Abstract
Background:
Diffusion-weighted magnetic resonance imaging (DW-MRI) in renal diseases is an evolving field and its potential is yet to be fully realized.
Purpose:
To study the relationship between apparent diffusion coefficient (ADC) values of renal parenchyma and serum markers of renal function and stage of chronic kidney disease (CKD).
Materials and Methods:
A retrospective review was performed of all adult patients who underwent DW-MRI (at b-values of 0 and 500 s/mm2) for renal lesions from January 2009 to September 2010 and revealed 88 patients, of which 22 patients had renal dysfunction and 66 had normal renal function. Of these 22, 15 patients were known cases of CKD and were staged depending on disease severity. ADC values were determined for renal parenchyma and compared. Receiver operating characteristic (ROC) curves were drawn to establish cut-off ADC values. Pearson's correlation coefficient (R) was calculated between ADC and renal function parameters.
Results:
ADC values in patients with renal dysfunction were significantly lower than in patients with normal renal function (2.1133 ± 0.2851 vs. 2.3198 ± 0.1246 (×10-3 mm2/s)). ADC values lower than 2.0354 (×10-3 mm2/s) were seen only with renal dysfunction and higher than 2.4516 (×10-3 mm2 /s) were seen only with normal function. There was significant inverse correlation between ADC and serum creatinine (R = -0.530), blood urea (R= -0.502), and significant linear correlation (R = 0.784) with estimated glomerular filtration rate (eGFR). ADC values showed a statistically significant decreasing trend with increasing stage of CKD.
Conclusion:
ADC values may serve as an additional marker for the presence and degree of renal dysfunction.
Keywords: Apparent diffusion coefficient, chronic kidney disease, diffusion-weighted MRI, kidney, renal dysfunction
Introduction
Chronic kidney disease (CKD) is a common global public health problem and the average incidence of end-stage renal disease in developing countries is 150 per million population, which is lower than that in the developed world.[1] Since renal parenchymal disease is accompanied by renal dysfunction, monitoring renal function permits assessment of disease progression, and periodic assessment of renal function is necessary for optimal management of a patient with suspected/proven renal disease. Serum creatinine (S Cr), blood urea (BU), and estimated glomerular filtration rate (eGFR) derived from creatinine clearance are useful for monitoring renal function; however, these indirect measures of renal filtration are imperfect and cannot assess single kidney function.[2,3]
Keeping in view the limitations of serum markers, imaging may play an important role in the evaluation of renal parenchymal disease. Ultrasonography (USG) and computed tomographic (CT) scan provide good anatomic images but limited functional information. Although USG may show changes in renal echogenicity, it suffers from operator dependency and lacks objectivity. In addition to exposure to ionizing radiation, CT scan requires use of iodinated contrast material, which is undesirable in patients with renal dysfunction. Magnetic resonance imaging (MRI) has the unique ability to show both structure and function objectively without any radiation exposure to the patient. Functional MRI techniques such as diffusion-weighted imaging (DWI), blood oxygen level-dependent (BOLD) imaging, and contrast-enhanced MRI renography have potential utility in the evaluation of renal function.[4]
Diffusion-weighted MRI (DW-MRI) is a non-invasive modality to characterize tissues based on Brownian motion of water molecules within them. Apparent diffusion coefficient (ADC) is a quantitative parameter calculated from DWI that combines the effects of capillary perfusion and water diffusion. DW-MRI in kidneys makes sense because of the organ's high blood flow and role in water filtration. DW-MRI in renal diseases is an evolving field and previous investigators have attempted to evaluate its utility in the characterization of focal renal lesions,[5–11] renal parenchymal disease,[7,12–16] and renal infections.[17–19] There is paucity of literature investigating the relationship between ADC values and eGFR as well as with different stages of CKD.[14–16] No cut-off ADC values have ever been proposed to identify renal dysfunction.
The purpose of this study was to investigate the relationship between ADC values of renal parenchyma and serum markers of renal function and stage of CKD. We also intended to establish cut-off ADC values to identify renal dysfunction.
Materials and Methods
Subject population and data collection
This was a single-institution retrospective study approved by the institutional ethics committee and the need to obtain informed consent was waived. We have been doing DW-MRI at our institute as part of protocol for focal renal lesions since 2008. A review was performed of all adult patients who underwent DW-MRI for renal lesions from January 2009 to September 2010 and revealed 88 patients (55 men, 33 women, mean age 45.1 years, age range 18-85 years). The patients were classified based on the presence of renal dysfunction, which was defined as S Cr > 1.5 mg/dl and/or BU > 40 mg/dl.[20] Twenty-two patients were found to have renal dysfunction whereas 66 had normal serum markers. Mean S Cr for the renal dysfunction study group was 4.03 mg/dl (range 1.6-18.4 mg/dl) and mean BU was 90.5 mg/dl (range 43-209 mg/dl). Out of these 22, 15 patients were known cases of CKD whereas the remaining had acutely deranged renal function. CKD patients were classified into stages based on disease severity, as per the K/DOQI CKD (kidney disease outcome quality initiative) classification.[21] For the cohort of CKD patients, eGFR was calculated by Cockcroft-Gault's equation for creatinine clearance. All data including demographic information, clinical, and laboratory profile were collected by one author.
MRI
All patients underwent MRI on a 1.5-T scanner (Siemens, Avanto, Erlangen, Germany) (maximum gradient strength 45 mTm−1, maximum slew rate 200 mTm−1 s−1) using a phased array body coil with the patient in supine position. Two six-element body matrix coils were placed anteriorly and used in conjunction with two posterior spine clusters (three channels each) to optimize the signal-to-noise ratio (SNR). The imaging protocol included True Fast Imaging and Steady Precession (True FISP) axial and coronal sequences, which served as localizer for planning further sequences. Then conventional MRI sequences, T1W axial (in and opposed phase) and fat-suppressed (FS) T2W axial and coronal sequences, were acquired.
DW MR imaging
Respiratory triggered FS (spectral fat suppression) spin echo-echo planar imaging (SE-EPI) axial diffusion-weighted sequence at b-values of 0 and 500 s/mm2 was done using parallel imaging based on generalized auto-calibrating partially parallel acquisition (GRAPPA) using a twofold acceleration factor and diffusion gradients applied in all three orthogonal directions separately. The following parameters were used: EPI factor = 95, TR/TE = 1600/62 ms, flip angle = 90 degrees, slice thickness = 7 mm, distance factor = 30%, number of averages = 6, receiver bandwidth = 1735 Hz/pixel, field of view = 249 × 380, matrix = 94 × 192, acquisition time = 2-4 min (depending on patient's respiratory cycle). The DW sequence was respiratory triggered using the navigator-triggered prospective acquisition correction technique (PACE) in which the diaphragmatic position is assessed periodically by navigator echoes. Trace DW images and ADC maps were derived automatically on a voxel-by-voxel basis. Good-quality DW images and ADC maps could be obtained in all the patients.
Image analysis
Regions of interest (ROIs) for quantitative measurement of ADC were placed on a commercial workstation by a single radiologist, blinded to the renal function parameters of the patients. To measure the ADC of renal parenchyma, circular ROIs of size 1 cm2 were placed on the normal renal parenchyma of the side contralateral to the focal renal lesion, without any preference for cortex/medulla. Three such ROIs were placed-one each in the upper pole, inter-polar region, and lower pole-and the mean of these three values was calculated. The ADC values were expressed as mean ± standard deviation in the form of A × 10−3 mm2/s up to four decimal places. We did not evaluate ADC values in the renal cortex and medulla separately because as pointed out by previous studies, it may be difficult to position the ROI cursor accurately in these areas.[6,16,22]
Statistical analysis
It was performed using the SPSS software (version 17.0; SPSS; Chicago, Illinois, USA). Unpaired Student's t-test and one-way analysis of variance (ANOVA) were used to evaluate the difference between ADC values of two or more groups. Box-and-whisker plots were drawn based on median and interquartile ranges to highlight the difference between the groups and variation within the groups. Receiver operating characteristic (ROC) curves were drawn to find out area under the curve (AUC) for differentiation of two groups and cut-off ADC values were calculated so as to achieve the highest average sensitivity and specificity. To investigate the relationship between ADC values and S Cr/BU/eGFR, Pearson's correlation coefficient was calculated by bivariate correlation. All P values <0.05 were taken as statistically significant.
Observations and Results
Patient characteristics
The study cohort of renal dysfunction included 22 patients (7 men, 15 women, mean age 39.5 years), of which 15 had CKD (4 men, 11 women, mean age 36.8 years). Of these 15, 4 patients had diabetes mellitus, 4 had hypertension, 3 had renal calculus disease, 1 each had systemic lupus erythematosus (SLE) and antiphospholipid (APL) antibody syndrome, and 2 had no obvious background clinical disease to which renal dysfunction could be attributed. As per the K/DOQI CKD classification, 6 patients had stage-3, 5 had stage-4, and 4 had stage-5 disease.
ADC values and renal function
The mean ADC value of renal parenchyma in patients with renal dysfunction (n = 22) was significantly lower than in patients with normal renal function (n = 66) (2.1133 ± 0.2851 vs. 2.3198 ± 0.1246 (× 10−3 mm2/s); P = 0.000) [Figure 1 and Figure 2]. There was no significant difference between the ADC values of patients with chronic renal dysfunction (n = 15) and those with acutely deranged renal function (n = 7) (1.9777 ± 0.3427 vs. 2.1912 ± 0.2572 (× 10−3 mm2/s), respectively; P = 0.173). We performed ROC analysis for ADC in differentiating patients with renal dysfunction from those with normal functioning kidneys. For detection of renal dysfunction, AUC was 0.720, SE = 0.081, and P = 0.006. For a cut-off ADC value of 2.2499 (× 10−3 mm2/s), sensitivity was 58.8%, specificity was 79.4%, and 95% confidence intervals = (0.562, 0.878) (values below cut-off indicated renal dysfunction). In addition, ADC values lower than 2.0354 (× 10−3 mm2/s) were seen only with renal dysfunction (100% specificity) and higher than 2.4516 (× 10−3 mm2/s) were seen only with normal renal function (100% sensitivity).
Figure 1.
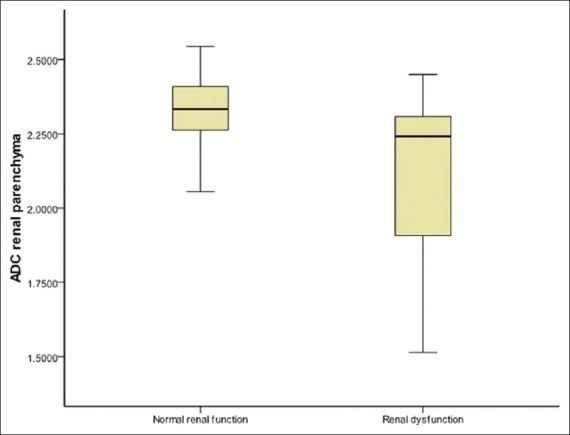
Box-and-whisker plot of renal parenchymal ADC in patients with renal dysfunction and those with normal renal function showing lower ADC values associated with renal dysfunction. The lines within boxes represent median value. The top and bottom of boxes (hinges) represent the 25th and 75th percentiles of the data values. The T-bars that extend from the boxes (whiskers) are expected to include approximately 95% of the data (assuming normal distribution)
Figure 2 (A, B).
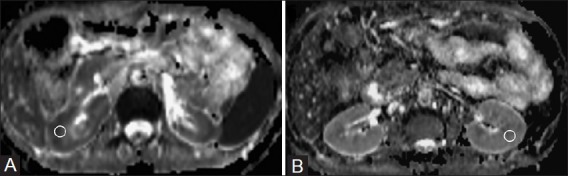
ADC map (derived from DW-MRI) (A) in a patient of CKD (stage-5) showing restricted diffusion in the renal parenchyma bilaterally with a mean ADC value of 1.4172 × 10−3 mm2/s). ADC map (B) in a patient with normal renal function showing no restriction of diffusion and mean ADC value was 2.3415 (×10−3 mm2/s). The circles depict examples of ROI placement
ADC values and serum markers of renal function
There was a significant inverse correlation between ADC values of renal parenchyma and S Cr levels (Pearson's correlation coefficient R = - 0.530; P = 0.000). Similarly, a significant inverse correlation was also observed between ADC values of renal parenchyma and BU levels (R = - 0.502; P = 0.000). Within the CKD study group (n = 15), a significant linear correlation was found between renal parenchymal ADC values and eGFR (R = 0.784; P = 0.003) [Figure 3].
Figure 3.
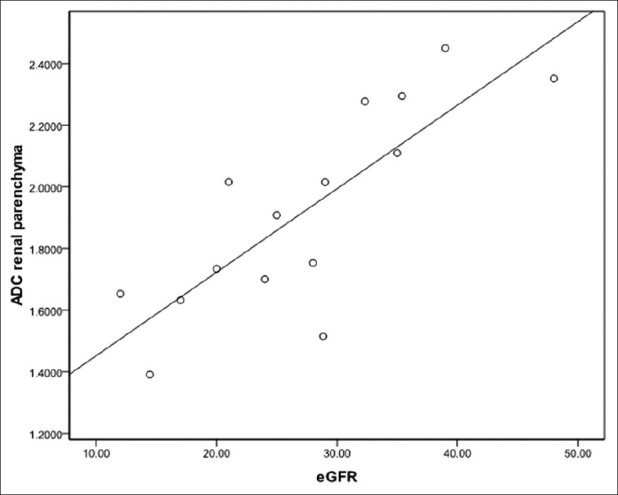
Scatter plot with interpolation line showing linear correlation between renal parenchymal ADC and eGFR
ADC values and stages of CKD
The mean ADC values of different stages of CKD were significantly different from each other (P = 0.001) and showed decreasing trend with increasing stage (2.2964 ± 0.1243 (× 10−3 mm2/s) for stage-3, 1.8413 ± 0.2117 (× 10−3 mm2/s) for stage-4, and 1.5218 ± 0.1853 (× 10−3 mm2/s) for stage-5) [Figure 4A and B]. On applying post-hoc analysis, the difference was statistically significant between stage-3 and-4, as well as between stage-4 and-5 (P = 0.003 and 0.05, respectively).
Figure 4 (A, B).
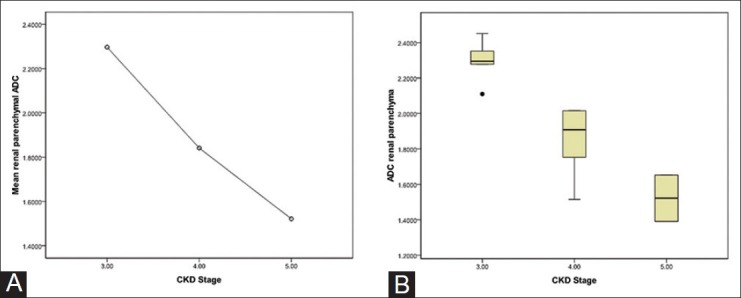
Means plot of renal parenchymal ADC in different stages of CKD showing decreasing trend of mean ADC values with increasing stage of CKD. Box-and-whisker plot (B) of renal parenchymal ADC in different stages of CKD showing decreasing ADC values with increasing stage of CKD. The points denote outliers
Discussion
The mean ADC value of renal parenchyma in patients with renal dysfunction was significantly lower than in patients with normal renal function. Similar results have also been reported by previous investigators.[7,12–15] Low ADC values in renal parenchymal disease can be explained by reduced perfusion as well as reduced water diffusion. Glomerulosclerosis, tubular atrophy, and interstitial fibrosis restrict the free movement of water molecules in both the extracellular and intracellular space, leading to lower ADC values. ADC values lower than 2.0354 (×10−3 mm2/s) were seen only with renal dysfunction and higher than 2.4516 (×10−3 mm2/s) were not seen in renal dysfunction. No cut-off ADC values have ever been proposed in the literature. Hence, we propose that population and equipment/protocol-based cut-off ADC values may serve as an additional marker for identifying renal dysfunction. No significant difference was observed between the ADC values of patients with chronic and acute renal dysfunction.
A significant inverse correlation was found between ADC values of renal parenchyma and S Cr/BU levels. This is in agreement with previous studies.[12,15] Also, a significant linear correlation was found between renal parenchymal ADC values and eGFR in CKD patients. This is in concordance with the results of Xu, et al.[14] who found a positive correlation between ADC and split renal GFR. Toya, et al.,[16] however, did not find any significant correlation between ADC values and eGFR.
The mean ADC values of different stages of CKD were significantly different from each other and showed a decreasing trend with increasing stage. For similar cut-off eGFR values, Toya, et al.[16] found a significant difference between stage-4 and-5 but not between stage-3 and -4 disease. On the other hand, in our study the difference was more striking between stage-3 and-4. Xu, et al.[15] found a mild negative correlation between ADC and stage of CKD; however, the difference between ADC values of different stages of CKD was not evaluated. A limitation of our study was that we did not have any patient with stage-1 and -2 CKD. Because of the small number of patients in different stages of CKD, cut-off ADC values were not calculated to distinguish among different stages.
Considering the inverse correlation of ADC values with S Cr and BU, positive correlation with eGFR, and taking into account the observation that ADC values showed a statistically significant decreasing trend with increasing stage of CKD, we propose that ADC values may be employed to estimate and monitor the degree of renal dysfunction. Comparison with the baseline ADC values may enable non-invasive monitoring of parenchymal disease progression. Similar to eGFR, cut-offs may be established for ADC values for distinction between various stages of CKD.
There are several limitations of our study. First, the patients underwent DW-MRI for characterization of focal renal lesions and not primarily for evaluation of renal function. Thus the sample size of our study group was small because we did not specifically recruit patients with renal dysfunction. Also, we did not recruit healthy volunteers for comparison with the renal dysfunction group. Rather we evaluated ADC values in patients with normal renal function, who otherwise had focal renal lesions. Care was taken to exclude any potential changes in renal parenchymal ADC (due to the renal lesion) by evaluating the contralateral kidney. Second, we did not investigate the correlation of ADC values with the function of each kidney. We evaluated eGFR by individual patient, not by each kidney. We also acknowledge that a standardized protocol for renal DW-MRI has not yet been established and this contributes to variation in the absolute ADC values in different studies; nevertheless, the trends of ADC values are reproducible.
To conclude, ADC values may serve as an additional paradigm to identify and estimate the degree of renal dysfunction. This may be especially useful in patients undergoing DW-MRI for other purposes (where it may lead to incidental detection of renal dysfunction) as well as in established CKD patients to monitor disease progression. Assessment of renal dysfunction by DWI may help guide the decision to inject a gadolinium-based contrast into patients not previously known to have renal disease. Population- and protocol-based cut-off ADC values may be established to identify renal dysfunction and distinguish between different stages of CKD, and comparison with the baseline ADC values may enable detection of disease worsening/improvement. The advantages of DW-MRI as indicator of renal function include short time of acquisition, non-invasive nature, and no exposure to ionizing radiation/contrast material, whereas the drawbacks include availability and cost. It must be borne in mind that DW-MRI is in no way a substitute to serum markers or renal scintigraphy for assessment of renal dysfunction; rather it is an additional tool, incorporation of which within existing MRI protocols provides additional functional information with minimal increase in imaging time. This functional information provided by DWI, along with morphological information of the kidneys, pelvicalyceal system (MRI urography), and renal vasculature (MRI angiography), may contribute toward making MRI a one-stop modality for comprehensive renal evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Agarwal SK, Srivastava RK. Chronic kidney disease in India: Challenges and solutions. Nephron Clin Pract. 2009;111:c197–203. doi: 10.1159/000199460. [DOI] [PubMed] [Google Scholar]
- 2.Bauer JH, Brooks CS, Burch RN. Clinical appraisal of creatinine clearance as a measurement of glomerular filtration rate. Am J Kidney Dis. 1982;2:337–46. doi: 10.1016/s0272-6386(82)80091-7. [DOI] [PubMed] [Google Scholar]
- 3.Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Chandarana H, Lee VS. Renal functional MRI: Are we ready for clinical application? AJR Am J Roentgenol. 2009;192:1550–7. doi: 10.2214/AJR.09.2390. [DOI] [PubMed] [Google Scholar]
- 5.Squillaci E, Manenti G, Di Stefano F, Miano R, Strigari L, Simonetti G. Diffusion weighted MR imaging in the evaluation of renal tumours. J Exp Clin Cancer Res. 2004;23:39–45. [PubMed] [Google Scholar]
- 6.Cova M, Squillaci E, Stacul F, Manenti G, Gava S, Simonetti G, et al. Diffusion weighted MRI in the evaluation of renal lesions: Preliminary results. Br J Radiol. 2004;77:851–7. doi: 10.1259/bjr/26525081. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, Seo Y, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187:1521–30. doi: 10.2214/AJR.05.0778. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: Characterization with diffusion-weighted MR imaging-A preliminary experience. Radiology. 2008;247:458–64. doi: 10.1148/radiol.2472070823. [DOI] [PubMed] [Google Scholar]
- 9.Taouli B, Thakur R, Mannelli L, Babb JS, Kim S, Hecht EM, et al. Renal lesions: Characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009;251:398–407. doi: 10.1148/radiol.2512080880. [DOI] [PubMed] [Google Scholar]
- 10.Kilickesmez O, Inci E, Atilla S, Tasdelen N, Yetimoglu B, Yencilek F, et al. Diffusion-weighted imaging of the renal and adrenal lesions. J Comput Assist Tomogr. 2009;33:828–33. doi: 10.1097/RCT.0b013e31819f1b83. [DOI] [PubMed] [Google Scholar]
- 11.Sandrasegaran K, Sundaram CP, Ramaswamy R, Akisik FM, Rydberg MR, Lin C, et al. Usefulness of diffusion-weighted imaging in the evaluation of renal masses. AJR Am J Roentgenol. 2010;194:438–45. doi: 10.2214/AJR.09.3024. [DOI] [PubMed] [Google Scholar]
- 12.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832–37. doi: 10.1002/(sici)1522-2586(199906)9:6<832::aid-jmri10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases-initial experience. Radiology. 2005;235:911–7. doi: 10.1148/radiol.2353040554. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: Preliminary experience. J Magn Reson Imaging. 2007;26:678–81. doi: 10.1002/jmri.20979. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Fang W, Ling H, Chai W, Chen K. Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: Initial study. Eur Radiol. 2010;20:978–83. doi: 10.1007/s00330-009-1619-8. [DOI] [PubMed] [Google Scholar]
- 16.Toya R, Naganawa S, Kawai H, Ikeda M. Correlation between estimated glomerular filtration rate (eGFR) and apparent diffusion coefficient (ADC) values of the kidneys. Magn Reson Med Sci. 2010;9:59–64. doi: 10.2463/mrms.9.59. [DOI] [PubMed] [Google Scholar]
- 17.Chan JH, Tsui EY, Luk SH, Fung SL, Cheung YK, Chan MS, et al. MR diffusion-weighted imaging of kidney-differentiation between hydronephrosis and pyonephrosis. Clin Imag. 2001;25:110–3. doi: 10.1016/s0899-7071(01)00246-7. [DOI] [PubMed] [Google Scholar]
- 18.Verswijvel G, Vandecaveye V, Gelin G, Vandevenne J, Grieten M, Horvath M, et al. Diffusion-weighted MR imaging in the evaluation of renal infection: Preliminary results. JBR-BTR. 2002;85:100–3. [PubMed] [Google Scholar]
- 19.Goyal A, Gadodia A, Sharma R. Xanthogranulomatous pyelonephritis: An uncommon pediatric renal mass. Pediatr Radiol. 2010;40:1962–3. doi: 10.1007/s00247-010-1828-y. [DOI] [PubMed] [Google Scholar]
- 20.Knochel JP. Biochemical alterations in advanced uremic failure. In: Jacobson HR, Striker GE, Klahr S, editors. The Principles and Practice of Nephrology. 1st ed. Philadelphia: BC Decker; 1991. pp. 682–4. [Google Scholar]
- 21.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 22.Fukuda Y, Ohashi I, Hanafusa K, Nakagawa T, Ohtani S, Annaka Y, et al. Anisotropic diffusion in kidney: Apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging. 2000;11:156–60. doi: 10.1002/(sici)1522-2586(200002)11:2<156::aid-jmri12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


