Abstract
Phenolyl cobamides are unique members of a class of cobalt-containing cofactors that includes vitamin B12 (cobalamin). Cobamide cofactors facilitate diverse reactions in prokaryotes and eukaryotes. Phenolyl cobamides are structurally and chemically distinct from the more commonly used benzimidazolyl cobamides such as cobalamin, as the lower axial ligand is a phenolic group rather than a benzimidazole. The functional significance of this difference is not well understood. Here we show that in the bacterium Sporomusa ovata, the only organism known to synthesize phenolyl cobamides, several cobamide-dependent acetogenic metabolisms have a requirement or preference for phenolyl cobamides. The addition of benzimidazoles to S. ovata cultures results in a decrease in growth rate when grown on methanol, 3,4-dimethoxybenzoate, H2 plus CO2, or betaine. Suppression of native p-cresolyl cobamide synthesis and production of benzimidazolyl cobamides occur upon the addition of benzimidazoles, indicating that benzimidazolyl cobamides are not functionally equivalent to the phenolyl cobamide cofactors produced by S. ovata. We further show that S. ovata is capable of incorporating other phenolic compounds into cobamides that function in methanol metabolism. These results demonstrate that S. ovata can incorporate a wide range of compounds as cobamide lower ligands, despite its preference for phenolyl cobamides in the metabolism of certain energy substrates. To our knowledge, S. ovata is unique among cobamide-dependent organisms in its preferential utilization of phenolyl cobamides.
INTRODUCTION
Cobamides function as enzyme cofactors for a variety of metabolic processes in most animals, protists, and prokaryotes, although their biosynthesis is limited to a subset of prokaryotes. Vitamin B12 (cobalamin) (Fig. 1A) is the best-studied cobamide and is a required micronutrient in humans. Two coenzyme forms of cobalamin exist, in which the upper ligand is either a methyl group (methylcobalamin) or 5′-deoxyadenosine (adenosylcobalamin). Methylcobalamin facilitates methyl transfer reactions involved in processes such as acetogenesis, methanogenesis, and methionine synthesis (1). Adenosylcobalamin facilitates radical-based rearrangements and cleavage reactions in the catabolism of substrates such as glycerol, ethanolamine, and various amino acids (2). The role of the upper ligand in catalysis by cobamide-dependent enzymes has been well studied. However, the significance of structural variability in other parts of the cobamide molecule is less clear. Differences have been found within the nucleotide loop (3) and in the lower ligand (Fig. 1). Variations in the lower ligand are the main source of diversity in cobamide structure, as 16 cobamides with different lower ligands have been reported (4), yet many questions remain regarding the effect of the lower ligand on the function of the cofactor.
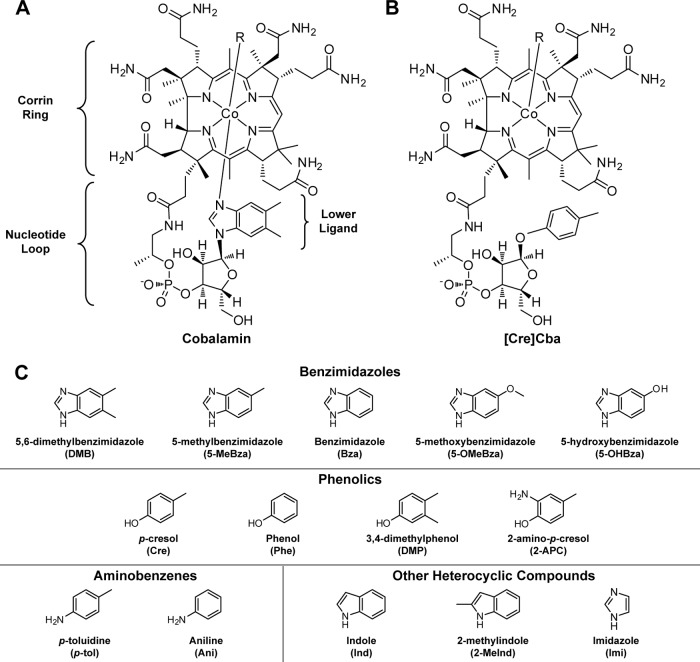
Fig 1.
Structures of cobamides and lower ligands. Chemical structures of cobalamin (A), [Cre]Cba (B), and lower ligand bases (C) studied in this work are shown. R represents the upper ligand, which may be a methyl or 5′-deoxyadenosine group in the cofactor forms or a cyano group in the vitamin form. Full chemical names of each lower ligand base are shown in panel C, with their abbreviations used in the text in parentheses. Corrinoids are defined as compounds that contain a corrin ring. Cobamides (Cba), corrinoids that have an upper and lower axial ligand, are discussed in the text with the abbreviation for the lower ligand in brackets, for example, [Cre]Cba for p-cresolyl cobamide.
Variations in lower ligand structure can influence the Km for the cofactor in cobamide-dependent enzymes. For example, the Km of the methylmalonyl coenzyme A (CoA) mutase enzyme of Propionibacterium freudenreichii subsp. shermanii, glutamate mutase of Clostridium tetanomorphum, and methionine synthase of Arthrobacter platensis are influenced by the structure of the lower ligand (5–7). In addition, coordination of the lower ligand to the central cobalt ion can affect the reactivity of the cofactor (8, 9). Coordination to the cobalt ion depends on the structural conformation of the cobamide when bound by the enzyme. One subset of cobamide-dependent enzymes, which includes diol dehydratase and ribonucleotide reductase, binds the cobamide in the “base-on” form, in which the lower ligand is coordinated to the cobalt ion via a lone pair of electrons from a nitrogen atom, as shown in Fig. 1A (10, 11). In enzymes that utilize the cobamide in the “base-off” form, such as methionine synthase and methylmalonyl-CoA mutase, the lower ligand is bound by the enzyme but is not coordinated to the cobalt ion (12, 13). Instead, a histidine residue in the protein is often coordinated to the cobalt ion (12, 14).
Many cobamides, including cobalamin, can exist in either the base-on or base-off form, and the structure of the lower ligand affects the equilibrium between the two configurations in solution (15, 16). An exception is the phenolyl cobamides, which exist exclusively in the base-off form because the lower ligand lacks a lone pair of electrons and thus is unable to coordinate to the cobalt ion (Fig. 1B). This inability to coordinate to the cobalt ion limits the reactions that phenolyl cobamides can catalyze. For example, phenolyl cobamides do not support the growth of Salmonella enterica on 1,2-propanediol or ethanolamine, which require enzymes that function with the cobamide in the base-on configuration (17). Furthermore, the phenolyl cobamide p-cresolyl cobamide ([Cre]Cba) (Fig. 1B) acts as a competitive inhibitor in vitro for the enzymes diol dehydratase, glycerol dehydratase, and ethanolamine ammonia lyase, all of which bind cobamides in the base-on form (18, 19).
To date, the acetogenic bacterium Sporomusa ovata is the only organism reported to produce phenolyl cobamides (20, 21). S. ovata is capable of metabolizing a variety of substrates to acetate (22), and many of these metabolisms appear to be cobamide dependent. S. ovata was proposed to use a cobamide cofactor for the metabolism of methanol and the phenyl methyl ether 3,4-dimethoxybenzoate (Fig. 2) (23, 24). Growth on each of these substrates is associated with the production of distinct corrinoid-containing proteins (23, 24). These proteins are thought to catalyze the transfer of methyl groups from methanol or 3,4-dimethoxybenzoate to tetrahydrofolate (THF) to form 5-methyltetrahydrofolate (Me-THF) (Fig. 2) (24). The subsequent steps of methanol and 3,4-dimethoxybenzoate metabolism in S. ovata have not been characterized. However, based on studies of the metabolism of similar substrates in other acetogens (25–30), it is likely that the Me-THF is then used for the synthesis of acetyl-CoA via the Wood-Ljungdahl pathway, which requires a corrinoid iron-sulfur protein (CFeSP) (31). Thus, the cobamide-dependent reactions that catalyze methyl transfer to THF effectively substitute for the “methyl branch” of the Wood-Ljungdahl pathway (Fig. 2) (31). In this model, Me-THF is additionally required to generate the reducing equivalents necessary for the “carbonyl branch” of the Wood-Ljungdahl pathway via oxidation to CO2 (29). Homoacetogenic growth on H2 plus CO2 also uses the Wood-Ljungdahl pathway (Fig. 2) (31).
Fig 2.
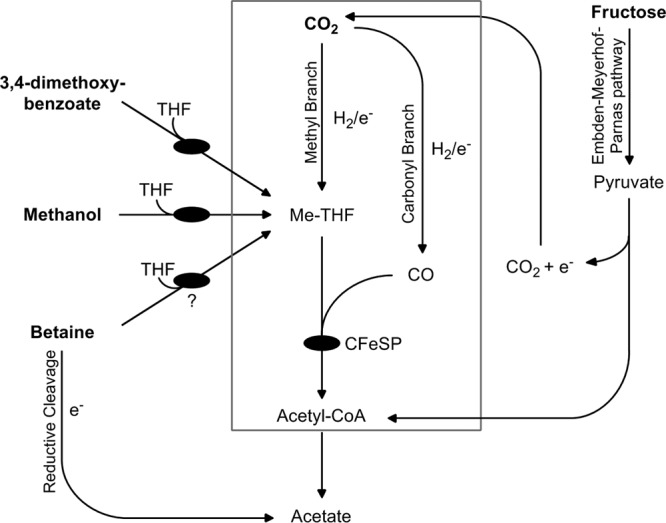
Model for cobamide requirements in acetogenic metabolism in S. ovata. The metabolism of methanol, 3,4-dimethoxybenzoate, H2 plus CO2, and fructose to acetate requires the Wood-Ljungdahl pathway (box). The “methyl branch” of the pathway is bypassed in the metabolism of methanol and 3,4-dimethoxybenzoate by substrate-specific sets of enzymes that catalyze the transfer of methyl groups to THF to form Me-THF. Betaine metabolism is also proposed to involve methyl transfer to THF. Me-THF oxidation (not shown) provides the reducing equivalents (e−) necessary for the reduction of CO2 to CO (“carbonyl branch”) during methanol and 3,4-dimethoxybenzoate metabolism to acetate. H2 serves as the electron donor in the Wood-Ljungdahl pathway during homoacetogenic growth on H2 plus CO2. The oxidation of Me-THF is also required to produce the reducing equivalents for the reductive cleavage of betaine to acetate. Fermentation of fructose to pyruvate requires the Embden-Meyerhof-Parnas pathway. The CO2 and reducing equivalents generated from the conversion of pyruvate to acetyl-CoA can be utilized in the Wood-Ljungdahl pathway. Proposed cobamide-dependent enzymes are represented by black ovals. This model is based on metabolic studies of S. ovata and other acetogenic bacteria (22, 25–30, 33, 47).
Unlike methanol, 3,4-dimethoxybenzoate, and H2 plus CO2, the metabolism of fructose does not appear to be entirely dependent on cobamides (Fig. 2). The fermentation of fructose likely occurs via the Embden-Meyerhof-Parnas pathway, which does not require cobamides (32). This pathway produces pyruvate, which is subsequently converted into acetyl-CoA. However, the CO2 and reducing equivalents generated during this reaction can also be converted to acetate via the cobamide-dependent Wood-Ljungdahl pathway (Fig. 2) (29).
The role of cobamides in betaine metabolism by acetogens is less clear. It was proposed that S. ovata reductively cleaves betaine to form acetate (22), which does not appear to require cobamides (Fig. 2). The reducing equivalents necessary for this reaction are thought to be generated via methyl transfer from a fraction of the betaine to THF followed by oxidation of Me-THF to CO2, similar to the metabolism of methanol and 3,4-dimethoxybenzoate (22, 33).
In each of these proposed metabolisms, the cobamide-dependent reactions involve the transfer of a methyl group. Crystallographic and biochemical studies have shown that cobamide-dependent enzymes that catalyze methyl transfer reactions, such as methionine synthase of Escherichia coli and those involved in methanol metabolism in Moorella thermoacetica and Methanosarcina barkeri, bind their respective benzimidazolyl cobamides in the base-off conformation (12, 25, 34), suggesting that a phenolyl cobamide would be a suitable cofactor for these metabolisms in S. ovata. A sequenced genome would enable a more detailed analysis of the cobamide-dependent enzymes present in S. ovata.
In the present work, we have analyzed the cobamide requirements of S. ovata in the metabolism of a variety of growth substrates. We found that S. ovata is capable of synthesizing benzimidazolyl cobamides when provided a benzimidazole base, yet these cobamides do not support growth on 3,4-dimethoxybenzoate and result in impaired growth on methanol, H2 plus CO2, and betaine. To our knowledge, this is the first observation of a cobamide-dependent organism for which phenolyl cobamides function more effectively than benzimidazolyl cobamides.
MATERIALS AND METHODS
Media and growth conditions.
S. ovata DSM 2662 was grown anaerobically under an atmosphere of 80% N2 and 20% CO2 at 30°C without agitation in medium adapted from the betaine standard medium described previously by Möller et al. (22). Energy substrates were provided at the following concentrations: 124 mM methanol, 20 mM 3,4-dimethoxybenzoate, 50 mM betaine, and 50 mM fructose. Vitamin B12 was omitted, and the media were reduced with a cysteine-sulfide solution at 0.01% (wt/vol). Media for homoacetogenic growth were prepared under an atmosphere of 80% H2 and 20% CO2. 5-Hydroxybenzimidazole (5-OHBza) was a gift from Terence Crofts.
Growth assays.
Cultures of S. ovata were prepared with a 1% inoculum of stationary-phase cells. Optical density at 600 nm (OD600) values of cultures with methanol were measured following growth to saturation after 64 h of incubation. The OD600 values of 200-μl aliquots from each culture were measured in a 96-well plate on a BioTek Synergy 2 microplate reader, and values were normalized to a path length of 1 cm. Dose-response curves and 50% inhibitory concentration (IC50) values were generated with a sigmoidal curve fit in KaleidaGraph v4.0 (Synergy Software).
Corrinoid extraction and analysis.
Cells were collected in early stationary phase for the extraction of corrinoids (64 h of incubation with methanol, 75 h with 3,4-dimethoxybenzoate, 72 h with H2 plus CO2, 50 h with betaine, and 62 h with fructose). Extraction of corrinoids from S. ovata cultures was performed as described previously, adjusting for culture volume and cell pellet weight (35). Analysis of corrinoid extractions was performed on an Agilent Technologies 1200 series high-performance liquid chromatography (HPLC) system equipped with a diode array detector. Samples were injected onto a Zorbax SB-Aq column (5 μm, 4.6 by 150 mm) at a flow rate of 1 ml min−1 at 30°C. Mobile phases used were 0.1% formic acid in water (solvent A) and 0.1% formic acid in methanol (solvent B). Samples were separated by a gradient of 25 to 50% solvent B over 3 min, followed by a gradient of 50 to 75% solvent B over 8 min (method 1), or by a gradient of 25 to 34% solvent B over 11 min, 34 to 50% solvent B over 2 min, and 50 to 75% solvent B over 8 min (method 2). Concentrations of cobamides were determined based on integrated peak areas at 525 nm in comparison to standard curves generated by using purified cobamides. Concentrations of the purified cobamides were determined spectrophotometrically by measuring the absorbance at 361 nm and using a molar extinction coefficient of 28,060 mol−1 cm−1 (36).
Liquid chromatography-tandem mass spectrometry (LC/MS/MS) analysis of corrinoids was performed on an Agilent Technologies 6410 liquid chromatograph-triple quadrupole mass spectrometer. Samples were injected onto a Zorbax SB-Aq column (5 μm, 4.6 by 150 mm) and separated at 0.5 ml min−1 using method 2 as described above. Phenolyl cobamides and p-toluidinyl cobamide ([p-tol]Cba) were detected by using an MS2 scan with the fragmentor set at 135 V. Benzimidazolyl cobamides were detected by multiple-reaction monitoring (MRM) with a collision energy of 45 V. Signature transitions unique to each cobamide were monitored. For cobalamin, a transition from a precursor ion of m/z 678.3 to a product ion of m/z 147.1 was monitored. The precursor and product ions for the other benzimidazolyl cobamides are as follows: 671.3 and 133.1 for 5-methylbenzimidazolyl cobamide ([5-MeBza]Cba), 664.3 and 119.1 for benzimidazolyl cobamide ([Bza]Cba), 679.3 and 149.1 for 5-methoxybenzimidazolyl cobamide ([5-OMeBza]Cba), and 672.3 and 135.1 for 5-hydroxybenzimidazolyl cobamide ([5-OHBza]Cba), respectively.
RESULTS
Benzimidazoles inhibit S. ovata growth on methanol by inhibiting [Cre]Cba synthesis.
A previous study showed that S. ovata could not grow with methanol as the energy substrate in the presence of 500 μM 5,6-dimethylbenzimidazole (DMB), the lower ligand of cobalamin (Fig. 1) (37). Based on this result, it is likely that this growth inhibition is due to the production of cobalamin and that cobalamin does not support the cobamide-dependent functions of S. ovata. Moreover, if the toxicity of DMB were due to its incorporation into a cobamide, a similar effect would likely be observed with other benzimidazoles. To test these hypotheses, growth of S. ovata on methanol was measured in the presence of a range of concentrations of each of the five benzimidazoles shown in Fig. 1C. As expected, all of the benzimidazoles inhibited growth in a dose-dependent manner (Fig. 3A). Different concentrations of each benzimidazole were required to inhibit growth, with IC50 values ranging from nearly 500 μM for 5-OHBza to less than 1 μM for 5-MeBza and DMB (Fig. 3A).
Fig 3.
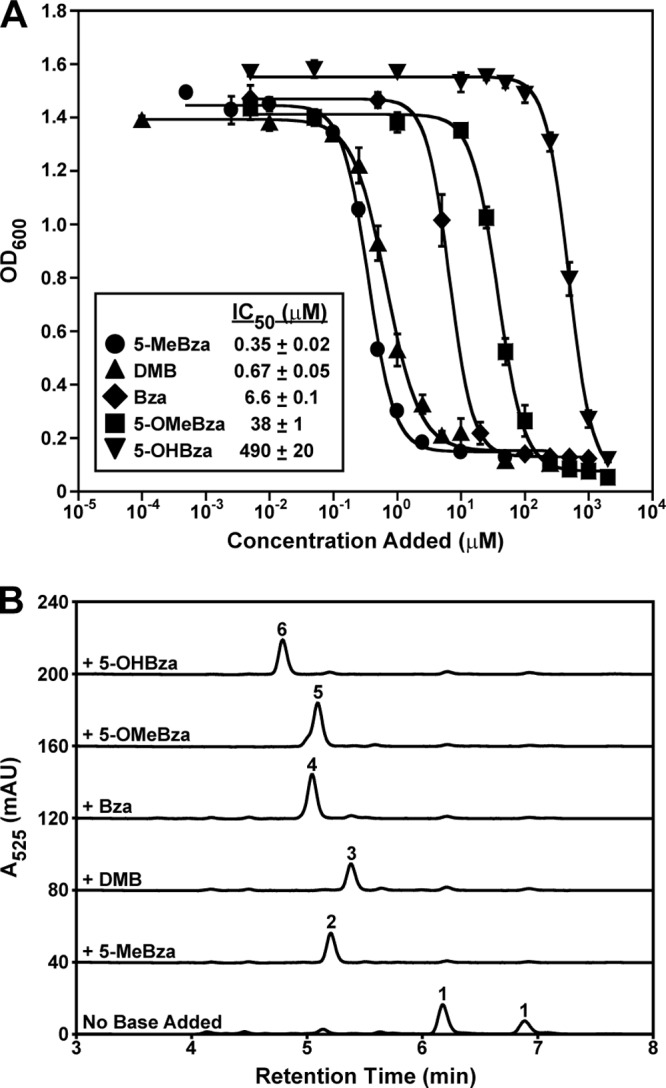
Addition of benzimidazoles results in growth inhibition and production of benzimidazolyl cobamides. (A) Growth of S. ovata was measured as the OD600 values of cultures grown for 64 h on methanol in the presence of a range of concentrations of each benzimidazole. The IC50 values for each benzimidazole are listed in the inset. Each point represents the average of three independent experiments, and error bars represent standard errors. (B) HPLC traces of corrinoid extractions from S. ovata grown with and without each of the five benzimidazoles tested in panel A are shown. Benzimidazoles were added to S. ovata at the following concentrations: 5 μM 5-MeBza, 100 μM DMB, 500 μM Bza, 2 mM 5-OMeBza, and 4 mM 5-OHBza. The identities of the corrinoids confirmed by LC/MS/MS are labeled as follows: 1, [Cre]Cba; 2, [5-MeBza]Cba; 3, cobalamin; 4, [Bza]Cba; 5, [5-OMeBza]Cba; 6, [5-OHBza]Cba. mAU, milli-absorbance units.
To determine whether benzimidazolyl cobamides are produced when S. ovata is grown with benzimidazoles, corrinoids were extracted from cultures provided with inhibitory concentrations of each benzimidazole and analyzed by HPLC and LC/MS/MS (Fig. 3B). In the absence of a benzimidazole, S. ovata synthesizes [Cre]Cba as its primary corrinoid, which can be seen by HPLC analysis as a pair of peaks (Fig. 3B), as observed previously (21). Although the production of both [Cre]Cba and phenolyl cobamide ([Phe]Cba) by S. ovata was reported previously (21), only [Cre]Cba was detected under our growth conditions. When inhibitory concentrations of benzimidazoles were added to S. ovata cultures, [Cre]Cba was almost completely absent in every case, and a benzimidazolyl cobamide was produced (Fig. 3B). These results show that S. ovata can attach benzimidazoles to form benzimidazolyl cobamides and suggest that these cobamides do not support growth on methanol.
Dose-dependent shifts in cobamide production result in growth inhibition on methanol.
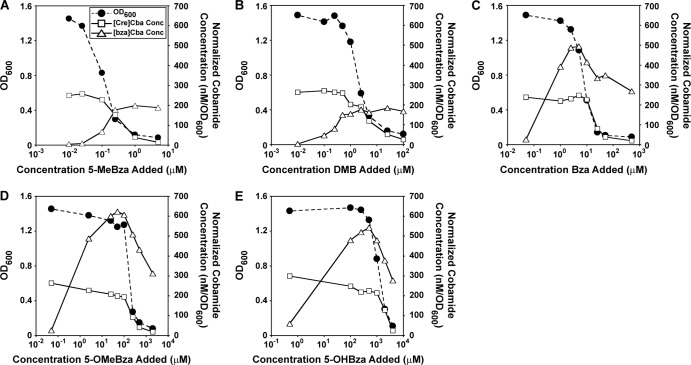
The link between cobamide production and growth inhibition was further investigated by examining the levels of each cobamide when S. ovata was grown on methanol in the presence of various concentrations of each benzimidazole. In each case, a dose-dependent inhibition of growth with increasing benzimidazole concentrations was accompanied by a parallel decrease in the concentration of [Cre]Cba (Fig. 4). Additionally, when either 5-MeBza or DMB was added, the concentration of the corresponding benzimidazolyl cobamide increased as the concentration of [Cre]Cba decreased (Fig. 4A and B). A similar result was observed with the addition of Bza, 5-OMeBza, or 5-OHBza, except that an increase in the corresponding benzimidazolyl cobamide occurred at subinhibitory concentrations (Fig. 4C to E). These results indicate that growth inhibition by the addition of benzimidazoles can be explained by the loss of [Cre]Cba production and, in some cases, possibly by an inhibitory effect of benzimidazolyl cobamides.
Fig 4.
Correlation between growth inhibition and cobamide content in S. ovata grown on methanol with benzimidazole bases. OD600 values of cultures grown for 64 h with a range of concentrations are shown for the following benzimidazoles: 5-MeBza (A), DMB (B), Bza (C), 5-OMeBza (D), and 5-OHBza (E). The concentrations of [Cre]Cba and the benzimidazolyl cobamide corresponding to the benzimidazole added ([bza]Cba) are shown for each culture.
Addition of Cre rescues growth and restores [Cre]Cba production.
The inhibition of growth on methanol upon addition of benzimidazoles led us to hypothesize that restoring the synthesis of [Cre]Cba in the presence of a benzimidazole would result in the rescue of growth. Indeed, we observed that the addition of Cre restored growth on methanol in media containing an inhibitory concentration of 5-MeBza in a dose-dependent manner (Fig. 5A, filled circles). Specifically, in the presence of 20 μM 5-MeBza, the addition of at least 5 μM Cre completely restored growth. Analysis of the corrinoid content of these cultures showed an increase in [Cre]Cba production with increasing Cre concentrations that was accompanied by a nearly complete loss of [5-MeBza]Cba (Fig. 5A, open symbols). This was a reversal of the phenotype seen when S. ovata was grown with increasing concentrations of the benzimidazoles. The addition of 10 μM Cre to the media was also sufficient to restore growth and [Cre]Cba production on methanol in the presence of inhibitory concentrations of each of the other four benzimidazoles tested (Fig. 5B and C).
Fig 5.
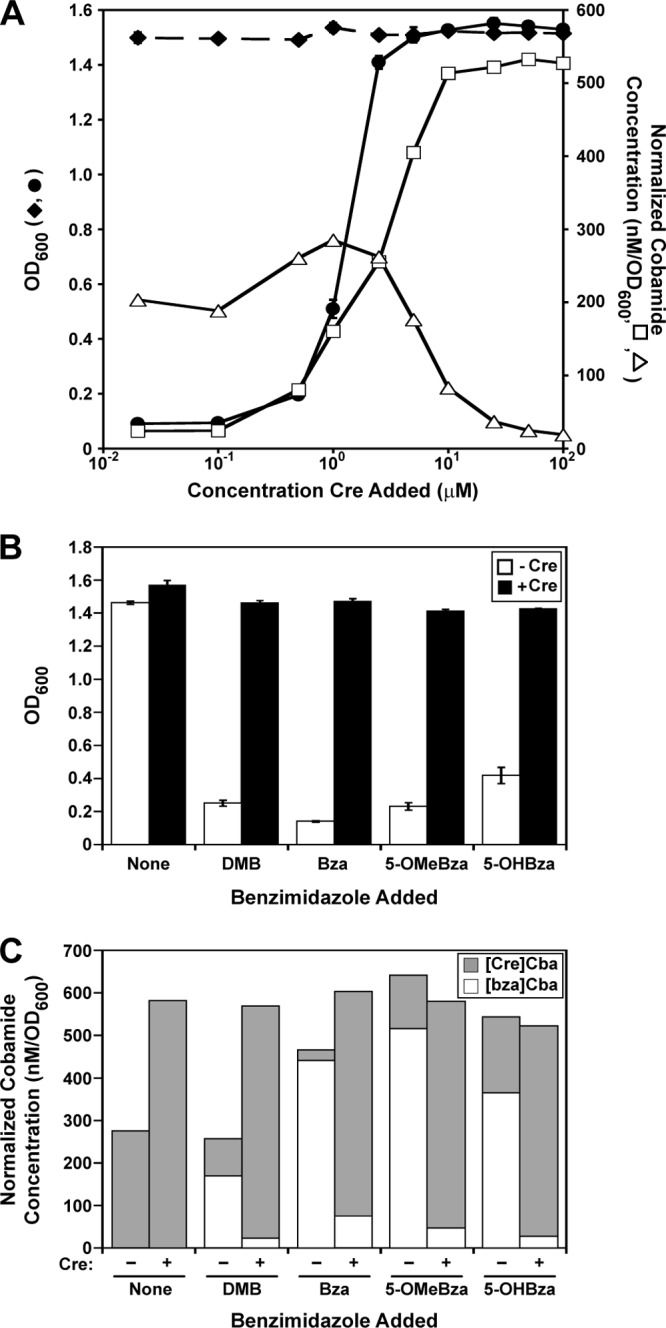
Rescue of benzimidazole-induced growth inhibition by addition of Cre. (A) OD600 values of cultures grown for 64 h on methanol with a range of concentrations of Cre in the absence (♦) or presence (●) of 20 μM 5-MeBza. Concentrations of [Cre]Cba (□) and [5-MeBza]Cba (△) are shown for the cultures containing 5-MeBza. (B) OD600 values for S. ovata cultured with inhibitory concentrations of benzimidazoles in the absence or presence of 10 μM Cre. Concentrations of the benzimidazoles are as follows: 10 μM DMB, 100 μM Bza, 250 μM 5-OMeBza, and 1 mM 5-OHBza. OD600 values for each point in panels A and B represent the averages of three independent experiments, and error bars represent standard errors. (C) Concentrations of [Cre]Cba and benzimidazolyl cobamides in the cultures from panel B. −, no Cre added; +, 10 μM Cre added. Gray bars show concentrations of [Cre]Cba; white bars show concentrations of benzimidazolyl cobamides corresponding to the benzimidazole added ([bza]Cba). Corrinoids in panels A and C were extracted from the culture with the median OD600 value of the three independent experiments.
These results suggest that it should also be possible to rescue growth by providing S. ovata with its native corrinoid, [Cre]Cba. A previous study showed that S. ovata may be capable of importing corrinoids, as corrinoids derived from exogenously supplied 57Co-labeled [5-OHBza]Cba were detected in the cell-associated fraction (38). However, under our growth conditions, the addition of [Cre]Cba at levels as high as 1.4 μM, five times higher than the endogenous level, did not rescue growth, and [Cre]Cba was detected only in culture supernatants (data not shown).
Growth on other substrates is inhibited by benzimidazoles to various degrees.
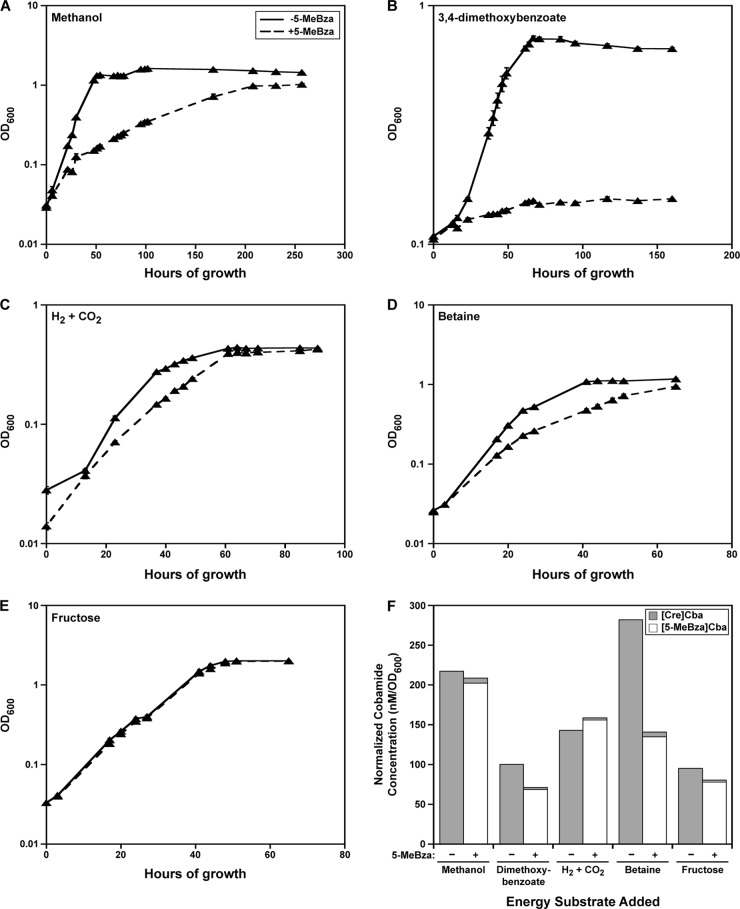
S. ovata is capable of utilizing a variety of growth substrates, many of which are thought to include steps that require cobamides (Fig. 2). To determine whether the addition of benzimidazoles also leads to an inhibition of growth on other substrates, we performed growth curve analyses of S. ovata grown on four other substrates in the presence and absence of 5-MeBza, the benzimidazole that we found has the greatest effect on growth on methanol (Fig. 3A). For comparison, we also analyzed growth on methanol, and as seen previously, growth was significantly affected by the addition of 5-MeBza (Fig. 6A). Specifically, when 5-MeBza was present, the growth rate was approximately 5-fold lower than that in the absence of 5-MeBza, but the cultures reached a final OD600 level nearly as high as that of cultures lacking 5-MeBza. Growth on 3,4-dimethoxybenzoate was more severely impacted by the addition of 5-MeBza, as stationary phase was reached at a considerably lower cell density than in the absence of 5-MeBza (Fig. 6B). Growth on H2 plus CO2 was modestly affected by the addition of 5-MeBza, with a 1.5-fold reduction in the growth rate and little effect on the final OD600 level in stationary phase (Fig. 6C). A similar result was seen for growth on betaine, in which the addition of 5-MeBza resulted in a 1.4-fold reduction in the growth rate (Fig. 6D). In contrast, growth on fructose was essentially unaffected by the addition of 5-MeBza (Fig. 6E).
Fig 6.
Effect of 5-MeBza on growth and cobamide production on different energy substrates. (A to E) Growth curves of S. ovata grown in the absence or presence of 25 μM 5-MeBza on the following energy substrates: methanol (A), 3,4-dimethoxybenzoate (B), H2 plus CO2 (C), betaine (D), and fructose (E). Each point represents the average of three independent experiments, and error bars represent standard errors. (F) [Cre]Cba and [5-MeBza]Cba levels in cells taken from early-stationary-phase cultures of S. ovata grown on the different energy substrates listed (64 h of growth with methanol, 75 h with 3,4-dimethoxybenzoate, 72 h with H2 plus CO2, 50 h with betaine, and 62 h with fructose). −, no 5-MeBza added; +, 25 μM 5-MeBza added.
The corrinoids present in cultures grown on each of these substrates were analyzed to determine whether [5-MeBza]Cba was produced, as we observed for growth on methanol. In each case, the addition of 5-MeBza resulted in the production of [5-MeBza]Cba and loss of [Cre]Cba (Fig. 6F). However, during growth on betaine, the concentration of [5-MeBza]Cba produced was approximately half of the level of [Cre]Cba produced in the absence of 5-MeBza, introducing the possibility that a decrease in overall cobamide levels was the cause of the growth defect. We ruled out this possibility by analyzing growth on betaine with the addition of Bza, which resulted in the production of [Bza]Cba at 50% higher levels than [Cre]Cba. A similar inhibition of growth was seen during exponential phase, indicating that the growth defect on betaine in the presence of 5-MeBza can be explained by the production of a benzimidazolyl cobamide rather than a reduction in the overall cobamide level (data not shown). Together, these results show that benzimidazolyl cobamides have various effects on different metabolic pathways in S. ovata, likely due to differences in the required cobamide-dependent enzymes.
Can S. ovata synthesize and use cobamides containing other natural and unnatural lower ligands?
Since our results showed that the structure of the lower ligand affects cobamide function in S. ovata, we next investigated the range of lower ligand structures that could be accommodated. Because S. ovata is unable to import cobamides efficiently from the environment under our growth conditions, and because we do not have a mechanism to inhibit the endogenous synthesis of Cre, we tested whether S. ovata could synthesize and utilize cobamides containing alternative bases as lower ligands by determining whether compounds other than Cre could rescue growth in the presence of a benzimidazole. The compounds tested include those that are structurally similar to either Cre or benzimidazoles, with various sizes and chemical properties (Fig. 1C). Growth on methanol was monitored in cultures containing each of these compounds in the presence of an inhibitory concentration of 5-OMeBza. We saw that only Phe and 3,4-dimethylphenol (DMP), the two compounds most similar to Cre, rescued the growth of S. ovata in the presence of 5-OMeBza (Fig. 7A). Similar results were obtained with inhibitory concentrations of 5-MeBza, DMB, and Bza (data not shown). An analysis of corrinoid extracts from these cultures showed that Phe and DMP were incorporated as cobamide lower ligands (Fig. 7B).
Fig 7.

Use of alternative substrates as lower ligands in S. ovata. (A) OD600 values of S. ovata grown on methanol in the presence of 250 μM 5-OMeBza and the following compounds: 10 μM Cre, 1mM Phe, 100 μM DMP, 2mM 2-APC, 2 mM p-tol, 1 mM Ani, 1 mM Ind, 500 μM 2-MeInd, and 1mM Imi. Each bar represents the average of three independent experiments, and error bars represent standard errors. (B) HPLC analysis of corrinoid extracts of S. ovata grown on methanol with the substrates shown. The identities of the corrinoids were confirmed by LC/MS and are labeled as follows: 1, [Cre]Cba; 2, [5-OMeBza]Cba; 3, [Phe]Cba; 4, [DMP]Cba; 5, [2-APC]Cba. A novel corrinoid (peak 6) was detectable only by HPLC in extracts from the culture grown with p-tol.
The remaining six compounds tested were unable to rescue growth (Fig. 7A). This could be either because they are not efficiently attached to form the corresponding cobamides or because they do not function as cofactors in methanol metabolism. To distinguish between these two possibilities, we first analyzed the corrinoids present in the cultures shown in Fig. 7A to determine whether the compounds can be attached as lower ligands. HPLC analysis revealed that no novel corrinoids were produced, suggesting that the compounds were not attached (data not shown). However, in the absence of 5-OMeBza, attachment of 2-amino-p-cresol (2-APC) was observed, although [Cre]Cba was not eliminated from the cultures (Fig. 7B). The addition of p-tol in the absence of 5-OMeBza also resulted in small amounts of a novel corrinoid that was detectable by HPLC (Fig. 7B). This experiment could not test whether [2-APC]Cba or [p-tol]Cba can be used by S. ovata, as neither compound is attached efficiently enough to be the major corrinoid present. Aniline (Ani), indole (Ind), 2-methylindole (2-MeInd), and imidazole (Imi) were not attached at any appreciable level (data not shown), and thus, it was not possible to determine whether S. ovata can utilize cobamides containing these compounds as lower ligands.
DISCUSSION
Although S. ovata has long been known to produce phenolyl cobamides, the functional significance of this unusual class of corrinoid cofactors remains poorly understood. Here we have shown that S. ovata is capable of attaching externally supplied benzimidazoles to form benzimidazolyl cobamides, yet these cobamides function poorly compared to phenolyl cobamides under most growth conditions. At least three cobamide-dependent methyltransferase enzymes are predicted to be required for the acetogenic metabolisms investigated here. Our results suggest that these enzymes are adapted for the use of phenolyl cobamides to various degrees in S. ovata. To our knowledge, S. ovata is unusual in its preference for phenolyl cobamides, as benzimidazolyl or purinyl cobamides are used by all other organisms known to require a cobamide cofactor for methyl transfer reactions.
Studies of several other acetogens have shown that growth on H2 plus CO2 requires the cobamide-dependent CFeSP enzyme in the Wood-Ljungdahl pathway, while growth on methanol and phenyl methyl ethers is each thought to require both the CFeSP and another cobamide-dependent methyltransferase enzyme (Fig. 2) (26, 27, 29, 30, 39). The phenotype of S. ovata grown on H2 plus CO2 with 5-MeBza suggests that S. ovata CFeSP functions best with phenolyl cobamides but can also use benzimidazolyl cobamides. The greater effect of 5-MeBza on growth with methanol than on H2 plus CO2 likely also reflects a preference for phenolyl cobamides by a cobamide-dependent enzyme that catalyzes methyl transfer from methanol to THF (Fig. 2). Likewise, the dramatic effect of 5-MeBza on growth on 3,4-dimethoxybenzoate may be due to a stringent requirement for a phenolyl cobamide by a cobamide-dependent enzyme that catalyzes the demethylation of 3,4-dimethoxybenzoate (Fig. 2). Similar methyltransferases involved in the metabolism of methanol and phenyl methyl ethers have been characterized in other organisms, but unlike in S. ovata, these enzymes have been shown to utilize benzimidazolyl cobamides in vivo or are capable of using cobalamin in vitro (25, 27, 28, 40–42).
We expected the cobamide requirements for growth on fructose to be similar to the requirements for growth on H2 plus CO2 because fructose metabolism is thought to be dependent on the CFeSP of the Wood-Ljungdahl pathway (Fig. 2). However, unlike growth on H2 plus CO2, the addition of 5-MeBza did not impair growth (Fig. 6E). This may be explained by a lower dependence on cobamides in fructose metabolism, as only one of the six carbon atoms of fructose is thought to be metabolized via the Wood-Ljungdahl pathway (29).
Although a requirement for cobamides in betaine metabolism has not been shown previously in acetogens, the modest effect of 5-MeBza on growth on betaine suggests that cobamides are involved. Reductive cleavage of betaine to acetate requires reducing equivalents generated from the oxidation of Me-THF (22, 33). Although the majority of the carbon in betaine is converted to acetate by reductive cleavage (22), the formation of Me-THF suggests that a fraction of the acetate could be produced by the Wood-Ljungdahl pathway, which requires a cobamide for the CFeSP (Fig. 2). Additionally, the methyl transfer step from betaine to Me-THF is analogous to cobamide-requiring steps in methanol and 3,4-dimethoxybenzoate metabolism, so a cobamide-dependent methyltransferase could also be involved in betaine metabolism. Together, these results support the model shown in Fig. 2, in which at least three cobamide-dependent enzymes with varying dependence on phenolyl cobamides function in acetogenic metabolism in S. ovata.
The inability of S. ovata to use benzimidazolyl cobamides as effectively as phenolyl cobamides in several acetogenic metabolisms is intriguing given that benzimidazolyl cobamides, like the phenolyl cobamides, are able to function in the base-off configuration. The requirement of S. ovata for phenolyl cobamides could be due to a role of the phenolic lower ligand in binding of the cobamide to the enzyme, as is the case for DMB in the binding of cobalamin to glutamate mutase of C. tetanomorphum (43). The cobamide-dependent enzymes in S. ovata may be adapted for binding cobamides with smaller phenolic lower ligands and may not bind the bulkier benzimidazole bases. Indeed, we found that three phenolic compounds, Cre, Phe, and DMP, were the only compounds that function as cobamide lower ligands in methanol-dependent growth of S. ovata. In addition to facilitating binding to the enzyme, phenolic lower ligands may also have another, as-yet-unidentified, role in methyl transfer reactions in S. ovata.
It is interesting that benzimidazoles are readily attached as cobamide lower ligands by S. ovata, as their structures differ considerably from those of the phenolic compounds. The ability of S. ovata to produce both phenolyl and benzimidazolyl cobamides is likely due to the activity of ArsAB, a CobT enzyme homolog shown to activate DMB in addition to Cre and Phe for attachment to a cobamide precursor (17). The various levels of attachment of different lower ligands that we observed in this work may reflect differences in the substrate specificity of ArsAB or another enzyme involved in lower ligand activation or attachment or differences in the ability of these compounds to enter the cell. Our results suggest that phenolics or aminobenzenes containing a p-methyl group are attached more readily than the corresponding compounds lacking a p-methyl group. In addition, the preference for phenolic compounds compared to aminobenzenes indicates that replacing the hydroxyl group with an amino group significantly hinders attachment to the corrin ring. Notably, aminobenzenes, which are more structurally and chemically similar to phenolics than are benzimidazoles, are attached more poorly than benzimidazoles. This analysis may contribute to the understanding of the mechanism of the ArsAB enzyme, which has the unique ability to catalyze the formation of both O- and N-glycosidic bonds in the activation of lower ligand bases.
It is curious that S. ovata has the ability to produce benzimidazolyl cobamides when provided a benzimidazole base, given the detrimental effect of benzimidazolyl cobamides under some growth conditions. The absence of a mechanism to exclude benzimidazoles from incorporation into cobamides may indicate that the natural habitat of S. ovata does not contain concentrations of free benzimidazoles sufficient to alter the levels of cobamides produced. Alternatively, S. ovata may have retained the ability to synthesize benzimidazolyl cobamides because the environments that it inhabits are abundant in substrates whose metabolisms are not particularly affected by benzimidazolyl cobamides, such as betaine and fructose.
Although S. ovata is the only organism known to produce phenolyl cobamides, these cobamides are abundant in mixed microbial communities, including those in which S. ovata has not been detected. For example, [Cre]Cba was found to comprise 16% of the corrinoids present in human feces (44), 20 to 34% of the corrinoids in bovine rumen (45, 46), and 70% of the corrinoids present in a trichloroethene-degrading enrichment community (Y. Men, E. C. Seth, S. Yi, T. S. Crofts, R. H. Allen, M. E. Taga, and L. Alvarez-Cohen, unpublished data). Given the ubiquity of phenolyl cobamides and their unique chemical properties, it will be important to understand the functions of this class of cobamides both at the enzymatic level and in the context of their functions in microbial communities.
ACKNOWLEDGMENTS
This work was supported by NIH grant R00-GM083303 and NSF grant MCB1122046 to M.E.T.
We thank Yujie Men, Shan Yi, and Lisa Alvarez-Cohen for assistance with LC/MS/MS; Cameron Thrash for assistance with anaerobic culturing; Sydney Kustu, Steven Lindow, and John Coates for helpful discussions; and members of the Taga laboratory for critical reading of the manuscript.
Footnotes
Published ahead of print 15 February 2013
REFERENCES
- 1. Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 72:209–247 [DOI] [PubMed] [Google Scholar]
- 2. Marsh EN, Holloway DE. 2000. Adenosylcobalamin-dependent enzymes. Subcell. Biochem. 35:351–403 [DOI] [PubMed] [Google Scholar]
- 3. Kräutler B, Fieber W, Ostermann S, Fasching M, Ongania KH, Gruber K, Kratky C, Mikl C. 2003. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo-B12, a new type of a natural corrinoid. Helv. Chim. Acta 86:3698–3716 [Google Scholar]
- 4. Renz P. 1999. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids, p 557–576 In Banerjee R. (ed), Chemistry and biochemistry of B12. John Wiley & Sons, New York, NY [Google Scholar]
- 5. Barker HA, Smyth RD, Weissbach H, Toohey JI, Ladd JN, Volcani BE. 1960. Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5,6-dimethylbenzimidazole. J. Biol. Chem. 235:480–488 [PubMed] [Google Scholar]
- 6. Lengyel P, Mazumder R, Ochoa S. 1960. Mammalian methylmalonyl isomerase and vitamin B12 coenzymes. Proc. Natl. Acad. Sci. U. S. A. 46:1312–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanioka Y, Miyamoto E, Yabuta Y, Ohnishi K, Fujita T, Yamaji R, Misono H, Shigeoka S, Nakano Y, Inui H, Watanabe F. 2010. Methyladeninylcobamide functions as the cofactor of methionine synthase in a cyanobacterium, Spirulina platensis NIES-39. FEBS Lett. 584:3223–3226 [DOI] [PubMed] [Google Scholar]
- 8. Kräutler B. 1990. Chemistry of methylcorrinoids related to their roles in bacterial C1 metabolism. FEMS Microbiol. Rev. 7:349–354 [DOI] [PubMed] [Google Scholar]
- 9. Kräutler B. 2009. Organometallic chemistry of B12 coenzymes, p 1–51 In Sigel A, Sigel H, Sigel RKO. (ed), Metal ions in life sciences, vol 6 Royal Society of Chemistry, Cambridge, United Kingdom: [DOI] [PubMed] [Google Scholar]
- 10. Lawrence CC, Gerfen GJ, Samano V, Nitsche R, Robins MJ, Rétey J, Stubbe J. 1999. Binding of Cob(II)alamin to the adenosylcobalamin-dependent ribonucleotide reductase from Lactobacillus leichmannii. Identification of dimethylbenzimidazole as the axial ligand. J. Biol. Chem. 274:7039–7042 [DOI] [PubMed] [Google Scholar]
- 11. Shibata N, Masuda J, Tobimatsu T, Toraya T, Suto K, Morimoto Y, Yasuoka N. 1999. A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase. Structure 7:997–1008 [DOI] [PubMed] [Google Scholar]
- 12. Drennan CL, Huang S, Drummond JT, Matthews RG, Lidwig ML. 1994. How a protein binds B12: a 3.0 Å X-ray structure of B12-binding domains of methionine synthase. Science 266:1669–1674 [DOI] [PubMed] [Google Scholar]
- 13. Mancia F, Keep NH, Nakagawa A, Leadlay PF, McSweeney S, Rasmussen B, Bösecke P, Diat O, Evans PR. 1996. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 Å resolution. Structure 4:339–350 [DOI] [PubMed] [Google Scholar]
- 14. Matthews RG. 2009. Cobalamin- and corrinoid-dependent enzymes, p 53–114 In Sigel A, Sigel H, Sigel RKO. (ed), Metal ions in life sciences, vol 6 Royal Society of Chemistry, Cambridge, United Kingdom: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamza MSA, Zou X, Banka R, Brown KL, van Eldik R. 2005. Kinetic and thermodynamic studies on ligand substitution reactions and base-on/base-off equilibria of cyanoimidazolylcobamide, a vitamin B12 analog with an imidazole axial nucleoside. Dalton Trans. 2005:782–787 [DOI] [PubMed] [Google Scholar]
- 16. Pol A, Gage RA, Neis JM, Reijnen JWM, van der Drift C, Vogels GD. 1984. Corrinoids from Methanosarcina barkeri: the β-ligands. Biochim. Biophys. Acta 797:83–93 [Google Scholar]
- 17. Chan CH, Escalante-Semerena JC. 2011. ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis. Mol. Microbiol. 81:952–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poppe L, Bothe H, Bröker G, Buckel W, Stupperich E, Rétey J. 2000. Elucidation of the coenzyme binding mode of further B12-dependent enzymes using a base-off analogue of coenzyme B12. J. Mol. Catal. 10:345–350 [Google Scholar]
- 19. Poppe L, Stupperich E, Hull WE, Buckel T, Rétey J. 1997. A base-off analogue of coenzyme-B12 with a modified nucleotide loop. 1H-NMR structure analysis and kinetic studies with (R)-methylmalonyl-CoA mutase, glycerol dehydratase, and diol dehydratase. Eur. J. Biochem. 250:303–307 [DOI] [PubMed] [Google Scholar]
- 20. Stupperich E, Eisinger HJ, Kräutler B. 1988. Diversity of corrinoids in acetogenic bacteria. P-Cresolylcobamide from Sporomusa ovata, 5-methoxy-6-methylbenzimidazolylcobamide from Clostridium formicoaceticum and vitamin B12 from Acetobacterium woodii. Eur. J. Biochem. 172:459–464 [DOI] [PubMed] [Google Scholar]
- 21. Stupperich E, Eisinger HJ, Kräutler B. 1989. Identification of phenolyl cobamide from the homoacetogenic bacterium Sporomusa ovata. Eur. J. Biochem. 186:657–661 [DOI] [PubMed] [Google Scholar]
- 22. Möller B, Oßmer R, Howard BH, Gottschalk G, Hippe H. 1984. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch. Microbiol. 139:388–396 [Google Scholar]
- 23. Stupperich E, Aulkemeyer P, Eckerskorn C. 1992. Purification and characterization of a methanol-induced cobamide-containing protein from Sporomusa ovata. Arch. Microbiol. 158:370–373 [DOI] [PubMed] [Google Scholar]
- 24. Stupperich E, Konle R. 1993. Corrinoid-dependent methyl transfer reactions are involved in methanol and 3,4-dimethoxybenzoate metabolism by Sporomusa ovata. Appl. Environ. Microbiol. 59:3110–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Das A, Fu ZQ, Tempel W, Liu ZJ, Chang J, Chen LR, Lee D, Zhou WH, Xu H, Shaw N, Rose JP, Ljungdahl LG, Wang BC. 2007. Characterization of a corrinoid protein involved in the C1 metabolism of strict anaerobic bacterium Moorella thermoacetica. Proteins 67:167–176 [DOI] [PubMed] [Google Scholar]
- 26. Engelmann T, Kaufmann F, Diekert G. 2001. Isolation and characterization of a veratrol:corrinoid protein methyl transferase from Acetobacterium dehalogenans. Arch. Microbiol. 175:376–383 [DOI] [PubMed] [Google Scholar]
- 27. Kaufmann F, Wohlfarth G, Diekert G. 1997. Isolation of O-demethylase, an ether-cleaving enzyme system of the homoacetogenic strain MC. Arch. Microbiol. 168:136–142 [DOI] [PubMed] [Google Scholar]
- 28. Naidu D, Ragsdale SW. 2001. Characterization of a three-component vanillate O-demethylase from Moorella thermoacetica. J. Bacteriol. 183:3276–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pierce E, Xie G, Barabote RD, Saunders E, Han CS, Detter JC, Richardson P, Brettin TS, Das A, Ljungdahl LG, Ragsdale SW. 2008. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ. Microbiol. 10:2550–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Meijden P, Drift C, Vogels GD. 1984. Methanol conversion in Eubacterium limosum. Arch. Microbiol. 138:360–364 [Google Scholar]
- 31. Ragsdale SW, Pierce E. 2008. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 1784:1873–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gottschalk G. 1986. Bacterial metabolism, p 249–252 Springer-Verlag, New York, NY [Google Scholar]
- 33. Kamlage B, Boelter A, Blaut M. 1993. Spectroscopic and potentiometric characterization of cytochromes in two Sporomusa species and their expression during growth on selected substrates. Arch. Microbiol. 159:189–196 [Google Scholar]
- 34. Hagemeier CH, Krüer M, Thauer RK, Warkentin E, Ermler U. 2006. Insight into the mechanism of biological methanol activation based on the crystal structure of the methanol-cobalamin methyltransferase complex. Proc. Natl. Acad. Sci. U. S. A. 103:18917–18922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yi S, Seth EC, Men YJ, Stabler SP, Allen RH, Alvarez-Cohen L, Taga ME. 2012. Versatility in corrinoid salvaging and remodeling pathways supports corrinoid-dependent metabolism in Dehalococcoides mccartyi. Appl. Environ. Microbiol. 78:7745–7752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pratt JM. 1972. Inorganic chemistry of vitamin B12. Academic Press, New York, NY [Google Scholar]
- 37. Stupperich E, Eisinger HJ, Schurr S. 1990. Corrinoids in anaerobic bacteria. FEMS Microbiol. Rev. 87:355–359 [Google Scholar]
- 38. Stupperich E, Eisinger HJ. 1989. Function and the biosynthesis of unusual corrinoids by a novel activation mechanism of aromatic compounds in anaerobic bacteria. Adv. Space Res. 9:117–125 [Google Scholar]
- 39. Ljungdahl LG. 1986. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 40:415–450 [DOI] [PubMed] [Google Scholar]
- 40. Siebert A, Schubert T, Engelmann T, Studenik S, Diekert G. 2005. Veratrol-O-demethylase of Acetobacterium dehalogenans: ATP-dependent reduction of the corrinoid protein. Arch. Microbiol. 183:378–384 [DOI] [PubMed] [Google Scholar]
- 41. Studenik S, Vogel M, Diekert G. 2012. Characterization of an O-demethylase of Desulfitobacterium hafniense DCB-2. J. Bacteriol. 194:3317–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Meijden P, Heythuysen HJ, Pouwels A, Houwen F, van der Drift C, Vogels GD. 1983. Methyltransferases involved in methanol conversion by Methanosarcina barkeri. Arch. Microbiol. 134:238–242 [DOI] [PubMed] [Google Scholar]
- 43. Tollinger M, Eichmüller C, Konrat R, Huhta MS, Marsh EN, Kräutler B. 2001. The B12-binding subunit of glutamate mutase from Clostridium tetanomorphum traps the nucleotide moiety of coenzyme B12. J. Mol. Biol. 309:777–791 [DOI] [PubMed] [Google Scholar]
- 44. Allen RH, Stabler SP. 2008. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am. J. Clin. Nutr. 87:1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girard CL, Berthiaume R, Stabler SP, Allen RH. 2009. Identification of cobalamin and cobalamin analogues along the gastrointestinal tract of dairy cows. Arch. Anim. Nutr. 63:379–388 [DOI] [PubMed] [Google Scholar]
- 46. Girard CL, Santschi DE, Stabler SP, Allen RH. 2009. Apparent ruminal synthesis and intestinal disappearance of vitamin B12 and its analogs in dairy cows. J. Dairy Sci. 92:4524–4529 [DOI] [PubMed] [Google Scholar]
- 47. Kamlage B, Blaut M. 1993. Isolation of a cytochrome-deficient mutant strain of Sporomusa sphaeroides not capable of oxidizing methyl groups. J. Bacteriol. 175:3043–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]