Abstract
Background
Black recipients undergoing liver transplantation (LT) for hepatitis C virus (HCV) have decreased patient and graft survival compared to white recipients, a finding which is primarily limited to black recipients of livers from white donors. The cause(s) for these discrepant outcomes are unclear but may be related to HCV disease recurrence. The rates of HCV-related disease recurrence and liver fibrosis progression among black and white LT recipients have not been investigated.
Methods
In this study, we compared liver fibrosis progression between 105 black and 364 white recipients after HCV-related LT in a multi-site cohort study and assessed the impact of donor race.
Results
At 6, 12 and 24 months after LT, there was a significantly higher percentage in the black (B) recipient/white (W) donor group with severe fibrosis, defined as stage 3 or 4 (F3/F4), compared to all other recipient/donor race combinations. The adjusted odds ratio of developing F3/F4 for the B/W group was 2.54 (1.49–4.69; reference group W/W). Black recipients with black donors had a similar rate of progression to F3/F4 as white recipients. Patient survival was also decreased in the B/W group compared to other recipient/donor race combinations.
Conclusion
African American recipients with white donors have more severe fibrosis progression after HCV related LT. The mechanisms responsible for accelerating fibrosis progression in this high-risk race-mismatched group need to be investigated.
Keywords: Hepatitis C virus (HCV), liver transplantation, race, survival
Introduction
Hepatitis C virus (HCV) infection is a significant public health problem, affecting roughly two percent of the United States (US) population (1). Approximately 20% will progress to cirrhosis within 2 decades of acquiring the virus (2), and end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) are significant causes of death in this population (3). Liver transplantation is the only therapeutic option for many of these patients. Unfortunately, recurrent HCV infection in LT recipients is inevitable among recipients viremic at the time of transplantation, and liver failure due to HCV has emerged as an important cause of graft loss leading to retransplantation or death (4–8).
Recent reports have shown that following LT for HCV, blacks as a group have significantly reduced patient and graft survival (9–12). However, not all blacks experience poor outcomes. Recipient/donor race matching appears to be a major determinant of outcomes, as it is only the subgroup of black recipients who have received livers from white donors who experience excess graft loss and mortality (12, 13). Black LT recipients with HCV who receive livers from black donors have graft and patient survival rates similar to white recipients (12, 13). The mechanisms by which recipient/donor race mismatch impacts outcomes after LT for HCV are not known. Paradoxically, in the non-transplant setting, HCV follows a less aggressive course in blacks compared to whites (14–15).
One explanation for these findings is that HCV disease recurrence and fibrosis progression may be more severe in the black recipient/white donor group. Using United Network for Organ Sharing (UNOS) data, we have shown that HCV recurrence is more often reported as the cause of graft failure this group compared to the black recipient/black donor group (13). To investigate the hypothesis that there may be race-related differences in HCV fibrosis progression after LT, we used post-transplantation biopsy data from five transplant centers to look for the development of severe fibrosis during the first two years after LT stratified by donor/recipient race combinations.
Results
Study population
714 white and black recipients underwent LT for HCV at the 5 study sites between 1999 and 2008. Of this population, 469 had at least one biopsy during the first two years post-transplantation and met all other inclusion criteria. There was no difference in the percent of whites and blacks who had biopsies and were thus included in the final study population (64.4% vs 69.8%, NS). The study population included 364 (77.6%) white recipients and 105 (22.4%) black recipients. Table 1 displays the demographics for study participants. Black recipients had significantly lower pre-transplant albumin scores, higher creatinine levels and more often required a combined renal transplantation. They also more often received induction therapy with an anti-lymphocyte agent but displayed a trend toward lower pre-transplant HCV viral loads.
Table 1.
Demographics by recipient race
| VARIABLE | WHITE RECIPIENTS (N=364) | BLACK RECIPIENTS (N=105) | P-value |
|---|---|---|---|
| Recipient Age | 52.4± 7.8 | 51.8 ± 6.8 | NS |
| Recipient Female Gender | 24.7% | 33.3% | 0.08 |
| Recipient BMI | 28.4 ± 5.4 | 29.1 ± 6.2 | NS |
| Recipient Albumin | 2.9 ± 0.6 | 2.5 ± 0.7 | <0.001 |
| Recipient Creatinine | 1.5 ± 1.2 | 2.1 ± 2.4 | <0.001 |
| Hepatocellular Carcinoma | 20.8% | 18.0% | NS |
| Alcohol cirrhosis co-morbidity | 44.5% | 43.1% | NS |
| HBV (Surface Antigen positive) | 2.1% | 2.0% | NS |
| Recipient Diabetes | 22.0% | 23.0% | NS |
| % living related transplant | 5.8% | 5.7% | NS |
| Combined Kidney Transplant | 4.4% | 14.3% | 0.001 |
| Anti-Lymphocyte Induction | 33.5% | 53.0% | <0.001 |
| Recipient pre-transplant log viral load | 5.6 ± 0.9 (N=134) | 5.3 ± 1.4 (N=42) | 0.08 |
| % treated for HCV recurrence post LT | 34.6% | 30.5% | NS |
| % treated for acute rejection in 1st two years | 25.0% | 28.6% | NS |
| Donation after cardiac death | 3.0% | 1.2% | NS |
| Cold Ischemia Time (In hours) | 6.7 ± 2.5 | 7.3 ± 5.1 | 0.2 |
| Donor Age | 39.2 ± 15.1 | 38.1 ± 15.5 | NS |
| Donor Female Gender | 64.0% | 61.0% | NS |
| Donor Race (% B/W) | 86.4%/13.7% | 67.6%/32.4% | <0.001 |
| CMV High Risk (D+/R−) | 20.2% | 8.9% | 0.01 |
BMI: body mass index; HCV: Hepatitis C Virus; LT: Liver Transplantation; B/W: Black/White; D+/R−: Donor positive, recipient negative; NS: Non-significant
Predictors of severe liver fibrosis
Within the first 2 years after LT, 21.5% (101/469) of the study population developed F3 or F4 fibrosis. Table 2 displays differences between recipients that developed stage F3/F4 during the first 2 years after LT (n=101) to those that did not (n=368). Although blacks were only 22% of the total study population, they made up almost 35% of those with F3/F4 fibrosis within 2 years after LT. Examining this group more closely, it was black recipients who had received livers from white donors who were disproportionately affected; 39.4% (28/71) of this group developed F3/F4 fibrosis compared to 20.5% (7/34) of blacks with black donors, 19% (60/314) of whites with white donors and 12% (6/50) of whites with black donors. Of note, patients with F3/F4 had more often been treated for acute rejection, and had a lower mean age. There were no significant differences between groups with respect to comorbidities, use of anti-lymphocyte induction or cold ischemia time. For the 55.5% of the study population for whom we had human leukocyte antigen (HLA) data, we found no association between the level of mismatching at the A, B, or DR loci and the level of fibrosis.
Table 2.
Differences in patients with and without F3/F4
| Variable | No F3/F4 during first two years (N=368) | F3/F4 during first two year (N=101) | P-value |
|---|---|---|---|
| Recipient Race (% Black) | 19.0% | 34.7% | 0.002 |
| Donor Race (% Black) | 19.3% | 12.9% | 0.1 |
| Recipient/Donor Race Combination | 69.0% W/W (N=254) 12.0% W/B (N=44) 7.3% B/B (N=27) 11.7% B/W (N=43) |
59.4% W/W (N=60) 5.9% W/B (N=6) 6.9% B/B (N=7) 27.7% B/W (N=28) |
0.001 |
| Recipient Gender (% Female) | 24.2% | 35.6% | 0.02 |
| Donor Gender (% Female) | 62.5% | 66.3% | NS |
| CMV High Risk (D+/R−) | 16.3% | 21.8% | 0.2 |
| Combined Kidney transplant | 6.0% | 8.9% | NS |
| Living Related transplant | 5.7% | 5.9% | NS |
| Donation after cardiac death | 2.7% | 2.2% | NS |
| HBV Surface Antigen Positive | 2.1% | 2.1% | NS |
| Recipient Diabetes | 24.0% | 16.0% | 0.1 |
| Hepatocellular Carcinoma | 21.0% | 17.2% | NS |
| Alcohol Cirrhosis Comorbidity | 45.0% | 41.7% | NS |
| Treated for acute rejection | 22.6% | 37.6% | 0.002 |
| Treated for HCV recurrence | 32.3% | 38.6% | 0.2 |
| Recipient Albumin | 2.9 ± 0.6 | 2.8 ± 0.7 | NS |
| Recipient INR | 1.6 ± 0.9 | 1.7 ± 0.9 | NS |
| Recipient Creatinine | 1.6 ± 1.5 | 1.6 ± 1.6 | NS |
| Recipient Pre-transplant Log Viral Load | 5.5 ± 1.0 (N=133) | 5.8 ± 0.9 (N=43) | 0.1 |
| Anti-Lymphocyte Induction | 37.5% | 39.2% | NS |
| Recipient Age | 52.7 ± 7.4 | 50.7 ± 8.1 | 0.02 |
| Donor Age | 38.3 ± 15.7 | 41.4 ± 14.8 | 0.07 |
| Recipient BMI | 29.5 ± 14.1 | 29.7 ± 17.8 | NS |
| Cold Ischemia Time (In Hours) | 6.8 ± 2.4 | 7.0 ± 5.0 | NS |
BMI: body mass index; HCV: Hepatitis C Virus; LT: Liver Transplantation; B/W: Black/White; D+/R−: Donor positive, recipient negative; NS: Non-significant; F3/F4: Fibrosis stage 3 or 4
Fibrosis stage by time and race
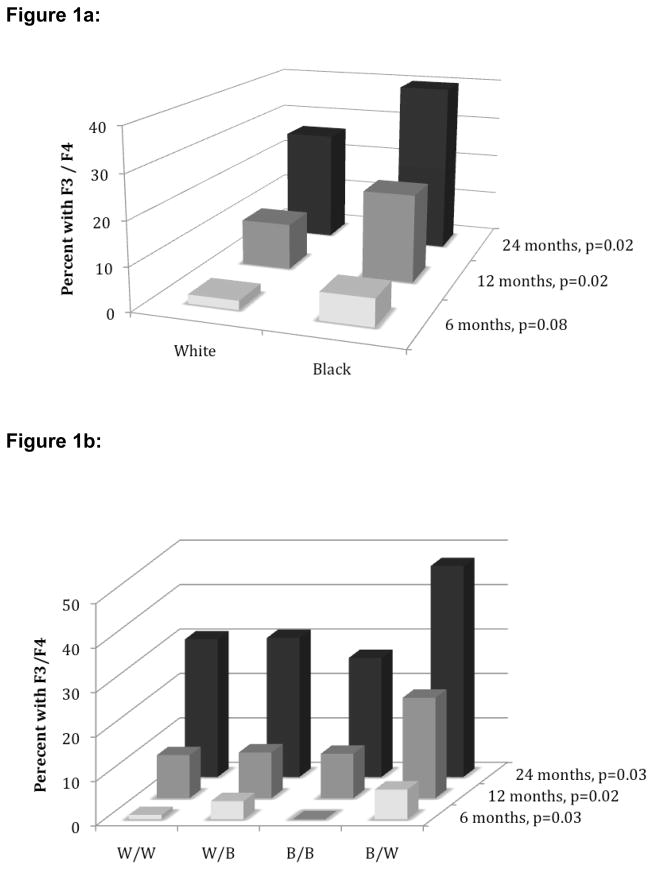
To discern the timing of severe fibrosis after LT, the percentage of patients with F3/F4 fibrosis at 6 (N = 302), 12 (N = 357) and 24 months (N = 230) after transplantation were assessed. Figure 1a demonstrates that at each of the three biopsy time points, a higher percentage of black recipients had a score of F3 or F4 compared to white recipients. At 6 months, the difference in the rate of F3 or F4 fibrosis between whites and blacks did not quite reach significance (2.1% vs 6.3% for white and black recipients, respectively [p=0.08]), but the differences at 12 and 24 months were significant (10.8% vs 20.5% (p=0.02) and 26.3% vs 40.0% (p=0.02), respectively). These differences could not be explained by differences in biopsy rates by race; among patients who reached each time point, similar percentages of whites and blacks underwent liver biopsy (65.4% of whites vs 60.9% of blacks at 6 months, 79.0% vs 78.7%, respectively, at 12 months and 59.4% vs 66.7%, respectively, at 24 months). Figure 1b depicts the percent of patients with F3/F4 fibrosis by recipient/donor combinations. At each biopsy time point, black recipients with white donors constituted the highest proportion of those with scores of F3/F4. By 12 and 24 months, 22.6% and 47.5%, respectively, of black recipients with white donors had fibrosis scores of F3 or F4, significantly higher than all other recipient/donor combinations. Black recipients with black donors had rates of F3/F4 fibrosis similar to white recipients at all time points.
Figure 1.
Figure 1a: Percent of patients with fibrosis stage 3/4 by recipient race
Figure 1b: Percent of patients with fibrosis stage 3/4 by recipient/donor race combination
W: White; B: Black; F3/F4: Fibrosis stage 3 or 4
Impact of recipient/donor race matching on severe fibrosis development
Table 3a illustrates that black recipients were greater than two times more likely to develop an F3 or F4 score during the first two years after LT compared to white recipients (odds ratio [OR]=2.26, p=0.001). In addition, receipt of a liver from a black donor tended to protect against the development of severe fibrosis, with an odds ratio of 0.62 (0.33–1.67; p=0.14), regardless of recipient race. Furthermore, analyzing the effect of recipient/donor race mismatch on the development of severe fibrosis showed that recipients with a race-mismatched liver were 67% more likely to develop F3/F4 fibrosis (OR=1.67 [1.04–2.67], p=0.03). However, an interaction was identified (p=0.03) that indicated the impact of recipient/donor race mismatching varied depending on the race of the recipient. To assess the specific differences, recipient/donor race categories were created and examined for their effect on the development of F3/F4 fibrosis (Table 3a). Black recipients with white donors were the only group (using white recipients with white donors as a reference group) that had significantly greater likelihood of developing severe fibrosis (OR=2.76 [1.59–4.79], p<0.001).
Table 3a.
Univariable logistic regression: F3/F4 during first two years post-transplantation by donor/recipient race
| Variable | Odds Ratio | 95% C.I | P-value |
|---|---|---|---|
| Black Recipient Race (compared to White recipient race) | 2.26 | 1.39–3.67 | 0.001 |
| Black Donor race (compared to White recipient race) | 0.62 | 0.33–1.17 | 0.14 |
| Recipient/Donor Mismatch | 1.67 | 1.04–2.67 | 0.03 |
| Interaction between Recipient Race and Mismatch | Not applicable | Not applicable | 0.03 |
| Recipient/Donor race combination Reference group: W/W | |||
| W/B vs W/W | 0.58 | 0.24–1.42 | 0.28 |
| B/B vs W/W | 1.10 | 0.46–2.64 | 0.84 |
| B/W vs W/W | 2.76 | 1.59–4.79 | <0.001 |
To adjust for other factors that could impact the development of severe fibrosis, multivariable logistic regression analysis was performed with the results shown in Table 3b. After controlling for recipient and donor age, recipient gender, recipient creatinine and albumin, recipient diabetes, CMV high risk status, cold ischemic time, treatment for acute rejection and treatment for HCV recurrence, the adjusted OR for black recipients with white donors compared to white recipients of white donors was 2.54 for the development of an F3/F4 score (1.49–4.69; p<0.001). The other two recipient/donor race combinations had likelihoods of developing severe fibrosis similar to the white recipient/white donor group.
Table 3b.
Multivariable adjusted odd’s ratio: F3/F4 during first two years post-transplantation by donor/recipient race
| Variable | Odds Ratio | 95% C.I | P-value |
|---|---|---|---|
| Recipient/Donor race combination Reference group: W/W | |||
| W/B vs W/W | 0.57 | 0.23–1.43 | 0.24 |
| B/B vs W/W | 1.13 | 0.46–2.76 | 0.78 |
| B/W vs W/W | 2.54 | 1.49–4.69 | <0.001 |
controlled for CMV high-risk status, recipient creatinine and albumin, anti-lymphocyte induction therapy, donor and recipient age, recipient gender, cold ischemia time, treatment for acute rejection, and treatment for HCV recurrence
The multivariable analysis did not adjust for pre-transplant viral load, given the low number of patients for whom these data were available (N = 176). A sub-analysis run with the categorical recipient/donor race variable and pre-transplant viral load showed that recipient/donor race mismatch remained significant, with an OR of 3.0 (1.2–7.4; p=0.02).
Because the treatment rate for acute rejection was significantly different between those developing F3/F4 fibrosis and those who did not, a multivariable analysis excluding patients treated for acute rejection was also performed. This did not markedly alter the results and yielded an OR for the development of a fibrosis score of F3/F4 of 3.12 (2.57–6.20; p=0.001) for the black recipient/white donor group. Also, because treatment for HCV recurrence could impact fibrosis progression, a multivariable analysis excluding patients treated for HCV recurrence was performed with similar results; the adjusted OR for black recipients with white donors for the development of severe fibrosis was 2.96 (1.47–6.21; p=0.001). Again, no differences in ORs were noted in other recipient/donor combinations compared to the white recipient/white donor group.
Finally, we assessed for variation in HLA mismatching across the different donor/recipient race pairings in the sub-population for whom these data were reported. There existed no difference in the degree of mismatching at the A, B, or DR loci, or when considering all loci combined according to donor/recipient race combinations.
Impact of recipient/donor race matching and severe fibrosis on patient mortality
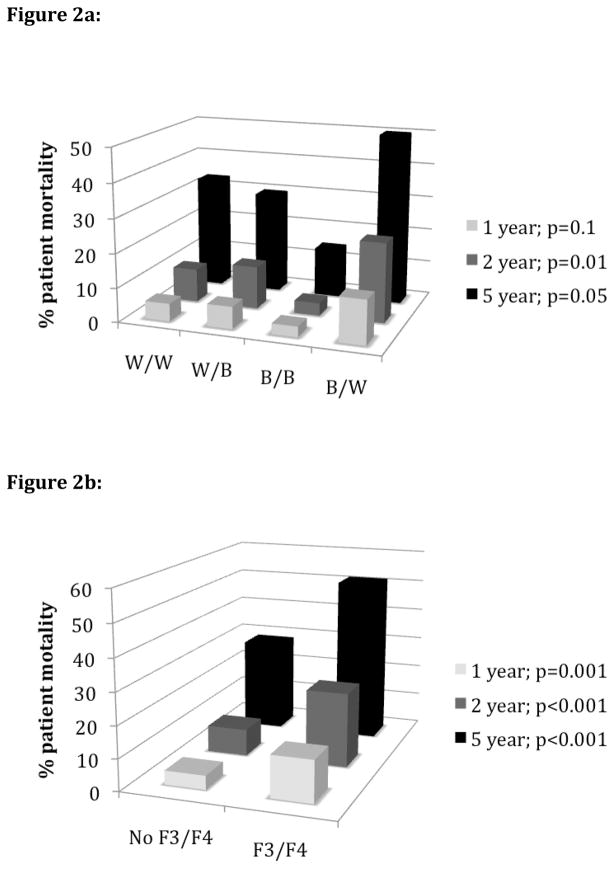
Figures 2a depicts the one, two, and five-year mortality rates based on recipient and donor rates. Black recipients of white donors had one, two and five year mortality rates of 12.9%, 23.3% and 50.0%, respectively, which was significantly higher than the other recipient/donor combinations at two (p=0.01) and five (p=0.05) years. The development of F3/F4 at some point during the first two years also significant increased mortality rates. For those withithout F3/F4, the one, two, and five year mortality rates were 4.4%, 8.5%, and 28.9% compared to 13.2%, 23.5%, and 51.6% for those with F3/F4 (p<0.001 for all time points).
Figure 2.
Figure 2a: One, two, and five-year patient mortality rate post-transplant by recipient/donor race
Figure 2b: One, two, and five-year patient mortality rate post-transplant based on fibrosis progression
W: White, B: Black; F3/F4: Fibrosis stage 3 or 4
In a patient survival analysis using multivariable Cox regression (controlling for all variables listed in Table 3b footnote), black recipients with white donors had the lowest patient survival, with a hazard ratio (HR) for death of 1.7 (1.04–2.7; p=0.03) compared to the other recipient/donor race combinations. The development of stage F3 or F4 fibrosis was also significantly associated with patient death, with a HR of 2.2 (1.5–3.3; p<0.001). Interestingly, when the development of F3/F4 was controlled for, the impact of recipient/donor race combination on patient death was reduced. The HR for black recipients of white donors after controlling for an F3/F4 fibrosis score was 1.4 (0.92–2.1; p=0.10). However, when controlling for recipient/donor race combination, having an F3/F4 fibrosis score remained significant, with a HR of 2.0 (1.4–3.1; p=0.001). Thus, it appears that the development of F3/F4 has a greater impact on patient death than recipient/donor race combination. Because black recipients with white donors consistently had the highest rates of F3/F4, survival was significantly lower in this group.
Discussion
Several studies have shown lower patient and graft survival for blacks who undergo HCV-related liver transplantation compared to whites recipients (9–13). Donor race also impacts outcomes, although this is not a uniform association (16). Recent data suggest that this may be largely limited to black recipients of livers from white recipients (12, 13), findings derived from analyses of UNOS data. Given the limitations of the UNOS database, it is not possible from these studies to adequately identify reasons for these disparate outcomes.
In the present study we hypothesized that the severity of HCV recurrence and fibrosis progression was greater in the first two years post-transplantation among black recipients, and particularly among black recipients with white donors. We found that while black recipients had overall higher rates of fibrosis progression at 6, 12 and 24 months post-transplantation, this was primarily limited to black recipients with white donors. That these results mirror prior studies showing the negative impact of black recipient/white donor race on patient survival suggests that lower patient survival in this group may be driven, in part, by the accelerated fibrosis progression. Indeed, when patient survival was analyzed, controlling for both recipient/donor combination and severe fibrosis during the first two years, only severe fibrosis remained a significant predictor of survival. For reasons as yet unexplained, black recipients with white donors have higher rates of severe fibrosis.
Many variables can impact patient and/or graft survival (12, 13, 16–23). In this study, we found recipient and donor age, female recipient gender, and treatment for acute rejection were also associated with severe fibrosis. There was also a trend for higher pre-transplant viral loads and CMV high-risk status to be associated with severe fibrosis. However, controlling for variables that varied across the race groups or that were associated with severe fibrosis did not significantly change the association between black recipient/white donor race and the development of severe fibrosis nor did it change the association of these variables with patient mortality.
Why blacks who are transplanted for HCV with livers from white donors have more severe fibrosis compared to other recipient/donor race combinations is unclear. It suggests, however, that both donor- and recipient-related factors are important. Genetic polymorphisms are associated with racial differences in response to HCV treatment with pegylated interferon and ribavirin, and with spontaneous clearance of the virus in acute HCV infection (24–27). Very recent studies suggest that polymorphisms in recipients and donors may impact disease recurrence after HCV-related liver transplantation (28–30). Differences in these genetic polymorphisms across race groups in the setting of liver transplantation have not been fully examined, however.
There are notable strengths to this study including its multicenter design, analysis of both donor and recipient characteristics and the racially diverse patient population; nationwide in 2008, 10.2% of LT recipients were black (31), while blacks comprised over 20% of our study population. The study also has several limitations which warrant discussion. While we had basic information on whether patients were treated for acute rejection and/or HCV treatment recurrence, we lacked detailed information about timing of the treatments and dosing. To account for this, we examined sub-groups that were not treated for acute rejection or HCV recurrence and found no differences in the results. To better account for these variables, prospective studies that collect detailed information on these events need to be performed. Furthermore, given the retrospective multi-site design, liver biopsies were not performed at all time points on all patients. However, even studies that incorporate protocol biopsies are often limited by non-compliance with the performance of those biopsies (32,33). Despite these limitations, this is the first multi-site study to illustrate a significant effect of donor/recipient race combination on fibrosis progression after HCV-related LT. Future prospective studies are needed to examine the potential mechanisms that could account for differences in disease progression after HCV-related LT.
Methods
Study Design and Patient Population
A retrospective multi-site cohort study was performed involving adults who underwent primary liver transplantation for HCV between 1999–2008 at 5 U.S. transplant centers: University of Illinois Medical Center, Chicago, IL; Henry Ford Hospital, Detroit, MI; University of Wisconsin Hospital, Madison, WI; University of Chicago Medical Center, Chicago, IL; Loyola University Medical Center, Maywood, IL. Institutional review board approval was obtained at each study site. All HIV-uninfected white and black adults (aged ≥ 18 years) who underwent primary liver transplantation for HCV-related ESLD between 1999–2008, lived more than 30 days after LT and had ≥ 1 liver biopsy performed within the first two years post-transplantation were included. Race was self-identified and was congruent with what was reported for the UNOS database. Hispanics were excluded to avoid bias; recent reports have suggested improved survival outcomes among Hispanics compared to whites after HCV-related LT (9). Standard immunosuppression protocols were employed at each institution, and there was no protocol-specific adjustment in calcineurin inhibitor dosing based on HCV recurrence severity. Decisions regarding treatment of acute rejection and HCV recurrence were at the discretion of the treating physicians at each institution.
Outcomes
The primary outcome was fibrosis stage (F0 to F4) assessed by the Batts and Ludwig scoring system (34) at 6, 12 and 24 months post-transplantation. Severe fibrosis was defined as F3 or F4 at any time point. A window of two months on either side of the time point was deemed acceptable for biopsies (i.e., for the 6 month post-LT time point, a biopsy that was performed between 4–8 months after LT was included). If a patient had a biopsy score of F0 at 12 months or 24 months, it was assumed that the fibrosis stage was F0 at six months as well. Biopsy fibrosis stage scores were determined by pathologists at each site at the time of the liver biopsy. Patient survival was also analyzed and patients were followed until death, re-transplantation or last follow-up.
Study Data
Medical charts and transplant databases at each site were reviewed to obtain: 1) recipient data, including age at transplant, race, gender, body mass index (BMI), pre-transplant laboratory values, and whether the patient had the pre-transplant comorbid conditions of alcohol-related cirrhosis, hepatitis B virus ([HBV]; defined as surface antigen positive), and hepatocellular carcinoma (HCC) 2) donor data, including age, gender, race and CMV serostatus 3) transplant data, including type of transplant (deceasedvs living donor), simultaneous renal transplant, cold ischemia time, and whether the patient received an anti-lymphocyte agent for induction immunosuppression 4) post-transplant data, including treatment for acute rejection with steroids, treatment for HCV recurrence, and date of last follow-up, death, or re-transplantation. Additionally, we examined donor-recipient HLA matching for those patients whose HLA data were reported (55.5% of the total study population).
Statistical Methods
Baseline demographics were compared between recipient race groups using the student t-test for continuous variables and Pearson’s chi-square analyses for categorical variables after normalcy was assessed. The same statistical tests were used to compare differences between those with and without severe fibrosis within the first two years post-transplantation. Differences in fibrosis stage score at 6,12 and 24 months were compared between recipient race groups and recipient/donor race combinations using chi-square testing. Uni- and multivariable binary logistic regression were used to calculate unadjusted and adjusted odds ratios, with the dichotomous outcome being an F3/F4 score during the first two years post transplantation. Additional logistic regression models were run excluding patients that were treated for acute rejection and treatment for HCV recurrence. Uni and multi-variable Cox proportional hazards regression models were used to analyze time to patient death with a time-dependent Cox proportional hazard model used to account for the timing to F3/F4 progression. All data were analyzed using SPSS 17.0 (SPSS Inc, Chicago, IL).
Acknowledgments
Financial Support: This project was supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number KL2RR029878 (JEL) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health
Abbreviations
- HCV
Hepatitis C Virus
- HBV
Hepatitis B Virus
- HBsAg
Hepatitis B Surface Antigen
- HCC
Hepatocellular Carcinoma
- HLA
Human leukocyte antigen
- LT
Liver Transplantation
- US
United States
- ESLD
End Stage Liver Disease
- BMI
Body Mass Index
- HR
Hazard Ratio
- MELD
Model for End Stage Liver Disease
- UNOS
United Network for Organ Sharing
Footnotes
Author Contributions:
JEL, SJC, MRL, TJL, NMC: participated in research design, performance of research, analysis and writing of manuscript KAB, HST, SE, CF: participated in research design, performance of research and analysis
Disclosures: The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999 Aug 19;341(8):556–6. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Di Bisceglie AM, Goodman ZD, Ishak KG, et al. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–74. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 3.Atler 1997 Epidemiology of Hepatitis C. Hepatology. 1997 Sep;26(3 Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M. Hepatitis C infection in liver transplantation. Am J Transplant. 2001 Sep;1(3):197–203. doi: 10.1034/j.1600-6143.2001.001003197.x. [DOI] [PubMed] [Google Scholar]
- 5.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996 Mar 28;334(13):815–20. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 6.Forman LM, Lewis JD, Berlin JA, et al. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002 Apr;122(4):88. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Ferrell L, Watson J, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000 Apr;32(4):673–84. doi: 10.1016/s0168-8278(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 8.Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009 Aug;9(8):1707–12. doi: 10.1111/j.1600-6143.2009.02702.x. [DOI] [PubMed] [Google Scholar]
- 9.Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the MELD era. Am J Gastroenterol. 2008 Apr;103(4):901–10. doi: 10.1111/j.1572-0241.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 10.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002 Jan 26;359(9303):287–93. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 11.Velidedeoglu E, Mange K, Frank A, et al. Factors differentially correlated with the outcome of liver transplantation in HCV+ and HCV− recipients. Transplantation. 2004;77(12):1834–42. doi: 10.1097/01.tp.0000130468.36131.0d. [DOI] [PubMed] [Google Scholar]
- 12.Pang PS, Kamal A, Glenn J. The effect of donor race on the survival of Black Americans undergoing liver transplantation for chronic hepatitis C. Liver Transpl. 2009 Sep;15(9):1126–32. doi: 10.1002/lt.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Layden JE, Cotler SC, Grim SA, Fischer MJ, Lucey MR, Clark NM. The impact of donor and recipient race on survival after hepatitis C-related liver transplantation. Transplantation. 2012 Feb 27;93(4):444–9. doi: 10.1097/TP.0b013e3182406a94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling RK, Stravitz RT, Luketic VT, et al. A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans. ClinGastroenterolHepatol. 2004 Jun;2(6):469–73. doi: 10.1016/s1542-3565(04)00164-8. [DOI] [PubMed] [Google Scholar]
- 15.Wiley TE, Brown J, Chan J. Hepatitis C infection in African Americans: its natural history and histological progression. Am J Gastroenterol. 2002 Mar;97(3):700–6. doi: 10.1111/j.1572-0241.2002.05555.x. [DOI] [PubMed] [Google Scholar]
- 16.Asrani SK, Lim YS, Therneau TM, et al. Donor race does not predict graft failure after liver transplantation. Gastroenterology. 2010 Jun;138(7):2341–7. doi: 10.1053/j.gastro.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burak KW, Kremers WK, Batts KP, et al. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transpl. 2002 Apr;8(4):362–9. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 18.Berenguer M, Crippin J, Gish R, et al. A model to predict severe HCV-related disease following liver transplantation. Hepatology. 2003 Jul;38(1):34–41. doi: 10.1053/jhep.2003.50278. [DOI] [PubMed] [Google Scholar]
- 19.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28(3):823–30. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 20.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transpl Surg. 1999 Jul;5(4 Suppl 1):S107–1. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 21.Sugo H, Balderson GA, Crawford D, et al. The influence of viral genotypes and rejection episodes on the recurrence of hepatitis C after liver transplantation. Surg Today. 2003;33(6):421–5. doi: 10.1007/s10595-002-2537-5. [DOI] [PubMed] [Google Scholar]
- 22.Charlton M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transpl. 2003 Nov;9(11):S58–62. doi: 10.1053/jlts.2003.50245. [DOI] [PubMed] [Google Scholar]
- 23.Ciccorossi P, Maina AM, Oliveri F, et al. Viral load 1 week after liver transplantation, donor age and rejections correlate with the outcome of recurrent hepatitis C. Liver Int. 2007 Jun;27(5):612–9. doi: 10.1111/j.1478-3231.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 24.Ge D, Fellay J, Thompson A, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009 Sep 17;461(7262):399–40. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009 Oct 8;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010 Apr;138(4):1338–45. 1345.e1–7. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009 Oct;41(10):1105–9. doi: 10.1038/ng.449. Epub 2009 Sep 13. [DOI] [PubMed] [Google Scholar]
- 28.Lange CM, Moradpour D, Doehring A, et al. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2010 Dec 10; doi: 10.1016/j.jhep.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Charlton MR, Thompson A, Veldt BJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011 Jan;53(1):317–24. doi: 10.1002/hep.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eurich D, Boas-Knoop S, Ruehl, et al. Relationship between the interleukin-28b gene polymorphism and the histological severity of hepatitis C virus-induced graft inflammation and the response to antiviral therapy after liver transplantation. Liver Transpl. 2011 Mar;17(3):289–98. doi: 10.1002/lt.22235. [DOI] [PubMed] [Google Scholar]
- 31.UNOS DATABASE. < http://www.unos.org/
- 32.Berenguer M, Aguilera V, San Juan F, et al. Effect of calcineurin inhibitors in the outcome of liver transplantation in hepatitis C virus-positive recipients. Transplantation. 2010 Dec 15;90(11):1204–9. doi: 10.1097/TP.0b013e3181fa93fa. [DOI] [PubMed] [Google Scholar]
- 33.Firpi RJ, Abdelmalek MF, Soldevila-Pico C, Cabrera R, Shuster JJ, Theriaque D, Reed AI, Hemming AW, Liu C, Crawford JM, Nelson DR. One-year protocol liver biopsy can stratify fibrosis progression in liver transplant recipients with recurrent hepatitis C infection. Liver Transpl. 2004 Oct;10(10):1240–7. doi: 10.1002/lt.20238. [DOI] [PubMed] [Google Scholar]
- 34.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J SurgPathol. 1995;19(12):1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]