Abstract
Biofilm is a complex aggregate of cells that adhere to each other and produce an extracellular matrix. In Bacillus subtilis, an extracellular polysaccharide (EPS) and amyloid fiber (TasA), synthesized by the epsA-epsO and tapA-sipW-tasA operons, respectively, are the primary components of the extracellular matrix. In the current study, we investigated the functional role of the previously uncharacterized veg gene in B. subtilis. Overproduction of Veg, a small protein highly conserved among Gram-positive bacteria, stimulated biofilm formation via inducing transcription of the tapA-sipW-tasA operon. Moreover, overproduced Veg restored the impairment of biofilm formation in mutants carrying a deletion of of sinI, slrA, or slrR, encoding an antirepressor of SinR that acts as the master regulator of biofilm formation, while biofilm morphology in the absence of SinR was not affected by either additional veg deletion or overproduction, indicating that Veg negatively regulates SinR activity independently of the known antirepressors. Expression of sinR was not affected in Veg-overproducing cells, and amounts of SinR were similar in cells expressing different levels of Veg, strongly suggesting that Veg modulates the repressor activity of SinR. Interestingly, the results of in vivo pulldown assays of the SinR complex indicate that Veg inhibits the interactions between SinR and SlrR. Based on these findings, we propose that Veg or a Veg-induced protein acts as an antirepressor of SinR to regulate biofilm formation.
INTRODUCTION
Bacillus subtilis is able to differentiate into a variety of cellular states in response to environmental changes. When nutrients are abundant, cells grow as a free-floating planktonic form. Under nutrient-limiting conditions, developmental cellular differentiation processes, such as competence of DNA uptake, sporulation, and biofilm formation, proceed appropriately in response to different environments. These processes are accompanied by dramatic changes in gene expression, i.e., a new set of genes is turned on and nonessential genes for differentiation are turned off. Thus, B. subtilis presents a good model organism to study these physiological mechanisms in complex lifestyles.
Biofilm formation is a developmental process in which bacteria switch from a free-living to a surface-associated multicellular state, and subsequent growth results in three-dimensional structured communities composed of different cell types (1–3). This complex structure is supported by a self-produced extracellular matrix that surrounds the cells (4). The matrix is not only responsible for adhesion of planktonic cells to solid environmental surfaces, adhesion of cells in the biofilm, and stabilization of the three-dimensional biofilm architecture but also serves as a nutrient source to provide carbon-, nitrogen-, and phosphorus-containing compounds for utilization by the biofilm community (5, 6). Biofilm formation is a serious problem during infection by pathogenic bacteria, because the matrix plays a central role in protection from host defenses and various antimicrobial agents (7–9). The matrix additionally functions as a signal to trigger sporulation in B. subtilis, allowing matrix-producing cells to continue sporulation development at the tips of aerial structures in mature biofilms (10).
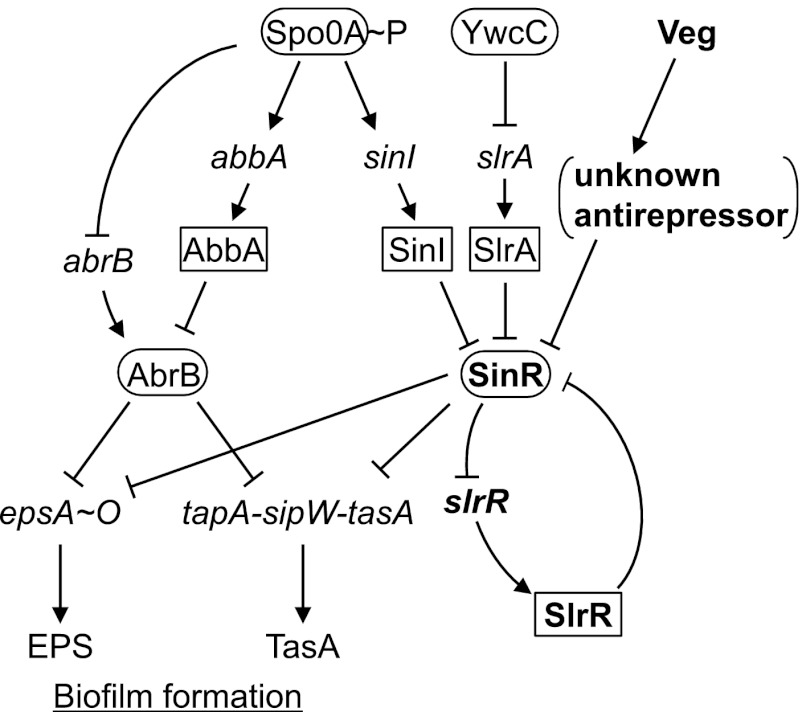
In B. subtilis, an extracellular polysaccharide (EPS) synthesized from the epsA-epsO (epsA-O) 15-gene operon (eps operon) and amyloid fiber formed by the TasA protein, encoded by the tapA-sipW-tasA operon (tasA operon), bind cells together in biofilm and constitute the primary components of the extracellular matrix (11). B. subtilis matrix production during biofilm formation is governed by multiple physiological and population signals. AbrB and SinR, the main repressors, bind promoters of the eps and tasA operons independently to block biofilm formation (12–14) (Fig. 1). These repressors are under the negative control of phosphorylated Spo0A, the key factor in the decision-making process for spore formation or biofilm formation (15, 16). Activation of Spo0A via phosphorylation (Spo0A∼P) suppresses expression of abrB and induces the antirepressor, AbbA, which binds to AbrB and prevents interactions with DNA (17, 18). On the other hand, the small protein antagonist of SinR, SinI, which lacks the N-terminal DNA binding domain but contains a C-terminal oligomerization domain similar to that of SinR, binds directly to SinR via protein-protein interactions to derepress the SinR regulon, and its transcription is directly switched on by Spo0A∼P. In addition to the Spo0A/SinI pathway, YwcC/SlrA, another pathway of SinR regulation, has been identified (19–21). YwcC is a TetR-type repressor that suppresses the divergently transcribed slrA gene, while SlrA is a SinI paralog that functions as another antirepressor of SinR. Based on observation that mutations of late flagellar genes, such as motA, affect expression of SlrR/SlrA-regulated genes, the pathway is proposed to be related to flagellum-associated regulation (21), although the precise underlying mechanisms remain to be clarified.
Fig 1.
Regulatory pathways for biofilm formation in B. subtilis. The arrows indicate activation, and T bars indicate repression. Contributions of AbrB and SinR to transcriptional repression of the eps and tasA operons are indicated with T bars. Transcriptional regulators and antirepressors are presented in ellipses and squares, respectively.
Furthermore, SinR activity is regulated by SlrR (Fig. 1). SlrR is a SinR homolog composed of both the C-terminal oligomerization domain and the N-terminal DNA-binding domain. Its transcription is repressed by SinR (12, 21, 22). Importantly, when slrR expression is derepressed via inactivation of SinR by SinI and/or SlrA, induced SlrR binds to SinR and inhibits the ability of SinR to bind to the control region of slrR. Thus, SinR, slrR, and SlrR form a self-reinforcing, double-negative feedback loop in which SlrR antagonizes SinR, thereby stimulating derepression of slrR (Fig. 1) (23). SlrR is additionally involved in the regulation of motility and cell separation as a repressor via direct interactions with regulatory regions of the hag, lytABC operon, and lytF genes, although these interactions are substantially stimulated by the presence of SinR (23).
The B. subtilis veg gene, encoding a small, conserved protein, is transcribed at high levels during both exponential growth and sporulation and thus widely applied for investigation of the transcription mechanism (24, 25). A previous study showed that the Veg protein localizes on the entire nucleoid, and its disruption results in delayed spore germination, although the exact function is yet to be elucidated (26). Interestingly, according to the MBGD database (27), veg is highly conserved in two phyla, Firmicutes (low-GC Gram-positive bacteria) and Synergistetes (anaerobic Gram-negative bacteria), as well as a family of Actinobacteria (Coriobacteridae), indicating that conservation of veg is not restricted to spore-forming bacteria (such as Bacillus and Clostridium) but also extends to non-spore-forming bacteria (Lactobacillus and Synergistetes). This finding further suggests an important role of Veg during growth phases other than sporulation.
In the present study, we demonstrated that Veg acts as an additional regulatory protein that contributes to the control of matrix production. Our results indicate that Veg stimulates biofilm development via transcriptional activation of matrix genes through repression of SinR activity independently of the SinI, SlrA, and SlrR pathways.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The B. subtilis strains used in the present study, specifically, the laboratory strain 168 BFA, undomesticated wild-type strain NCIB3610, and derivatives, are listed in Table S1 in the supplemental material. The procedures used to construct mutant strains are described in Methods in the supplemental material. Plasmids and primers used in the present study are listed in Tables S2 and S3, respectively. The Escherichia coli strains DH5α and C600 were used for plasmid construction. All bacterial strains were grown in Luria-Bertani (LB), MSgg (11), or Spizizen's minimal medium (SMM) (28) at 37°C or 30°C, as indicated. Antibiotics were used at the following concentrations: ampicillin, 50 μg ml−1; chloramphenicol, 5 μg ml−1; kanamycin, 10 μg ml−1; spectinomycin, 100 μg ml−1; tetracycline, 10 μg ml−1; erythromycin plus lincomycin, 0.5 μg ml−1 and 25 μg ml−1, respectively.
Assay of colony and pellicle formation.
Strains were grown in LB to an optical density at 600 nm (OD600) of 1.0. For the colony assay, 3 μl of culture was spotted onto LB, SMM, or MSgg agar (1.5% agar), and plates were incubated at 30°C for various times. For the pellicle assay, 9 μl of culture was added to 9 ml of MSgg medium contained within a well of a 6-well microtiter dish (BD Falcon), and the dishes were incubated without agitation at 30°C for indicated times. Photographs were obtained using a Nikon camera (D70S).
β-Galactosidase assay.
To monitor β-galactosidase activity in liquid medium, cells were grown in LB at 37°C with shaking, and 1 ml of culture was collected to the determine specific β-galactosidase activity, as described previously (29). To monitor β-galactosidase activity on solid medium, colonies were grown on MSgg plates containing 40 μg ml−1 X-Gal (5-bromo-4-chloro-3indolyl-β-d-galactopyranoside) at 30°C. Images were obtained using an Epson scanner (GT-X900) or a Nikon camera (D70S).
Antibody production.
Anti-Veg antibody was raised in rabbits via injection of purified Veg-12×His (Veg protein fused to 12 histidine residues) prepared from E. coli DH5α cells harboring pO-veg12His, as described in a previous report (13).
Western blot analysis.
Cells were grown in SMM to an OD600 of 0.4 to 0.5, and 10 ml culture was harvested by centrifugation at 6,000 × g for 5 min. Cells were washed once with 1 ml chilled killing buffer (30), resuspended in lysis buffer (25 mM Tris-HCl [pH 8.0], 10 mM EDTA, 50 mM glucose, 0.1 mg ml−1 lysozyme, and 1 mM phenylmethylsulfonyl fluoride [PMSF]), and incubated for 10 min at 37°C. The cell lysate was separated on a precast polyacrylamide gel, specifically, a 16% (wt/vol) Novex Tricine gel (Life Technologies) for SinR/SlrR detection and 10 to 20% (wt/vol) SuperSep Ace gel (Wako) for Veg detection and transferred to Immobilon-PSQ membranes (Millipore) for 1 h at 10 V in a semidry transfer apparatus (Bio-Rad). Transferred proteins were examined by incubation with anti-SinR antiserum (a generous gift from R. Losick [20]) at a 1/8,000 dilution or anti-Veg antibody (1/20,000 dilution) as the primary antibody, followed by secondary anti-rabbit (1/8,000 dilution) conjugated to horseradish peroxidase. Detection of proteins was performed using the ECL-Plus system (Amersham), followed by exposure to X-ray film.
In vivo pulldown assay of protein complexes.
To cross-link proteins, cells were treated with formaldehyde (0.1%, final concentration) for 30 min at an OD600 of 0.4 to 0.5 and stored at −80°C after washing twice with Tris-buffered saline (50 mM Tris-HCl [pH 7.5] and 0.15 M NaCl). Cells were disrupted by sonication on ice using an Astrason ultrasonic processor XL (Misonix) for 10 min (4 s “ON” and 10 s “OFF” at an output level of 5.0) in 3 ml of UT buffer (0.1 M HEPES, 0.5 M NaCl, 10 mM imidazole [pH 7.5], 8 M urea, 1% [vol/vol] Triton X-100, and 1 mM PMSF). After centrifugation at 6,000 × g for 15 min at 4°C, 100 μl of MagneHis beads (Promega) were added to the supernatant, followed by overnight incubation at room temperature with gentle rotating. MagneHis beads were washed five times with 1.5 ml UT buffer. Bound proteins were eluted with elution buffer (0.1 M Tris-HCl [pH 7.5], 0.5 M imidazole [pH 7.5], 1% [wt/vol] SDS, and 10 mM dithiothreitol [DTT]). Cross-linked proteins were dissociated by heating for 40 min at 90°C and separated on a 16% (wt/vol) Tricine gel, followed by Western blotting as described above.
RESULTS
Biofilm formation is stimulated by overproduction of Veg and inhibited in its absence.
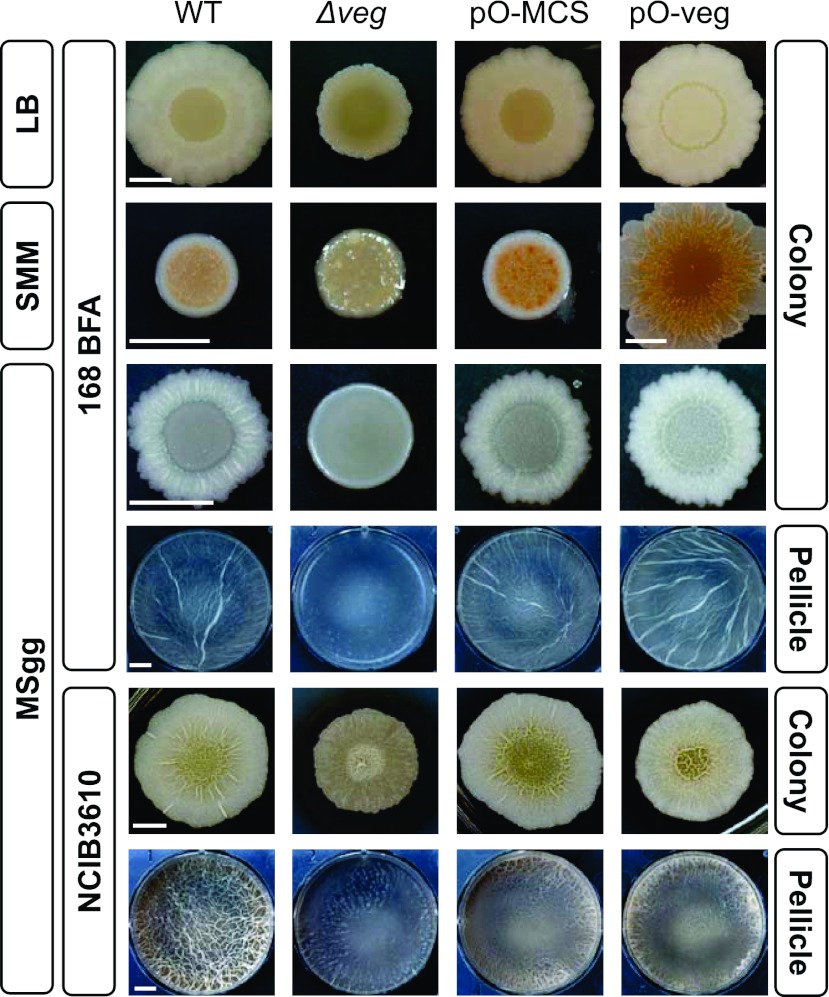
To explore the function of Veg, we created its deletion mutant using B. subtilis strain 168 BFA (31), which has been used as a wild-type strain in our laboratory. The growth rate and cell morphology of the veg deletion mutant (Δveg) were similar to those of the parent strain. Interestingly, although veg is highly transcribed, the translated protein in wild-type cells was barely detectable with Western blot analysis owing to rapid degradation (see Fig. S1A in the supplemental material see also Fig. 6). Accordingly, we constructed a Veg-overexpressing strain harboring the multicopy plasmid pO-veg, in which expression is inducible by isopropyl-β-d-thiogalactopyranoside (IPTG). The Veg protein became detectable in Veg-overexpressing cells grown in rich (LB), minimum (SMM), or biofilm-forming (MSgg) medium containing IPTG (see Fig. S1B). Interestingly, the colony morphology of this strain was distinguishable from that of the parent strain. Thus, we speculated that Veg might be involved in biofilm formation.
Assessment of colony morphology revealed for the first time a clear phenotype of the Δveg mutant, deficient in steric structure on agar plates containing LB, SMM, or MSgg medium compared to that of the parent strain (Fig. 2). On the other hand, overproduction of Veg seemed to enhance biofilm formation on the agar plates compared to those of the parent and a strain harboring a control plasmid without the veg gene insert (pO-MCS), although the effect was not clear compared to that of veg deletion.
Fig 2.
Effects of Veg inactivation and overproduction on the architecture of colonies and pellicles. Wild-type (WT) (168 BFA or NCIB3610), Δveg (LY011 [168 BFA harboring Δveg] or LY048 [NCIB3610 harboring Δveg]), pO-veg (LY016 [168 BFA harboring pO-veg] or LY058 [NCIB3610 harboring pO-veg]) and pO-MCS (LY015 [168 BFA harboring pO-MCS] or LY057 [NCIB3610 harboring pO-MCS]) strains were grown in the indicated media for 72 h (168 background strains) or 48 h (NCIB3610 background strains) at 30°C. The LY015, LY016, LY057, and LY058 strains were grown in the presence of 1 mM IPTG. Scale bar, 0.5 cm.
We additionally investigated the formation of pellicle, another biofilm form that develops at the liquid-air interface of standing cultures (32). Pellicle formation at the surface of a standing MSgg culture was severely impaired in the Δveg strain, while the effect of Veg overexpression on pellicle formation was again not clear (Fig. 2). It is possible that expression levels of biofilm formation genes are already elevated under the biofilm-forming condition and thus their further increase was not induced by Veg overproduction.
Some laboratory 168 strains have been reported to lose the ability to form clear biofilm architecture compared to findings for the undomesticated wild-type NCIB3610 strain (31–33). Therefore, we examined biofilm formation using strains derived from the NCIB3610 strain (Fig. 2). In the NCIB3610 genetic background, the Δveg mutant strain also showed reduced biofilm formation on MSgg plates and pellicle formation in standing culture, albeit to a moderate extent compared to that of the 168 BFA-derived strain. These results demonstrate that Veg impacts biofilm formation, even in the NCIB3610 genetic background. However, the clear inhibition of biofilm formation observed in the 168 BFA-derived Δveg strain appeared to be partly masked by strong biofilm formation ability in the NCIB3610 genetic background. In addition, introduction of the control plasmid (pO-MCS) resulted in reduced pellicle formation, and thus, the precise effects of Veg overproduction could not be examined (Fig. 2). Although the exact reason is unclear at present, it is proposed that the pO-MCS plasmid acts negatively on the 80-kb plasmid harboring a gene related to colony morphology in the NCIB3610 strain (31). Thus, in subsequent experiments, we used derivatives of the 168 BFA strain.
Transcription of the tapA-sipW-tasA operon is reduced in the absence of Veg and induced by its overproduction.
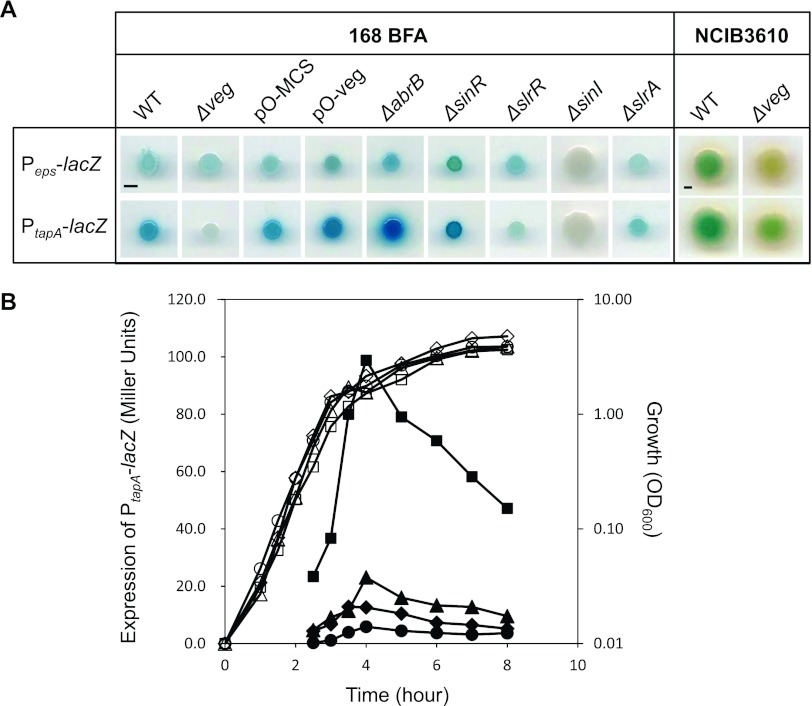
To determine the mechanisms of involvement of Veg in biofilm formation, transcriptional activities of the promoters of biofilm extracellular matrix operons, epsA-O (eps) and tapA-sipW-tasA (tasA), were examined on solid MSgg medium containing X-Gal (Fig. 3A). We introduced into the 168 BFA strain the lacZ gene under the control of Peps or PtapA at the amyE locus (amyE::Peps-lacZ or amyE::PtapA-lacZ) to monitor expression of eps and tasA operon promoters and epsH deletion mutation to suppress the aerial structure that interferes with color comparison in the colonies (34). The Δveg mutation, Veg overexpression (pO-veg), and control plasmid (pO-MCS) were introduced into the resultant strains. For comparison, LacZ activities in deletion mutants of known biofilm regulators, abrB, sinR, sinI, slrA, and slrR, were examined.
Fig 3.
Overproduction and deletion of Veg induces and suppresses transcription of the tasA operon. (A) Expression of Peps-lacZ and that of PtapA-lacZ were compared after cultivation for 72 h (168 BFA background strains) or 24 h (NCIB3610 background strains) at 30°C on solid MSgg medium containing X-Gal and 1 mM IPTG, for strains with a mutation(s) and/or plasmid, indicated at the top of each picture. All strains contain an epsH mutation to abolish cell aggregation, and strains in the top and bottom panels have insertion of Peps-lacZ and PtapA-lacZ at the amyE loci, respectively. Scale bar, 0.2 cm. (B) Growth and expression of PtapA-lacZ in wild-type (LY032, diamonds), Δveg (LY041, circles), pO-veg (LY039, squares) and pO-MCS (LY038, triangles) strains in LB liquid medium, indicated with open and closed symbols, respectively, were measured. The LY036, LY037, LY038, and LY039 strains were grown in the presence of 1 mM IPTG.
Since the master repressors, AbrB and SinR, act in an additive manner to repress matrix operons (12), intensity of the blue color of colonies containing the PtapA-lacZ and Peps-lacZ reporters in the ΔsinR and ΔabrB strains increased, indicating derepression of eps and tasA in the absence of SinR or AbrB (Fig. 3A). In addition, colonies containing either of the reporters were not colored in the mutant carrying a deletion of sinI, encoding an antagonist of SinR. Thus, it appears that deletion leads to constitutive repression of these operons (14, 35, 36). The ΔslrA strain color for the PtapA-lacZ reporter was slightly weaker (Fig. 3A), indicating that SlrA plays a minor role in suppressing the expression of matrix genes, as observed in the NCIB3610 strain (20). SlrR has been shown to induce transcription of tasA but not the eps operon (12). Consistently, the ΔslrR strain containing the Peps-lacZ reporter was faint blue in color, similar to the wild-type background, while PtapA-lacZ expression was markedly impaired. Thus, effects of transcriptional regulators on the eps and tasA operons appear to be essentially similar in both 168 BFA and NCIB3610 strains. Consistent with the finding that the inactivation of Veg impairs biofilm formation, its deletion resulted in severe blockage of PtapA-lacZ expression, although that of Peps-lacZ was not clearly detected (Fig. 3A). Conversely, its overexpression enhanced the expression of PtapA-lacZ to some extent (Fig. 3A; see also Fig. S3 in the supplemental material).
To quantitatively evaluate the effects of Veg on transcriptional activity of the tasA operon from exponential phase to stationary phase, expression levels of PtapA-lacZ were measured in the Veg-overproducing and deletion strains. Liquid LB medium was selected, since tasA is not highly induced in LB, and the effect was thus expected to be clearly detectable (16). Consistent with the LacZ plate assay data, transcriptional activity of tasA in LB liquid medium was dramatically increased upon overproduction of Veg from the exponential growth phase (Fig. 3B). On the other hand, tasA expression in the Δveg mutant was decreased, but only to a slight extent, since tasA is induced at a very low level in LB liquid medium (Fig. 3B). Based on these results, we conclude that Veg promotes biofilm formation on MSgg plates through transcriptional activation of the tasA operon, while prevention of biofilm formation in the Δveg strain is caused mainly by a transcriptional block of the tasA operon.
We additionally examined the involvement of Veg in regulation of expression of matrix genes in the NCIB3610 strain. We found that expression levels of both Peps-lacZ and PtapA-lacZ were reduced by the veg deletion (Fig. 3A). It should be noted that although expression of Peps-lacZ was not affected in the 168 BFA strain by veg deletion or overexpression, it is reduced by the veg deletion in the NCIB3610 strain.
Veg induces biofilm formation independently of AbrB.
Biofilm formation is governed by two master repressors, AbrB and SinR. Accordingly, we investigated whether Veg regulates biofilm formation via inhibition of either of these repressors. To this end, we examined effects of veg deletion and overexpression on colony architecture and Peps-lacZ and PtapA-lacZ expression in mutants of regulators for biofilm formation (Fig. 4). In addition, effects on pellicle formation were also examined (see Fig. S2 in the supplemental material).
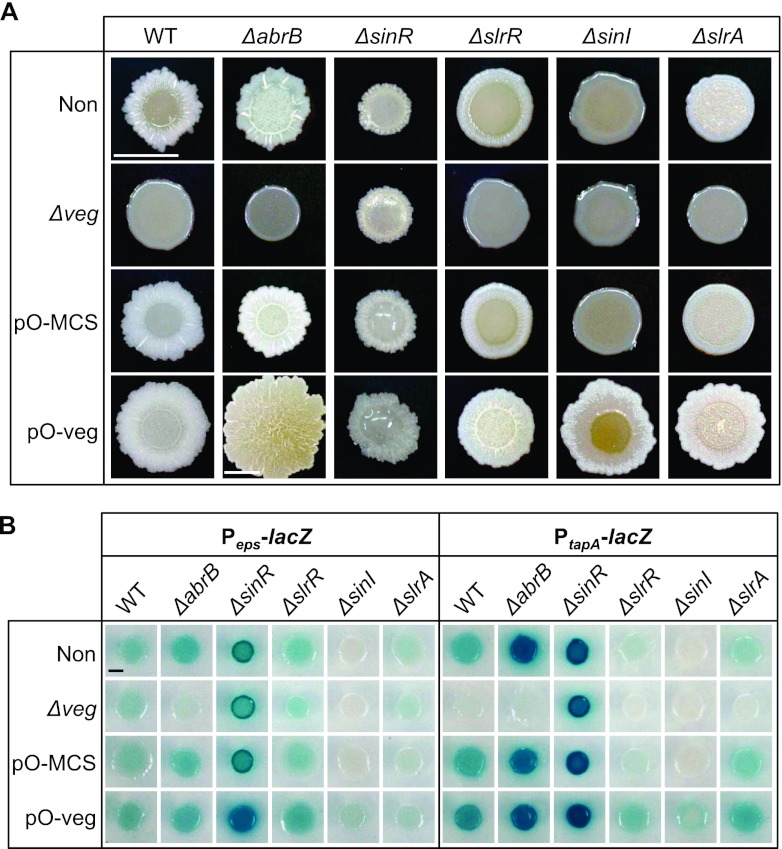
Fig 4.
Comparison of the architecture of colonies formed by mutants of regulators for biofilm formation and the transcription of the tasA and eps genes. (A) Colonies of strains with a mutation(s) and/or plasmid, indicated at the top and the left side of each picture, were formed on solid MSgg medium for 48 h at 30°C. Strains harboring a plasmid were cultured in the presence of 1 mM IPTG. Scale bar, 0.5 cm. (B) Expression of Peps-lacZ and that of PtapA-lacZ were compared after cultivation for 48 h at 30°C on solid MSgg medium containing X-Gal and 1 mM IPTG. All strains contain an epsH mutation, and strains in the left and right panels have insertion of Peps-lacZ and PtapA-lacZ at the amyE loci, respectively. Scale bar, 0.2 cm.
On biofilm-forming medium, expression of Peps-lacZ and PtapA-lacZ was increased in the ΔabrB strain (Fig. 4B). Also, the center of the colony showed more complicated structure than was seen with the parent strain (Fig. 4A), although modulation of pellicle formation was not clearly observed (see Fig. S2 in the supplemental material). In contrast, both transcription of the eps and tasA operons and biofilm formation on solid and liquid media were suppressed upon additional deletion of veg (Fig. 4; see also Fig. S2). These results are similar to the genetic relationship previously reported in the ΔabrB-ΔsinI double mutant (36). They strongly suggest that Veg induces biofilm formation through upregulation of matrix genes independently of AbrB. The effect of Veg on Peps-lacZ expression was not detected in the presence of AbrB, suggesting that Veg function in regulation of the eps promoter was masked by AbrB in the Δveg strain.
The effect of Veg overproduction was examined by comparing mutants harboring the control plasmid (pO-MCS) to mutants carrying the Veg-overexpressing plasmid (pO-Veg) (Fig. 4; see also Fig. S2 in the supplemental material). For unknown reasons, the ΔabrB strain harboring the control plasmid showed a different colony morphology and a decrease in PtapA-lacZ expression compared to the parent strain. When a Veg-overexpressing plasmid was introduced into the ΔabrB strain, its colony size doubled and the surface of the colony was colored compared to findings for the ΔabrB strain harboring the control plasmid (Fig. 4A). However, the effect of Veg overproduction on expression of matrix genes was not evident (Fig. 4B). It is possible that slightly increased matrix gene expression undetectable by the LacZ assay leads to the alteration of colony morphology. However, it seems more plausible that in the absence of AbrB, expression levels of matrix genes are already elevated close to the maximum level on the biofilm-forming medium and thus their further increase was not induced by Veg overproduction. If this hypothesis is correct, Veg might affect colony morphology in the absence of AbrB through direct regulation of an unknown gene(s), as discussed later.
We additionally examined the effects of Veg overexpression on other AbrB-regulated genes by comparing genome-wide transcriptional profiles in both overexpressing and control strains during the mid-exponential phase using the Affymetrix tiling array as described in Methods in the supplemental material. Transcriptome analysis revealed that transcription of the eps and tasA operons, which is not induced in the control strain, was induced (see Table S4), while other AbrB regulons were not affected by Veg overexpression in exponentially growing cells (see Table S5). These results further indicate that Veg has an ability to induce matrix genes independently of AbrB.
Veg induces biofilm formation through inhibition of SinR activity.
Next, we examined the possibility that Veg inhibits the ability of SinR to suppress expression of the eps and tasA operons, either directly or indirectly. In the absence of SinR, transcriptional levels of the eps and tasA operons were increased, leading to highly structured colonies and pellicles in the NCIB3610 genetic background (36). In the 168 BFA background, the ΔsinR deletion also enhanced expression of these matrix genes (Fig. 4B). However, unexpectedly, the induction of matrix gene expression by the ΔsinR deletion did not enhance the biofilm formation in the 168 BFA background. Instead, the ΔsinR strain formed a smaller colony than the parent strain, having a smaller structured periphery and being covered by transparent mucoid on the central area (Fig. 4A). Furthermore, pellicle formation was impaired (see Fig. S2 in the supplemental material). While the precise reason for this phenotype of the ΔsinR mutant in the 168 BFA background is unclear, it is possible that this phenotype is related to mutations of sfp, swrA, and degQ in the 168 strain that impair not only biofilm formation but also swarming motility (31). For example, the phenotype may be caused by further impairment of motility enhanced by sinR deletion even in the presence of increased matrix materials (36, 37). Downsizing of the colony was also observed in the reporter strains and affected intensities of the blue color of the colonies. To avoid this problem, we compared the color intensities of ΔsinR strains after cultivation for 24 h before the time of the apparent morphological change among the strains (see Fig. S3).
Next, we compared the phenotypes of the ΔsinR and ΔsinR-Δveg double mutants. Colony morphology of the double mutant was similar to that of the ΔsinR mutant (Fig. 4A), indicating that the deficiency of biofilm formation in the absence of Veg is partially restored by the additional deletion of sinR through derepression of transcription of eps and tasA operons (Fig. 4). Conversely, expression levels of eps and tasA operons and colony morphology in the ΔsinR mutant were not affected by additional inactivation or overproduction of Veg (Fig. 4). Additional inactivation or overproduction of Veg had no impact on the pellicle formation of the ΔsinR mutant, although pellicle formation was stimulated by introduction of the control plasmid (see Fig. S2 in the supplemental material). These results strongly suggest that Veg is involved in negative control of the SinR activity.
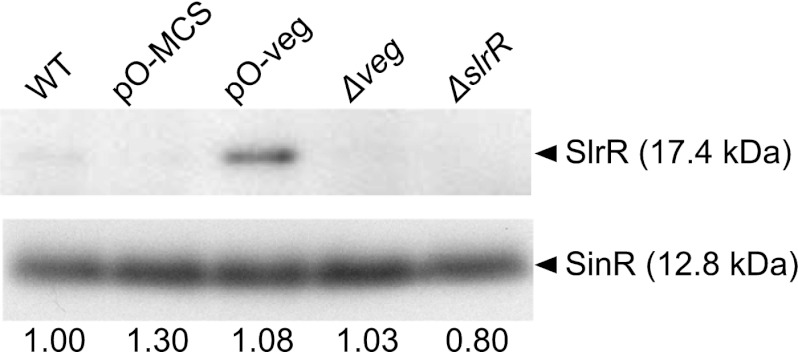
Our transcriptome results showed that sinR expression is not affected in Veg-overproducing cells (see Table S6 in the supplemental material). Furthermore, wild-type, Δveg, Veg-overexpressing, and control strain cells displayed similar protein levels during the exponential growth phase (Fig. 5). Interestingly, SlrR, whose transcription is blocked by SinR under these conditions, was induced only upon overproduction of Veg (Fig. 5). Consistently, slrR expression was elevated in Veg-overexpressing cells (see Table S6). Thus, all biofilm-associated genes repressed by SinR (not only the eps and tasA operons but also slrR) were derepressed upon overexpression of Veg, with no corresponding alterations in the SinR levels, strongly suggesting that Veg inhibits the repressor activity of SinR either directly or indirectly.
Fig 5.
Induction of SlrR upon overproduction of Veg. WT (168 BFA), Δveg (LY011), pO-veg (LY016), pO-MCS (LY015) and ΔslrR (LY013) strains were grown to mid-logarithmic phase in SMM at 37°C, and SinR in cells equivalent to 0.1 OD600 units was detected by Western blotting with an anti-SinR antibody that cross-reacts with SlrR (20). The LY015 and LY016 strains were grown in the presence of 1 mM IPTG. The signal intensities of SinR bands on X-ray films were quantified using the NIH-Imagine software program, and the signal intensities relative to that of WT cells are shown below the bands.
Veg inhibits SinR activity independently of known antirepressors.
SinI, SlrA, and SlrR are antirepressors of SinR which exert their activities via protein-protein interactions (12, 20, 21, 36). To determine the relationship between Veg and known antirepressors, we next determined whether the Veg-induced increase in transcription of slrR is responsible for inhibition of SinR activity. Deletion of slrR led to partial biofilm-deficient phenotypes, formation of weak structured colonies, and thin and flat pellicles, possibly owing to the remaining inhibitory activities of SinI and SlrA on SinR (Fig. 4A; see also Fig. S2 in the supplemental material). Similar to the Δveg mutant, a double mutant carrying ΔslrR and Δveg showed reduced expression of PtapA-lacZ and exhibited a more severe biofilm-deficient colony morphology than the ΔslrR mutant (Fig. 4). Conversely, overexpression of Veg induced expression of Peps-lacZ and PtapA-lacZ and rescued the biofilm-deficient phenotype of the ΔslrR strain, forming a more complicated colony structure and hyperwrinkled pellicles (Fig. 4; see also Fig. S2). These results indicate that Veg inhibits SinR activity independently of SlrR.
Deletion of SinI leads to a more severe biofilm-deficient phenotype than single deletion of other antirepressors of SinR (34, 36). Consistently, the ΔsinI mutant strain showed the most severe biofilm-deficient phenotype, i.e., transcription of the eps and tasA operons was almost completely blocked (Fig. 4B), thus forming smooth and featureless colonies and no pellicles (Fig. 4A; see also Fig. S2 in the supplemental material). Overproduction of Veg derepressed the expression of Peps-lacZ and PtapA-lacZ (Fig. 4B) and partially restored biofilm deficiency of the ΔsinI mutant, leading to the formation of a complicated structure in the peripheral zones of colonies and thin pellicles (Fig. 4A; see also Fig. S2). These results imply that Veg functions independently of SinI.
Consistent with the results of the LacZ assay for matrix genes (Fig. 4B), deletion of slrA impaired biofilm formation mildly to form a colony with moderate structure and thick but less wrinkled pellicles (Fig. 4A; see also Fig. S2). The ΔslrA Δveg double mutant showed severely reduced expression of PtapA-lacZ (Fig. 4B) and displayed severely impaired colony and pellicle morphology, similar to the Δveg mutant (Fig. 4A; see also Fig. S2). Overexpression of Veg derepressed the expression of PtapA-lacZ (Fig. 4B) and bypassed the biofilm formation deficiency in ΔslrA cells (Fig. 4A), indicating that Veg inhibits SinR activity independently of SlrA.
Based on the collective results, we conclude that Veg controls SinR activity not through effects on the known antirepressor SlrR, SinI, or SlrA but via a novel, unknown pathway.
Examination of physical interactions between Veg and SinR.
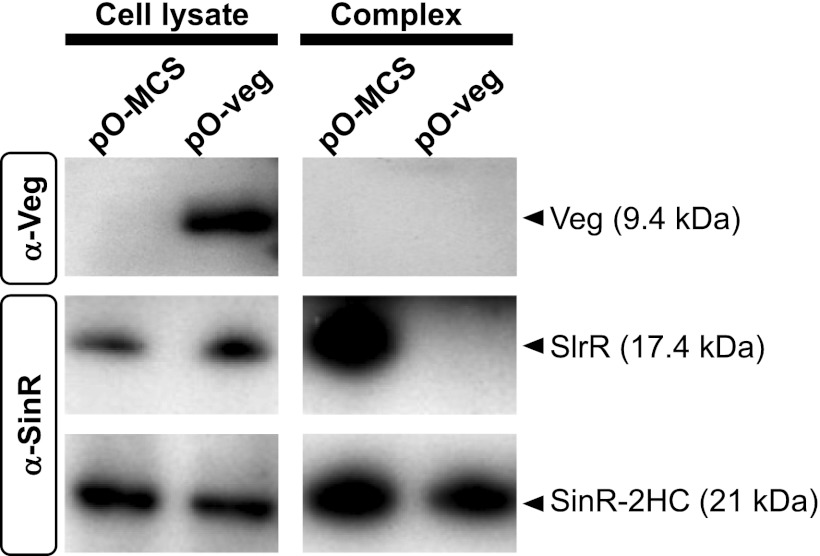
While our results showed that SinR-repressed genes, including tapA-sipW-tasA and slrR, are induced by Veg independently of antirepressors on MSgg medium, it was unclear whether SinR activity is directly or indirectly inhibited by Veg. To resolve this issue, proteins interacting with SinR in Veg-overproducing and control cells were examined. In brief, cells expressing SinR-2HC (SinR C-terminally tagged with 12 histidines plus a chitin-binding domain [8.2 kDa, total]) were grown to mid-exponential phase in minimal medium and treated with formaldehyde to stabilize complexes among proteins, followed by affinity purification of SinR complexes, as described previously (38). Notably, the expression level of SlrR seemed to be higher in SinR-2HC-expressing cells (Fig. 6) than in 168 BFA cells (Fig. 5), suggesting that repressor activity of SinR is partly impaired by the C-terminal 2HC-tag fusion. However, the SinR-2HC-expressing strain showed a colony morphology intermediate between those observed for the wild-type and ΔsinR strains (see Fig. S4 in the supplemental material), indicating that SinR-2HC retained the repressor activity, albeit partially. Upon examination of SinR complexes with the anti-Veg antibody, Veg was not detected despite its clear presence in the Veg-overexpressing cells (Fig. 6), suggesting that Veg does not interact directly with SinR. Interestingly, SlrR was clearly detected in the SinR complex purified from the control cells but not in the complex purified from the Veg-overexpressing cells, suggesting that Veg prevents SlrR binding to SinR directly or indirectly (Fig. 6).
Fig 6.
Examination of physical interactions between Veg and SinR. SinR-2HC harboring plasmid pO-veg (LY085) or pO-MCS (LY084) was grown to mid-logarithmic phase in SMM medium at 37°C in the presence of 1 mM IPTG. SinR-2HC complexes were purified as described in Materials and Methods and detected by Western blotting, together with crude cell lysates, using anti-Veg (α-Veg) and anti-SinR (α-SinR) antibodies. SinR complexes and crude cell lysates equivalent to 0.8 and 0.1 OD600 units, respectively, were applied on SDS-PAGE gels.
DISCUSSION
In the present investigation, comprehensive analysis of the genetic interactions of regulators of biofilm formation in B. subtilis revealed that Veg stimulates biofilm formation by inhibiting SinR repressor activity to induce gene expression for matrix synthesis. Additionally, SinR activity is inhibited by Veg through a novel pathway that is independent of the three known antirepressors, SlrR, SinI, and SlrA.
Biofilm morphology in the absence of SinR was not affected by either veg deletion or overproduction, whereas the biofilm-deficient phenotype of the sinI, slrA, or slrR deletion mutant was partially restored by Veg overproduction, supporting the theory that Veg controls the ability of SinR to induce biofilm formation independently of the antirepressors. This conclusion was further supported by transcriptome analysis showing that transcription of the Spo0A regulon, including not only sinI and abrB but also other genes, such as spoIIGA-sigE, spoIIE, and spoIIAA-spoIIAB-sigF, involved in sporulation (39), was not affected by overexpression of Veg (see Table S5 in the supplemental material). Earlier studies have reported that transcription of slrA is regulated by YwcC (20, 21), and increased transcription induces the genes required for biofilm formation and reduces the entire σD regulon (40). However, in our experiments, Veg overproduction did not alter expression of ywcC, slrA, or the σD regulon (see Tables S5 and S6) (41). These results collectively suggest that Veg controls SinR activity through a novel mechanism independent of the Spo0A-sinI-SinI and YwcC-slrA-SlrA pathways (Fig. 1).
We found that not only deletion of veg but also single deletion of slrR and slrA specifically reduce transcription of the tasA operon but not that of the eps operon (Fig. 3A and 4B). Thus, the differential effect on the expression of matrix genes will be common for antirepressors of SinR. On the other hand, transcriptome analysis demonstrated that Veg overexpression induces transcription of the eps and tasA operons in cells exponentially growing in SMM medium. Furthermore, we found that veg deletion decreases both Ptas-lacZ and Peps-lacZ expression in the ΔabrB mutant. These results may suggest that transcriptional regulation of biofilm extracellular matrix genes is modulated by the expression levels of regulators. Further systematic and quantitative expression analysis of regulators involved in biofilm formation is necessary to understand the complex regulatory system for biofilm formation.
Expression of sinR was not affected in Veg-overproducing cells (see Table S6 in the supplemental material), and SinR levels were similar in cells expressing different levels of Veg (Fig. 5), strongly suggesting that Veg modulates the repressor activity of SinR. Interestingly, although Veg was not detected in the SinR complex purified from Veg-overproducing cells, interactions between SinR and SlrR were inhibited upon overexpression of Veg. These results suggest that an unknown antirepression factor for SinR that competes with SlrR via direct binding to SinR is induced or activated by Veg. However, considering that Veg is extremely unstable, it is possible that the protein interacts directly with SinR and is degraded due to proteolysis during the purification process.
The veg gene is constitutively transcribed at a high level, during not only the exponential phase but also the stationary phase, in rich (LB) and minimum (SMM) media (42). Our experiments showed that although the Veg protein is extremely unstable (see Fig. S1 in the supplemental material) and undetectable on a Western blot (Fig. 6) at the exponential phase, increasing Veg expression to a detectable level leads to induction of biofilm matrix genes (Fig. 3; see also Fig. S1B). The results suggest that rather than transcriptional induction of the veg gene, stabilization of the Veg protein leads to activation of genes that promote biofilm development. However, experimental demonstration of this idea is unsuccessful at the moment. The Veg protein has not been specifically detected as a band distinguishable from nonspecific proteins for stationary-phase cells grown in LB, SMM, and MSgg media.
Thus, it is expected that activity of the protease(s) responsible for Veg degradation is decreased during the biofilm development process to regulate biofilm formation in response to an unknown signal(s). In B. subtilis, protease-dependent regulation of activity has been identified for the Spx protein. Spx, a known global regulator that controls transcription through interactions with the C-terminal domain of the RNA polymerase α subunit (α-CTD), is maintained at very low levels by activity of the ClpXP protease (43). However, under disulfide stress conditions, the Spx concentration increases, partly due to a reduction in ClpXP-catalyzed proteolysis, to activate genes for detoxification of stress and induce Spx-dependent transcriptional repression. Although the protease(s) involved in proteolysis of Veg are yet to be identified, one possible explanation is that in order to survive stress conditions, an unknown signal stabilizes the Veg protein to stimulate biofilm formation. The specific protease(s) contributing to Veg instability and times of Veg stabilization are under investigation.
Transcriptome analysis results showed that in addition to biofilm matrix genes (the eps and tasA operons), several genes in the prophage regions of PBSX and SPβ are induced by Veg (see Table S4 in the supplemental material). Interestingly, the same gene clusters on PBSX are highly expressed in biofilm-forming cells (44) but are not induced in sinR deletion mutant cells (34). Our results suggest that during biofilm formation, the gene cluster on PBSX may be induced in a Veg-dependent, SinR-independent manner. Earlier reports have demonstrated that phage genes are induced in a Pseudomonas aeruginosa biofilm and have proposed that phage induction participates in gene transfer or exclusion of other strains (45). Although the exact biological roles of phage-related genes in B. subtilis cells have not been established yet, one possibility is a function similar to that reported for P. aeruginosa. Another proposal is that restricted phage-related genes are induced to develop a defense machinery to protect against phage infection for survival. Furthermore, Veg has been shown to play an important role during the sporulation phase in mature spore development for normal germination (26). Thus, Veg appears to function in the regulation of other genes in addition to SinR.
Finally, the fundamental question of whether Veg activates target proteins through induction of expression or modulates activities through protein-protein interactions remains to be answered. Our preliminary chromatin immunoprecipitation (ChIP)-chip analysis failed to detect specific Veg binding sites on the genome. Thus, interactions of Veg with DNA, if any, are possibly nonspecific. Proteome analyses to identify the proteins interacting with Veg have not been successful to date.
In summary, in addition to the two known pathways, Spo0A-sinI-SinI and YwcC-slrA-SlrA, we have identified a novel third pathway triggered by Veg to induce biofilm formation (Fig. 1). All three pathways act in parallel, leading to inhibition of SinR to stimulate a self-reinforcing double-negative feedback loop of SinR-slrR-SlrR and derepress the SinR-repressed eps and tasA operons, which facilitates biofilm development. Moreover, we suggest that Veg is involved in the regulation of genes other than SinR during biofilm and spore formation, although further studies are necessary to elucidate the entire spectrum of Veg-induced gene activities.
Supplementary Material
ACKNOWLEDGMENTS
We thank Y. Chai, F. Chu, D. B. Kearns, S. S. Branda, R. Kolter, and R. Losick for the anti-SinR antibody and B. subtilis lacZ strains and M. Yoshimura for sharing the pCm::Em and pTc::Sp plasmids.
This work was supported by the Advanced Low Carbon Technology Research and Development Program (ALCA) of the Japan Science and Technology Agency (JST). N.O. was supported by a Japanese Government Scholarship for Foreign Research Students.
Footnotes
Published ahead of print 1 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02201-12.
REFERENCES
- 1. Kuchma SL, O'Toole GA. 2000. Surface-induced and biofilm-induced changes in gene expression. Curr. Opin. Biotechnol. 11:429–433 [DOI] [PubMed] [Google Scholar]
- 2. Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R. 2008. Biofilm development with an emphasis on Bacillus subtilis. Curr. Top. Microbiol. Immunol. 322:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209 [DOI] [PubMed] [Google Scholar]
- 4. Branda SS, Vik S, Friedman L, Kolter R. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20–26 [DOI] [PubMed] [Google Scholar]
- 5. Abee T, Kovacs AT, Kuipers OP, van der Veen S. 2011. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 22:172–179 [DOI] [PubMed] [Google Scholar]
- 6. Flemming HC, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- 7. Anderson GG, O'Toole GA. 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 322:85–105 [DOI] [PubMed] [Google Scholar]
- 8. Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40 [DOI] [PubMed] [Google Scholar]
- 9. López D, Vlamakis H, Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398 doi:10.1101/cshperspect.a000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aguilar C, Vlamakis H, Guzman A, Losick R, Kolter R. 2010. KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms. mBio 1(1):e00035–10 doi:10.1128/mBio.00035-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59:1229–1238 [DOI] [PubMed] [Google Scholar]
- 12. Chu F, Kearns DB, McLoon A, Chai Y, Kolter R, Losick R. 2008. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol. Microbiol. 68:1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chumsakul O, Takahashi H, Oshima T, Hishimoto T, Kanaya S, Ogasawara N, Ishikawa S. 2011. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res. 39:414–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamon MA, Stanley NR, Britton RA, Grossman AD, Lazazzera BA. 2004. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol. Microbiol. 52:847–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamon MA, Lazazzera BA. 2001. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 42:1199–1209 [DOI] [PubMed] [Google Scholar]
- 16. López D, Vlamakis H, Kolter R. 2009. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 33:152–163 [DOI] [PubMed] [Google Scholar]
- 17. Banse AV, Chastanet A, Rahn-Lee L, Hobbs EC, Losick R. 2008. Parallel pathways of repression and antirepression governing the transition to stationary phase in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 105:15547–15552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strauch M, Webb V, Spiegelman G, Hoch JA. 1990. The Spo0A protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. U. S. A. 87:1801–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chai Y, Beauregard PB, Vlamakis H, Losick R, Kolter R. 2012. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio 3(4):e00184-12 doi:10.1128/mBio.00184-12 (Erratum, 4(1):e00555–12, 2013. doi:10.1128/mBio.00555-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chai Y, Kolter R, Losick R. 2009. Paralogous antirepressors acting on the master regulator for biofilm formation in Bacillus subtilis. Mol. Microbiol. 74:876–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi K. 2008. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol. Microbiol. 69:1399–1410 [DOI] [PubMed] [Google Scholar]
- 22. Colledge VL, Fogg MJ, Levdikov VM, Leech A, Dodson EJ, Wilkinson AJ. 2011. Structure and organisation of SinR, the master regulator of biofilm formation in Bacillus subtilis. J. Mol. Biol. 411:597–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chai Y, Norman T, Kolter R, Losick R. 2010. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 24:754–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Grice SF, Shih CC, Whipple F, Sonenshein AL. 1986. Separation and analysis of the RNA polymerase binding sites of a complex Bacillus subtilis promoter. Mol. Gen. Genet. 204:229–236 [DOI] [PubMed] [Google Scholar]
- 25. Ollington JF, Losick R. 1981. A cloned gene that is turned on at an intermediate stage of spore formation in Bacillus subtilis. J. Bacteriol. 147:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukushima T, Ishikawa S, Yamamoto H, Ogasawara N, Sekiguchi J. 2003. Transcriptional, functional and cytochemical analyses of the veg gene in Bacillus subtilis. J. Biochem. 133:475–483 [DOI] [PubMed] [Google Scholar]
- 27. Uchiyama I, Higuchi T, Kawai M. 2010. MBGD update 2010: toward a comprehensive resource for exploring microbial genome diversity. Nucleic Acids Res. 38:D361–D365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus Subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. U. S. A. 44:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Youngman P, Perkins JB, Sandman K. 1985. Use of Tn917-mediated transcriptional gene fusions to lacZ and cat-86 for the identification and study of spo genes in Bacillus subtilis, p 44–54 In Hoch JA, Setlow P. (ed), Molecular biology of microbial differentiation. American Society for Microbiology, Washington, DC [Google Scholar]
- 30. Völker U, Engelmann S, Maul B, Riethdorf S, Volker A, Schmid R, Mach H, Hecker M. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140(Part 4):741–752 [DOI] [PubMed] [Google Scholar]
- 31. McLoon AL, Guttenplan SB, Kearns DB, Kolter R, Losick R. 2011. Tracing the domestication of a biofilm-forming bacterium. J. Bacteriol. 193:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 98:11621–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stanley NR, Lazazzera BA. 2005. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-γ-DL-glutamic acid production and biofilm formation. Mol. Microbiol. 57:1143–1158 [DOI] [PubMed] [Google Scholar]
- 34. Chu F, Kearns DB, Branda SS, Kolter R, Losick R. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 59:1216–1228 [DOI] [PubMed] [Google Scholar]
- 35. Chai Y, Chu F, Kolter R, Losick R. 2008. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 67:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kearns DB, Chu F, Branda SS, Kolter R, Losick R. 2005. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55:739–749 [DOI] [PubMed] [Google Scholar]
- 37. Fredrick K, Helmann JD. 1996. FlgM is a primary regulator of σD activity, and its absence restores motility to a sinR mutant. J. Bacteriol. 178:7010–7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ishikawa S, Kawai Y, Hiramatsu K, Kuwano M, Ogasawara N. 2006. A new FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in Bacillus subtilis. Mol. Microbiol. 60:1364–1380 [DOI] [PubMed] [Google Scholar]
- 39. Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 50:1683–1701 [DOI] [PubMed] [Google Scholar]
- 40. Cozy LM, Phillips AM, Calvo RA, Bate AR, Hsueh YH, Bonneau R, Eichenberger P, Kearns DB. 2012. SlrA/SinR/SlrR inhibits motility gene expression upstream of a hypersensitive and hysteretic switch at the level of σD in Bacillus subtilis. Mol. Microbiol. 83:1210–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serizawa M, Yamamoto H, Yamaguchi H, Fujita Y, Kobayashi K, Ogasawara N, Sekiguchi J. 2004. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene 329:125–136 [DOI] [PubMed] [Google Scholar]
- 42. Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, Manabe K, Kanaya S, Ara K, Ozaki K, Ogasawara N. 2008. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 15:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zuber P. 2004. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J. Bacteriol. 186:1911–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stanley NR, Britton RA, Grossman AD, Lazazzera BA. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.