: 6126–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa KC, Wong PM, Wang T, Lie TJ, Dodsworth JA, Swanson I, Burn JA, Hackett M, Leigh JA. 2010. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc. Natl. Acad. Sci. U. S. A. 107: 11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendrickson EL, Leigh JA. 2008. Roles of coenzyme F420-reducing hydrogenases and hydrogen- and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J. Bacteriol. 190: 4818–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lupa B, Hendrickson EL, Leigh JA, Whitman WB. 2008. Formate-dependent H2 production by the mesophilic methanogen Methanococcus maripaludis. Appl. Environ. Microbiol. 74: 6584–6590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lie TJ, Costa KC, Lupa B, Korpole S, Whitman WB, Leigh JA. 2012. Essential anaplerotic role for the energy-converting hydrogenase Eha in hydrogenotrophic methanogenesis. Proc. Natl. Acad. Sci. U. S. A. 109: 15473–15478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Major TA, Liu Y, Whitman WB. 2010. Characterization of energy-conserving hydrogenase B in Methanococcus maripaludis. J. Bacteriol. 192: 4022–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Porat I, Kim W, Hendrickson EL, Xia Q, Zhang Y, Wang T, Taub F, Moore BC, Anderson IJ, Hackett M, Leigh JA, Whitman WB. 2006. Disruption of the operon encoding Ehb hydrogenase limits anabolic CO2 assimilation in the archaeon Methanococcus maripaludis. J. Bacteriol. 188: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore BC, Leigh JA. 2005. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J. Bacteriol. 187: 972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haydock AK, Porat I, Whitman WB, Leigh JA. 2004. Continuous culture of Methanococcus maripaludis under defined nutrient conditions. FEMS Microbiol. Lett. 238: 85–91 [DOI] [PubMed] [Google Scholar]
- 21. Leigh JA. 2011. Growth of methanogens under defined hydrogen conditions. Methods Enzymol. 494: 111–118 [DOI] [PubMed] [Google Scholar]
- 22. Sarmiento FB, Leigh JA, Whitman WB. 2011. Genetic systems for hydrogenotrophic methanogens. Methods Enzymol. 494: 43–73 [DOI] [PubMed] [Google Scholar]
- 23. Yoon SH, Reiss DJ, Bare JC, Tenenbaum D, Pan M, Slagel J, Moritz RL, Lim S, Hackett M, Menon AL, Adams MW, Barnebey A, Yannone SM, Leigh JA, Baliga NS. 2011. Parallel evolution of transcriptome architecture during genome reorganization. Genome Res. 21: 1892–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Sieprawska-Lupa M, Whitman WB, White RH. 2010. Cysteine is not the sulfur source for iron-sulfur cluster and methionine biosynthesis in the methanogenic archaeon Methanococcus maripaludis. J. Biol. Chem. 285: 31923–31929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dodsworth JA, Leigh JA. 2006. Regulation of nitrogenase by 2-oxoglutarate-reversible, direct binding of a PII-like nitrogen sensor protein to dinitrogenase. Proc. Natl. Acad. Sci. U. S. A. 103: 9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudnick H, Hendrich S, Pilatus U, Blotevogel K. 1990. Phosphate accumulation and the occurrence of polyphosphates and cyclic 2,3-diphosphoglycerate in Methanosarcina frisia. Arch. Microbiol. 154: 584–588 [Google Scholar]
- 27. Yu JP, Ladapo J, Whitman WB. 1994. Pathway of glycogen metabolism in Methanococcus maripaludis. J. Bacteriol. 176: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendrickson EL, Kaul R, Zhou Y, Bovee D, Chapman P, Chung J, Conway de Macario E, Dodsworth JA, Gillett W, Graham DE, Hackett M, Haydock AK, Kang A, Land ML, Levy R, Lie TJ, Major TA, Moore BC, Porat I, Palmeiri A, Rouse G, Saenphimmachak C, Soll D, Van Dien S, Wang T, Whitman WB, Xia Q, Zhang Y, Larimer FW, Olson MV, Leigh JA. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J. Bacteriol. 186: 6956–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hendrickson EL, Liu Y, Rosas-Sandoval G, Porat I, Soll D, Whitman WB, Leigh JA. 2008. Global responses of Methanococcus maripaludis to specific nutrient limitations and growth rate. J. Bacteriol. 190: 2198–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia Q, Hendrickson EL, Zhang Y, Wang T, Taub F, Moore BC, Porat I, Whitman WB, Hackett M, Leigh JA. 2006. Quantitative proteomics of the archaeon Methanococcus maripaludis validated by microarray analysis and real time PCR. Mol. Cell. Proteomics 5: 868–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zirngibl C, Van Dongen W, Schworer B, Von Bunau R, Richter M, Klein A, Thauer RK. 1992. H2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase without iron-sulfur clusters in methanogenic archaea. Eur. J. Biochem. 208: 511–520 [DOI] [PubMed] [Google Scholar]
- 32. de Poorter LM, Geerts WJ, Keltjens JT. 2005. Hydrogen concentrations in methane-forming cells probed by the ratios of reduced and oxidized coenzyme F420. Microbiology 151: 1697–1705 [DOI] [PubMed] [Google Scholar]
- 33. May HD, Schauer NL, Ferry JG. 1986. Molybdopterin cofactor from Methanobacterium formicicum formate dehydrogenase. J. Bacteriol. 166: 500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hochheimer A, Hedderich R, Thauer RK. 1998. The formylmethanofuran dehydrogenase isoenzymes in Methanobacterium wolfei and Methanobacterium thermoautotrophicum: induction of the molybdenum isoenzyme by molybdate and constitutive synthesis of the tungsten isoenzyme. Arch. Microbiol. 170: 389–393 [DOI] [PubMed] [Google Scholar]
- 35. Wood GE, Haydock AK, Leigh JA. 2003. Function and regulation of the formate dehydrogenase genes of the methanogenic archaeon Methanococcus maripaludis. J. Bacteriol. 185: 2548–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buurman ET, Teixeira de Mattos MJ, Neijssel OM. 1991. Futile cycling of ammonium ions via the high-affinity potassium uptake system (Kdp) of Escherichia coli. Arch. Microbiol. 155: 391–395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.