Abstract
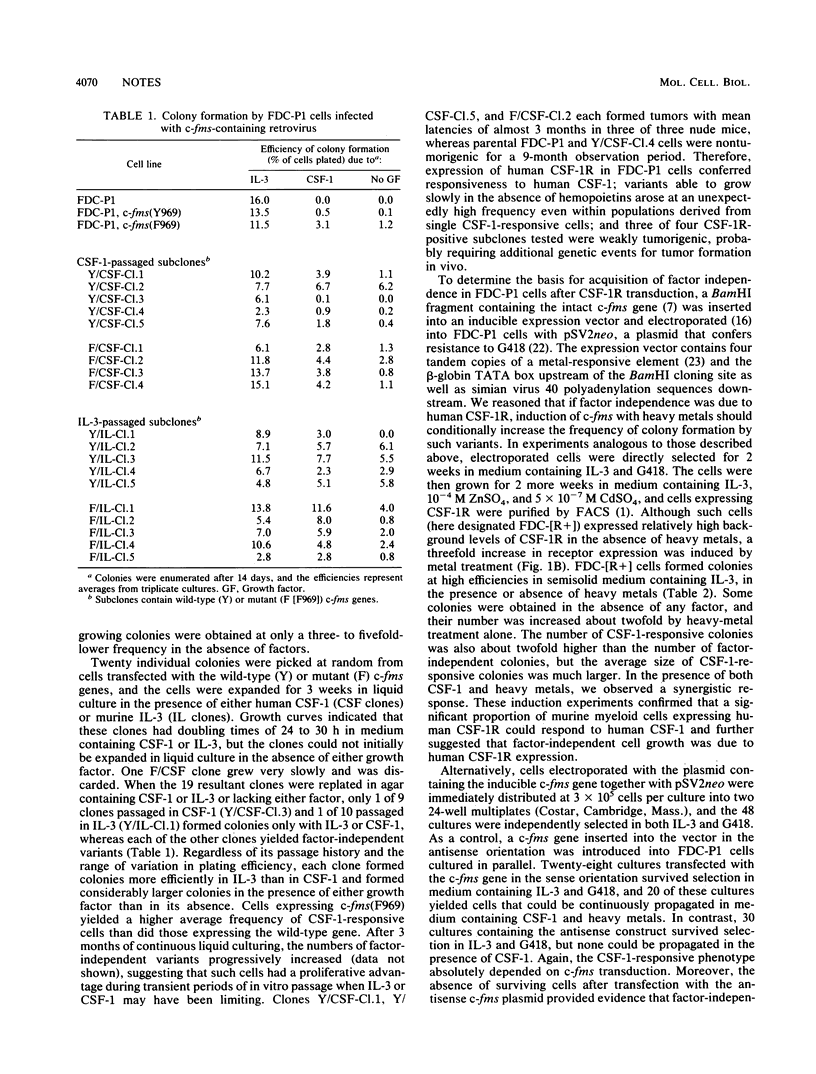
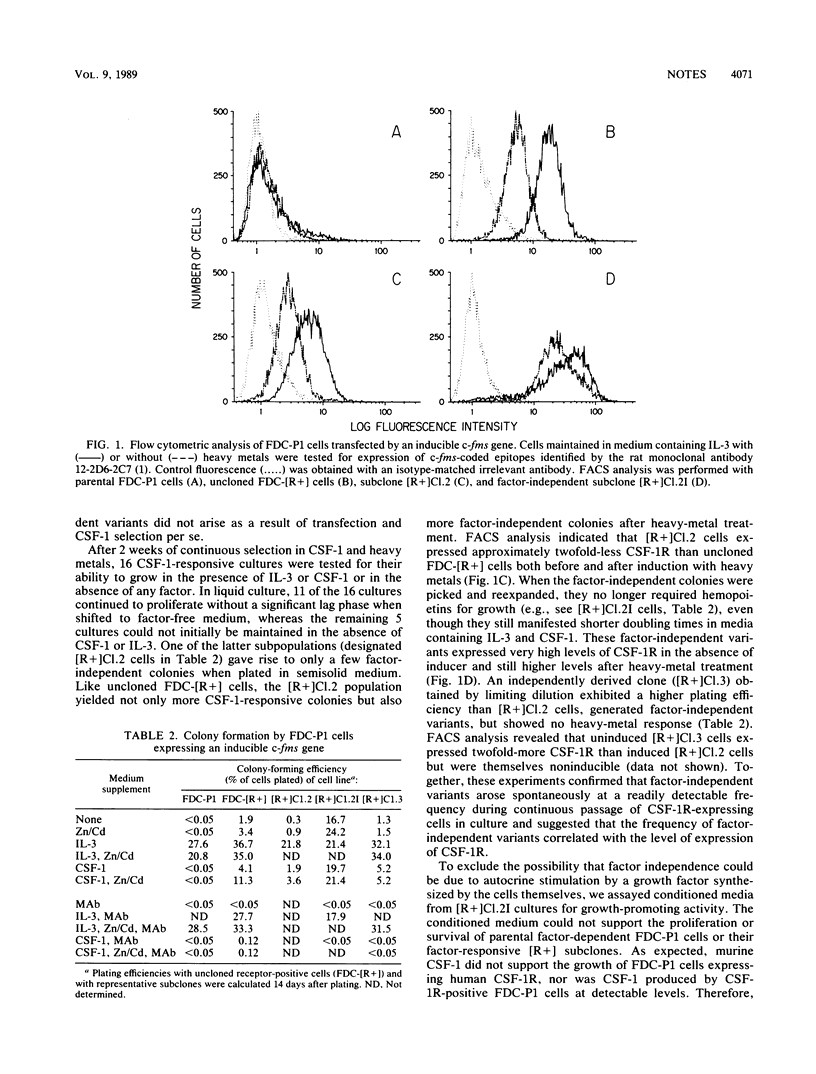
A retroviral vector encoding the receptor for human colony-stimulating factor-1 (CSF-1) was introduced into murine myeloid FDC-P1 cells which require interleukin-3 (IL-3) for their proliferation and survival in culture. Cells expressing the CSF-1 receptor (CSF-1R), selected by fluorescence-activated cell sorting in the continued presence of murine IL-3, formed colonies in semisolid medium and were able to proliferate continuously in liquid cultures containing human recombinant CSF-1. Thus, although they do not synthesize endogenous murine CSF-1R, FDC-P1 cells express the downstream components of the CSF-1 mitogenic pathway necessary for its signal-response coupling. After receptor transduction, slowly proliferating factor-independent variants that produced neither CSF-1 nor growth factors able to support the proliferation of parental FDC-P1 cells also arose. When the human CSF-1R was expressed in FDC-P1 cells under the control of an inducible metallothionein promoter, the frequencies of both CSF-1-responsive and factor-independent variants increased after heavy-metal treatment. In addition, a monoclonal antibody to human CSF-1R arrested colony formation by both the CSF-1-dependent and factor-independent cells but did not affect their growth in response to IL-3. Therefore, the induction of both the CSF-1-dependent and factor-independent phenotypes depended on expression of the transduced human CSF-1R.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmun R. A., Look A. T., Roberts W. M., Roussel M. F., Seremetis S., Ohtsuka M., Sherr C. J. Monoclonal antibodies to the human CSF-1 receptor (c-fms proto-oncogene product) detect epitopes on normal mononuclear phagocytes and on human myeloid leukemic blast cells. Blood. 1989 Feb 15;73(3):827–837. [PubMed] [Google Scholar]
- Bartelmez S. H., Stanley E. R. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: assay of hemopoietin-1. J Cell Physiol. 1985 Mar;122(3):370–378. doi: 10.1002/jcp.1041220306. [DOI] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chen B. D., Clark C. R. Interleukin 3 (IL 3) regulates the in vitro proliferation of both blood monocytes and peritoneal exudate macrophages: synergism between a macrophage lineage-specific colony-stimulating factor (CSF-1) and IL 3. J Immunol. 1986 Jul 15;137(2):563–570. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht S., Fichelson S., Sola B., Bordereaux D., Hampe A., André C., Galibert F., Tambourin P. Frequent c-fms activation by proviral insertion in mouse myeloblastic leukaemias. Nature. 1987 Sep 17;329(6136):259–261. doi: 10.1038/329259a0. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Heard J. M., Sola B., Martial M. A., Fichelson S., Gisselbrecht S. Long-term culture of bone marrow-derived preleukemic cells from F-MuLV-infected mice. Blood. 1986 Jul;68(1):193–199. [PubMed] [Google Scholar]
- Ihle J. N. The molecular and cellular biology of interleukin-3. Year Immunol. 1989;5:59–102. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Palaszynski E. W., Ihle J. N. Evidence for specific receptors for interleukin 3 on lymphokine-dependent cell lines established from long-term bone marrow cultures. J Immunol. 1984 Apr;132(4):1872–1878. [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Ashmun R. A., Downing J. R., Ohtsuka M., Quan S. G., Golde D. W., Roussel M. F. Inhibition of colony-stimulating factor-1 activity by monoclonal antibodies to the human CSF-1 receptor. Blood. 1989 May 15;73(7):1786–1793. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]


