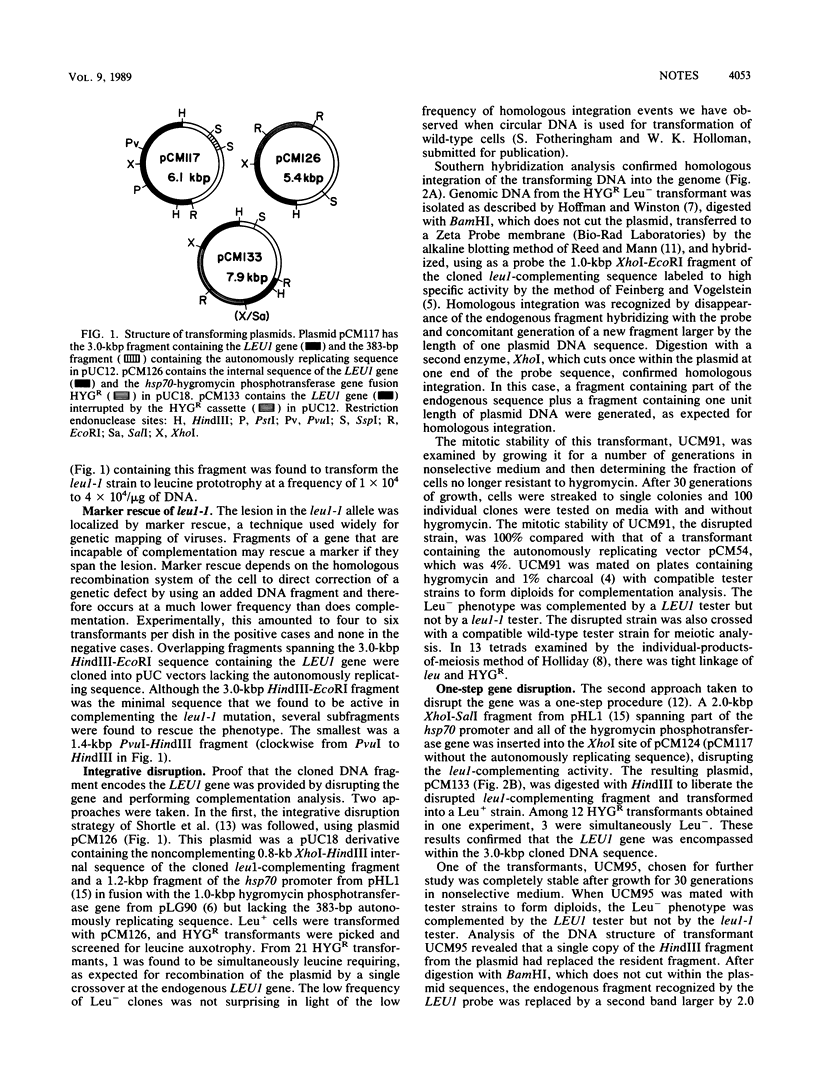
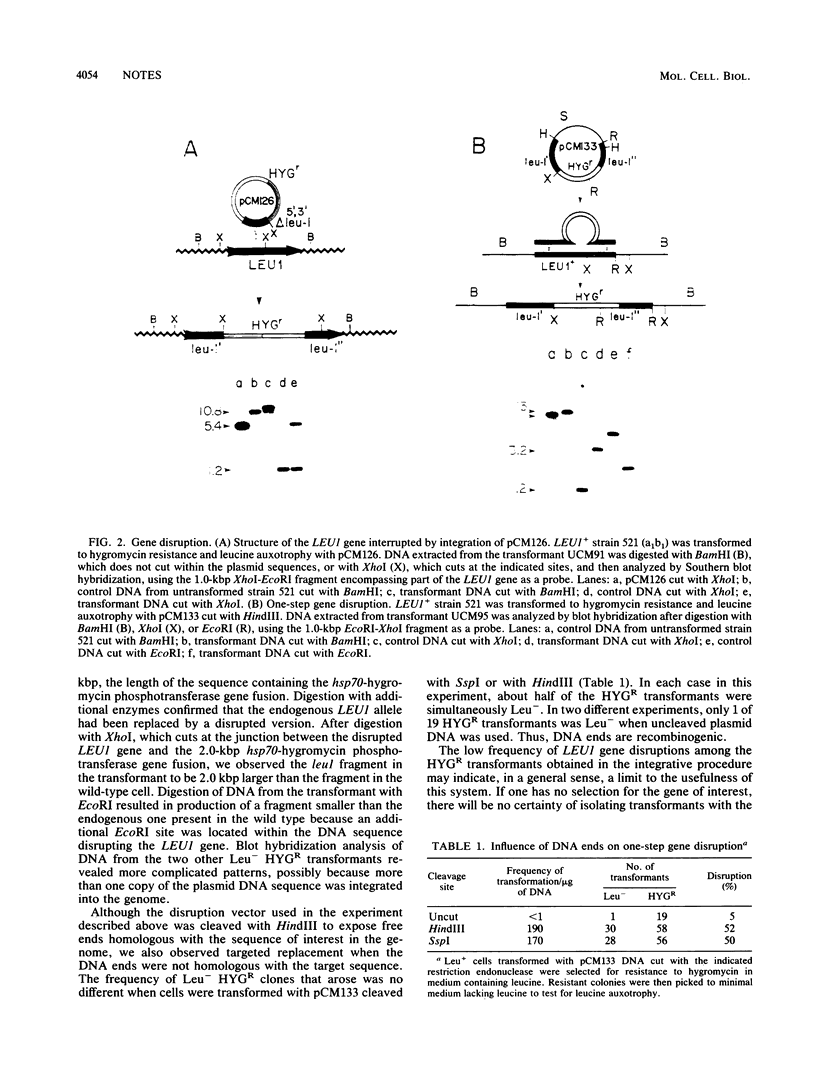
Abstract
We have demonstrated that genes from Ustilago maydis can be cloned by direct complementation of mutants through the use of genomic libraries made in a high-frequency transformation vector. We isolated a gene involved in amino acid biosynthesis as an illustrative example and showed that integrative and one-step disruption methods can be used to create null mutations in the chromosomal copy of the gene by homologous recombination. The results of this investigation make it clear that one-step gene disruption will be of general utility in investigations of U. maydis, since simple, precise replacement of the sequence under study was readily achieved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Taylor S. Y. Cloning of the PYR3 gene of Ustilago maydis and its use in DNA transformation. Mol Cell Biol. 1988 Dec;8(12):5417–5424. doi: 10.1128/mcb.8.12.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Day P. R., Anagnostakis S. L. Corn smut dikaryon in culture. Nat New Biol. 1971 May 5;231(18):19–20. doi: 10.1038/newbio231019a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Holliday R. Radiation sensitive mutants of Ustilago maydis. Mutat Res. 1965 Dec;2(6):557–559. doi: 10.1016/0027-5107(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Homologous pairing of DNA molecules promoted by a protein from Ustilago. Cell. 1982 Jun;29(2):367–374. doi: 10.1016/0092-8674(82)90153-2. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Holden D. W., Leong S. A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci U S A. 1988 Feb;85(3):865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]