Dear Editor:
As recently published in your Journal, the LILAC prospective cohort study showed a decrease in self-reported adherence between pregnancy and postpartum in South America.1 Interestingly, our smaller study with a longitudinal measure and analysis of adherence to antiretroviral treatment confirms these findings.
Antiretroviral therapy (ART) during pregnancy and postpartum involves two issues: prophylaxis of perinatal transmission and treatment of maternal infection.2 Adherence, defined as “the process by which patients take their medications as prescribed,” is crucial to address these concerns.3,4
Pregnancy and the first months of motherhood cause important changes in a women's life, which might impact ART adherence. Most frequently cited barriers to adherence were competition with other issues including family obligation and hectic lifestyle. Conversely, the baby's health was cited as a motivator for adherence.5
During postpartum, women tend to miss more medical visits, and adherence tends to be lower than during pregnancy.1,6–10 Between 10% and 50% of the women stopped their ART after childbirth, part of them on their own without physician's approval.7–10 Most of published studies are based on pill count or self-reported adherence. To our knowledge this is the first study assessing electronically the dynamics of adherence in a continuum from pregnancy to postpartum along with clinical data.
This exploratory, retrospective study was approved by the Swiss Ethic Commission (Vaud) for clinical research. It aimed at comparing adherence to the entire ART—during pregnancy and postpartum—using continuous electronic drug monitoring data.
The community Pharmacy of the Department of ambulatory care and community medicine in Lausanne has been running an adherence-enhancing program since 2004, which combines electronic drug monitoring and semistructured, repeated motivational interviews.4 All pregnant HIV-positive women having taken part in the program between 2004 and 2012 were retrieved.
The maximal observation period extended from first adherence visit after last menstruation to 6 months after childbirth. Sociodemographic and clinical data were collected from the Swiss HIV Cohort Study database. Percent of attended visits were compared between pregnancy and postpartum using the McNemar test.
Electronic data on medication adherence, extracted from the adherence-enhancing program database, were reconciled with pill count and interview notes in order to include reported pocket doses. For each woman, adherence was described with a binary variable (1=correct number of daily opening(s) of all electronic monitors; 0=less daily openings than prescribed). Data were analyzed via a piecewise logistic mixed effect model. Mixed effect models are methods of choice for analyzing data with repeated measures for each participant. They take into account the interindividual variability of the measures and can deal with unbalanced data due to missing values. In particular, a piecewise logistic mixed effect model allowed us to estimate the probability of taking ART as prescribed, for each day d of pregnancy and postpartum periods.11 Adherence at days 0–3 from childbirth has been replaced by missing values before entering the model, because of the bias due to hospitalization.
Analysis of change in CD4 over time was performed using a piecewise polynomial mixed effect model. A qualitative inspection of viral RNA individual trajectories was made only graphically, as the large interindividual variability and the small number of data prevented the application of a mixed effect model. We used the Stata/IC software (v12.0, StataCorp, College Station, TX) for data description and the R system for computation and graphic of mixed effect models (v2.12.1, www.r-project.org/, library “nlme”).
Among 400 patients referred to the adherence program, 29 pregnant women (7%) were screened and 25 (86%) were included. Three women who did not use electronic monitors were excluded, and one underwent an early pregnancy interruption. Median age was 29 (interquartile range [IQR]: 26.5, 32.0). Seventeen women (68%) were black and 11 (44%) were ART naive. ART included protease and nucleoside reverse transcriptase inhibitors (n=24; 96%).
Six women (24%) stopped the program during pregnancy, 3 (12%) at childbirth and 4 (16%) during the postpartum period. Among these 13 women, 8 (62%) were kept on ART, 3 stopped ART after childbirth according to guidelines, and 2 stopped ART without physician's agreement. All these women were considered in the analysis. The percentage of unattended visits increased from 17% during pregnancy to 38% during postpartum (p=0.001).
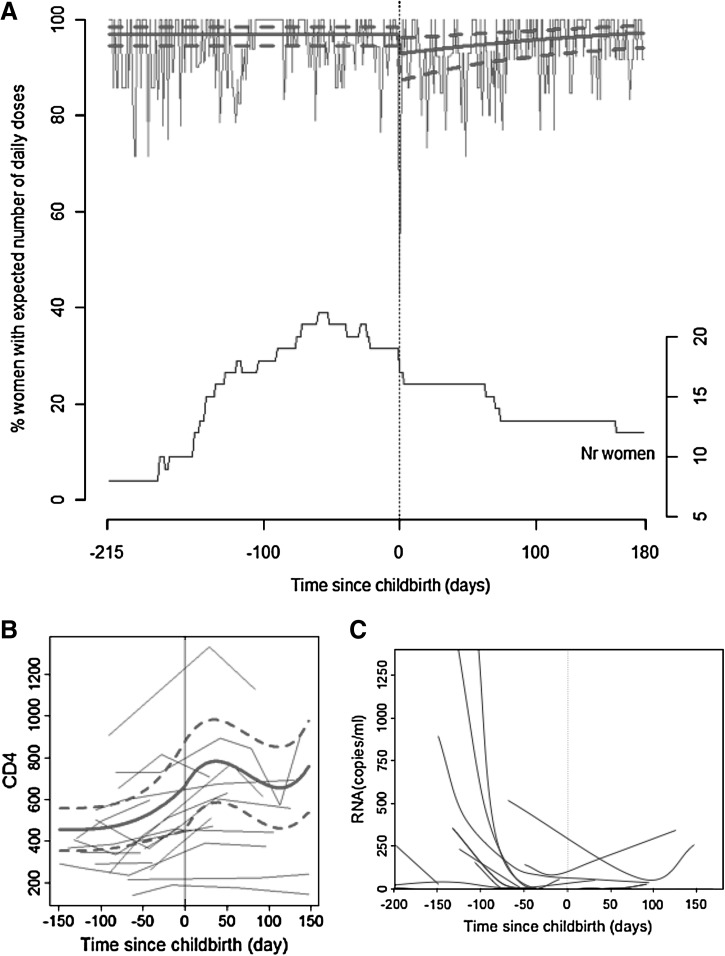
Probability of correct ART intake was continuously high during pregnancy (97% [95% CI: 0.94–0.98]), decreased to 93% (95% CI: 0.87–0.96) at childbirth (p of the difference=0.006), and increased again during postpartum (p of the slope coefficient=0.009) (Fig. 1A) to reach the same probability than during pregnancy after 164 days. A sensitivity analysis with the 10 women who were continuously monitored during pregnancy and postpartum confirmed the decrease in ART intake after childbirth.
FIG. 1.
Change in drug intake, viral load, and CD4 count over time. (A) Percentage of women with correct number of daily opening(s) of the electronic monitors over time. Curves in bold represent the prediction±95% confidence interval, (days 0–3 at birth have been excluded from calculation of the model as data are biased due to hospitalization). Curve in the lower part of the graph represents number of participating women over time. (B) Modeled change in CD4 count over time. (C) Change in RNA (copies per milliliter) over time per woman.
CD4 count increased during pregnancy, decreased around 40 days after childbirth then increased again (p of the linear, quadratic and cubic coefficient <0.005; Fig. 1B). Individual ARN curves decreased during pregnancy. Within the 13 continuing women after childbirth, 4 (31%) had at least one worsening viral load (range, 25–344 copies per milliliter; Fig. 1C).
In summary, our data describe adherence during pregnancy and postpartum from a real-life medication adherence clinic. Our model predicts that 97 women of 100 take all doses of ART during pregnancy versus 93 women 3 days after childbirth. Our results also mean that missing doses increased from 1 per month (i.e., 3 per 100 days) during pregnancy to 2 per month (i.e., 7 per 100 days) at beginning of postpartum. We could show a lag time during postpartum before women were able to reach the same probability of adherence than during pregnancy.
Strength of our analysis results from: (1) a day-by-day adherence monitoring to entire ART, (2) reconciliation of electronic data with pill count and interview to reflect actual drug intake, and (3) the use of advanced statistics.
Our main limitation is due to the fact that 9 women stopped the program before or at childbirth. Fortunately, it did not impact the adherence level during pregnancy as confirmed by a sensitivity analysis. External validity is limited by the fact that women were included in an adherence-enhancing program. Drop in adherence during standard care might be more significant.
Our data show that a major event such as childbirth impacts adherence transiently, which may result in viral rebound. Of note, 4 women of 13 had at least one worsening viral load. These preliminary results need to be confirmed by larger studies. Although much attention in clinical care is devoted to pregnant women, emphasis should be put on postpartum care as well.
Acknowledgments
This study was O.M.'s master's thesis work. M.C., M.P.S., O.B., O.M., and A.G. were responsible for study design. O.M. was responsible for data collection. M.R. was responsible for the Swiss HIV Cohort database extraction. I.L., A.G., O.M., and M.P.S. did the statistical analyses. A.G., M.P.S., and M.C. wrote the manuscript. All authors read and approved the article.
The authors would like to thank Pilar Zuniga Rojas, Maria Del, and Séverine Gorgerat for their help in the retrospective data search.
Part of the data extraction was possible thanks to the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant # 33CS30_134277).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kreitchmann R. Harris DR. Kakehasi F, et al. Antiretroviral adherence during pregnancy and postpartum in Latin America. AIDS Patient Care STDs. 2012;26:486–495. doi: 10.1089/apc.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Transmission PoToH-IPWaPoP. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2012. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. [Oct 30;2012 ]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf
- 3.Vrijens B. De Geest S. Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummenacher I. Cavassini M. Bugnon O. Schneider MP. An interdisciplinary HIV-adherence program combining motivational interviewing and electronic antiretroviral drug monitoring. AIDS Care. 2011;23:550–561. doi: 10.1080/09540121.2010.525613. [DOI] [PubMed] [Google Scholar]
- 5.Ciambrone D. Loewenthal HG. Bazerman LB. Zorilla C. Urbina B. Mitty JA. Adherence among women with HIV infection in Puerto Rico: The potential use of modified directly observed therapy (MDOT) among pregnant and postpartum women. Women Health. 2006;44:61–77. doi: 10.1300/j013v44n04_04. [DOI] [PubMed] [Google Scholar]
- 6.Nassali M. Nakanjako D. Kyabayinze D. Beyeza J. Okoth A. Mutyaba T. Access to HIV/AIDS care for mothers and children in sub-Saharan Africa: Adherence to the postnatal PMTCT program. AIDS Care. 2009;21:1124–1131. doi: 10.1080/09540120802707467. [DOI] [PubMed] [Google Scholar]
- 7.Nachega JB. Uthman OA. Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-, middle and high income countries: A systematic review and meta-analysis. AIDS. 2012;26:2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellins CA. Chu C. Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–968. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- 9.Ickovics JR. Wilson TE. Royce RA, et al. Prenatal and postpartum zidovudine adherence among pregnant women with HIV: Results of a MEMS substudy from the Perinatal Guidelines Evaluation Project. J Acquir Immune Defic Syndr. 2002;30:311–315. doi: 10.1097/00126334-200207010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Cohn SE. Umbleja T. Mrus J. Bardeguez AD. Andersen JW. Chesney MA. Prior illicit drug use and missed prenatal vitamins predict nonadherence to antiretroviral therapy in pregnancy: Adherence analysis A5084. AIDS Patient Care STDs. 2008;22:29–40. doi: 10.1089/apc.2007.0053. [DOI] [PubMed] [Google Scholar]
- 11.Naumova EN. Must A. Laird NM. Tutorial in Biostatistics: Evaluating the impact of 'critical periods' in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol. 2001;30:1332–1341. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]