Abstract
The sympathetic nervous system regulates human immune system functions through epinephrine (Epi) and norepinephrine (NE) activation of adrenergic receptors (AR) expressed on immunocompetent cell populations. The anti-inflammatory effects that are most often attributed to increased sympathetic activity have been shown to occur through β2- and α2-AR stimulation. However, dichotomous AR effects on immune system function are becoming increasingly apparent. Reports of α1-AR expression on immune cell populations have been conflicting due to a lack of specific antibodies or subtype-selective receptor ligands. This has made α1-AR identification difficult and further characterization of α1-AR subtype expression limited. Nevertheless, there is some evidence suggesting an induction of α1-AR expression on immunocompetent cells under certain physiological conditions and disease states. Also, the function of α1-AR activation to modulate immune responses is just beginning to emerge in the literature. Changes in the secretion of inflammatory mediators as well as increased cell migration and differentiation have been described following α1-AR stimulation on immunocompetent cells. These observations demonstrate the significance of α1-AR activity in immune cell biology and emphasize the importance for understanding α1-AR effects on the immune system.
I. Introduction
The endogenous catecholamines epinephrine (Epi) and norepinephrine (NE) are critical for initiating the “fight or flight” response of the sympathetic nervous system. Epi and NE are released from peripheral neurons and the adrenal medulla in response to physical as well as psychological stress to regulate a number of physiological functions including energy metabolism, cardiovascular homeostasis and thermal adaptation. There are extensive interactions of the central nervous system with the immune system and all immune organs are innervated by post-ganglionic sympathetic fibers. Furthermore, sympathetic nerve terminals are located in the vicinity of immune cells that comprise both the innate and adaptive immune system. Moreover, macrophages have recently been shown to synthesize and release catecholamines in vivo (Flierl et al., 2007). Consequently, the close propinquity of catecholamines release to cells of the immune system introduces an opportunity for these endogenous AR agonists to regulate immune cell functions.
AR-mediated sympathetic responses to stress are a result of receptor agonist stimulation caused by the increased release of Epi and NE. The AR family is classified according to type (α1-, α2- and β-AR), which can be further characterized into nine distinct receptor subtypes (α1A-, α1B-, α1D; α2A-, α2B-, α2C-; β1-, β2- and β3-AR; see review by (Guimarães and Moura, 2001). All three AR types are expressed in the immune system and like glucocorticoid receptors are considered immunosuppressive when activated by Epi or NE. However, there is a growing body of evidence to suggest that AR activation influences the immune response in a less monochromatic way.
AR activation serves many functions in the immune system including modifying the number or proportion of cells participating in an immune response as well as altering individual immune cell responsiveness (Calcagni and Elenkov, 2006; Bao et al., 2007; Pesic et al., 2009). In addition, a variety of immune cell activities are modulated by AR stimulation including cell proliferation, cytokine production, lytic activity, migration and antibody production (Maestroni, 2000; Seiffert et al., 2002; Pesic et al., 2009; Grisanti et al., 2010). Studies examining the β-AR family are the most extensive, with the “anti-inflammatory” β2-AR subtypes thought to be the predominant AR expressed in the immune system (Elenkov et al., 2000). However, there is growing evidence to suggest a “pro-inflammatory” function of β-AR activation, which is mediated through the β1-AR subtype (Grisanti et al., 2010). The α2-AR family has also been extensively investigated and again is regarded as having anti-inflammatory effects when activated (Elenkov et al., 2000). The α1-AR family is the least characterized AR in the immune system, which is likely due to conflicting reports of their expression as well as function on immune cells (Ricci et al., 1999; Elenkov et al., 2000; Tayebati et al., 2000).
II. α1-Adrenergic Receptor Expression in the Immune System
The three characterized α1-AR subtypes (α1A-, α1B- and α1D-) are differentially expressed in many organs and cells of the immune system. Investigation of α1-AR expression in immune tissues has relied heavily on RT-PCR analysis. Little information is known about α1-AR subtype localization at the protein level in the immune system since commercially available antibodies have been shown to be non-selective in wild-type and transgenic animal models (Jensen et al., 2009). Therefore, most studies have been performed utilizing PCR techniques, which is prone to contamination or radioligand binding studies that used non-selective ligands. α1-AR expression is found in murine hematopoietic stem cell progenitor cells during all stages of development from bone marrow to monocytes/macrophages (Muthu et al., 2007). Murine bone marrow expresses α1A- and α1B-AR mRNA while human bone marrow transcriptionally expresses the α1B- and α1D-AR subtypes (Maestroni et al., 1992; Kavelaars, 2002). High levels of α1A- and α1B-AR mRNA for the are present in the human spleen (Price et al., 1993; Faure et al., 1995), while others have reported the expression for all three α1-AR subtypes (Kavelaars, 2002).
The majority of studies examining immune cell α1-AR expression have been performed on peripheral blood mononuclear cell (PMBC) preparations, which include several blood cell types including T cells, B cells, NK cells, monocytes and macrophages. Reported α1-AR expression on PMBC preparations as well as many of the individual cell populations is contradictory. There are numerous reports documenting an absence of α1-AR expression using PMBC preparations (Casale and Kaliner, 1984; Kavelaars, 2002). Conversely, others have shown no genomic α1-AR expression on PMBC preparations under normal culturing conditions, but expression could be induced for all three α1-AR subtypes following phytohemagglutinin (PHA) or lipopolysaccharide (LPS) stimulation (Rouppe van der Voort et al., 2000). In situ hybridization techniques have been used to show that the majority of cells in a PMBC preparation are positive for α1B- and α1A-AR expression with α1D-AR subtypes found to a lesser extent (Tayebati et al., 2000). Immunocytochemistry analysis in this same study confirmed a majority of PMBCs expressing the mature α1B-AR protein with fewer cells expressing the α1A- and α1D-AR subtypes. In other immune cell types, genomic expression of the α1A-AR subtype has been shown in RNA isolated from rat microglia, the resident macrophage of the brain (Mori et al., 2002). Radioligand binding analysis has described the representative expression of all three AR families on human NK cells (Jetschmann et al., 1997). Immature dendritic cells have been shown to express α1B-AR mRNA, which is lost upon maturation (Maestroni, 2000).
Studies which characterize α1-AR expression on monocytes have been controversial. The human monocytic cell line, THP-1, has been shown to endogenously express α1B- and α1D-AR mRNA, while genomic α1A-AR expression could be induced following treatment with tissue necrosis factor (TNF)-α or interleukin (IL)-1β (Heijnen et al., 2002). Furthermore, evidence for functional α1-AR expression on primary monocytes isolated from human blood has been described (Takahashi et al., 2005). Conversely, other reports have documented no detectible α1-AR mRNA from human monocytes (Rouppe van der Voort et al., 1999). However, this same comprehensive study demonstrated that monocytes cultured in the presence of a glucocorticoid, dexamethasone, or the β2-AR agonist, terbutaline, resulted in the induced expression of α1B- and α1D-AR mRNA. In addition, upregulation of cAMP-dependent protein kinases using dibutyryl cAMP specifically increased α1B-AR mRNA expression. These authors also utilized immunoblot techniques and radioligand binding analysis to confirm these changes in α1-AR expression at the protein level.
The thymus, an important organ of the adaptive immune system where T cell maturation and differentiation occurs, has been reported to have low mRNA expression for all three α1-AR subtypes (Kavelaars, 2002). Further translational analysis using immunohistochemistry techniques confirmed α1-AR thymus expression, predominantly in the subcapsulary/subtrabecular cortex and cortico-medullary junctions, but rarely in the thymic medulla (Pesic et al., 2009). α1-AR expression was found in this study primarily on thymic epithelial cells, but also was shown on cluster of differentiation (CD)68+ cells, a monocyte/macrophage marker. Only a small portion of T cell precursors or thymocytes, showed mature α1-AR protein expression and of this population, the majority were CD3− cells with lower expression found on CD3low and CD3high thymocytes. Others have demonstrated that matured thymocytes or lymphocytes, isolated from the blood of healthy human patients express mRNA for all three α1-AR subtypes, with α1B-AR expression being the highest (Ricci et al., 1999). Radioligand binding analysis in this investigation confirmed mature translational expression on lymphocytes with a calculated [3H]-prazosin affinity (Kd) value of 0.65±0.05 nmol/L for these α1-ARs with a total receptor density (Bmax) of 175±20 fmol/106 cells.
III. The Role of α1-Adrenergic Receptors in the Innate Immune System
The human innate immune system is a non-specific means of defense against a pathogenic challenge. This generic means of defense is thought to be a more primitive or non-specific means of protection than the adaptive immune system. Protective systems initiated by innate immune responses include recruitment of immunocompetent cells to sites of infection through production of chemical mediators such as cytokines, activation of the complement cascade to identify and clear invading pathogens, the removal of foreign substances by white blood cells and activation of the adaptive immune system through antigen presentation. Physical barriers such as epithelial cells as well as homeostatic mechanisms such as peristaltic movement, tears and mucus production help to prevent colonization and expedite removal of invading pathogens. Furthermore, innate inflammatory responses create a biological barrier through the release of chemical factors from injured cells, which establishes an additional obstruction against the spread of infection, while promoting healing by increasing pathogen clearance. Cells of the innate immune system provide the first line of defense against invading pathogens through recognition of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs), which initiates cellular and humoral responses. The complement system is a protease C3-convertase cascade, which when activated leads to the recruitment of inflammatory cells while at the same time opsonizing infected cells for destruction through disruption of the plasma membrane resulting in cytolysis.
A. Monocytes
Monocytes are a type of white blood cell responding rapidly to inflammatory signals by moving into the affected tissue and differentiating into macrophages and dendritic cells. Monocytes are involved in phagocytosis, antigen presentation and are major producers of pro-inflammatory cytokines. Reported α1-AR expression on monocytes has been variable. Using RT-PCR to examine transcriptional expression levels in the human monocytic cell line, THP-1, it was shown that these cells express mRNA for α1B- and α1D-AR subtypes only (Heijnen et al., 2002). Conversely, other reports using primary human monocytes demonstrated no detectable levels for any α1-AR mRNA (Rouppe van der Voort et al., 1999). There is some evidence suggesting that monocyte α1-AR expression levels change during certain culturing conditions, possibly explaining the variable literature reports. For example, culturing primary monocytes in the presence of dexamethasone or terbutaline induces α1B- and α1D-AR mRNA expression (Rouppe van der Voort et al., 1999). In other reports, addition of the pro-inflammatory cytokines TNF-α or IL-1β into the media induced genomic α1A-AR expression, while at the same time decreasing α1D-AR subtype expression (Heijnen et al., 2002).
Little evidence is found in the literature as to the function of α1-AR activation on monocytes. There has been some suggestion that inhibition of α1-AR signaling on monocytes regulates migration. For example, migration of THP-1 or PMBCs in response to monocyte chemotactic protein-1 (MCP-1) is dose-dependently attenuated by administration of the α1-AR antagonists doxazosin or phenoxybenzamine (Kintscher et al., 2001). However, this inhibitory effect on the monocyte migratory response was suggested to occur independent of α1-AR blockade, possibly through enhanced expression of tissue inhibitor of metalloproteinases 1 (TIMP-1). There is also compelling evidence suggesting that α1-AR stimulation by Epi and NE enhance compliment synthesis. Studies have shown that enhanced complement component 2 (C2) was synthesized from PBMCs treated with increasing concentrations of the selective α1-AR agonist, phenylephrine (PE), but not when selective β-AR agonist, isoproterenol was used (Lappin and Whaley, 1982). Furthermore, the non-selective α-AR antagonist, phentolamine as well as the selective α1-AR antagonist, prazosin but not the selective α2- or β-AR antagonists, yohimbine and propranolol respectively, abrogated the increased C2 synthesis observed in monocytes treated with Epi, NE and PE. Moreover, other complement cascade components including C4, C3, C5, factor B, properdin, C3bINA and β1H were also observed to be increased following PE treatment. Inhibition of monocyte α1-AR activation also influences expression of signaling components related to T cell activation. Preparations of PBMCs treated with the quinazoline-based α1-AR antagonists doxazosin, prazosin and terazosin, induced the expression of intercellular adhesion molecule-1 (ICAM-1) and CD40 in a concentration-dependent manner (Takahashi et al., 2005). This study also demonstrated decreased production of the pro-inflammatory cytokine IL-18 from PBMCs using these same receptor antagonists. Alternatively using selective α2-, β1- or β2-AR antagonists did not change levels of ICAM-1, CD40 or IL-18. Recent studies have shown that murine hematopoietic progenitor cells, ER-MP20+, which are monocyte committed cells, express α1-ARs on their cell surface that function to increase LPS-mediated TNF-α secretion (Muthu et al., 2007).
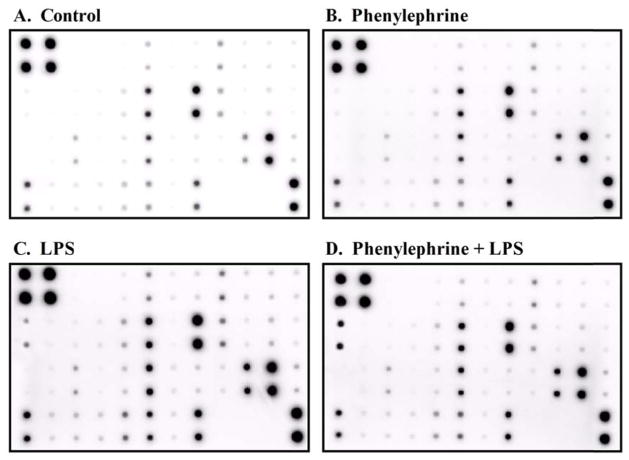
Our laboratory has also examined the influence of α1-AR activation to modulate pro-inflammatory cytokine production from pathogenically challenged human monocytes. Using LPS, which is a component of Gram negative bacterial cell walls and a potent endotoxin, to model inflammation, we investigated changes in the cytokine profile generated from monocytes concurrently treated with PE. An antibody array containing 39 specific antibodies for known mediators of inflammation immobilized on a membrane support (table 1; RayBiotech, Norcross, GA) was used to screen for modulated cytokines/chemokines in response to individual or combined 3 h treatments with 10 μM PE and 25 ng/mL LPS as described previously (fig 1; (Grisanti et al., 2010)). Expression levels of inflammatory mediators from quiescent THP-1 cells were relatively low with the strongest basal expression observed for IL-8 and macrophage inflammatory protein (MIP)-1β (fig 1A). Although a majority of inflammatory mediators did not change for monocytes treated with PE when compared to control, minor qualitative changes in the levels of MIP-1α, MIP-1β and IL-8 were observed (fig 1B). As expected, treatment with LPS qualitatively increased monocyte secretion of many inflammatory mediators including IL-1β, TNF-α, IL-6, the IL-6 receptor, IL-8, IL-10, ICAM-1, TIMP-2 and RANTES when compared to basal levels (fig 1C). Finally, there were characteristic changes in the inflammatory protein expression pattern secreted from monocytes treated concomitantly with PE and LPS (fig 1D). There was a significant qualitative increase in the levels of IL-1β released from PE plus LPS treated monocytes when compared to LPS alone. Conversely, there was a qualitative loss of LPS mediated TNF-α, IL-8 and MIP-1β levels in the presence of PE when compared to monocytes treated with LPS only. These results initially characterize the functional regulation of α1-AR activation for multiple inflammatory factors from LPS-challenged monocytes and demonstrate the utility of antibody arrays to analyze several mediators of the innate immune response simultaneously.
TABLE 1.
Map of Cytokine/Chemokine Antibody Array
| POS | POS | NEG | NEG | EOTAXIN | EOTAXIN-2 | GCSF | GM-CSF | ICAM-1 | IFN-γ | I-309 | IL-1α |
| POS | POS | NEG | NEG | EOTAXIN | EOTAXIN-2 | GCSF | GM-CSF | ICAM-1 | IFN-γ | I-309 | IL-1α |
| IL-1β | IL-2 | IL-3 | IL-4 | IL-6 | IL-6Sr | IL-7 | IL-8 | IL-10 | IL-11 | IL-12 p40 | IL-12 p70 |
| IL-1β | IL-2 | IL-3 | IL-4 | IL-6 | IL-6Sr | IL-7 | IL-8 | IL-10 | IL-11 | IL-12 p40 | IL-12 p70 |
| IL-13 | IL-15 | IL-16 | IL-17 | IP-10 | MCP-1 | MCP-2 | M-CSF | MIG | MIP-1α | MIP-1β | MIP-1δ |
| IL-13 | IL-15 | IL-16 | IL-17 | IP-10 | MCP-1 | MCP-2 | M-CSF | MIG | MIP-1α | MIP-1β | MIP-1δ |
| RANTES | TGF-β1 | TNF-α | TNF-β | s TNF RI | s TNF RII | PDGR-BB | TIMP-2 | BLANK | BLANK | NEG | POS |
| RANTES | TGF-β1 | TNF-α | TNF-β | s TNF RI | s TNF RII | PDGR-BB | TIMP-2 | BLANK | BLANK | NEG | POS |
Positions on membrane support of antibodies that recognize specific mediators of inflammation.
Abbreviations used are POS, positive biotinylated protein control; NEG, negative BSA control; GCSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; IL-6Sr, interleukin-6 soluble receptor; IL-12 p40, interleukin-12 p40 subunit; IL-12 p70, interleukin-12 p70 subunit; IP-10, IFN receptor inducible protein 10; MCP, monocyte chemoattractant protein; M-CSF, macrophage-colony stimulating factor, MIG, monokine induced by γ-interferon; MIP, macrophage inflammatory protein; TGF, tissue growth factor; TNF, tissue necrosis factor; s TNF R, soluble tumor necrosis factor receptor; PDGR, platelet-derived growth factor; TIMP, tissue inhibitor of metalloproteinases.
Figure 1.
Representative antibody array membrane for specific inflammatory cytokines (see Table 1 for layout) incubated with conditioned media taken from THP-1 cells treated with (A) culture media, (B) 10 μM PE, (C) 25 ng/mL LPS or (D) 10 μM PE plus 25 ng/mL LPS.
B. Macrophages
Macrophages are phagocytes residing in tissues functioning as antigen presentation cells to stimulate responses of the adaptive immune system. They also have an important regulatory role in the development of innate immune responses by producing chemical substances including complement proteins, cytokines, chemokines and proteolytic enzymes. There are limited investigations characterizing the expression profile or function of α1-ARs on macrophages. The previously described ER-MP20+ monocyte committed progenitor cells in mice, which only differentiate into monocytes and macrophages, express functional α1-ARs that lead to increases in TNF-α secretion through a cooperative mechanism with Toll-like receptor (TLR)4 (Muthu et al., 2007). In the rat thymus, immunohistochemistry techniques co-localized α1-AR expression with the monocyte/macrophage marker CD68 (Pesic et al., 2009). Functional α1-ARs were identified on murine RAW264 macrophages when PE and other protein kinase C (PKC) activating agents were used to initiate cell spreading (Petty, 1989). In other studies, PE was also shown to increase primary peritoneal macrophage phagocytosis (Javierre et al., 1975). Kupffer cells are resident macrophages of the liver, which are important for cell-cell communication and are essential for the physiological immune response of the liver. Kupffer cell preparations from control and tumor bearing rats increased production of prostaglandin (PG)E2 when treated with PE, which could be blocked by the administration of prazosin (Seelaender et al., 1999). Resident macrophages of the brain or microglia in the rat have been shown to transcriptionally express the α 1A-AR subtype (Mori et al., 2002) This studies also demonstrated that culturing microglial with LPS and L-serine then treating with PE significantly decreased TNF-α and IL-6 at the transcriptional and translational levels as well as inhibited production of nitric oxide (NO). PE treatments have also been shown to have an inhibitory effect on LPS-induced NO production in the murine N9 microglia cell line (Chang and Liu, 2000). Osteoclasts are resident bone macrophages that function to resorb old bone formations. Using the receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) ligand (RANKL) to differentiate murine macrophage RAW264 cells into osteoclasts, investigations have characterized α1A-AR mRNA expression in macrophages with higher levels observed in osteoclasts (Suga et al., 2010). Conversely, transcriptional expression of the α1B-AR subtype was only observed in RAW264 cells and was not found in RANKL differentiated osteoclasts (Suga et al., 2010). This study also examined a postulated α1-AR-mediated neuro-osteogenic network using primary murine superior cervical ganglia and RANKL differentiated osteoclasts co-cultures. Neurite activation was evoked by treatment with scorpion venom (SV), which subsequently resulted in osteoclast activation as measured by Ca2+ mobilization. Treatment of osteoclast alone with SV exhibited no response. Pretreatment of co-cultures with prazosin did not affect SV-mediated neurite activation but did inhibit the osteoclast Ca2+ response. Subsequent treatment of RANKL differentiated osteoclasts with PE increased the synthesis of IL-6 validating functional α1-AR expression on these cells.
C. Dendritic Cells
Dendritic cells (DCs) are important components of the innate immune system, which process and present antigen material to activate cells of the adaptive immune system. Immature murine dendritic cells express α1B-AR subtype mRNA that is lost upon maturation in the lymph nodes (Maestroni, 2000). Correspondingly, migration of immature Langerhans cells, skin DCs, to the lymph nodes in response to NE was inhibited by prazosin, but not by propranolol. Interestingly, pretreatment with yohimbine had the opposite effect by increasing NE-mediated Langerhans cell migration. Conversely, other investigations have identified α1A-AR mRNA expression in murine Langerhans cells as well as in a DC line (Seiffert et al., 2002).
D. Neutrophils
Neutrophils are the most abundant white blood cell in mammals and are vital to the innate immune response. Under normal conditions, neutrophils reside in the blood and upon initiation of inflammation migrate towards the site of injury. An important neutrophil function at the site of injury is to release cytokines, which amplify the inflammatory response. Additionally, neutrophils are phagocytes that ingest and destroy microorganisms or cell debris. Like many cells of the innate immune system literature reports of neutrophil α1-AR expression is mixed. Early radioligand binding investigations of polymorphonuclear leukocyte (PMN) preparations isolated from human blood, which is a mixed population of cells comprised of eosinophils, basophils and neutrophils, demonstrated no specific binding for [3H]-prazosin (Casale and Kaliner, 1984). While there is little evidence to suggest functional α1-ARs on neutrophils, α1-AR activation has a positive effect on the number of circulating cells in the blood or neutrophilia. For example, LPS dramatically increases neutrophilia in rats 3h following injection, while pretreating these animals with reserpine, which depletes catecholamine levels by blocking the vesicular monoamine transporter, significantly decreased blood neutrophilia as a result of LPS (Altenburg et al., 1997). Similarly, pretreatment with phentolamine or prazosin inhibited the LPS-induced increase in blood neutrophil counts, while use of PE alone caused neutrophilia in the absence of LPS. The LPS effects on neutrophilia in rats were not affected by pretreating animals with yohimbine or propranolol.
E. Mast Cells
Mast cells are resident immune cells that contain numerous secretory granules and are best known for their role in allergic and anaphylactic responses. However, mast cells also play an important role in wound healing and host defense mechanisms against pathogens. Activation of mast cells by cross-linking of immunoglobulin (Ig)E receptors or complement proteins, causes cell degranulation, releasing inflammatory mediators into the interstitium. Mature α1-AR expression has been shown on mast cells from cultured neonatal rat heart cells using immunocytochemistry techniques (Schulze and Fu, 1996). In a murine mast cell line, increasing concentrations of PE or NE treatments could increase 14C-histamine release, which could be blocked by pre-incubation with phentolamine (Moroni et al., 1977). Other investigations have shown a correlation between decreased amounts of the degranulation marker, mast cell peroxidase (MPO) and the protective effects of NE pretreatment on a rat model of heart ischemia-reperfusion injury (Parikh and Singh, 1999). However, prazosin administration during ischemia following NE pretreatment reversed the decrease in MPO observed in ischemic-NE preconditioned animals.
F. Natural Killer Cells
Natural killer (NK) cells are a large, granular type of cytotoxic lymphocyte that plays a major role in rejection of tumors and viral infected cells through the release of cytotoxic granules (Paust et al., 2010). They were named natural killer cells because they do not require any preceding pathogenic stimulation to initiate cell killing. Activation of NK cells by cytokines, Fc portion of antibodies binding to Fc receptors or other activating or inhibiting receptors leads to the release of cytoplasmic granules containing perforin and granzyme causing the target cell to die by apoptosis. Radioligand binding has been performed on CD16+ cells isolated from human blood, which identified α1-, α2- and β-AR expression on NK cells (Jetschmann et al., 1997). Expression of α-ARs on NK cells appears to vary depending on external stimuli. For example, infusion of Epi decreased expression of β2- and α1- but not α2-ARs while NE had no effect on AR expression (Jetschmann et al., 1997). Morphine exposure suppresses splenic NK activity, which occurs, in part through α1-ARs. In lymphocyte populations isolated from mouse spleen, phentolamine administration suppresses morphine-induced NK activity suppression (Carr et al., 1993). Prazosin administration had the same effect on NK activity as phentolamine, while yohimbine showed no change from morphine administration alone.
To circumvent problems with non-specific antibodies and non-selective ligands, our laboratory used α1A-AR-enhanced green fluorescent protein (EGFP) tagged transgenic mice to assess lymphocyte populations (Papay et al., 2006). These mice are under the control of the endogenous promoter and therefore, express the α1A-AR subtype in all naturally occurring cell types throughout the body (Rorabaugh et al., 2005). We focused our investigations on the liver because of the bright green cells present in the sinusoids, the key role in systemic innate immunity regulated by the liver, and that this organ harbors a large population of innate immune cells.
DX5 antibody recognizes the CD49b antigen (Arase et al., 2001) that is expressed on the vast majority of mouse NK cells as well as on 5% of CD8+ cytotoxic T cells (Kambayashi et al., 2001). DX5+ NK cells also display an increased cytotoxicity and indicate that functional subsets exist among NK cell population (Arase et al., 2001). B (bone marrow-derived) lymphocytes (cells) not only play a pivotal role in humoral immunity through the production of antibodies, but also are involved in antigen presentation and regulation of T-cell function (LeBien and Tedder, 2008). CD19 is a specific B cell marker and is present on the earliest B lineage cells during development. CD3 is a general marker for most T cells as it is part of the T cell receptor (TCR) complex present on adult T cells. Both T and B cells also possess the ability to remember encounter antigens in the form of memory cells, which mediate adaptive immunity (i.e., the subsequent immune response to an encounter is different than the first).
We found that the α1A-AR subtype were not expressed in CD3+ T cells (fig 2A), but instead was expressed in CD19+ B cells (fig 2B) and in DX5+ NK cells (fig 2C). As expected the α1A-AR subtype was also highly expressed in liver vasculature (fig 2D). Hepatic NK cells are located in the sinusoids as indicated in figure 2C and high levels are present in the liver, more than any other organ (Nemeth et al., 2009). Since the liver is a target organ for the metastasis of many cancers and for innate immunity, high levels of NK cells may have an effective anti-tumor effect (Subleski et al., 2006). Localization of the α1A-AR in liver vasculature and immune cells may also account for the high level of this subtype expression in liver membrane preparations analyzed by ligand binding (Rorabaugh et al., 2005) even though the α1B-AR subtype is dominant in the rodent liver (Yang et al., 1998). B cells are of low abundance in the liver comprising less than 10% of the lymphocyte population (Nemeth et al., 2009). However, recent evidence suggests that B cell lymphopoiesis occurs in the liver sinusoids by endothelial cells and we therefore speculate this may account for the high degree of co-localization in smaller cells expressing the α1A-AR (Wittig et al., 2010).
Figure 2.
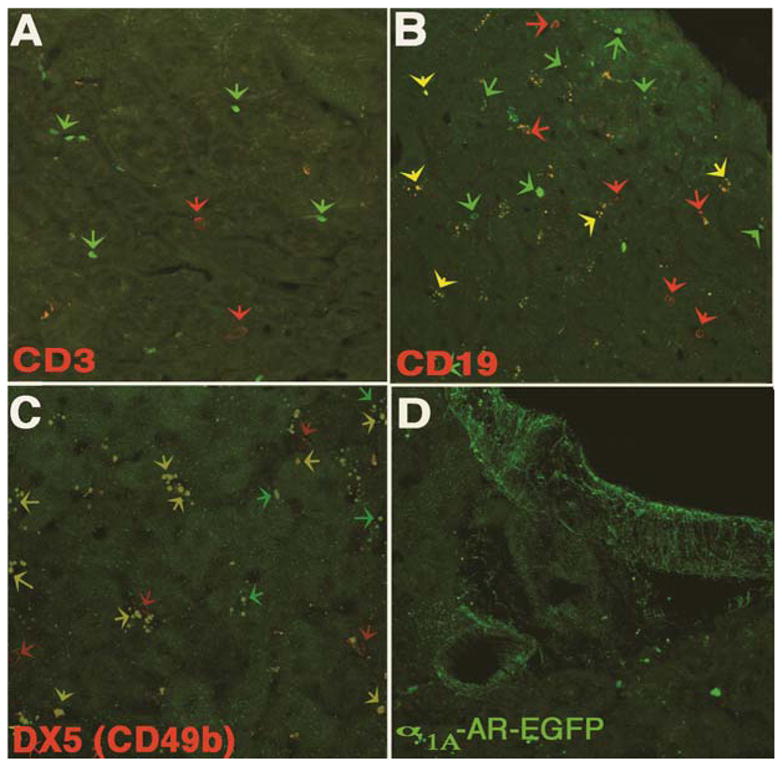
Immunohistochemistry of α1A-AR-EGFP liver tissue sections reacted with (A) CD3 antibody (T cells), (B) CD19 antibody (B cells), or (C) DX5 antibody (NK cells). (D) Designates α 1A-AR-EGFP expression in the liver vasculature.
IV. α1-Adrenergic Receptor Influences on the Adaptive Immune System
The highly specialized adaptive immune system allows the host to recognize and remember specific pathogens so that a strong attack can be mounted every time the pathogen is encountered. The system is highly pliant allowing a small number of host genes to generate huge numbers of diverse antigen receptors uniquely expressed on individual lymphocytes. The adaptive immune system functions to recognize specific non-self antigens to generate maximally effective responses tailored to eliminate specific pathogens or pathogen infected cells. Through this initial response the adaptive immune system also develops immunological memory by forming unique antibodies so that memory cells can be called upon to quickly eliminate the pathogen upon subsequent infections. Lymphocytes are the effector cells of the adaptive immune system of which there are two main types, B cells and T cells. Mature cells that have left the bone marrow or thymus and entered into the lymphatic system are naïve and have yet to encounter their cognate antigen. Upon activation by the B or T cell’s cognate antigen, they become effector cells, which are actively involved with eliminating the invading pathogen. Memory cells are long lived lymphocyte survivors of past infections that can recognize specific pathogenic antigens.
A. Peripheral Blood Mononuclear Cells
As stated previously, the majority of studies examining immune cell α1-AR expression have been performed on PMBC preparations, which includes a variety of blood cell types. In studies that induced PMBC α1-AR expression using PHA or LPS, subsequent addition of NE increased extracellular signal-regulated kinase (ERK) activation, which could be blocked by pretreating with the irreversible α1-AR antagonist benextramine, but not yohimbine (Rouppe van der Voort et al., 2000). The functional outcome of NE-initiated ERK activation was not further characterized in this investigation. PE treatment of PMBCs isolated from patients with juvenile rheumatoid arthritis induced the mature expression of IL-6, which is commonly secreted by T cells, when compared to preparations from healthy individuals that showed little change in cytokine levels (Heijnen et al., 1996).
B. T Lymphocytes
T (thymus-derived) lymphocytes or T cells are a type of white blood cell which plays an important role in the adaptive immune response. As stated previously, expression of the TCR complex is a marker that distinguished T cells from other lymphocytes. There are also several types of mature T cells including T helper cells (TH cells), cytotoxic T cells (TC cells), memory T cells, regulatory T cells (Treg cells), natural killer T cells (NKT cells) and δγ T cells (Chaplin, 2010). TH cells are CD4+ cells that aid other immunocompetent cells in their function such as maturation of B cells as well as activation of TC cells and macrophages. Activation of macrophage and TC cells occurs through the presentation of peptide antigens by major histocompatibility complex (MHC) class II molecules on the surface of TH and other antigen presenting cells. Activation results in rapid cell division as well as cytokine secretion which facilitate a variety of immune responses. TC cells are CD8+ cells that destroy tumor and viral infected cells through TCRs that recognize specific antigenic peptides bound to MHC class I and CD8 glycoproteins. Memory T cells are antigen-specific CD4+ or CD8+ cells that remain following termination of the infection, which can quickly expand into effector T cells (TH or TC) following re-exposure to their cognate antigen. Treg cells are crucial for immunological tolerance by functioning to end T cell-mediated immunity following an immune reaction as well as suppressing auto-reactive T cells that may escape the negative selection process in the thymus. NKT cells are unique in that they bridge the innate and adaptive immune responses. Unlike most T cells, which recognize peptide antigens presented by MHC molecules, NKT cells recognize glycolipid antigens presented by CD1d. Following activation, NKT cells have similar functions as TH and TC cells in that they contribute to both cytokine production and release of cytolytic molecules. δγ T cells are a small T cell subset primarily found in the gut mucosa having a distinct TCR made from one δ- and one γ-chain glycolipid.
While α1-AR expression is not commonly found on T lymphocytes under normal conditions, some investigations suggest α1-AR expression may be regulated in certain lymphoid compartments or under certain pathologic conditions. For example, lymphocytes from rat mesenteric lymph nodes have been shown to transcriptionally express the α1-AR (Bao et al., 2007). Activation of these lymphocytes with a T cell mitogen, concanavalin A (Con A), increased the quantity of α1-AR mRNA over resting lymphocytes. However, this study was unable to determine a potential function for these α1-AR transcripts in that PE had no effect on Con A-induced proliferation or interferon (IFN)-γ and IL-4 production. In other investigations, transcriptional α1A- and α1D-AR subtype expression was also detected in rat lymphocyte populations from the thymus, spleen and peripheral blood (Schauenstein et al., 2000). However, this study documented a decreased in α1A- and α1D-AR mRNA following peripheral blood lymphocyte (PBL) treatment with Con A. These investigators also showed no differences in the mRNA expression of α1A- and α1D-AR subtypes between CD4+ and CD8+ T cells. Radioligand binding studies found a correlation of [3H]-prazosin binding site densities between PBL preparations isolated from spontaneously hypertensive rats (SHR) and humans diagnosed with essential hypertension (Veglio et al., 2001). In this investigation, isolated PBL from both human hypertensive and SHR showed a significant decreases in the [3H]-prazosin Bmax when compared with Wistar-Kyoto (WKY) rat controls or normotensive individuals. Using subtype-selective α1-AR antagonists to characterize these specific [3H]-prazosin binding sites in humans, the authors described the mature expression for all three α1-AR subtypes in both hypertensive and normotensive subjects. The α1B-AR subtype was the highest expressed receptor in normal patients, while PBLs isolated from hypertensive individuals showed no change in the α1A-AR subtype density when compared to control. However, there was decreased α1B-AR subtype expression with an increase in the α1D-AR population from hypertensive patients when compared with normotensive individuals.
Early investigations also have demonstrated the role for α1-AR activation to inhibit proliferative T cell responses (Heilig et al., 1993). In this study, [3H]-thymidine incorporation of primary murine lymphocytes isolated from immunized animals was decreased with increasing concentrations of PE, which could be blocked with phentolamine. A more recent comprehensive study using flow cytometry has shown that 11.3% of isolated rat thymus cells express the α1-AR (Pesic et al., 2009). This α1-AR expressing cell population primarily consisted of the least mature CD3− (51.2%) and CD3low (33.2%) to the most mature CD3high (14.2%) differentiating and proliferating thymocytes prior to leaving the thymus. Chronic treatment with the selective α1-AR antagonist, urapidil, increased the absolute and relative thymic weight in these animals. This result correlated with both absolute and relative thymocyte numbers resulting from a decrease in cell apoptosis. There was also a greater frequency of the nuclear cell proliferation-associated antigen, Ki-67, on thymocytes after urapidil treatment, which is another indication of the negative regulatory function of α1-AR activation in T lymphocytes.
The overall number of progenitor TCRαβ− thymocytes, which give rise to the distinct CD4 or CD8 functional T cell subsets, was likewise increased in urapidil-treated rats (Pesic et al., 2009). In this group the number of CD4−CD8+ single-positive (SP) and CD4−CD8− double-negative (DN) cells remained unaltered. However, a rise in CD4+CD8+ double-positive (DP) and CD4+CD8−SP cell subsets was the reason for an increased number of urapidil-treated TCRαβ− thymocytes. Urapidil treatment also increased the number of immature TCRαβlow thymocytes undergoing the selection process. In this group, absolute numbers of CD4+CD8+DP and CD4+CD8−SP thymocyte subsets were increased while the cellularity of other subsets remained unaltered. Similarly, urapidil treatment increased the overall frequency of the most mature, post-selected TCRαβhigh cells, which is reflected as large increases in the CD4+CD8−SP subset, a decrease in CD4−CD8−DN thymocytes, while the CD4−CD8+SP and CD4+CD8+DP populations remained the same.
The Ig Thy-1 (CD90) has been shown to modulate TCRαβ signaling and selection thresholds (Hueber et al., 1997). In urapidil treated thymocytes, CD90 expression was increased in all TCRαβ groups examined over control, again supporting a role for the inhibition of T cell proliferation by α1-AR signaling (Pesic et al., 2009). The impact of urapidil treatment on Treg maturation in the thymus was similarly examined using this animal model. The unique “self-antigen” RT6.1 previously identified on peripheral T lymphocytes was used to distinguish maturing CD4+CD25+ Treg thymocytes from cells re-entering the thymus (Agus et al., 1991). In urapidil treated animals, the relative and absolute numbers of CD4+CD25+RT6.1− thymocytes was greater when compared to control. Finally, chronic urapidil treatment also increased both the relative and absolute numbers of maturing CD161+TCRαβ+ NKT cells relative to control animals. These results together point to an α1-AR mechanism which negatively regulates maturation of T lymphocytes in the thymus.
C. B Lymphocytes
B cells are the major producers of antibodies that circulate in the blood plasma and lymphatic system. Upon activation, B cells generate antibodies that recognize unique antigens which neutralize specific pathogens. Each B cell expresses a unique B cell receptor (BCR) that recognizes and binds a particular antigen. Upon antigen recognition, B cells differentiate into effector plasma cells. Plasma cells secrete these specific antibodies, which bind the unique pathogenic antigens on cells to initiate the complement cascade as well as targeting these antigenic cells for phagocytes. While there are varied reports of α1-AR expression on isolated PMBCs, a mixed cell preparation which contains B cells, there are no reports to suggest α1-AR expression specifically on B cells (Casale and Kaliner, 1984; Rouppe van der Voort et al., 2000; Tayebati et al., 2000).
V. α1-Adrenergic Receptors in Immune Tissues
A. Spleen
The spleen is an important immune system organ responsible for removing old red blood cells, maintaining a blood reserve, recycling elemental iron, synthesizing antibodies as well as retaining half the body’s monocytes, which allows them to move into injured tissues for differentiation into dendritic cells and macrophages. The spleen is richly innervated by the sympathetic nerves, which has an affect its physiology (Felten et al., 1987). Reports of high transcriptional expression for all three α1-AR subtypes in spleen have been published (Alonso-Llamazares et al., 1995; Kavelaars, 2002). However, the spleen was one of the first tissues described in which translational α1-AR homogeneity was demonstrated (Han et al., 1987). Subsequent radioligand binding analysis in bovine and guinea pig spleen demonstrated a homogenous α1B-AR subtype population (Buscher et al., 1996). Conversely, no specific [3H]-prazosin binding could be observed in murine strain (HLG) broken cell spleen preparation (Yang et al., 1998).
There is also evidence to suggest functional α1-AR expression in the spleen. Electrical stimulation (ES) of isolated murine spleen slices inhibits basal IL-6 secretion, which is attenuated by phentolamine (Straub et al., 1997). Application of the α1-AR agonist methoxamine mimicked the inhibitory response of ES on basal IL-6 levels. In other studies, NE treatment in the presence propranolol enhanced the murine IgM antibody response in primary spleen cells immunized with sheep erythrocytes in vitro (Sanders and Munson, 1984). In a subsequent investigation, methoxamine was used to demonstrate that early IgM increases from immunized murine spleen cells was mediated through α1-AR activation, while late IgM changes observed in the presence of clonidine were facilitated by α2-AR stimulation (Sanders and Munson, 1985). Additionally, there is evidence linking changes in spleen α1-AR activation with chronic inflammatory disease states (Straub et al., 2008). In this study, ES of the splenic nerve in an early type II collagen-induced arthritis (CIA) mouse model showed a decrease in IFN-γ secretion compared to control animals, which was partially reversed in the presence of the α1-AR antagonist benoxathian.
B. Thymus
The thymus is an organ important for T cell maturation and differentiation as well as contributing to the production and secretion of additional factors that influence immune system function. The thymus is comprised by a central medulla and peripheral cortex, which is entirely surrounded by an outer capsule. The peripheral cortex is where thymocyte development begins as well as TCR gene rearrangement and positive T cell selection occurs. Conversely, the medulla is the location of late T cell development where a majority of negative selection happens. There are two main thymus cell types; thymic stromal cells, which include cortical epithelial cells and thymic medullary epithelial cells; and cells of hematopoietic origin such as dendritic cells, thymocytes or T cell precursors. The thymus is innervated by post-ganglionic sympathetic nerve fibers which use NE as the primary neurotransmitter (Felten et al., 1987).
In addition to the previously described expression of α1-ARs on thymocytes, which are the hematopoietic progenitor T cell precursors (Pesic et al., 2009), mRNA for all three α1-AR subtypes is reportedly expressed in the human thymus (Kavelaars, 2002). Further analysis using immunohistochemistry delineated the location of α1-ARs predominantly in the subcapsulary/subtrabeculary cortex and cortico-medullary junction, with rare expression in the thymic medulla (Pesic et al., 2009). Cell specific α1-AR expression occurs primarily on thymic epithelial cells, but also can be found on CD68+ cells, a monocyte/macrophage marker, located in the outer cortex and cortico-medullary junction (Pesic et al., 2009).
α1-AR expression in the subcapsulary/subtrabeculary cortex and cortico-medullary junction suggests a role for these receptors in early T cell development and proliferation. For example, previous studies have demonstrated increased lymphopoiesis and greater mitogen reactivity over control from cultured fetal thymus explants incubated in the presence of PE (Singh, 1979). Additionally, α1-AR blockade using urapidil has been shown to decrease the proportional thymus weight in immature rat pups, which was the result of reduced total thymocyte number (Plećaš-Solarović et al., 2005). This decrease in thymocyte cell number was localized to the cortex, but not observed in the medullary compartment. Chronic urapidil treatment also resulted in a decreased population of CD4+CD8−SP cells with a concomitant increase in CD4−CD8+SP cells, supporting the idea that α1-ARs influence thymocyte proliferation.
In contrast to immature pups, chronic urapidil treatment increased absolute and relative thymic weigh in adult rats (Plećaš-Solarović et al., 2005; Pesic et al., 2009). Both absolute and relative thymocyte numbers were also increased in urapidil-treated rats (Pesic et al., 2009). Using annexin V as an indicator of cell death, this study showed a decreased frequency of annexin V+ thymocytes in urapidil-treated animals when compared with control. Using a marker of nuclear cell proliferation, Ki-67 immunostaining was a significantly greater in the subcapsular/subtrabecular cortex of rats treated with urapidil as compared to control. As described previously, this investigation also demonstrated an overall increase in thymocyte numbers, which resulted in proportional changes of CD4CD8 T cell populations.
C. Blood/Circulating Cytokines
α1-AR investigations using individual cell populations and isolated tissues provides a detailed account of their expression and potential function. However, the immune system is a complex network of interacting cells and it is therefore important to know how α1-ARs are affecting the immune response under physiological conditions in vivo. For example, prazosin treatment alone has no effect on murine basal plasma IL-1β levels, but prazosin pretreatment could block increases in plasma IL-1β as a result of intraperitoneal LPS injection (Dong et al., 2002). Similarly, another investigation demonstrated decreased TNF-α levels in mice pretreated with prazosin prior to LPS injection when compared to LPS treatment alone (Sugino et al., 2009). Conversely, both studies showed that prazosin pretreatment further increased levels of the anti-inflammatory cytokine IL-10 in LPS treated animals when compared LPS treatment only.
D. Non-Immune Tissue
α1-ARs in tissues not considered part of the immune system have been shown to influence immune processes in a number of different ways. In transfected α1A-AR rat fibroblasts, oligonucleotide microarray technology demonstrated that following Epi treatment that several mediators of inflammation, cell motility and adhesion were temporally altered, which was confirmed using RT-PCR and immunoblot analysis (Shi et al., 2006). Some notable observations included increased levels of IL-6 following 1 h Epi treatment, followed by a decreased level after 18 h that was still significantly above levels detected from non-stimulated control cells. Likewise, levels of the neutrophil chemoattractant CXC chemokine, Gro (CXCL1) were increased after 1 h Epi treatment, but alternatively dropped to levels significantly below basal after 18 h. No change was noted after 1 h for the T cell proliferation cytokine IL-15 and the inflammatory transcriptional regulator, high mobility group box 2 (Hmgb2) protein when compared to control, but after 18 h transcriptional levels for these targets were significantly decreased and increased, respectively. Similarly, hyaluronan synthase 2 that synthesizes a component of the extracellular matrix hyaluronan and the hyaluronan receptor (CD44), which mediates lymphocyte binding showed no change from basal levels after 1 h, but both were significantly increased after 18 h of Epi treatment. These changes in hyaluronan targets were also observed in treated a rat thoracic aorta smooth muscle cell line (A-10) as well as in DDT1-MF2 hamster smooth muscle cells transfected with the human α1A-AR subtype.
Increased IL-6 levels following α1-AR activation was also shown in primary murine neonatal cardiomyocytes (Perez et al., 2009). This study further elucidated the molecular mechanisms responsible for the observed increased cytokine levels, which included stabilization of the IL-6 transcript. In addition, unique α1-AR-mediated signaling pathways were found to be necessary for the increased cardiomyocyte IL-6 translation observed in this study. Specifically, pharmacological inhibition of p38 mitogen-activated protein kinase (MAPK) and NF-κB significantly decreased IL-6 levels when compared to Epi treatment. Transgenic mice over-expressing a constitutively active (CAM) α1A-AR construct under control of the endogenous mouse promoter also showed significantly increased IL-6 serum levels when compared with CAM α1B-AR transgenic or nontransgenic animals (Perez et al., 2009). Remarkably, these CAM α1A-AR mice showed no signs of inflammation and were protected against myocardial ischemia suggesting that increased IL-6 levels may be a beneficial and adaptive α1-AR cardioprotective mechanism (Rorabaugh et al., 2005).
V. α1-Adrenergic Receptors in Disease States
Given the summary of information to date, an immunomodulatory role under pathophysiological conditions can be brought forward for α1-ARs expressed in the immune system. For example, α1-AR activation has been proposed to be important in the development of experimental autoimmune encephalomyelitis (EAE), an inflammatory demyelinating disease of the central nervous system that is often used as a model for multiple sclerosis (Brosnan et al., 1985). Development of EAE results from T lymphocyte sensitization against the myelin basic protein (MBP) and is a typical inflammatory delayed-type hypersensitivity response. Rats sensitized to MBP were analyzed histologically for assessment of inflammatory cell infiltration as well as clinically (e.g., muscle weakness, ataxia, impaired respiration) using a scaled index to grade EAE development. In MBP sensitized male rats, maximal signs of the disease were observed at 13 days post-inoculation (dpi) with a peak clinical index score of 3.9. Conversely, in sensitized prazosin treated animals, there was a dose-dependent increase when peak clinical signs were observed (15 dpi) as well as a decreased peak clinical index (2.2). These observations were specific for α1-AR antagonism because treatment of MBP sensitized rats with yohimbine or propranolol had the opposite effect by increasing and extending the signs and duration of the disease. This study also described a dose-dependent decrease of brain and spinal cord immune cell infiltration from prazosin treated animals when compared with control. Other investigations demonstrated changes in the permeability of the blood-brain barrier following induction of EAE (Goldmuntz et al., 1986). In this study, immune cell infiltration occurred less rapidly in prazosin treated animals, with no differences from control in cell infiltration at the disease peak. As a result, it is unclear if prazosin effects EAE disease progression is the result of vascular changes or alterations in immune cells function.
There are several studies correlating α1-AR expression with disease onset or severity. For instance, PMBC preparations are most often reported to have little to no α1-AR expression. However, PMBCs isolated from patients with juvenile rheumatoid arthritis have increased IL-6 production following PE treatment, which was abolished in the presence of doxazosin (Heijnen et al., 1996). Conversely, PE treatment of PMBCs isolated from normal patients demonstrated a decrease in the generation of IL-6. Using a CIA animal model of autoimmune disease, other investigations have focused around the sympathetic nervous systems effect on splenic function in relation to INF-γ secretion (Straub et al., 2008). In this study, ES was used to release NE from splenic sympathetic nerve terminals in an isolated perfused slice preparation. ES significantly decreased basal IFN-γ levels in CIA mice, which was partially reversed in the presence of benoxathian, indicating that NE acts on α1-ARs to inhibit INF-γ secretion. In parallel experiments, T cell depletion with anti-CD3 antibodies completely eliminated the basal response from CIA spleens, indicating INF-γ secretion is a T cell-dependent process.
One of the most common causes of intensive care unit patient death is shock due to sepsis, in which cytokine overproduction by the immune system results in systemic vasodilation and circulatory failure due to decreased vasoconstrictor reactivity (Russell, 2006). Patients diagnosed with sepsis require increasing doses of NE in order to maintain blood pressure via α1-AR activation on vascular smooth muscle cells. In a rat model of LPS-induced endotoxemia, increased levels of TNF-α and IL-1β were associated with decreased mRNA levels for all three α1-AR subtypes (Bucher et al., 2003). Addition of TNF-α and IL-1β to rat renal cells decreased levels of α1B-AR subtype expression as assessed by [3H]-prazosin binding. In other studies, blocking vasculature α1-ARs using prazosin had the same cardiovascular effects on control mice as observed in cecal ligation and puncture (CLP)-induced septic mice (Schmidt et al., 2009). Using dexamethasone treatment or RNA interference technology to decrease levels of cytokine expression caused an attenuated cardiovascular effect and α1-AR downregulation in CLP mice when compared to control. Small interfering RNA treatment specific for NF-κB also prevented downregulation of α1-ARs and inhibited cardiovascular dysfunction of CLP mice in this study.
VI. Conclusions
α1-AR expression on various immunocompetent cell populations has been reported and has been shown to be regulated during pathophysiological processes. However, α1-AR function to modulate immune cell responses is just beginning to be understood. α1-AR activation appears to alter production of inflammatory mediators from certain cell types including monocytes, macrophages and myocytes. Additionally, α1-AR signaling plays a role in dendritic cell migration, lymphopoiesis and mast cell degranulation. A better understanding of how α1-ARs regulate immune system function could uncover potential therapeutic strategies for modulating pathophysiological responses in human diseases where chronic, inappropriate or hyperactive inflammation is an underlying etiology.
Contributor Information
Laurel A. Grisanti, Email: lgrisanti@medicine.nodak.edu, Department of Pharmacology, Physiology and Therapeutics, School of Medicine and Health Sciences, University of North Dakota, Grand Forks, North Dakota, 58202
Dianne M. Perez, Email: perezd@ccf.org, Department of Molecular Cardiology, Lerner Research Institute, The Cleveland Clinic Foundation, Cleveland, Ohio, 44195
James E. Porter, Email: james.porter@med.und.edu, Department of Pharmacology, Physiology and Therapeutics, School of Medicine and Health Sciences, University of North Dakota, Grand Forks, North Dakota, 58202
References
- Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. Journal of Experimental Medicine. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Llamazares A, Zamanillo D, Casanova E, Ovalle S, Calvo P, Chinchetru MA. Molecular cloning of α1d-adrenergic receptor and tissue distribution of three α1-adrenergic receptor subtypes in mouse. Journal of Neurochemistry. 1995;65:2387–2392. doi: 10.1046/j.1471-4159.1995.65062387.x. [DOI] [PubMed] [Google Scholar]
- Altenburg SP, Martins MA, Silva AR, Cordeiro RS, Castro-Faria-Neto HC. LPS-induced blood neutrophilia is inhibited by α1-adrenoceptor antagonists: a role for catecholamines. Journal of Leukocyte Biology. 1997;61:689–694. doi: 10.1002/jlb.61.6.689. [DOI] [PubMed] [Google Scholar]
- Arase H, Saito T, Phillips JH, Lanier LL. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (α2 integrin, very late antigen-2) Journal of Immunology. 2001;167:1141–1144. doi: 10.4049/jimmunol.167.3.1141. [DOI] [PubMed] [Google Scholar]
- Bao JY, Huang Y, Wang F, Peng YP, Qiu YH. Expression of α-AR subtypes in T lymphocytes and role of the α-ARs in mediating modulation of T cell function. Neuroimmunomodulation. 2007;14:344–353. doi: 10.1159/000129670. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Goldmuntz EA, Cammer W, Factor SM, Bloom BR, Norton WT. Prazosin, an α1-adrenergic receptor antagonist, suppresses experimental autoimmune encephalomyelitis in the Lewis rat. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:5915–5919. doi: 10.1073/pnas.82.17.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Kees F, Taeger K, Kurtz A. Cytokines down-regulate α1-adrenergic receptor expression during endotoxemia. Critical Care Medicine. 2003;31:566–571. doi: 10.1097/01.CCM.0000048621.36569.69. [DOI] [PubMed] [Google Scholar]
- Buscher R, Heeks C, Taguchi K, Michel MC. Comparison of guinea-pig, bovine and rat α1-adrenoceptor subtypes. British Journal of Pharmacology. 1996;117:703–711. doi: 10.1111/j.1476-5381.1996.tb15247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Annals of the New York Academy of Sciences. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Gebhardt BM, Paul D. Alpha adrenergic and mu-2 opioid receptors are involved in morphine-induced suppression of splenocyte natural killer activity. Journal of Pharmacology and Experimental Therapeutics. 1993;264:1179–1186. [PubMed] [Google Scholar]
- Casale TB, Kaliner M. Demonstration that circulating human blood cells have no detectable α1-adrenergic receptors by radioligand binding analysis. Journal of Allergy and Clinical Immunology. 1984;74:812–818. doi: 10.1016/0091-6749(84)90184-2. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Catecholamines inhibit microglial nitric oxide production. Brain Research Bulletin. 2000;52:525–530. doi: 10.1016/s0361-9230(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Chaplin DD. Overview of the immune response. Journal of Allergy and Clinical Immunology. 2010;125:S3–23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Mrabet O, Moze E, Li K, Neveu PJ. Lateralization and catecholaminergic neuroimmunomodulation: prazosin, an alpha1/alpha2-adrenergic receptor antagonist, suppresses interleukin-1 and increases interleukin-10 production induced by lipopolysaccharides. Neuroimmunomodulation. 2002;10:163–168. doi: 10.1159/000067178. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacological Reviews. 2000;52:595–638. [PubMed] [Google Scholar]
- Faure C, Gouhier C, Langer SZ, Graham D. Quantification of α1-adrenoceptor subtypes in human tissues by competitive RT-PCR analysis. Biochemical and Biophysical Research Communications. 1995;213:935–943. doi: 10.1006/bbrc.1995.2219. [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system: Structure and function. Immunological Reviews. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van RN, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Goldmuntz EA, Brosnan CF, Norton WT. Prazosin treatment suppresses increased vascular permeability in both acute and passively transferred experimental autoimmune encephalomyelitis in the Lewis rat. Journal of Immunology. 1986;137:3444–3450. [PubMed] [Google Scholar]
- Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. Pro-inflammatory responses in human monocytes are β1-adrenergic receptor subtype dependent. Molecular Immunology. 2010;47:1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães S, Moura D. Vascular adrenoceptors: An update. Pharmacological Reviews. 2001;53:319–356. [PubMed] [Google Scholar]
- Han C, Abel PW, Minneman KP. Heterogeneity of α1-adrenergic receptors revealed by chlorethylclonidine. Molecular Pharmacology. 1987;32:505–510. [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe vd V, de van PM, Kavelaars A. Cytokines regulate α1-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. Journal of Neuroimmunology. 2002;125:66–72. doi: 10.1016/s0165-5728(02)00034-6. [DOI] [PubMed] [Google Scholar]
- Heijnen CJ, Rouppe vd V, Wulffraat N, van der NJ, Kuis W, Kavelaars A. Functional α1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. Journal of Neuroimmunology. 1996;71:223–226. doi: 10.1016/s0165-5728(96)00125-7. [DOI] [PubMed] [Google Scholar]
- Heilig M, Irwin M, Grewal I, Sercarz E. Sympathetic regulation of T-helper cell function. Brain, Behavior, and Immunity. 1993;7:154–163. doi: 10.1006/brbi.1993.1017. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Bernard AM, Battari CL, Marguet D, Massol P, Foa C, Brun N, Garcia S, Stewart C, Pierres M, He HT. Thymocytes in Thy-1−/− mice show augmented TCR signaling and impaired differentiation. Current Biology. 1997;7:705–708. doi: 10.1016/s0960-9822(06)00300-9. [DOI] [PubMed] [Google Scholar]
- Javierre MQ, Pinto LV, Lima AO, Sassine WA. Immunologic phagocytosis by macrophages: effect by stimulation of alpha adrenergic receptors. Revista Brasileira de Pesquisas Medicas e Biologicas. 1975;8:271–274. [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedebergs Archives of Pharmacology. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetschmann JU, Benschop RJ, Jacobs R, Kemper A, Oberbeck R, Schmidt RE, Schedlowski M. Expression and in-vivo modulation of α- and β-adrenoceptors on human natural killer (CD16+) cells. Journal of Neuroimmunology. 1997;74:159–164. doi: 10.1016/s0165-5728(96)00221-4. [DOI] [PubMed] [Google Scholar]
- Kambayashi T, Assarsson E, Chambers BJ, Ljunggren HG. Expression of the DX5 antigen on CD8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. European Journal of Immunology. 2001;31:1523–1530. doi: 10.1002/1521-4141(200105)31:5<1523::AID-IMMU1523>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of α-1 adrenergic receptors in the immune system. Brain, Behavior, and Immunity. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Kon D, Wakino S, Goetze S, Graf K, Fleck E, Hsueh WA, Law RE. Doxazosin inhibits monocyte chemotactic protein 1-directed migration of human monocytes. Journal of Cardiovascular Pharmacology. 2001;37:532–539. doi: 10.1097/00005344-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Lappin D, Whaley K. Adrenergic receptors on monocytes modulate complement component synthesis. Clinical and Experimental Immunology. 1982;47:606–612. [PMC free article] [PubMed] [Google Scholar]
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ. Dendritic cell migration controlled by α1b-adrenergic receptors. Journal of Immunology. 2000;165:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A, Pedrinis E. Effect of adrenergic agents on hematopoiesis after syngeneic bone marrow transplantation in mice. Blood. 1992;80:1178–1182. [PubMed] [Google Scholar]
- Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, Maeda N, Sakanaka M, Tanaka J. Effects of norepinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology. 2002;43:1026–1034. doi: 10.1016/s0028-3908(02)00211-3. [DOI] [PubMed] [Google Scholar]
- Moroni F, Fantozzi R, Masini E, Mannaioni PF. The modulation of histamine release by alpha-adrenoceptors: evidences in murine neoplastic mast cells. Agents and Actions. 1977;7:57–61. doi: 10.1007/BF01964881. [DOI] [PubMed] [Google Scholar]
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB. Murine hematopoietic stem cells and progenitors express adrenergic receptors. Journal of Neuroimmunology. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Baird AW, O’Farrelly C. Microanatomy of the liver immune system. Seminars in Immunopathology. 2009;31:333–343. doi: 10.1007/s00281-009-0173-4. [DOI] [PubMed] [Google Scholar]
- Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM. Localization of the mouse α1A-adrenergic receptor (AR) in the brain: α1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. Journal of Comparative Neurology. 2006;497:209–222. doi: 10.1002/cne.20992. [DOI] [PubMed] [Google Scholar]
- Parikh V, Singh M. Possible role of adrenergic component and cardiac mast cell degranulation in preconditioning-induced cardioprotection. Pharmacological Research. 1999;40:129–137. doi: 10.1006/phrs.1999.0501. [DOI] [PubMed] [Google Scholar]
- Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunological Reviews. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DM, Papay RS, Shi T. α1-Adrenergic receptor stimulates interleukin-6 expression and secretion through both mRNA stability and transcriptional regulation: Involvement of p38 mitogen-activated protein kinase and nuclear factor-κB. Molecular Pharmacology. 2009;76:144–152. doi: 10.1124/mol.108.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesic V, Kosec D, Radojevic K, Pilipovic I, Perisic M, Vidic-Dankovic B, Leposavic G. Expression of α1-adrenoceptors on thymic cells and their role in fine tuning of thymopoiesis. Journal of Neuroimmunology. 2009;214:55–66. doi: 10.1016/j.jneuroim.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Petty HR. Regulation of RAW264 macrophage morphology and spreading: Studies with protein kinase C activators, inhibitors and a cyclic AMP analog. Biochimica et Biophysica Acta. 1989;1012:284–290. doi: 10.1016/0167-4889(89)90110-9. [DOI] [PubMed] [Google Scholar]
- Plećaš-Solarović B, Hristić-Živković I, Radojević K, Kosec D, Leposavić G. Chronic α1-adrenoreceptor blockade produces age-dependent changes in rat thymus structure and thymocyte differentiation. Histology and Histopathology. 2005;20:833–841. doi: 10.14670/HH-20.833. [DOI] [PubMed] [Google Scholar]
- Price DT, Lefkowitz RJ, Caron MG, Berkowitz D, Schwinn DA. Localization of mRNA for three distinct α1-adrenergic receptor subtypes in human tissues: Implications for human α-adrenergic physiology. Molecular Pharmacology. 1993;45:171–175. [PubMed] [Google Scholar]
- Ricci A, Bronzetti E, Conterno A, Greco S, Mulatero P, Schena M, Schiavone D, Tayebati SK, Veglio F, Amenta F. α1-adrenergic receptor subtypes in human peripheral blood lymphocytes. Hypertension. 1999;33:708–712. doi: 10.1161/01.hyp.33.2.708. [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, Perez DM. α1A- but not α1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovascular Research. 2005;65:436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. Neuroendocrine mediators up-regulate α1b- and α1d-adrenergic receptor subtypes in human monocytes. Journal of Neuroimmunology. 1999;95:165–173. doi: 10.1016/s0165-5728(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. Noradrenaline induces phosphorylation of ERK-2 in human peripheral blood mononuclear cells after induction of α1-adrenergic receptors. Journal of Neuroimmunology. 2000;108:82–91. doi: 10.1016/s0165-5728(00)00253-8. [DOI] [PubMed] [Google Scholar]
- Russell JA. Management of sepsis. New England Journal of Medicine. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Munson AE. Beta adrenoceptor mediation of the enhancing effect of norepinephrine on the murine primary antibody response in vitro. Journal of Pharmacology and Experimental Therapeutics. 1984;230:183–192. [PubMed] [Google Scholar]
- Sanders VM, Munson AE. Role of alpha adrenoceptor activation in modulating the murine primary antibody response in vitro. Journal of Pharmacology and Experimental Therapeutics. 1985;232:395–400. [PubMed] [Google Scholar]
- Schauenstein K, Felsner P, Rinner I, Liebmann PM, Stevenson JR, Westermann J, Haas HS, Cohen RL, Chambers DA. In vivo immunomodulation by peripheral adrenergic and cholinergic agonists/antagonists in rat and mouse models. Annals of the New York Academy of Sciences. 2000;917:618–627. doi: 10.1111/j.1749-6632.2000.tb05427.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Kurt B, Hocherl K, Bucher M. Inhibition of NF-κB activity prevents downregulation of α1-adrenergic receptors and circulatory failure during CLP-induced sepsis. Shock. 2009;32:239–246. doi: 10.1097/SHK.0b013e3181994752. [DOI] [PubMed] [Google Scholar]
- Schulze W, Fu ML. Localization of α1-adrenoceptors in rat and human hearts by immunocytochemistry. Molecular and Cellular Biochemistry. 1996;163–164:159–165. doi: 10.1007/BF00408653. [DOI] [PubMed] [Google Scholar]
- Seelaender MC, Kazantzis M, Costa Rosa LF. The effect of adrenaline and Walker-256 tumour-induced cachexia upon Kupffer cell metabolism. Cell Biochemistry and Function. 1999;17:151–156. doi: 10.1002/(SICI)1099-0844(199909)17:3<151::AID-CBF820>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Seiffert K, Hosoi J, Torii H, Ozawa H, Ding W, Campton K, Wagner JA, Granstein RD. Catecholamines Inhibit the Antigen-Presenting Capability of Epidermal Langerhans Cells. Journal of Immunology. 2002;168:6128–6135. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- Shi T, Duan ZH, Papay R, Pluskota E, Gaivin RJ, de la Motte CA, Plow EF, Perez DM. Novel α1-adrenergic receptor signaling pathways: Secreted factors and interactions with the extracellular matrix. Molecular Pharmacology. 2006;70:129–142. doi: 10.1124/mol.105.020735. [DOI] [PubMed] [Google Scholar]
- Singh U. Effect of catecholamines on lymphopoiesis in fetal mouse thymic explants. Journal of Anatomy. 1979;129:279–292. [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Herrmann M, Berkmiller G, Frauenholz T, Lang B, Scholmerich J, Falk W. Neuronal regulation of interleukin 6 secretion in murine spleen: Adrenergic and opioidergic control. Journal of Neurochemistry. 1997;68:1633–1639. doi: 10.1046/j.1471-4159.1997.68041633.x. [DOI] [PubMed] [Google Scholar]
- Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-γ secretion from T cells in early type II collagen-induced arthritis. Arthritis and Rheumatism. 2008;58:3450–3460. doi: 10.1002/art.24030. [DOI] [PubMed] [Google Scholar]
- Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Research. 2006;66:11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- Suga S, Goto S, Togari A. Demonstration of direct neurite-osteoclastic cell communication in vitro via the adrenergic receptor. Journal of Pharmacological Sciences. 2010;112:184–191. doi: 10.1254/jphs.09283fp. [DOI] [PubMed] [Google Scholar]
- Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:303–307. doi: 10.1016/j.pnpbp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Iwagaki H, Tamura R, Katsuno G, Xue D, Sugita S, Mori S, Yoshino T, Tanaka N, Nishibori M. α1-Adrenergic receptor antagonists induce production of IL-18 and expression of ICAM-1 and CD40 in human monocytes. Journal of Immunotherapy. 2005;28:40–43. doi: 10.1097/00002371-200501000-00005. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Bronzetti E, Morra Di Cella S, Mulatero P, Ricci A, Rossodivita I, Schena M, Schiavone D, Veglio F, Amenta F. In situ hybridization and immunocytochemistry of α1-adrenoceptors in human peripheral blood lymphocytes. Journal of Autonomic Pharmacology. 2000;20:305–312. doi: 10.1046/j.1365-2680.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Veglio F, Tayebati SK, Schiavone D, Ricci A, Mulatero P, Bronzetti E, Rabbia F, Amenta F. α1-adrenergic receptor subtypes in peripheral blood lymphocytes of essential hypertensives. Journal of Hypertension. 2001;19:1847–1854. doi: 10.1097/00004872-200110000-00020. [DOI] [PubMed] [Google Scholar]
- Wittig O, Paez-Cortez J, Cardier JE. Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells. Stem Cells and Development. 2010;19:341–350. doi: 10.1089/scd.2009.0300. [DOI] [PubMed] [Google Scholar]
- Yang M, Reese J, Cotecchia S, Michel MC. Murine alpha1-adrenoceptor subtypes. I. Radioligand binding studies. Journal of Pharmacology and Experimental Therapeutics. 1998;286:841–847. [PubMed] [Google Scholar]