Abstract
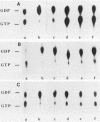
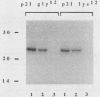
Residues 32 to 40, which are conserved among ras proteins from different species, are likely to participate in interactions with the p21 effector system. With the goal of understanding the structural basis of the regulatory functions of c-Ha-ras p21, we produced rabbit antisera against a synthetic peptide corresponding to amino acids 33 to 42 of the protein. The affinity-purified antibodies interacted specifically with p21 and with the antigenic peptide. The epitope recognized by the antibodies appeared to be centered on threonine 35. The antibodies inhibited both in vitro p21-induced production of cyclic AMP in detergent extracts of RAS-defective yeast membranes and GAP-stimulated GTPase activity. However, monoclonal anti-ras antibodies Y13-259 and Y13-238 were not capable of specifically inhibiting interactions of p21 with these two putative effector proteins. The apparent inhibitory effect of Y13-259 on stimulation of p21 by GAP was due to a greatly reduced rate of exchange of nucleotides in the binding pocket of the protein. These findings provide additional support for the essential role of the residue 32 to 40 domain as the true effector site and further evidence of the involvement of GAP as a cellular effector of ras proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Clark S. G., McGrath J. P., Levinson A. D. Expression of normal and activated human Ha-ras cDNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Oct;5(10):2746–2752. doi: 10.1128/mcb.5.10.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vendittis E., Vitelli A., Zahn R., Fasano O. Suppression of defective RAS1 and RAS2 functions in yeast by an adenylate cyclase activated by a single amino acid change. EMBO J. 1986 Dec 20;5(13):3657–3663. doi: 10.1002/j.1460-2075.1986.tb04696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Denèfle P., Kovarik S., Guitton J. D., Cartwright T., Mayaux J. F. Chemical synthesis of a gene coding for human angiogenin, its expression in Escherichia coli and conversion of the product into its active form. Gene. 1987;56(1):61–70. doi: 10.1016/0378-1119(87)90158-2. [DOI] [PubMed] [Google Scholar]
- Deshpande A. K., Kung H. F. Insulin induction of Xenopus laevis oocyte maturation is inhibited by monoclonal antibody against p21 ras proteins. Mol Cell Biol. 1987 Mar;7(3):1285–1288. doi: 10.1128/mcb.7.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFRIEND T. L., LEVINE L., FASMAN G. D. ANTIBODIES TO BRADYKININ AND ANGIOTENSIN: A USE OF CARBODIIMIDES IN IMMUNOLOGY. Science. 1964 Jun 12;144(3624):1344–1346. doi: 10.1126/science.144.3624.1344. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Marsico-Ahern J. D., Scolnick E. M., Sigal I. S. Inhibition of yeast adenylate cyclase by antibodies to ras p21. Biochem J. 1988 May 15;252(1):289–292. doi: 10.1042/bj2520289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Allard W. J., Sigal I. S., Scolnick E. M. Purification of ras GTPase activating protein from bovine brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5026–5030. doi: 10.1073/pnas.85.14.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Clanton D. J., Satoh T., Nakamura S., Kaziro Y., Kawakita M., Shih T. Y. Neutralizing monoclonal antibody against ras oncogene product p21 which impairs guanine nucleotide exchange. Mol Cell Biol. 1987 May;7(5):1999–2002. doi: 10.1128/mcb.7.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan Hand P., Vilasi V., Caruso A., Schlom J. Absolute values of ras p21 defined by direct binding liquid competition radioimmunoassays. Biochim Biophys Acta. 1987 Feb 27;908(2):131–142. doi: 10.1016/0167-4781(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Smith M. R., Bekesi E., Manne V., Stacey D. W. Reversal of transformed phenotype by monoclonal antibodies against Ha-ras p21 proteins. Exp Cell Res. 1986 Feb;162(2):363–371. doi: 10.1016/0014-4827(86)90341-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. Monoclonal antibody Y13-259 recognizes an epitope of the p21 ras molecule not directly involved in the GTP-binding activity of the protein. Mol Cell Biol. 1986 Apr;6(4):1002–1009. doi: 10.1128/mcb.6.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989 Jan 13;56(1):5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Scolnick E. M. Identification of effector residues and a neutralizing epitope of Ha-ras-encoded p21. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4725–4729. doi: 10.1073/pnas.83.13.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Di Donato A., Lacal J. C. H-ras mutants lacking the epitope for the neutralizing monoclonal antibody Y13-259 show decreased biological activity and are deficient in GTPase-activating protein interaction. Mol Cell Biol. 1989 Apr;9(4):1779–1783. doi: 10.1128/mcb.9.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Matsumoto K., Toh-E A. IRA1, an inhibitory regulator of the RAS-cyclic AMP pathway in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):757–768. doi: 10.1128/mcb.9.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Papageorge A. G., Kung H. F., Bekesi E., Robins T., Johnsen M., Vass W. C., Lowy D. R. Mutational analysis of a ras catalytic domain. Mol Cell Biol. 1986 Jul;6(7):2646–2654. doi: 10.1128/mcb.6.7.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]