Abstract
Neisseria gonorrhoeae uses a type IV secretion system (T4SS) to secrete chromosomal DNA into the surrounding milieu. The DNA is effective in transforming gonococci in the population, and this mechanism of DNA donation may contribute to the high degree of genetic diversity in this species. Similar to other F-like T4SSs, the gonococcal T4SS requires a putative membrane protein, TraG, for DNA transfer. In F-plasmid and related systems, the homologous protein acts in pilus production, mating pair stabilization, and entry exclusion. We characterized the localization, membrane topology, and variation of TraG in N. gonorrhoeae. TraG was found to be an inner-membrane protein with one large periplasmic region and one large cytoplasmic region. Each gonococcal strain carried one of three different alleles of traG. Strains that carried the smallest allele of traG were found to lack the peptidoglycanase gene atlA but carried a peptidoglycan endopeptidase gene in place of atlA. The purified endopeptidase degraded gonococcal peptidoglycan in vitro, cutting the peptide cross-links. Although the other two traG alleles functioned for DNA secretion in strain MS11, the smallest traG did not support DNA secretion. Despite the requirement for a mating pair stabilization homologue, static coculture transformation experiments demonstrated that DNA transfer was nuclease sensitive and required active uptake by the recipient, thus demonstrating that transfer occurred by transformation and not conjugation. Together, these results demonstrate the TraG acts in a process of DNA export not specific to conjugation and that different forms of TraG affect what substrates can be transported.
INTRODUCTION
Neisseria gonorrhoeae encodes a type IV secretion system (T4SS) that acts to secrete chromosomal DNA into the environment (1). The secreted DNA is taken up by other gonococci and is incorporated into their genomes through the process of natural transformation (1–3). Thus, DNA secretion works as a method of DNA donation for natural transformation without requiring lysis and death of the donor cell (3–6). Frequent natural transformation has made the gonococcal population structure panmictic, or completely sexual (7), increasing genetic diversity and spreading advantageous genes including antibiotic resistance markers and variant alleles for surface antigens (reviewed in reference 5). The T4SS genes are located on a 57-kb genetic island known as the gonococcal genetic island (GGI) found in 80% of N. gonorrhoeae strains (2, 4). Several of the genes for DNA donation are similar to conjugation genes of the Escherichia coli F-plasmid, suggesting that DNA secretion by the gonococcal T4SS may work by a method similar to plasmid conjugation or to chromosome mobilization in E. coli Hfr strains (3, 4). An important difference between these systems is that gonococcal DNA is secreted into the medium, whereas F-plasmid is conjugated directly from one cell to another through a mating bridge (8). The mechanisms for requiring contact for conjugation or those allowing contact independence in N. gonorrhoeae secretion are not understood.
The subject of the present study is N. gonorrhoeae TraG, a homologue of the mating pair formation protein TraG found in E. coli F-plasmid and the similar TraG proteins in other F-like T4SSs, including those of the integrative conjugative elements SXT in Vibrio cholerae and R391 in Providencia rettgeri (1, 9, 10). Gonococcal TraG is 23% identical to F-plasmid TraG over an 843-amino-acid region. F-plasmid TraG is necessary for conjugative pilus production (11), mating pair stabilization (12), and entry exclusion (13, 14). The functions of the TraG homologues are poorly understood, although it is known that they are inner-membrane proteins (11, 15) and that they recognize inner-membrane proteins in cells that already carry the plasmid or element to prevent DNA transfer to those cells (10, 14, 15). It is unclear how inner-membrane proteins in two different cells could interact with each other, but these results led to the hypothesis that the TraG proteins or a TraG cleavage product may be transported out of the donor cell (10, 14). Since gonococci do not use the T4SS to transfer DNA directly from cell to cell by conjugation (2), do not require the T4SS pilin for DNA secretion (16), and do not prevent entry of DNA into recipients (1, 2), it is unclear what the function of gonococcal TraG would be. It was shown that an insertion mutation in traG diminished DNA secretion and DNA donation for natural transformation (1). It is also known that more than one allele of traG exists in the gonococcal population, with each GGI+ strain carrying one traG allele (2, 6).
To gain a better understanding of the role of gonococcal TraG in DNA secretion, we characterized TraG for subcellular localization, membrane topology, and sequence variability. Also, the different traG alleles were tested for function in DNA secretion. TraG was found to be an inner-membrane protein with one large domain in the periplasm. One traG allele was always associated with the gene for a putative peptidoglycan endopeptidase, and we found that this peptidase degraded peptidoglycan in vitro. Two of the traG alleles were functional for DNA secretion, whereas the third traG did not support DNA secretion. Coculture DNA transfer experiments in static liquid cultures confirmed that chromosomal marker transfer among gonococci occurs by DNA export and natural transformation and not by mating pair formation and conjugation. Together, these data indicate that the gonococcal TraG and, possibly, all of the TraG homologues have an essential function in secretion beyond those involved in mating and entry exclusion.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids are described in Table 1. N. gonorrhoeae strains were constructed using gonococcal strain MS11 as the parent, except as noted for two strains derived from PID2059 and one strain derived for JC1. All assays were carried out using piliated, transparent gonococci except for the analysis of gonococcal peptidoglycan, which was carried out using nonpiliated gonococci. N. gonorrhoeae was grown at 37°C on GCB agar plates containing Kellogg's supplements (17) in 5% CO2 or in GCB liquid (GCBL) medium containing Kellogg's supplements and 0.042% NaHCO3 (18). For DNA secretion assays, gonococci were grown in GW medium (19). E. coli strains were constructed using E. coli strain TAM1 (active motif), and proteins were expressed for purification in BL21 STAR cells (Invitrogen). traG fusion constructs made using the transposon system were constructed in E. coli strain CC118. Strain construction details are supplied in the supplemental material. E. coli was grown in Luria-Bertani (LB) broth or on LB agar plates (20). For N. gonorrhoeae, chloramphenicol (Cm) was used at 10 μg/ml, spectinomycin (Sp) was used at 75 μg/ml, streptomycin (Sm) was used at 100 μg/ml, and erythromycin (Em) was used at 10 μg/ml, except that 2 μg of Em/ml was used for strains PID2059 and JC1. For E. coli, Cm was used at 25 μg/ml, Em was used at 500 μg/ml, and ampicillin (Ap) was used at 100 μg/ml.
Table 1.
Strains and plasmids
| Strain or plasmid | Propertiesa | Source or reference |
|---|---|---|
| Plasmids | ||
| pHH18 | EcoRI/SpeI fragment of traG from pKS43 in pKC1 (Ermr/Strs) | This study |
| pHH36 | traG in pKH9 (Cmr) | This study |
| pHH42 | pilTK136Q in pIDN1 | 66 |
| pIDN1 | Insertion duplication and cloning vector (Ermr) | 1 |
| pIDN3 | Insertion duplication and cloning vector (Ermr) | 1 |
| pKC1 | Insertion/duplication, positive/negative selection vector (Ermr)/(Strs) | 67 |
| pKH9 | Complementation vector (Cmr) | 4 |
| pKH35 | Complementation vector (Cmr) | 4 |
| pKH37 | Complementation vector (Cmr) | 6 |
| pKH39 | lacZ in pKH35 (Cmr) | 68 |
| pKH116 | phoA from pUI1158 in pIDN1 (Emr) | This study |
| pKH117 | ′traG479-′phoA in pKH116 (Cmr) | This study |
| pKH119 | phoA from pUI1160 in pIDN1 (Emr) | This study |
| pKH120 | ′traG524-′phoA in pKH119 (Cmr) | This study |
| pKH123 | ′traG661-′phoA in pKH116 (Cmr) | This study |
| pKH128 | ′traG656-′phoA in pKH116 (Cmr) | This study |
| pKH163 | ′lacZ no RBS in pKH35 (Cmr) | This study |
| pKH164 | ′traG479-′lacZ in pKH163 (Cmr) | This study |
| pKH165 | ′traG645-′lacZ in pKH163 (Cmr) | This study |
| pKH166 | ′traG439-′lacZ in pKH163 (Cmr) | This study |
| pKS43 | 479 bp Sau3AI fragment of traG cloned into pIDN1 (Ermr) | 1 |
| pPK53 | atlA in pKH35 for complementation | 6 |
| pPK84 | 3′ traG fragment in pTYB12 for purification of ′TraG | This study |
| pPK1005 | traG in pIDN3 (Ermr) | This study |
| pPK1023 | Plasmid for construction ΔtraG strain (Ermr) | This study |
| pPK1040 | traG in pKH9 for complementation (Cmr) | This study |
| pPK1017 | traG828-′lacZ in pKH37 (Cmr) | This study |
| pPK1018 | traG75-′lacZ in pKH37 (Cmr) | This study |
| pPK1019 | traG647-′lacZ in pKH37 (Cmr) | This study |
| pPK1020 | traG862-′lacZ in pKH37 (Cmr) | This study |
| pPK1025 | traG310-′phoA in pUI1156 (Cmr) | This study |
| pPK1027 | traG′-′phoA fusion from pPK1025 in pKH37 (Cmr) | This study |
| pPK1031 | ′phoA cloned from pUI1156 in pKH37 (Cmr) | This study |
| pPK1032 | traG310-′lacZ in pKH39 (Cmr) | This study |
| pPK1033 | traG971-′phoA in pKH35 (Cmr) | This study |
| pPK1034 | traG609-′phoA in pKH35 (Cmr) | This study |
| pPK1035 | traG87-′phoA in pPK35 (Cmr) | This study |
| pPK1036 | traG125-′phoA in pPK35 (Cmr) | This study |
| pPK1037 | traG49-′phoA in pPK35 (Cmr) | This study |
| pPK1038 | traG430-′lacZ in pKH39 (Cmr) | This study |
| pPK1039 | traG387-′phoA in pUI1156 (Cmr) | This study |
| pPK1041 | traG31-′phoA in pUI1156 (Cmr) | This study |
| pPK1042 | ′traG430-′phoA in pUI1158 (Cmr) | This study |
| pPK1043 | ′traG387-′lacZ in pKH39 (Cmr) | This study |
| pPK1044 | traG′-′phoA fusion from pPK1041 in pKH37 (Cmr) | This study |
| pPK1045 | ′traG′-′phoA fusion from pPK1042 in pKH37 (Cmr) | This study |
| pPK1047 | traG31-′lacZ in pKH39 (Cmr) | This study |
| pSI7 | traG and surrounding region in pIDN1 (Ermr) | This study |
| pTYB12 | Expression vector for protein purification | New England Biolabs |
| pUI1156 | Plasmid for constructing translational fusions with ′PhoA (Apr) | 69 |
| pUI1158 | Plasmid for constructing translational fusions with ′PhoA (Apr) | 69 |
| pUI1160 | Plasmid for constructing translational fusions with ′PhoA (Apr) | 69 |
| N. gonorrhoeae strains | ||
| FA1090 | Wild-type N. gonorrhoeae | 70 |
| HH513 | PID2059 transformed with pHH18 (Ermr/Strs) | This study |
| HH526 | MS11 transformed with HH513 chromosomal DNA (Ermr/Strs) | This study |
| HH528 | HH526 derivative containing traG3-eppA locus from PID2059 replacing traG1-atlA region of MS11 (Erms/Strr) | This study |
| HH530 | JC1 transformed with pHH18 (Ermr/Strs) | This study |
| HH533 | MS11 transformed with HH530 chromosomal DNA (Ermr/Strs) | This study |
| HH534 | HH533 derivative containing traG2 from JC1 replacing traG1 of MS11 (Erms/Strr) | This study |
| JC1 | N. gonorrhoeae DGI isolate | R. Hull |
| KH530 | MS11 pacAH329Q | 26 |
| KH582 | PK202 transformed with pKH117, traG479-′phoA+ (Cmr) | This study |
| KH583 | PK202 transformed with pKH120, traG524-′phoA+ (Cmr) | This study |
| KH586 | PK203 transformed with pKH123, traG661-′phoA+ (Cmr) | This study |
| KH589 | PK198 transformed with pKH128, traG656-′phoA+ (Cmr) | This study |
| KH621 | PK181 transformed with pKH166, traG439-′lacZ+ (Cmr) | This study |
| KH622 | PK181 transformed with pKH164, traG479-′lacZ+ (Cmr) | This study |
| KH623 | PK181 transformed with pKH165, traG645-′lacZ+ (Cmr) | This study |
| LI1653 | N. gonorrhoeae low-passage clinical isolate | 71 |
| LI1656 | N. gonorrhoeae low-passage clinical isolate | 2 |
| LI1660 | N. gonorrhoeae low-passage clinical isolate | 71 |
| LI1665 | N. gonorrhoeae low-passage clinical isolate | 71 |
| LI1667 | N. gonorrhoeae low-passage clinical isolate | 71 |
| LI1673 | N. gonorrhoeae low-passage clinical isolate | 71 |
| MS11 | Wild-type N. gonorrhoeae (Strr) | 72 |
| MS11Spc | MS11 (Spcr) | 2 |
| ND500 | MS11 ΔGGI | 4 |
| NT04 | MS11Spc transformed with pHH42 (pilTK136Q) | This study |
| PID2004 | N. gonorrhoeae low-passage clinical isolate | 71 |
| PID2025 | N. gonorrhoeae low-passage clinical isolate | 71 |
| PID2059 | N. gonorrhoeae low-passage clinical isolate | 71 |
| PK161 | HH528 complemented with atlA by transformation with pPK53 | This study |
| PK162 | PID2059 complemented with atlA by transformation with pPK53 | This study |
| PK166 | MS11 transformed with pKH37, cat+ (Cmr) | This study |
| PK180 | MS11 transformed with pKH39, lacZ+ (Cmr) | This study |
| PK181 | PK180 transformed with pPK1017, traG828-′lacZ+ (Cmr) | This study |
| PK182 | PK180 transformed with pPK1018, traG75-′lacZ+ (Cmr) | This study |
| PK184 | PK180 transformed with pPK1019, traG647-′lacZ+ (Cmr) | This study |
| PK185 | PK180 transformed with pPK1020, traG862-′lacZ+ (Cmr) | This study |
| PK186 | MS11 ΔtraG | This study |
| PK189 | MS11 transformed with pPK1032, traG310-′lacZ+ (Cmr) | This study |
| PK190 | MS11 transformed with pPK1031, ′phoA+ (Cmr) | This study |
| PK191 | MS11 ΔtraG complemented with traG by transformation with pPK1040 (Cmr) | This study |
| PK195 | PK190 transformed with pPK1035, traG87-′phoA+ (Cmr) | This study |
| PK196 | PK190 transformed with pPK1036, traG125-′phoA+ (Cmr) | This study |
| PK197 | PK190 transformed with pPK1037, traG49-′phoA+ (Cmr) | This study |
| PK198 | PK190 transformed with pPK1034, traG609-′phoA+ (Cmr) | This study |
| PK199 | PK181 transformed with pPK1038, traG430-′lacZ+ (Cmr) | This study |
| PK200 | PK181 transformed with pPK1043, traG387-′lacZ+ (Cmr) | This study |
| PK201 | PK190 transformed with pPK1044, traG31-′phoA+ (Cmr) | This study |
| PK202 | PK198 transformed with pPK1045, traG430-′phoA+ (Cmr) | This study |
| PK203 | PK198 transformed with pPK1027, traG310-′phoA+ (Cmr) | This study |
| PK204 | PK180 transformed with pPK1047, traG31-′lacZ+ (Cmr) | This study |
| PK205 | PK198 transformed with pPK1033, traG971-′phoA+ (Cmr) | This study |
Cmr, chloramphenicol resistance; Ermr, erythromycin resistance; Spcr, spectinomycin resistance; Apr, ampicillin resistance; Strr, streptomycin resistance. Gene subscripts indicate mutations, e.g., “pilTK136Q” indicates “pilT with mutation K136Q”.
Southern blot analysis.
Chromosomal DNA was prepared from N. gonorrhoeae as previously described and digested with ClaI and EcoRI (2). DNA for the traG coding region probe was prepared by PCR using primers 21F and TraG2R (2). Sequences of PCR primers used in these studies are shown in Table 2. The probe was labeled with 32P by random-primed labeling. Southern blotting was carried out according to standard procedures (20).
Table 2.
PCR primers used in this study
| Primer | Sequence (5′–3′)a |
|---|---|
| 6R | GTCTTCCACTTGATATTCCACG |
| 21F | CAAGCTGCTGCCTTATTTGG |
| 22R | CTGCTTCAATACTTTCCCCAATTTC |
| 23F Xho | GCTCGAGGATTCGTTCTGCTGCTGAGGC |
| EPatL5′NheI | GTTCGCTAGCCTATCGCCCAAAGAA |
| EPEcoRIR | CTGAATTCCAATATGGGCAATGCTCATGATATG |
| EPSpeIF | GAACTAGTAGCATAACGGCAGCCAAACAGT |
| lacZ3′Mfe | GAGCCAATTGGGCCTGCCCGGTTATTATTA |
| lacZ5′Pst | GCGACTGCAGCACACAGGAAACAGCTATGA |
| lacZnoRBSXma | TCACCCGGGATGACCATGATTACGGATTC |
| newEP3′XhoI | AATGCTCGAGATATGTACCCCTCTCTATTT |
| NhetraGR | GATCGTGCTAGCCCGTTGCCTCCATATGAGGTGG |
| SpeItrunctraGF | GACACTAGTATGACTTCATCGGCCAAATCAAC |
| traG2R | TTCCTGCCGAATTTGTACCC |
| traG439 Xma | CATCCCGGGGGCTGCTGCCATCAATAACG |
| traG479 Xma | CATCCCGGGGGTTGATTTGGCCGATGAAGTC |
| traG645 Xma | CATCCCGGGTGCGATGGCAGATATAACTGC |
| traGdelF | TAAGGAGAGATGTAACCCCGTTGCGTAATGCC |
| traGdelR | CAACGGGGTTACATCTCTCCTTAGAAACGGTTCATCG |
| traGdown | CGAGATATCGGATGCTAAAGCCGGGTTGC |
| traGlacZ1R | CAGTCAAGCTTGATCCTGATATTGTGGCAACGCC |
| traGlacZ2R | GACTCAAGCTTGCTTGACGAATAACCTCAACCATC |
| traGlacZ3R | GTCAGCCTGCAGCCGTTGGCGGCTTCCACG |
| traGlacZ4R | CTGGTAGAAGCTTAATAACCGGAATGC |
| traGup | CGAATGCATGCTCAGCCTTTGTGGATACC |
| XhotraGF | GAGGCCTCGAGCTCCATGACTTTTGATTCGATGAACC |
The sequences of the restriction sites in the primers are underlined.
DNA sequencing and analysis.
DNA sequencing was carried out using the BigDye method according to the manufacturer's instructions at the University of Wisconsin-Madison DNA sequencing center. The sequence of the traG3-eppA region of strain PID2059 was submitted to GenBank under accession number DQ835990. Incomplete N. gonorrhoeae genome sequences were accessed via the Broad Institute (http://www.broad.mit.edu). Sequence alignments and similarities were determined using the BLAST program (21).
DNA secretion assay.
The DNA secretion assay was carried out as described previously (3). Briefly, gonococcal strains were grown in GW medium for 2 h from a starting optical density at 540 nm (OD540) of 0.18. Cultures were vortexed for 1 min, and 0.6 ml of gonococcal culture was added to 2.4 ml of fresh GW medium. Strains were allowed to grow for 2.5 h, and culture supernatants were collected at 0 and 2.5 h. Supernatants were treated with the fluorescent, DNA-binding dye, PicoGreen (Invitrogen) and compared to DNA standards to determine the concentration of DNA. The amount of DNA in each culture was standardized to the amount of protein in the cell pellet as determined by the Bradford assay (Bio-Rad) (22). The fluorescence background was determined by performing the assay using GGI deletion strain ND500, and this value was subtracted from the values for all strains.
Expression and purification of proteins for antibody production.
For expression and purification of the 436-amino-acid C-terminal fragment of TraG (′TraG), a traG fragment was PCR amplified with primers SpeItrunctraGF and 6R, digested with SpeI and EcoRI, and ligated into expression vector pTYB12 to make pPK84. Induction with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at room temperature was used to overexpress ′TraG fused to a chitin-binding domain and a self-cleavable intein. ′TraG was purified using chitin resin (New England BioLabs) according to the manufacturer's instructions, and polyclonal anti-′TraG antisera were generated in rats (Harlan Bioproducts).
Protein overproduction and purification for in vitro assays.
His6-EppA protein was overproduced in E. coli BL21(λDE3) cells. Cells were grown at 25°C in 3 liters of LB medium containing 40 μg of kanamycin/ml until the OD600 reached 0.5 to 0.7. The temperature was reduced to 15°C; after 1.5 to 2 h, IPTG was added to a final concentration of 100 μM. Cells were grown an additional 16 h and harvested by centrifugation. Affinity chromatography with a zinc-chelating resin (G Biosciences) was used to purify His6-EppA. Cells were resuspended in histidine (His) tag purification buffer (50 mM sodium phosphate buffer [pH 7.5], 300 mM NaCl, 10% glycerol) and lysed by two passages through a French pressure cell (1,200 lb/in2). The cell extract containing 10 mM imidazole was incubated with 1 ml of the resin with gentle shaking for 1.5 h on ice. The resin was collected by centrifugation, loaded into a column, and washed with His tag purification buffer containing 10 mM imidazole. Stepwise elutions with His tag purification buffer containing 20, 40, 60, 100, and 250 mM imidazole were performed. Fractions containing purified protein as determined by SDS-PAGE analysis were pooled and dialyzed overnight at 4°C in dialysis buffer (50 mM sodium phosphate buffer [pH 7.5], 100 mM NaCl, 10% glycerol); the next day, the buffer was changed, and the protein was dialyzed for an additional 4 h. Purified protein was flash frozen in liquid nitrogen and stored at −80°C. Protein concentrations were determined using the calculated molar extinction coefficient (14,650 M−1 cm−1).
Zymogram analysis.
Zymogram analysis of His6-EppA peptidoglycanase activity was performed as previously described with slight modifications (23, 24). Peptidoglycan was isolated from gonococcal strain FA1090 as described earlier (25). Various amounts of purified His6-EppA were loaded onto a 12% polyacrylamide gel containing either 0.2% (wt/vol) lyophilized Micrococcus lysodeikticus cells or 700 μl of the gonococcal peptidoglycan preparation. Gels were electrophoresed at 75 V at 4°C, washed in distilled water, and then washed with renaturation buffer (25 mM Tris-HCl [pH 7.5], 0.5% Triton X-100). After overnight incubation in the renaturation buffer at room temperature, the gels were put in fresh buffer and incubated at 37°C for 2 h. The gels were stained at room temperature for 1 h with 0.1% methylene blue dissolved in 0.01% KOH, destained with water, and then visualized to see zones of clearing. To visualize the protein, the gels were stained with Coomassie brilliant blue.
Peptidoglycan solubilization assays with His6-EppA.
For the high-pressure liquid chromatography (HPLC)-based assays, reaction mixtures contained 50 mM sodium phosphate buffer (pH 7.5), 25 μl of purified FA1090 peptidoglycan prepared as described above, 1.5 μM His6-EppA, and either 1 μM ZnSO4 or 3 mM EDTA in a total reaction volume of 150 μl. The reactions were incubated on a rotator overnight at 37°C. The insoluble material was removed by centrifugation, and the supernatants were applied to Centricon 10,000 molecular weight cutoff (MWCO) spin columns, which had been prewashed with 50 mM sodium phosphate buffer (pH 7.5). HPLC analysis was carried out using a Prevail (Alltech) C18 HPLC column (5 μm, 25 by 4.6 mm). Reaction products were separated using a 0 to 25% gradient of 60% acetonitrile–0.01% trifluoroacetic acid over 60 min at a flow rate of 1 ml/min, and the elution of the products was monitored at 210 nm. Reaction mixtures analyzed by LC/MS were set up as described above except that 75 μl of FA1090 peptidoglycan was used. The filtered reaction products were analyzed at the UW-Madison Biotechnology Center using a Zorbax SB-C18 column (1.8 μM, 2.1 by 50 mm) run on an Agilent 1200 HPLC with a linear gradient of 99.9% water–0.1% formic acid to 99.9% acetonitrile–0.1% formic acid over 60 min, at a flow rate of 0.25 ml/min. Peaks were analyzed using an Agilent LC/MSD TOF using electrospray ionization in positive-ion mode.
Analysis of His6-EppA reaction products digested with mutanolysin.
Reaction mixtures contained 50 mM sodium phosphate buffer (pH 7.5), 75 μl of purified KH530 peptidoglycan, 1.5 μM His6-EppA, and 10 μM ZnSO4 in a total reaction volume of 200 μl. The reactions were incubated on a rotator overnight at 37°C. The enzyme was heat killed by boiling for 5 min, and the insoluble material was removed by centrifugation. The supernatant was filtered using a Centricon 10,000-MWCO spin column, and the filtrate was divided into two aliquots. To one aliquot, 10 μl of a stock solution of mutanolysin (1 mg/ml) was added; to the second aliquot, 10 μl of water was added as a control. The reaction mixtures were incubated on a rotator at 37°C for 2 h. The samples were then filtered using a Centricon 10,000 MWCO spin column and analyzed by liquid chromatography-mass spectrometry (LC/MS) at the UW-Madison Biotechnology Center, following the same protocol described above.
Peptidoglycan solubilization assay.
Degradation of gonococcal peptidoglycan was measured in an in vitro assay as previously described (6). To prepare the substrate, N. gonorrhoeae was grown in the presence of [6-3H]glucosamine in gonococcal base liquid medium using pyruvate as the carbon source and omitting glucose. This method labels both the N-acetylglucosamine and the N-acetylmuramic acid of peptidoglycan. Gonococcal peptidoglycan was purified as described in reference 26.
Subcellular fractionation of N. gonorrhoeae.
Gonococcal strains were grown in 100-ml liquid cultures for 3 h starting at an OD540 of 0.2. Membrane and soluble fractions were collected using a method adapted from Gauthier et al. (27). Cells were collected by centrifugation and resuspended in 1 ml of cold 50 mM Tris-HCl, 20% sucrose, and 1× Complete Mini protease inhibitor cocktail (Roche). Then, 100 μl of a 1-mg/ml concentration of lysozyme (Sigma) in 250 mM EDTA was added to the cells, followed by incubation on ice for 15 min. The cells were broken by bead beating with 140- to 300-μm glass beads on a vortex mixer at 4°C for 20 min. Unbroken cells were collected by centrifugation at 16,000 × g. The suspension of broken cells was centrifuged at 50,000 × g for 1 h. The soluble fraction was collected and concentrated using Microcon centrifugal filter devices with a 10-kDa cutoff (Millipore). The membrane fractions were washed with cold 50 mM Tris-HCl and resuspended in 0.1% SDS and 0.5% N-lauroylsarcosine. The amount of protein in each sample was determined by the Lowry method (Bio-Rad DC protein assay) (28), and 300 μg of protein from each sample was used for Western blot analysis.
Western blot analysis.
Proteins were electrophoresed on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked in 5% milk in Tris-buffered saline with 0.5% Tween 20 (TTBS), followed by incubation with primary antibodies in 5% milk in TTBS for 2 to 18 h and then incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies in TTBS for 1 to 2 h. Blots were washed with TTBS, developed with Immobilon Western Chemiluminescent HRP substrate (Millipore), and exposed to film. Antisera were used at the following concentrations: anti-TraG, 1:400; anti-CAT (Sigma), 1:1,000; anti-LacZ (Santa Cruz Biotech), 1:500; anti-PhoA (Chemicon), 1:600; anti-PilQ (H. S. Seifert), 1:1,000; and anti-mouse-HRP, anti-rat-HRP, and anti-rabbit-HRP (all from Santa Cruz Biotech), 1:20,000.
β-Galactosidase and phosphatase assays.
β-Galactosidase assays were carried out as described previously (29) with the following modifications. Gonococcal strains were grown in GCBL for 3 h starting at an OD540 of 0.2. Then, 1 ml of culture was diluted into 2 ml of fresh medium, and the cultures were induced with 0.33 mM IPTG and grown for 2 h. The cultures were vortexed for 1 min, and the OD600 of each culture was measured. Next, 0.5 ml of culture was used for the assay and diluted into 0.5 ml of Z buffer (29).
Phosphatase assays were carried out as described previously (30) with minor modifications. Briefly, gonococcal strains were grown as described above for β-galactosidase assays. The OD600 of each culture was measured before centrifugation of 0.5 ml of each culture. The cells were washed once in 10 mM Tris-HCl (pH 8.0) and 10 mM MgSO4 and resuspended in cold 1 M Tris-HCl (pH 8.0). The resuspended cells were added to 0.5 ml of 1 M Tris-HCl (pH 8.0)–0.1 mM ZnCl2. Two drops of CHCl3 and one drop of 0.1% SDS were added to each sample before vortexing and incubation at room temperature for 5 min. Next, 0.1 ml of 4 mg of p-nitrophenyl phosphate/ml in 1 M Tris-HCl (pH 8.0) was added to each reaction, and the reactions were incubated at room temperature. Phosphatase units were calculated after measurement of the OD420 and OD550.
Coculture transformation assays.
Coculture transformation was performed as described by Dillard and Seifert with minor modifications (2). Donor strain JD1545 carries a Cmr marker on the chromosome at cnp. It also carries the recA6 allele, which prevents the strain from serving as a recipient in transformation unless recA expression is induced. MS11Spc or MS11Spc with a mutation in pilT (NT04) was used as the recipient. Separate cultures of the donor and the recipient were grown with aeration in GCBL plus supplements to an OD540 of ∼1.0. Sufficient volumes of donor and recipient were mixed and diluted to a final volume of 2 ml using prewarmed GCBL with supplements such that each contributed an OD540 of 0.1 for a total OD540 of 0.2. The mixed cultures were placed in a T-25 tissue culture flask and incubated horizontally in a 5% CO2 atmosphere at 37°C for 2.5 h. The cultures were diluted in GCBL and plated on GCB agar plates containing 10 μg of Cm/ml, 75 μg of Sp/ml, or 8 μg of Cm/ml plus 75 μg of Sp/ml.
RESULTS
Membrane localization of TraG.
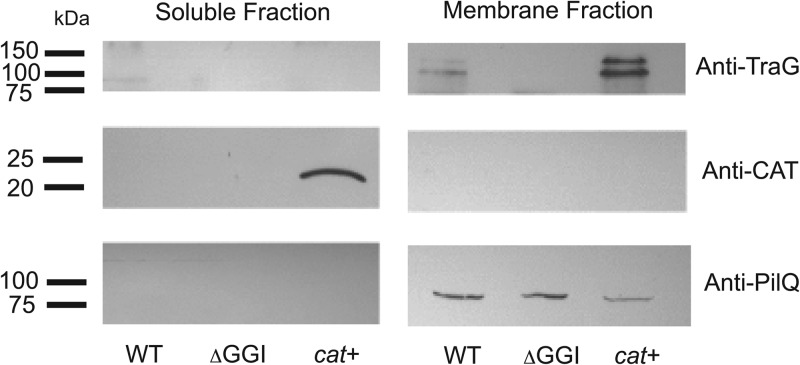
Gonococcal TraG is predicted to be an inner-membrane protein based on sequence characteristics, including the presence of at least five regions of hydrophobicity that may function as membrane spans. Also, experimental evidence indicates that TraG homologues in E. coli F-plasmid (11), V. cholerae, and P. rettgeri are localized to the inner membrane (10, 15). However, the existence of multiple traG alleles led us to wonder if TraG might be exposed on the cell surface and subject to immune surveillance (2). Furthermore, it has been suggested that the F-plasmid TraG may be secreted into the recipient cell during conjugation (9, 14). To examine the subcellular localization of N. gonorrhoeae TraG, we separated gonococcal cells into soluble and membrane fractions and probed immunoblots with anti-TraG antisera. TraG was detected only in the membrane fractions from gonococcal strains and was not detected in the soluble fraction or in a strain with a deletion of the GGI (Fig. 1). We detected two bands corresponding to TraG. One was near the predicted molecular mass of 107 kDa, and the second exhibited a larger apparent molecular weight. The reason for the presence of two bands is unknown but could be due to modification or processing of the protein, or to degradation of the protein during subcellular fractionation. We constructed a strain that expresses CAT, a known cytoplasmic protein, so that we could detect the presence of soluble proteins in the cell fractions. Immunoblots of both the soluble and membrane fractions indicated that CAT was only found in the soluble fraction of the strain expressing the protein (Fig. 1). In order to test for membrane proteins in the cell fractions, we probed blots with antibodies raised against PilQ, a known N. gonorrhoeae outer-membrane protein (31, 32). PilQ was found only in the membrane fractions of all three strains tested (Fig. 1). These results indicate that TraG is a membrane protein and confirm that our method for fractionating gonococci effectively separated membrane and soluble fractions.
Fig 1.
Subcellular fractionation and Western blot analysis of gonococcal strains. The wild-type strain MS11 (WT), the GGI deletion strain ND500 (ΔGGI), and strain PK166 expressing CAT (cat+) were separated into soluble and membrane fractions, and the fractions were subjected to Western blot analysis with anti-TraG, anti-CAT, and anti-PilQ antibodies.
Membrane topology of TraG.
We used three different computer algorithms to predict the membrane topology of TraG, TMHMM (33), HMMTOP (34), and SOSUI (35). All three programs predicted that the N terminus of TraG resides in the periplasm and recognized five putative membrane-spanning domains around amino acids 33 to 52, 59 to 82, 359 to 380, 385 to 407, and 441 to 461, respectively. However, TMHMM predicted a sixth transmembrane domain at amino acids 96 to 115, and HMMTOP predicted a sixth transmembrane domain at amino acids 414 to 434. TraG in gonococcal strain MS11 is a 1,000-amino-acid protein, so these predictions indicate that the C-terminal half of the protein consists of a large soluble domain.
To examine the topology of TraG in the membrane, we expressed translational fusions of segments of TraG (TraG′) with E. coli β-galactosidase (LacZ) or E. coli alkaline phosphatase lacking a signal sequence (′PhoA). Fusing an N-terminal fragment of a protein with these enzymes has been used widely to build models of the topologies of membrane proteins, including F-plasmid TraG (14). LacZ will exhibit activity if localized to the cytoplasm, and PhoA is only enzymatically active if it is folded in the periplasm. Thus, the enzymatic activity of PhoA or LacZ fused to an N-terminal fragment of a protein of interest will predict the location of the junction of the protein of interest with LacZ or ′PhoA (reviewed in reference 30). We first used phage-encoded transposon systems (TnphoA/In and TnlacZ/In) created by Manoil and Bailey (36) to construct traG′-lacZ or traG′-′phoA fusions in a nondirected manner. Using this method, we identified multiple traG fusions that, when expressed in E. coli, were positive for LacZ or PhoA activity. We also constructed directed fusions of traG with lacZ or ′phoA to allow us to examine regions of TraG that were missed using the transposon system. The isolation of multiple active fusions of TraG′ with LacZ and TraG′ with ′PhoA indicates that TraG is an inner-membrane protein when expressed in E. coli.
We transformed the TraG fusion constructs into N. gonorrhoeae in order to measure LacZ or PhoA activity in the natural species background. The units of activity of LacZ or PhoA we calculated were overall lower than the values observed in other systems, but this may be due to the construct used for expression. We considered all calculated activities of LacZ or PhoA to be positive if they were statistically different (P < 0.05 as determined by using the Student t test) from the value obtained for the parent strain that does not express PhoA or LacZ (strain MS11).
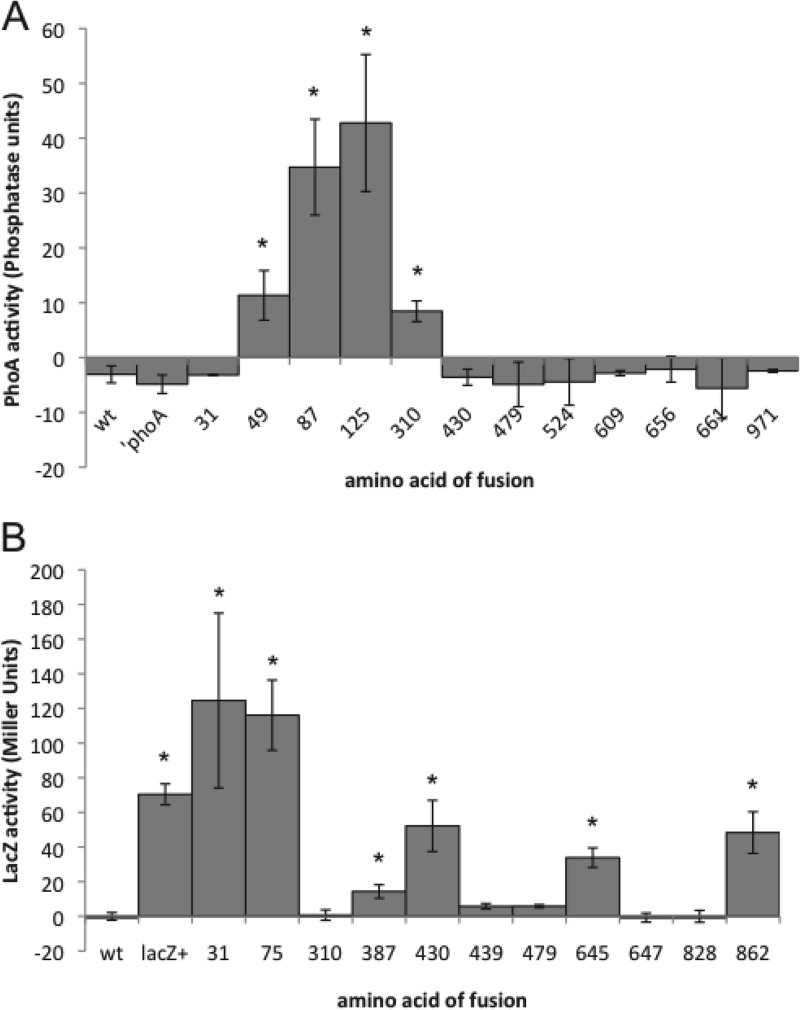
The expression of fusions of TraG′ and ′PhoA at amino acids 49, 87, 125, and 310 in gonococci resulted in positive phosphatase activity, indicating that ′PhoA was folded in the periplasm (Fig. 2A). In contrast, the expression of the TraG′-′PhoA fusions at amino acids 31, 430, and six more locations between amino acid 430 and the C terminus did not result in phosphatase activity. The absence of phosphatase activity suggests that ′PhoA did not fold in the periplasm, ′PhoA did not dimerize as necessary for activity, or the fusion was not expressed.
Fig 2.
(A) Quantitative analysis of the enzymatic activities of TraG′-′PhoA fusions expressed in N. gonorrhoeae. Strains were grown in log-phase in liquid medium and induced to express the TraG′-′PhoA fusion proteins. The enzymatic activity of ′PhoA was calculated and plotted in phosphatase units. (B) Quantitative analysis of the enzymatic activities of TraG′-′LacZ fusions expressed in N. gonorrhoeae. Strains were grown in log phase in liquid medium and induced to express the TraG′-′LacZ fusion proteins. The enzymatic activity of LacZ was calculated and plotted in Miller units. Each reported value is the result of three independent experiments and the reported errors are the standard deviations. *, P < 0.05 as determined by using the Student t test.
We carried out Western blot analysis of eight of the fusions using anti-PhoA antibodies to determine whether the TraG fusion proteins were expressed. For all fusions except the one at amino acid 310, we detected bands of increasing size as the size of the TraG′ fragment fused to ′PhoA increased (data not shown). These results indicate that most of the TraG fusion proteins were expressed even if their activity was not detected in the enzymatic assay.
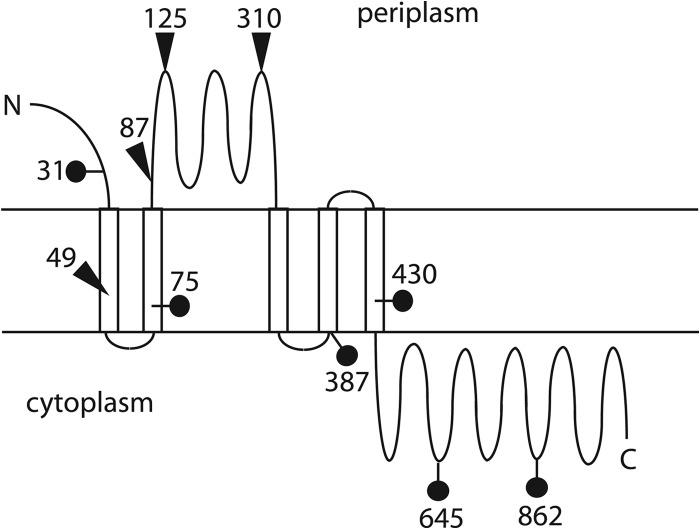
Expression of the TraG′-LacZ and TraG′-′PhoA fusions in gonococci, combined with membrane topology predictions allowed us to create a model of the topology of TraG in the inner membrane. With the exception of the fusion at amino acid 49, the TraG′-′PhoA fusion data are consistent with a model where the TraG N terminus is in the periplasm, there are five membrane-spanning regions giving two periplasmic loops from amino acids 82 to 358 and amino acids 408 to 414, and the C terminus is in the cytoplasm (Fig. 3). The ′PhoA fusion at 49 might disrupt the membrane span, leading to the PhoA+ phenotype, although this region would normally not be present in the periplasm in intact TraG.
Fig 3.
Topology model for TraG. The results from the fusion protein studies are consistent with a five membrane-span model. Active ′PhoA fusions are shown with triangles. Active LacZ fusions are shown with lollipop shapes. The numbers indicate the location of the TraG junction in amino acids.
The expression of fusions of TraG′ with LacZ at amino acids 31, 75, 387, 430, 645, and 862 in gonococci resulted in positive β-galactosidase activity, indicating that the LacZ portion of these fusion proteins remained in the cytoplasm (Fig. 2B). Western blot analysis for eight of these fusions using anti-LacZ antibodies showed that the TraG fusion proteins were expressed (data not shown). A strain expressing LacZ showed a specific band of the expected size not found in the parent strain MS11. Fusions at amino acids 647 and 828 showed fusion protein expression but no LacZ activity. The fusion of TraG′ to LacZ at amino acid 310 was expressed at a much lower level than the other TraG fusion proteins.
We incorporated the TraG′-′LacZ fusion data into the TraG topology model. The TraG′-to-LacZ fusion at amino acid 31 was expected to give positive LacZ activity. Since this fusion was constructed in a region of the protein before any predicted transmembrane domains, the truncated TraG protein is predicted to be localized to the cytoplasm regardless of the localization of this domain in the full-length form of TraG. The LacZ fusions are consistent with a model of TraG topology with five membrane spans, one large soluble domain in the periplasm, and the C-terminal soluble domain in the cytoplasm (Fig. 3).
The traG region of the GGI is variable among strains.
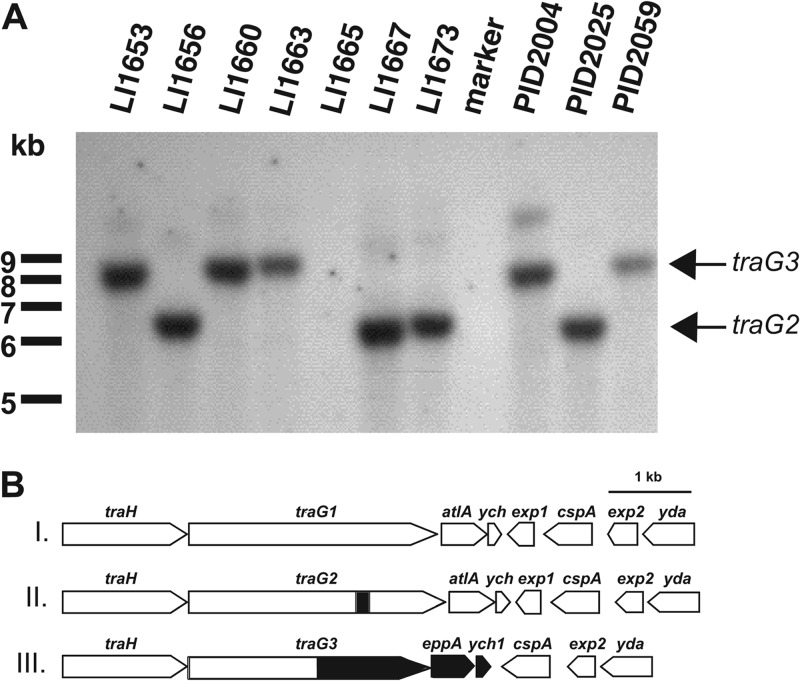
Significant variation within the GGI is found in the region surrounding traG. Directly downstream of traG is atlA, which encodes an enzyme that cleaves the glycan backbone of peptidoglycan strands (2, 6). It is hypothesized that peptidoglycanases in T4SSs create localized breaks in the peptidoglycan cell wall to allow for assembly of T4SS components or secretion of substrates across the cell wall (37). We have shown that the peptidoglycanase activity of atlA is essential for DNA secretion (6). Thus, it is curious that some gonococcal strains carry versions of the GGI that lack atlA (2). Also, some strains carry a different allele of traG, known as the sac-4 allele or traG2. The open reading frame of this traG allele encodes a 1,032-amino-acid protein with a predicted molecular mass of 111 kDa (2). We used Southern analysis and DNA sequencing to further examine the nature and extent of variation in this region. We used a portion of the 5′ end of traG as a probe in Southern blot analysis of 10 low-passage clinical isolates of N. gonorrhoeae that do not contain a traG1-atlA region like that of MS11, i.e., these strains either lacked atlA or carried the traG2 allele as determined by PCR (2). Southern blots carried out using the conserved part of traG as a probe revealed that either traG was absent because the GGI was not present (as in strain LI1665) or it was present on one of two different sized restriction fragments (Fig. 4A). The smaller restriction fragments are consistent with the size of the fragment containing traG2 and atlA, as is seen in the JC1 version of the GGI, and all of the strains containing traG on the smaller restriction fragment were sac-4+, as determined by PCR (2).
Fig 4.
(A) Southern blot analysis of EcoRI- and ClaI-digested chromosomal DNA from 10 low-passage gonococcal isolates. A region internal to the conserved portion of traG was used as the probe. traG is not present at all or is found on one of two different sized restriction fragments in these isolates. (B) Three different versions of the GGI. Class I is the version found in strain MS11 and contains traG1 and atlA. Class II contains both traG2 (traGsac-4) and atlA, while class III is the version found in strain PID2059. Class III contains traG3 and eppA but lacks atlA. The shaded areas indicate regions that are different from the nucleotide sequence of strain MS11.
To investigate the traG locus of a strain containing traG on the larger restriction fragment, we cloned and sequenced the traG region of strain PID2059 (GenBank accession number DQ835990). Sequence analysis confirmed that atlA is missing from the GGI of this strain but also revealed that traG from strain PID2059 shows differences from the MS11 version of traG (Fig. 4B). The MS11 allele is hereafter referred to as traG1. We called the PID2059 allele traG3. It encodes a 970-amino-acid protein with a predicted molecular mass of 104 kDa. The predicted amino acid sequence of TraG3 from PID2059 is almost identical to the predicted amino acid sequence of TraG1 from strain MS11 from amino acids 1 to 535, slightly more than half of the protein sequence. After that point the amino acid sequence still shows significant similarity to TraG by amino acid alignment but varies significantly from the MS11 version (E = 3e−32, 31% over 355 amino acids). After traG3, this strain carries a gene for a putative endopeptidase (EppA) similar to proteins of the M23 family (pfam01551), a family of zinc metallopeptidases. Many characterized M23 family members are putative peptidases and some, including lysostaphin, which cleaves Staphylococcus aureus peptidoglycan (38), are peptidoglycan endopeptidases. Lysostaphin cleaves pentaglycine chains that cross-link peptidoglycan in some Gram-positive bacteria (39). Gonococcal M23B family member NG1686 has endopeptidase and carboxypeptidase activity on peptidoglycan (25). Other M23 family members are peptidases that cleave protein substrates (40). Some members of the M23 family, however, are bacterial lipoproteins that have no demonstrated peptidase activity (41, 42).
The region downstream of traG3 and eppA in strain PID2059 was sequenced and was found to have several differences relative to the region downstream of traG1 in strain MS11 (Fig. 4B). In addition to lacking atlA, the ych gene of strain PID2059 was different from ych of strain MS11. The function of ych is not known, although it is not required for DNA secretion (our unpublished observation). The different allele in PID2059 was designated ych1. Sequence analysis showed that PID2059 lacks exp1, a gene found in the MS11 version of the GGI between ych and cspA. The exp1 open reading frame is oriented in the opposite direction as traG, atlA, and ych and encodes a protein that has been shown to be exported from the cytoplasm by N. gonorrhoeae and E. coli (2, 43). PID2059 also contains two genes directly adjacent to ych1, designated cspA and exp2, which are also found in the MS11 version of the GGI. cspA encodes a protein carrying a cold shock domain with similarity to other bacterial cold shock-like proteins (2). The products encoded by cspA in both the MS11 and PID2059 versions of the GGI are nearly identical. In the MS11 version of the GGI, the 5′ third of exp2 is identical to the 5′ third of exp1. Similar to cspA, the products encoded by exp2 in the MS11 and PID2059 versions of the GGI are almost identical. Microarray analysis indicated that N. meningitidis strain A22 contains genes of the GGI but lacks atlA (44). We sequenced the traG region in this strain and found sequences similar to traG3 and eppA of strain PID2059. To date, all N. meningitidis strains that carry the GGI and a traG allele, have traG3 and eppA (45).
The data suggesting that there are three different alleles of traG are also supported by recent N. gonorrhoeae genome sequence data. The completed NCCP11945 genome (GenBank accession no. CP001050) shows a strain with traG1 and atlA like MS11. Similarly, the partially completed sequence of strain PID1 is like that of MS11 for traG1 and atlA. The sac-4 allele of traG (traG2) is found in the sequences of strains FA19, PID332, and SK93-1035, and these strains also contain atlA. Interestingly, strains FA19 and PID332 do not contain exp1 or cspA, although SK93-1035 carries these genes. The variation in the presence of exp1 and cspA was previously reported by Dillard and Seifert (2). Strain FA19 does not secrete DNA (our unpublished observation), although the reason for the lack of secretion is not known. The genome sequence of PID18 shows the traG3 allele, eppA, and the absence of exp1, just as we found for strain PID2059. The genome sequences of strains FA19, MS11, PID18, PID332, and SK93-1035 are available elsewhere (http://www.broad.mit.edu).
EppA exhibits peptidoglycanase activity.
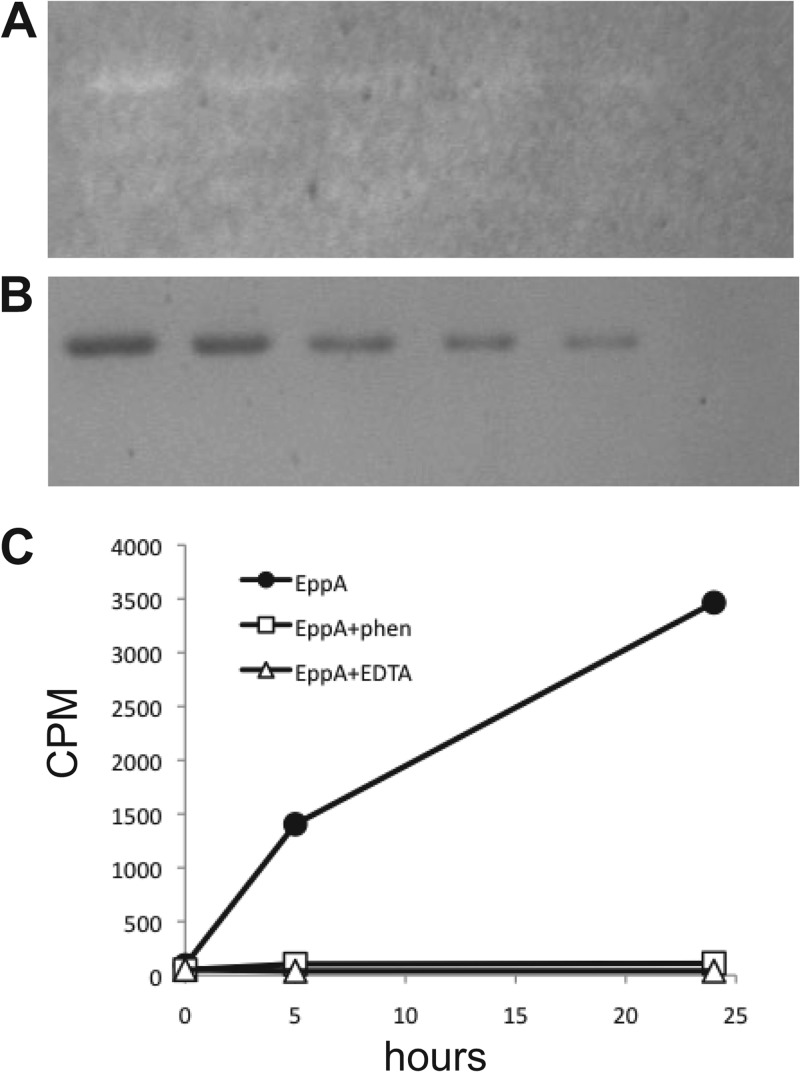
The similarity of EppA to peptidoglycan endopeptidases suggested that EppA might function to break down peptidoglycan. To test this idea, we overproduced EppA as a His-tagged protein in E. coli and purified it by immobilized metal ion affinity chromatography. The purified EppA was used in zymogram assays to look for peptidoglycan-binding or peptidoglycan-degrading activity. EppA produced a zone of clearing in zymogram gels containing gonococcal peptidoglycan (Fig. 5A and B) but not in zymogram gels containing Micrococcus lysodeikticus cells (data not shown). To further examine EppA function, we added EppA to 3H-labeled gonococcal sacculi and monitored the degradation of the peptidoglycan by measuring the release of 3H-labeled material into the soluble fraction. EppA was found to degrade gonococcal peptidoglycan (Fig. 5C). The addition of EDTA or 1,10-phenanthroline to the reaction abolished peptidoglycan degradation, suggesting that EppA requires a metal ion for function.
Fig 5.
EppA degrades peptidoglycan. (A) Zymogram of purified EppA electrophoresed through a polyacrylamide gel containing gonococcal peptidoglycan. Zones of clearing indicate areas where EppA bound to or degraded peptidoglycan. (B) Coomassie blue-stained gel showing quantities of EppA used for part A. Lane 1, 2.4 μg; lane 2, 1.8 μg; lane 3, 1.2 μg; lane 4, 0.9 μg; lane 5, 0.6 μg; lane 6, no EppA. C. EppA degraded 3H-labeled gonococcal sacculi producing soluble peptidoglycan fragments that were quantified by scintillation counting. This reaction was inhibited by addition of EDTA or 1,10-phenanthroline.
EppA is a peptidoglycan endopeptidase.
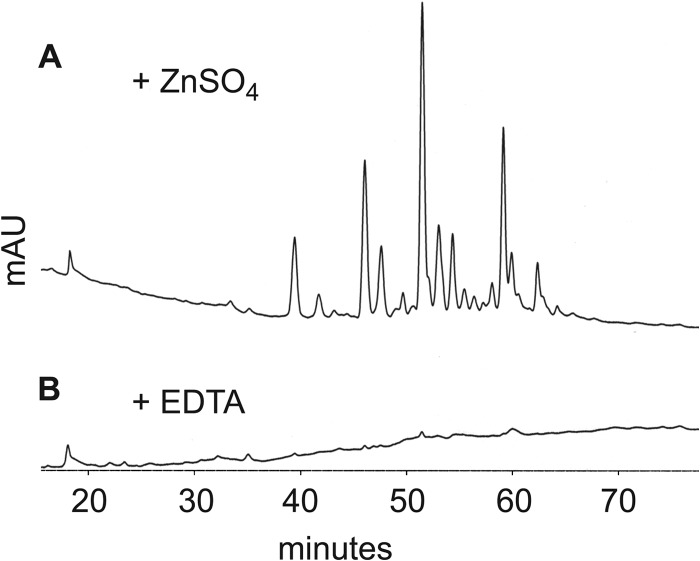
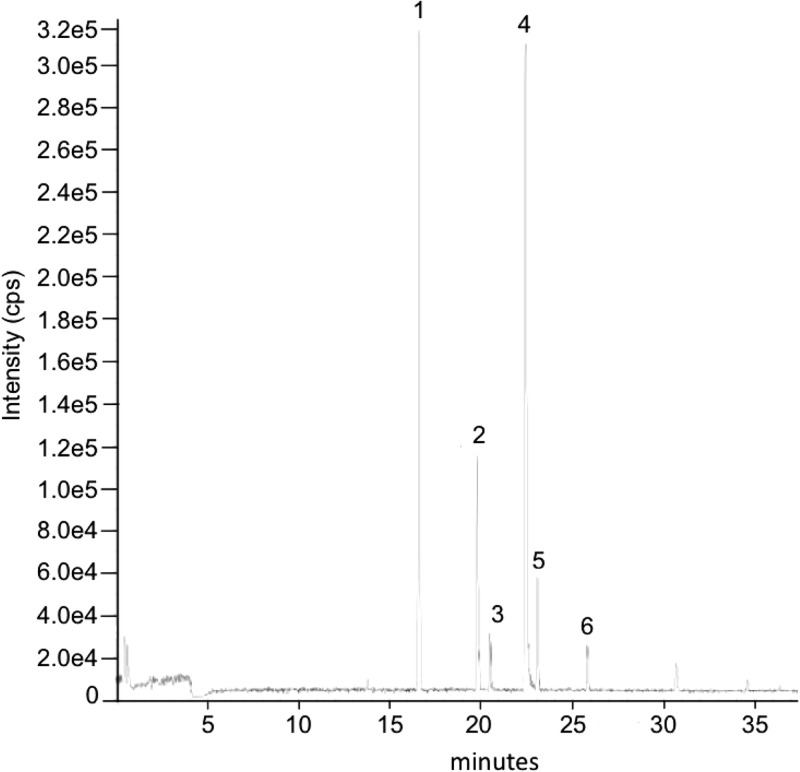
To examine the biochemical function of EppA, we digested gonococcal sacculi with EppA in vitro and characterized the products by HPLC and LC/MS. Digestion of sacculi with EppA produced a variety of products that could be detected by HPLC (Fig. 6A). However, the addition of EDTA prevented digestion, and no products were detected from this reaction (Fig. 6B). Characterization by LC/MS of the peptidoglycan fragments produced from gonococcal sacculi showed six major peaks (Fig. 7), representing monomeric, dimeric, and trimeric peptidoglycan fragments with each containing one 1,6-anhydro-N-acetylmuramic acid (Table 3).
Fig 6.
HPLC analysis shows EppA produces a variety of soluble peptidoglycan fragments from digestion of gonococcal peptidoglycan, but these peptidoglycan fragments are not produced in the presence of EDTA. Peptidoglycan fragments were detected spectrophotometrically by absorbance at 210 nm.
Fig 7.
LC/MS analysis of peptidoglycan fragments generated by EppA. Proposed structures for the peptidoglycan fragments numbered in the figure are listed in Table 3.
Table 3.
Proposed structures of His6-EppA reaction products revealed by LC/MS
| Peaka | Mass (Da) |
Proposed structureb | |
|---|---|---|---|
| Calculated | Observed | ||
| Monomer | |||
| 1 | 921.38 | 921.36 | Tetra (anh) |
| Dimers | |||
| 2 | 1,842.76 | 1,842.73 | Tetra-tetra (anh) |
| 3 | 1,913.80 | 1,913.80 | Penta-tetra (anh) |
| 4 | 1,884.77 | 1,884.77 | Tetra-tetra (anh) OAc |
| 5 | 1,955.81 | 1,955.81 | Penta-tetra (anh) OAc |
| Trimer | |||
| 6 | 2,848.17 | 2,848.17 | Tetra-tetra-tetra (anh) di-OAc |
Peaks correspond to those shown in Fig. 7.
Tetra, disaccharide tetrapeptide; Penta, disaccharide pentapeptide (disaccharide = N-acetylglucosamine-N-acetylmuramic acid); anh, 1,6-anhydro-muramic acid; OAc, O-acetylation on N-acetylmuramic acid.
If EppA is a peptidoglycan endopeptidase as predicted from its sequence homology, then any multimeric peptidoglycan fragments produced by EppA digestion of sacculi should be glycosidically linked, not peptide linked. To test this idea, we sequentially digested gonococcal sacculi with EppA and mutanolysin. Mutanolysin specifically cuts the MurNAc-β-(1,4)-GlcNAc bond in peptidoglycan, converting glycosidically linked peptidoglycan strands to monomers. For these experiments, sacculi from nonacetylating gonococcal strain KH530 was used in order to ease analysis of the products. The sequential digestion demonstrated that the multimers produced by EppA were glycosidically linked. The addition of mutanolysin eliminated all of the tetramers and trimers, and almost all of the dimers (Table 4). New species of monomers were seen resulting from mutanolysin digestion, and these were identified as reducing monomers, indicating that they were cleaved from longer peptidoglycan strands by mutanolysin digestion of glycosidic linkages. A very small amount of reducing tetrapeptide monomer was identified in the EppA alone digest (Table 4), but the addition of mutanolysin increased the amount of this product 48-fold (data not shown). These data demonstrate that peptidoglycan fragments released from sacculi have no peptide cross-links, indicating that EppA digests peptide cross-links (DAP–d-Ala or DAP-DAP) to release these fragments.
Table 4.
Reaction products from the sequential digestion of PG by His6-EppA and mutanolysina
| Product | Calculated mass (Da) | Observed mass (Da) |
|
|---|---|---|---|
| EppA + mutanolysin | EppA + water | ||
| Monomers | |||
| Tri (anh) | 850.3 | 850.4 | 850.4 |
| Tri (red) | 868.4 | 868.4 | ND |
| Tetra (anh) | 921.4 | 921.4 | 921.4 |
| Tetra (red) | 939.4 | 939.4 | 939.4 |
| Penta (anh) | 992.4 | 992.4 | 992.4 |
| Penta (red) | 1,010.4 | 1,010.5 | ND |
| Dimers | |||
| Tetra-tri (anh) or Tri-tetra (anh) | 1,771.7 | ND | 1,771.8 |
| Tetra-tetra (anh) | 1,842.8 | 1,842.8 | 1,842.8 |
| Penta-tetra (anh) or Tetra-penta (anh) | 1,913.8 | ND | 1,913.8 |
| Trimers | |||
| Tetra-tri-tri (anh)b | 2,622.1 | ND | 2,622.1 |
| Tetra-tetra-tri (anh)c | 2,693.1 | ND | 2,693.2 |
| Tetra-tetra-tetra (anh) | 2,764.1 | ND | 2,764.2 |
| Tetramer | |||
| Tetra-tetra-tetra-tetra (anh) | 3,685.5 | ND | 3,685.6 |
Tri, disaccharide tripeptide; Tetra, disaccharide tetrapeptide; Penta, disaccharide pentapeptide (disaccharide = N-acetylglucosamine-N-acetylmuramic acid); anh, 1,6-anhydro-muramic acid; red, N-acetylmuramic acid with reducing end; ND, not detected.
Could be Tetra-tri-tri (anh), Tri-tri-tetra (anh), or Tetra-tri-tetra (anh).
Could be Tetra-tetra-tri (anh), Tetra-tri-tetra (anh), or Tri-tetra-tetra (anh).
Function of different alleles of traG in DNA secretion.
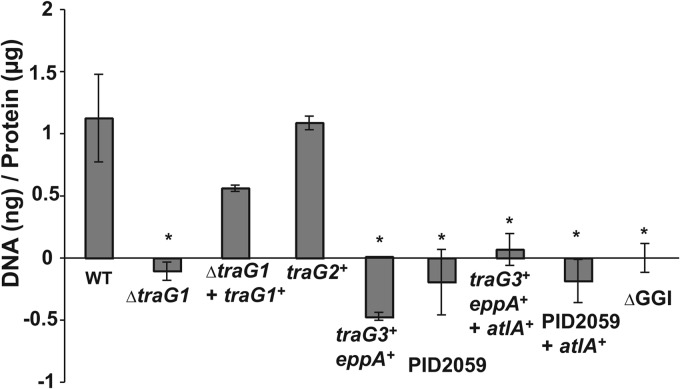
It was previously shown that an insertion mutation in traG1 in strain MS11 eliminated DNA secretion by gonococci (1). To better examine the requirement for traG for DNA secretion, we constructed an in-frame deletion in traG1, leaving only the start and stop codons. The deletion in traG eliminated DNA secretion, and complementation with a wild-type copy of traG1 restored DNA secretion. These results confirm that traG1 is necessary for DNA secretion (Fig. 8). We also examined the ability of traG2 to functionally replace traG1 in strain MS11. The traG1 locus of MS11 was replaced with the traG2 allele from strain JC1. The resulting strain secreted DNA at levels similar to wild-type MS11, indicating that traG2 is functional for DNA secretion (Fig. 8).
Fig 8.
Fluorometric detection of DNA secreted by gonococcal strains. The concentration of DNA in culture supernatants was measured by treating culture supernatants with a fluorescent, DNA-binding dye and comparing them to DNA standards of known concentrations. Secreted DNA was normalized to total protein in the cell pellet. The average background fluorescence value was subtracted from the values for all strains. Strains measured were MS11 (WT), traG1 deletion mutant PK186 (ΔtraG1), complemented traG1 strain PK191 (ΔtraG1 + traG1+), HH534 (traG2+), HH528 (traG3+ eppA+), PID2059, PK161 (traG3+ eppA+ + atlA+), PK162 (PID2059 + atlA+), and ND500 (ΔGGI). All strains are MS11 background except those labeled PID2059. Each reported value is the result of at least three separate experiments, and the errors are the standard deviations. *, P < 0.05 compared to wild type (as determined by using the Student t test).
The ability of EppA to degrade peptidoglycan suggested that this peptidoglycan endopeptidase might be able to functionally substitute for the peptidoglycan lytic transglycosylase activity of AtlA in the T4SS. To test this hypothesis, we replaced the traG1-atlA locus in strain MS11 with traG3 and eppA from strain PID2059, creating strain HH528. DNA secretion was eliminated in this strain, indicating that traG3 and eppA cannot functionally replace the traG1-atlA locus for DNA secretion (Fig. 8). We also found that wild-type strain PID2059 is deficient for DNA secretion. We hypothesized that expression of atlA might confer the ability to secrete DNA in strains containing traG3 and eppA in place of traG1 and atlA. We tested this hypothesis by complementing strains HH528 and PID2059 with atlA and examining the resulting strains for DNA secretion. The resulting strains did not exhibit DNA secretion, indicating that expression of atlA is not sufficient to allow DNA secretion in strains containing traG3 and eppA (Fig. 8). The atlA expression construct was previously shown to be effective in complementing an atlA mutant of strain MS11 (6). These results suggest that both atlA and either traG1 or traG2 are necessary for DNA secretion.
eppA has no effect on peptidoglycan fragment release.
Because EppA degrades peptidoglycan, we hypothesized that EppA might be involved in release of toxic peptidoglycan fragments by gonococci. N. gonorrhoeae releases cytotoxic peptidoglycan monomers into the extracellular milieu during growth (46, 47) that are identical to tracheal cytotoxin released by Bordetella pertussis (48) or to the proinflammatory peptidoglycan monomers released by Shigella flexneri (49). In Helicobacter pylori, peptidoglycan fragment transfer to host cells is dependent on expression of the cag T4SS (50). We examined the peptidoglycan fragments released during growth of the gonococcal strain containing traG3 and eppA in place of the traG-atlA locus. The peptidoglycan was metabolically labeled, and the peptidoglycan fragments in culture supernatants were separated by size-exclusion chromatography and compared to known peptidoglycan standards. The strain containing traG3 and eppA in place of the traG1-atlA locus in the MS11 background did not differ from wild-type strain MS11 in the release of peptidoglycan fragments (data not shown). This result suggests that traG3 and eppA are not involved in the degradation of the cell wall for release of cytotoxic peptidoglycan monomers by gonococci.
No evidence for mating pair formation.
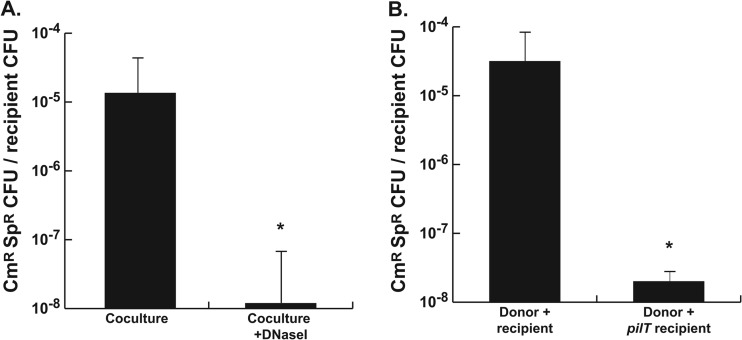
The homologues of TraG in other bacteria are involved in mating pair formation for conjugation. Previous studies have indicated that the gonococcal T4SS secretes DNA into the medium, since the DNA is readily detected in cell-free culture supernatants and DNA transfer is greatly diminished in the presence of DNase I or single-strand specific nucleases (1–3). However, the requirement for a mating pair formation homologue made us wonder whether conjugation is the normal method for chromosomal DNA transfer. The previous studies used cultures of N. gonorrhoeae grown in liquid medium aerated by rotation of the culture tubes. Perhaps mating pairs formed in these cultures but were ripped apart by the movement of liquid in the culture tubes. DNA might then still be released by the donors from these separated mating pairs. Therefore, we examined DNA transfer in static liquid cultures. Donor and recipient strains were mixed in equal amounts in liquid medium and were grown for 2 h in a shallow depth of medium in a tissue culture flask in a 5% CO2 atmosphere. This method allowed growth of gonococci in liquid culture without the need for aeration by shaking. As was found in previous studies (1–3), a chromosomal marker (cat) was efficiently transferred from a donor strain to a spectinomycin-resistant recipient strain (Fig. 9A). This transfer was sensitive to DNase I, indicating that the DNA was present in the medium even in the static liquid culture. We also tested whether chromosomal transfer required that the recipient be competent for natural transformation. We measured DNA transfer into an N. gonorrhoeae pilT mutant. PilT is required for DNA uptake for natural transformation (51). Static mixed culture produced transformants for wild-type recipients, but the pilT mutant strain did not produce doubly resistant isolates (Fig. 9B). These results confirm that chromosomal DNA transfer in N. gonorrhoeae occurs by DNA export and natural transformation and not by mating pair formation and conjugation.
Fig 9.
Coculture transformation in static liquid culture. (A) Strains JD1545 (cnp::cat, recA6) and MS11Spc (rpsE) were mixed and grown in shallow liquid culture without shaking for 2.5 h in the absence or presence of 25 μg of DNase I/ml. Transformation was assessed by enumerating the Cmr Spr transformants per Spr recipient. (B) Strain JD1545 (cnp::cat, recA6) was cocultured with MS11Spc or MS11Spc pilT (NT04), and transformation frequency was determined as in panel A.
DISCUSSION
TraG homologues are found in many type IV secretion systems related to the F-plasmid conjugation system. Some 111 different bacterial species have TraG homologues in the current sequence databases. The TraG of F-plasmid is the best characterized, and it has been shown to have multiple functions. F-plasmid TraG is necessary for conjugative pilus assembly (11), stabilization of mating pairs (12), and entry exclusion (13, 14). The same functions have been seen for TraG homologues in two other conjugative systems (10, 15). Furthermore, traG mutants in these systems are deficient in conjugative transfer, leading to the suggestion that TraG might play a more direct role in the DNA transfer mechanism (52–54). Our results demonstrate that gonococcal TraG is required for DNA secretion. The function of the gonococcal protein must be other than those involved in mating pair formation, mating pair stabilization, and entry exclusion, since gonococcal chromosomal DNA transfer via the T4SS does not involve any of those processes (Fig. 9) (2, 3).
We characterized TraG localization and topology in order to better understand its possible functions. Gonococcal TraG was found to be an inner-membrane protein with a large periplasmic domain and a large cytoplasmic domain (Fig. 1 and 3). The protein fusion data indicate that the region from amino acid 83 to amino acid 358 is in the periplasm, and the C-terminal region, starting from amino acid 435, is in the cytoplasm. The periplasmic region might interact with other proteins of the secretion apparatus. TraG of F-plasmid has been suggested to interact with TraN, another mating pair stabilization protein (11). N. gonorrhoeae encodes two proteins with similarity to F-plasmid TraN, named TraN and Ybi. traN was shown to be required for DNA secretion (4). The C-terminal, cytoplasmic domain of TraG might interact with other T4SS components in the cytoplasm, possibly the relaxosome or other substrate-interacting components. The topology model for N. gonorrhoeae TraG is consistent with those determined for P. rettgeri TraG and A. tumefaciens VirB6, but it differs slightly from that of E. coli F-plasmid TraG which localized the C-terminal soluble region of F-plasmid TraG to the periplasm (10, 14, 55). It should be emphasized that the topology mapping results represent a snapshot in time and that our results do not preclude the possibility that the C-terminal domain of TraG could be proteolytically cleaved and transported through the T4SS channel or extended across the T4SS channel upon receiving an activation signal, as is predicted for other TraG homologues (10, 14).
In addition to the TraG homologues in F-like systems, a distant homologue is found in the P-type systems such as that of A. tumefaciens (9). Named VirB6 in A. tumefaciens and the related systems, these homologues are also inner-membrane proteins and interact with other proteins of their T4SSs (55–57). A. tumefaciens VirB6 interacts directly with inner-membrane protein VirB8 and stabilizes VirB3 and VirB5 in the secretion apparatus (63, 64, 66, 67). A. tumefaciens VirB6 can be cross-linked to DNA using the TrIP method (58). Thus, it was suggested that VirB6 might be part of the secretion channel (58). Similarly, it has been suggested that TraG in the F-type systems might function as an inner-membrane component of the secretion channel (53). Another interesting similarity between gonococcal TraG and VirB6 is that there are multiple alleles of virB6 in some species. Rickettsia prowazekii, Rickettsia conorii, and Ehrlichia chaffeensis each show four or five virB6 alleles within each strain (59, 60). The reasons for the multiple virB6 alleles in these species are not known.
It was previously shown that at least three different versions of the GGI could be found, classified by differences in the traG-atlA region (2). One version contains traG1 and atlA like strain MS11. A second version carries a different allele of traG, known as traGsac-4 or traG2, and also carries atlA (Fig. 4). The third version was known to lack atlA but was otherwise uncharacterized (2). Using Southern blotting, cloning, and DNA sequencing, we found that strains in this third group carry a short allele of traG and instead of atlA, carry eppA, which encodes a peptidoglycan endopeptidase. Examination of recently completed N. gonorrhoeae genome sequences shows that the same three alleles of traG described here are also found in the newly sequenced strains. When we moved the different traG alleles into strain MS11, we found that traG2 could function in DNA secretion. However, traG3 and eppA did not functionally substitute for traG1 and atlA of strain MS11 for DNA secretion. Furthermore, a wild-type strain with the traG3 allele (PID2059) did not secrete DNA. Even when atlA was expressed in the traG3+ eppA+ derivative of strain MS11 or in strain PID2059, no DNA was secreted (Fig. 8). These data suggest that the traG allele affects what substrates are secreted and that TraG3 does not function for DNA secretion.
Approximately 13% of gonococcal strains carry the traG3 allele and eppA, as determined by the presence of traG and the absence of atlA (2). Furthermore, all meningococcal strains that carry the GGI and have the traG region, have the traG3 allele and eppA (45). It is unclear what the function of the T4SS would be in these gonococcal and meningococcal strains, since it appears that they cannot secrete DNA (Fig. 8) (45). It is possible that these strains may secrete proteins. Alternatively, the function of this system may be like that proposed for the trw T4SS in Bartonella tribocorum. That system is not thought to secrete substrates, but instead the apparatus itself may mediate host cell interactions, possibly by acting in adherence (61).
Biochemical characterization of EppA demonstrated that it is a peptidoglycan-degrading endopeptidase. Zymograms demonstrated that EppA was active against DAP-containing gonococcal cell walls but not against Lys-containing Micrococcus cell walls. EppA degraded gonococcal peptidoglycan to monomers, dimers, and trimers, and this reaction was inhibited by EDTA or phenanthroline, indicating that EppA is a zinc-dependent peptidoglycanase. Sequential digests with mutanolysin demonstrated that all multimeric peptidoglycan fragments liberated by EppA contained glycosidic, not peptide, cross-links. Thus, EppA digests peptidoglycan by cutting peptide cross-links. Like AtlA, EppA does not have an obvious signal sequence for transport to the periplasm, and it is possible that it is transported to the periplasm through part of the secretion apparatus (6, 62). It is not clear why gonococci would have different versions of the GGI encoding different types of peptidoglycanases, i.e., the lytic transglycosylase AtlA or the endopeptidase EppA. Peptidoglycanases associated with type III and type IV secretion systems are often lytic transglycosylases, and these enzymes are thought to create an opening in the cell wall to allow secretion system assembly (37, 63). A. tumefaciens and other P-type systems use a lytic transglycosylase named VirB1 (64). Conjugative plasmids such as F-plasmid also use a lytic transglycosylase, encoded by geneX in F-plasmid (53, 63). The GGI also carries a geneX homologue, ltgX, which is required for DNA secretion (6). B. pertussis is unusual in that it uses a glycohydrolase domain at the N terminus of its VirB8 homolog PtlE for peptidoglycan degradation (65). Although the biochemical difference in AtlA and EppA reactions is clear, the functional differences for making the secretion system are not. It is possible that lytic transglycosylases may make different-sized holes in the cell wall compared to endopeptidases, but as of yet, no one has been able to specifically examine the gap created by one of these secretion system peptidoglycanases.
Despite the requirement for the mating pair stabilization homologue TraG, transfer of chromosomal DNA in GGI+ strains was confirmed to occur by transformation, not conjugation. DNase I prevented transfer of a chromosomal marker in static liquid cultures. Thus, the presence of DNA in the medium and the transfer of chromosomal markers by T4SS+ strains are not due to breaking of mating pairs during a conjugation process. These data are consistent with studies that demonstrated that DNA secretion does not involve cell lysis or death, processes which might be expected to increase if mating pairs were disrupted by shear forces (4, 6). Furthermore, DNA transfer was demonstrated to require functional PilT, an ATPase which functions in pilus retraction and DNA uptake for natural transformation (51). The requirement seen here for a mating pair formation homologue for contact-independent secretion supports the contention of Frost that mating pair formation proteins are not just involved in mating but are also necessary components of the secretion apparatus (53).
In summary, the studies presented here indicate that traG encodes an inner-membrane protein that is necessary for DNA secretion by gonococci. Future studies focusing on the effects of different traG alleles on infection of host cells may identify other functions of the different traG alleles. In addition, identification of soluble loops of TraG will guide studies of potential areas of contact between TraG and other T4SS components.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grants AI047958 and AI072605 to J.P.D. P.L.K. was supported by NIH grant T32 AI055397 to the University of Wisconsin-Madison.
We thank J. Lindquist for the gift of M. luteus, H. S. Seifert for the gift of anti-PilQ antisera, R. Welch for E. coli strain CC118, and C. Manoil for generous gifts of lambda TnphoA/In and lambda TnlacZ/In. LC/MS analysis was performed at the UW Biotechnology Center, with the assistance of Amy Harms and Melissa Boersma. We thank Shelly Immel Schmoller for constructing the plasmid pSI7. We also thank J. V. Troll for helpful technical advice.
Footnotes
Published ahead of print 1 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02098-12.
REFERENCES
- 1. Hamilton HL, Schwartz KJ, Dillard JP. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dillard JP, Seifert HS. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263–277 [DOI] [PubMed] [Google Scholar]
- 3. Salgado-Pabón W, Jain S, Turner N, van der Does C, Dillard JP. 2007. A novel relaxase homologue is involved in chromosomal DNA processing for type IV secretion in Neisseria gonorrhoeae. Mol. Microbiol. 66:930–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol. Microbiol. 55:1704–1721 [DOI] [PubMed] [Google Scholar]
- 5. Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 59:376–385 [DOI] [PubMed] [Google Scholar]
- 6. Kohler PL, Hamilton HL, Cloud-Hansen K, Dillard JP. 2007. AtlA functions as a peptidoglycan lytic transglycosylase in the Neisseria gonorrhoeae type IV secretion system. J. Bacteriol. 189:5421–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maynard Smith J, Smith NH, O'Rourke M, Spratt BG. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. U. S. A. 90:4384–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durrenberger MB, Villiger W, Bachi T. 1991. Conjugational junctions: morphology of specific contacts in conjugating Escherichia coli bacteria. J. Struct. Biol. 107:146–156 [DOI] [PubMed] [Google Scholar]
- 9. Lawley TD, Klimke WA, Gubbins MJ, Frost LS. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1–15 [DOI] [PubMed] [Google Scholar]
- 10. Marrero J, Waldor MK. 2007. Determinants of entry exclusion within Eex and TraG are cytoplasmic. J. Bacteriol. 189:6469–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Firth N, Skurray R. 1992. Characterization of the F plasmid bifunctional conjugation gene, TraG. Mol. Gen. Genet. 232:145–153 [DOI] [PubMed] [Google Scholar]
- 12. Manning PA, Morelli G, Achtman M. 1981. traG protein of the F sex factor of Escherichia coli K-12 and its role in conjugation. Proc. Natl. Acad. Sci. U. S. A. 78:7487–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anthony KG, Klimke WA, Manchak J, Frost LS. 1999. Comparison of proteins involved in pilus synthesis and mating pair stabilization from the related plasmids F and R100-1: insights into the mechanism of conjugation. J. Bacteriol. 181:5149–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Audette GF, Manchak J, Beatty P, Klimke WA, Frost LS. 2007. Entry exclusion in F-like plasmids requires intact TraG in the donor that recognizes its cognate TraS in the recipient. Microbiology 153:442–451 [DOI] [PubMed] [Google Scholar]
- 15. Marrero J, Waldor MK. 2005. Interactions between inner membrane proteins in donor and recipient cells limit conjugal DNA transfer. Dev. Cell 8:963–970 [DOI] [PubMed] [Google Scholar]
- 16. Hamilton HL, Domínguez NM, Edwards JL, Apicella MA, Dillard JP. 2004. Interactions of Neisseria gonorrhoeae with the host: a role for the gonococcal genetic island-encoded type IV secretion system in infection, abstr B-194. p 66 Abstr. 104th Gen. Mtg. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 17. Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle CL. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillard JP. 2011. Genetic Manipulation of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. Chapter 4:Unit 4A2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wade JJ, Graver MA. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol. Lett. 273:35–37 [DOI] [PubMed] [Google Scholar]
- 20. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21. Altschul SF, Madden TL, Schafer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 23. Leclerc D, Asselin A. 1989. Detection of bacterial cell wall hydrolases after denaturing polyacrylamide gel electrophoresis. Can. J. Microbiol. 35:749–753 [DOI] [PubMed] [Google Scholar]
- 24. Jayaswal RK, Lee YI, Wilkinson BJ. 1990. Cloning and expression of a Staphylococcus aureus gene encoding a peptidoglycan hydrolase activity. J. Bacteriol. 172:5783–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stohl EA, Chan YA, Hackett KT, Kohler PL, Dillard JP, Seifert HS. 2012. Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology. J. Biol. Chem. 287:11222–11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dillard JP, Hackett KT. 2005. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 73:5697–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gauthier A, Puente JL, Finlay BB. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect. Immun. 71:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 29. Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 30. Manoil C. 1991. Analysis of membrane protein topology using alkaline phosphatase and beta-galactosidase gene fusions. Methods Cell Biol. 34:61–75 [DOI] [PubMed] [Google Scholar]
- 31. Drake SL, Koomey M. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975–986 [DOI] [PubMed] [Google Scholar]
- 32. Newhall WJ, Wilde CEIII, Sawyer WD, Haak RA. 1980. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect. Immun. 27:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 34. Tusnady GE, Simon I. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850 [DOI] [PubMed] [Google Scholar]
- 35. Hirokawa T, Boon-Chieng S, Mitaku S. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378–379 [DOI] [PubMed] [Google Scholar]
- 36. Manoil C, Bailey J. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250–263 [DOI] [PubMed] [Google Scholar]
- 37. Dijkstra AJ, Keck W. 1996. Peptidoglycan as a barrier to transenvelope transport. J. Bacteriol. 178:5555–5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schindler CA, Schuhardt VT. 1964. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. U. S. A. 51:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Browder HP, Zygmunt WA, Young JR, Tavormina PA. 1965. Lysostaphin: enzymatic mode of action. Biochem. Biophys. Res. Commun. 19:383–389 [DOI] [PubMed] [Google Scholar]
- 40. Ichimura T, Yamazoe M, Maeda M, Wada C, Hiraga S. 2002. Proteolytic activity of YibP protein in Escherichia coli. J. Bacteriol. 184:2595–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ichikawa JK, Li C, Fu J, Clarke S. 1994. A gene at 59 minutes on the Escherichia coli chromosome encodes a lipoprotein with unusual amino acid repeat sequences. J. Bacteriol. 176:1630–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lange R, Hengge-Aronis R. 1994. The nlpD gene is located in an operon with rpoS on the Escherichia coli chromosome and encodes a novel lipoprotein with a potential function in cell wall formation. Mol. Microbiol. 13:733–743 [DOI] [PubMed] [Google Scholar]
- 43. Boyle-Vavra S, Seifert HS. 1995. Shuttle mutagenesis: a mini-transposon for producing PhoA fusions with exported proteins in Neisseria gonorrhoeae. Gene 155:101–106 [DOI] [PubMed] [Google Scholar]
- 44. Snyder LA, Jarvis SA, Saunders NJ. 2005. Complete and variant forms of the “gonococcal genetic island” in Neisseria meningitidis. Microbiology 151:4005–4013 [DOI] [PubMed] [Google Scholar]
- 45. Woodhams KL, Benet ZL, Blonsky SE, Hackett KT, Dillard JP. 2012. Prevalence and detailed mapping of the gonococcal genetic island in Neisseria meningitidis. J. Bacteriol. 194:2275–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sinha RK, Rosenthal RS. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29:914–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. 2008. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J. Bacteriol. 190:5989–5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosenthal RS, Nogami W, Cookson BT, Goldman WE, Folkening WJ. 1987. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 55:2117–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nigro G, Fazio LL, Martino MC, Rossi G, Tattoli I, Liparoti V, De Castro C, Molinaro A, Philpott DJ, Bernardini ML. 2008. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell. Microbiol. 10:682–695 [DOI] [PubMed] [Google Scholar]
- 50. Viala J, Chaput C, Boneca IG, Cardona A, Giardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166–1174 [DOI] [PubMed] [Google Scholar]
- 51. Wolfgang M, Lauer P, Park HS, Brossay L, Hebert J, Koomey M. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330 [DOI] [PubMed] [Google Scholar]
- 52. Achtman M, Skurray RA, Thompson R, Helmuth R, Hall S, Beutin L, Clark AJ. 1978. Assignment of tra cistrons to EcoRI fragments of F sex factor DNA. J. Bacteriol. 133:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frost LS, Ippen-Ihler K, Skurray RA. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Rev. 58:162–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beaber JW, Hochhut B, Waldor MK. 2002. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J. Bacteriol. 184:4259–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jakubowski SJ, Krishnamoorthy V, Cascales E, Christie PJ. 2004. Agrobacterium tumefaciens VirB6 domains direct the ordered export of a DNA substrate through a type IV secretion System. J. Mol. Biol. 341:961–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Villamil Giraldo AM, Sivanesan D, Carle A, Paschos A, Smith MA, Plesa M, Coulton J, Baron C. 2012. Type IV secretion system core component VirB8 from Brucella binds to the globular domain of VirB5 and to a periplasmic domain of VirB6. Biochemistry 51:3881–3890 [DOI] [PubMed] [Google Scholar]
- 57. Judd PK, Mahli D, Das A. 2005. Molecular characterization of the Agrobacterium tumefaciens DNA transfer protein VirB6. Microbiology 151:3483–3492 [DOI] [PubMed] [Google Scholar]
- 58. Cascales E, Christie PJ. 2004. Definition of a bacterial type IV secretion pathway for a DNA substrate. Science 304:1170–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ohashi N, Zhi N, Lin Q, Rikihisa Y. 2002. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect. Immun. 70:2128–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bao W, Kumagai Y, Niu H, Yamaguchi M, Miura K, Rikihisa Y. 2009. Four VirB6 paralogs and VirB9 are expressed and interact in Ehrlichia chaffeensis-containing vacuoles. J. Bacteriol. 191:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seubert A, Hiestand R, de la Cruz F, Dehio C. 2003. A bacterial conjugation machinery recruited for pathogenesis. Mol. Microbiol. 49:1253–1266 [DOI] [PubMed] [Google Scholar]
- 62. Dillard JP, Seifert HS. 1997. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol. Microbiol. 25:893–901 [DOI] [PubMed] [Google Scholar]
- 63. Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A, Koraimann G, Högenauer G. 1995. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J. Bacteriol. 177:4279–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mushegian AR, Fullner KJ, Koonin EV, Nester EW. 1996. A family of lysozyme-like virulence factors in bacterial pathogens of plants and animals. Proc. Natl. Acad. Sci. U. S. A. 93:7321–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rambow-Larsen AA, Weiss AA. 2002. The PtlE protein of Bordetella pertussis has peptidoglycanase activity required for Ptl-mediated pertussis toxin secretion. J. Bacteriol. 184:2863–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zola TA, Strange HR, Dominguez NM, Dillard JP, Cornelissen CN. 2010. Type IV secretion machinery promotes Ton-independent intracellular survival of Neisseria gonorrhoeae within cervical epithelial cells. Infect. Immun. 78:2429–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cloud KA, Dillard JP. 2002. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 70:2752–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramsey ME, Hackett KT, Kotha C, Dillard JP. 2012. New complementation constructs for inducible and constitutive gene expression in Neisseria gonorrhoeae and Neisseria meningitidis. Appl. Environ. Microbiol. 78:3068–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ouchane S, Kaplan S. 1999. Topological analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 274:17290–17296 [DOI] [PubMed] [Google Scholar]
- 70. Nachamkin I, Cannon JG, Mittler RS. 1981. Monoclonal antibodies against Neisseria gonorrhoeae: production of antibodies directed against a strain-specific cell surface antigen. Infect. Immun. 32:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nowicki S, Ram P, Pham T, Goluszko P, Morse S, Anderson GD, Nowicki B. 1997. Pelvic inflammatory disease isolates of Neisseria gonorrhoeae are distinguished by C1q-dependent virulence for newborn rats and by the sac-4 region. Infect. Immun. 65:2094–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Swanson J. 1972. Studies on gonococcus infection II. Freeze-fracture, freeze-etch studies on gonococci. J. Exp. Med. 136:1258–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.