Abstract
Hypo-pituitarism results from impaired production of one or more of anterior pituitary trophic hormones. A rare cause of hypo-pituitarism is pituitary stalk transection syndrome. The MRI features of this condition in children and its association with hormonal deficiencies have been reported earlier. Reports on adults with this disorder are scarce, with only one small case series published in the recent literature. We studied the hormonal deficiency pattern and MRI findings of 12 patients with pituitary stalk transection syndrome who presented to our department between 2004 and 2011. Six patients were children and six were adults (≥18 years). This article compares the adult clinico-radiological phenotype of pituitary transection syndrome with the pediatric group of patients with same condition.
Keywords: Ectopic posterior pituitary, hypo-pituitarism, magnetic resonance imaging, pituitary hormones, stalk transaction syndrome
Introduction
Hypo-pituitarism results from impaired production of one or more of anterior pituitary trophic hormones.[1] Patients with hypo-pituitarism may have isolated pituitary hormone deficiency (IPHD) or multiple pituitary hormone deficiency (MPHD), and may present with varied clinical manifestations like growth failure, central hypothyroidism, or underdeveloped secondary sexual characters. A rare cause of hypo-pituitarism is pituitary stalk transection syndrome. This syndrome was recognized only after MRI became widely used for evaluation of children with hypo-pituitarism. The characteristics features encountered are small anterior pituitary gland, absent or ectopic high signal intensity of posterior pituitary, and absence or hypoplasia of pituitary stalk. The MRI features of this condition in children and its association with hormonal deficiencies has been reported earlier.[2–4] Reports on adults with this disorder are scarce, with only one small case series published in the recent literature.[5] This article describes the adult clinico-radiological phenotype of pituitary transaction syndrome, and compares this with the pediatric group of patients with same condition.
Aim
The aim of this study was to compare the adult clinico-radiological phenotype of pituitary transection syndrome with the pediatric group of patients with same condition.
Materials and Methods
We studied the hormonal deficiency pattern and MRI findings of 12 patients with pituitary stalk transection syndrome who presented to our department between 2004 and 2011. Six patients were children and six were adults (≥18 years). The children (4 males and 2 females) were in the age group 4-16 years (mean ± SD = 11.7 ± 4.3 years. Adults (3 males and 3 females) were in age group of 22-41 years (mean ± SD = 27.5 ± 7.4 years).
MRI Technique
Three-millimeter contiguous sagittal and coronal plain T1W and T2W and dynamic post-contrast T1W MRI images through the sella were obtained using a 1.5-T superconducting MRI unit (Magnetom; Siemens, Erlangen, Germany). The images were evaluated for central nervous system malformations, with specific attention to the location and size of the anterior pituitary, its stalk, and posterior pituitary bright spot. Radiological diagnosis of empty sella was made when the cerebrospinal fluid (CSF) space was seen invaginating into the sella with non-visualization of the pituitary gland. Hypoplastic pituitary gland was defined as crescentic glandular tissue seen at the floor of the sella, with a maximum measurable height of 2 mm.[6] The stalk was reported as thin or absent.
Observations and Results
Of the children, three presented with isolated growth hormone deficiency and three presented with MPHD, and of the six adults, only one presented with isolated cortisol deficiency and the rest presented with multiple hormone deficiencies [Tables 1–3]. Only one adult patient had reported an onset of symptom at 14 years (short stature, delayed puberty), whereas the others presented at ≥18 years. History of breech delivery was elicited in two children, one of whom had IPHD and the other had MPHD. Of the five adults with MPHD, two had breech deliveries and one had a history of perinatal asphyxia. The only adult with IPHD had cortisol deficiency, with a history of difficult delivery with shoulder dystocia. The MRI features in our cases are as shown in Tables 1, 2 and 4.
Table 1.
Correlation of hormone deficiencies and MRI findings in children
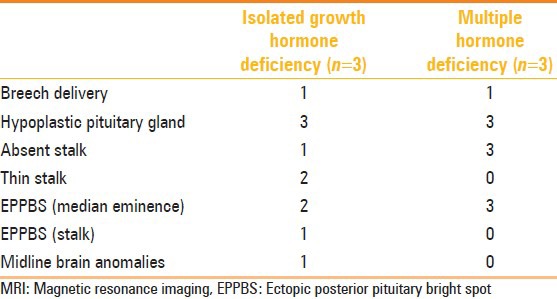
Table 3.
Pattern of multiple hormonal deficiency in children and adults
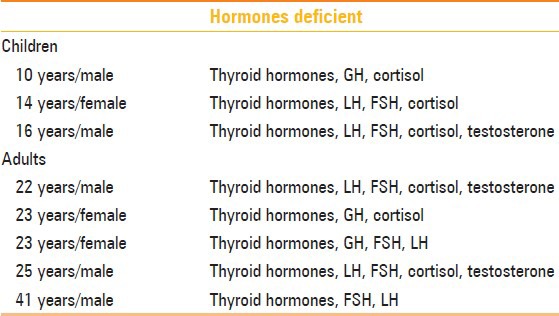
Table 2.
Correlation of hormone deficiencies and MRI findings in adults
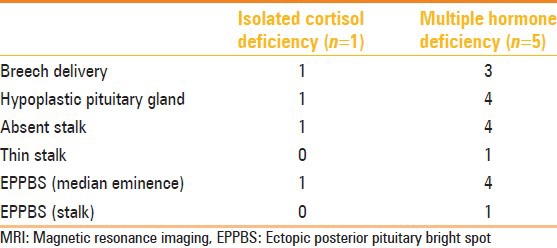
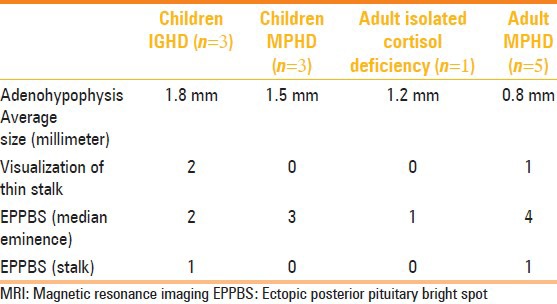
Table 4.
Comparison between MRI findings in children and adults
Discussion
Structural pituitary abnormalities have been reported in 50%-70% of patients with pituitary hormone deficiency.[7–12] These abnormalities are more prevalent in MPHD.[9] In both adults and children, ectopic posterior pituitary bright spot (EPPBS) at the median eminence was a universal finding in all patients, except one child who had the EPPBS in the stalk [Figure 1]. In general, subjects with multiple hormone deficiency did not have a stalk (3/3 (100%) in children; 4/5 (80%) in adults), and children with IPHD had a detectable thin stalk (2/3 (66.66%) in children) [Figure 2]. In our series, non-visualization of stalk was an important indicator of MPHD [Figures 3 and 4]. These findings are in consonance with previous studies.[13,14] The thin stalk was visualized only in post-contrast images in two children with IPHD and one adult with MPHD. Maghnie, et al.[15] and Genovese, et al.[16] have stressed the importance of contrast injection as a means of optimizing visualization of the thin stalk. They observed that in a previous study of similar patients evaluated with plain MRI, the thin stalks were deemed absent.[17] Visualization of an enhancing stalk on post-contrast images of patients with IGHD indicates partial preservation of the hypothalamo-hypophyseal portal vessels, and non-visualization in most patients with MPHD indicates progression of disease. Apparently the stalk becomes thinner as disease progresses from IPHD to MPHD. So the term ‘pituitary stalk hypoplasia or ‘thin stalk syndrome’ may be preferred over pituitary stalk transection syndrome. Using new MRI sequences like constructive interference in steady-state (CISS) may actually show the stalk in most cases of IPHD. The mean size of the adenohypohysis in children with IPHD was 1.86 ± 0.05 mm and the value in one adult with IPHD was 1.2 mm. The mean size of the adenohypophysis in children with MPHD was 1.5 ± 0.1 mm and in adults with MPHD it was 0.8 ± 0.74 mm [Table 4]. While the number of patients was too small to derive statistical significance, we hypothesize that patients progress from IPHD to MPHD, with subsequent hormone deficiencies appearing as they progress from childhood to adulthood. This is corroborated by our finding that the size of adenohypophysis seems to diminish, and also by the higher occurrence of MPHD in adults. This correlates with previous reports of hypo-pituitarism, where a small gland was found to be significantly related to MPHD after puberty.[18] Clearly a large multicenter study compiling patient data could answer this fascinating question. It is important therefore that the patients have long-term follow-up, with early detection and treatment of hormone deficiency as and when they manifest.
Figure 1 (A, B).
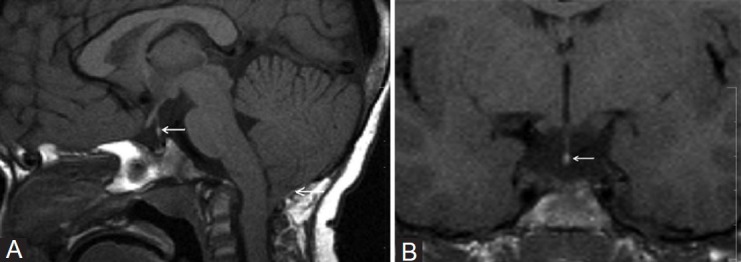
A 4-year-old child presented with IGHD. Plain sagittal (A) and coronal (B) T1W images show hypoplastic adenohypophysis, thin interrupted stalk, and EPPBS in mid-stalk (arrow) with type-1 Arnold-Chiari malformation (arrow)
Figure 2 (A-D).
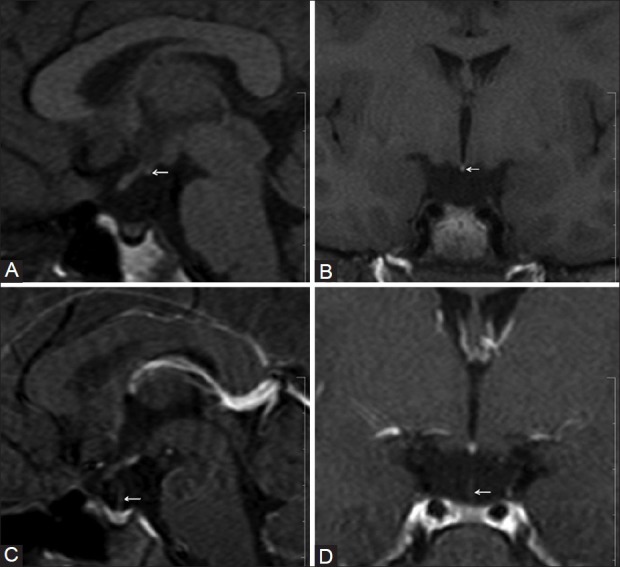
A 14-year-old female presented with IGHD. Plain sagittal (A) and coronal (B) T1W images show EPPBS at the median eminence (arrow), hypoplastic adenohypophysis, and absent stalk. However, in the contrast-enhanced, sagittal and coronal T1W images (D), a thin interrupted stalk was visualized (arrow)
Figure 3 (A-D).
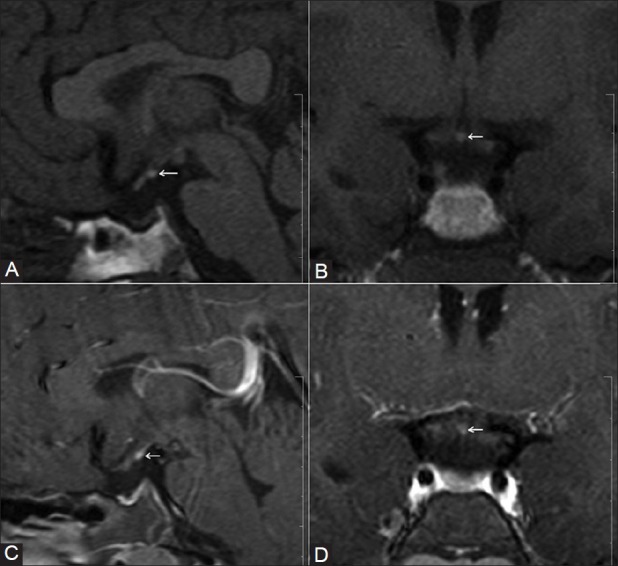
A 16-year-old male presented with MPHD. Plain sagittal (A) and coronal (B) T1W images, and contrast-enhanced sagittal (C) and coronal (D) T1W images show EPPBS at the median eminence (arrow), hypoplastic adenohypophysis, and absent stalk
Figure 4 (A, B).
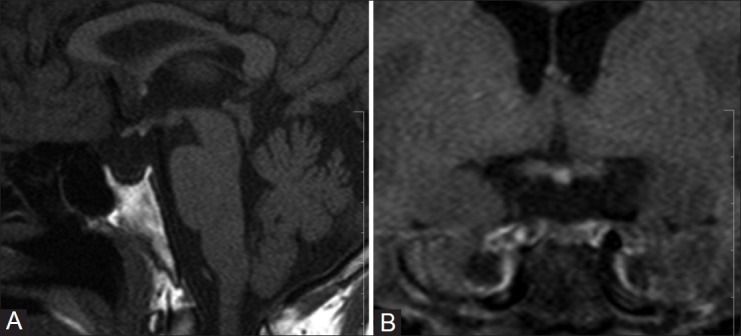
A 41-year-old male with a known case of panhypopituitarism (diagnosed at the age of 31 years) presented with hyponatremic seizures. Plain sagittal (A) and coronal (B) T1W images show EPPBS, a severely hypoplastic adenohypophysis, and absent stalk
Various hypotheses have been proposed to explain the pituitary stalk transection syndrome. One hypothesis is an ischemic insult to the pituitary stalk occurring because of trauma during breech delivery.[19] However, no pathological proof has been found and moreover these patients do not exhibit hypoxic damage to other structures sharing the same vascular supply. Another hypothesis is that head trauma associated with breech delivery causes mechanical rupture of the pituitary stalk as the stalk is stretched between the pituitary gland and the mobile brain.[20,21] Six out of 12 patients in our series had breech delivery and one patient had evidence of perinatal hypoxia. However, these two hypotheses do not explain the association of midline anomalies in these patients. A third conjecture therefore is that congenital hypoplasia or dysplasia of the pituitary gland is the cause of hypo-pituitarism. There is early fetal mal-development of midline structures, which results in failure of the neurohypophysis and its investing vascular plexus to descend completely into sella turcica. Anterior lobe hypoplasia and dysfunction result in decreased hormone secretion, which in turn results in increased incidence of breech presentation.[20,21] One of our children aged 4 years with IGHD had type-1 Chiari malformation [Figure 1].
Conclusion
This entity should be considered in the differential diagnosis in adults with hypo-pituitarism. The presence of MRI features suggestive of pituitary stalk transection syndrome should prompt a full pituitary hormonal evaluation. It is important to closely follow up these patients in the long-term so that their natural history of progressive radiological and hormonal deterioration can be ascertained and these patients can be managed better.
As patients progress from IPHD to MPHD, with subsequent hormone deficiencies appearing as they age from childhood to adulthood, the stalk and adenohypophysis become smaller, indicating that the term ‘pituitary stalk hypoplasia’ or ‘thin stalk syndrome’ is more appropriate than pituitary stalk transection syndrome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Melmed S, Jameson JL. Disorders of the anterior pituitary and hypothalamus. In: Lango DL, editor. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill Publishers; 2012. pp. 2876–902. [Google Scholar]
- 2.Fujisawa WM, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, et al. Transaction of pituitary stalk: Development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;166:487–9. doi: 10.1148/radiology.165.2.3659371. [DOI] [PubMed] [Google Scholar]
- 3.Kelly WM, Kucharczyk W, Kuharczyk J, Kjos B, Peck WW, Norman D, et al. Posterior pituitary ectopia: An MR feature of pituitary dwarfism. AJNR Am J Neuroradiol. 1988;9:453–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Kikuchi K, Fujisawa I, Momoi T, Yamanaka C, Kaji M, Nakano Y, et al. Hypothalamic pituitary function in growth hormone deficient patients with pituitary stalk transaction. J Clin Endocrinol Metab. 1988;67:817–23. doi: 10.1210/jcem-67-4-817. [DOI] [PubMed] [Google Scholar]
- 5.Ioachimescu AG, Hamrahian AH, Stevens M, Zimmerman RS. The pituitary transaction syndrome: Multifaceted presentation in adulthood. Pituitary. 2012;15:405–11. doi: 10.1007/s11102-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 6.Argyropoulou M, Perigram F, Brauner R, Brunelle F. Magnetic resonance imaging in diagnosis of growth hormone deficiency. J Pediatr. 1992;12:886–91. doi: 10.1016/s0022-3476(05)81955-9. [DOI] [PubMed] [Google Scholar]
- 7.Cacciari E, Zucchini S, Carla G, Pirazzoli P, Cicognini A, Mandini M, et al. Endocrine function and morphologic findings in patients with disorders of hypothalamo pituitary area: A study with magnetic resonance. Arch Dis Child. 1990;65:1199–202. doi: 10.1136/adc.65.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon D, Hadjiathanasiou C, Czernichow P, Leger J. Phenotypic variability in children with growth hormone deficiency associated with posterior pituitary ectopia. Clin Endocrinol. 2006;64:416–22. doi: 10.1111/j.1365-2265.2006.02484.x. [DOI] [PubMed] [Google Scholar]
- 9.Turton JP, Mehta A, Raza J, Woods KS, Tiulpakov A, Cassar J, et al. Mutations within the transcription factor PROP 1 are rare in cohort of patients with sporadic combined pituitary hormone deficiency (CPHD) Clin Endocrinol (Oxf) 2005;63:10–8. doi: 10.1111/j.1365-2265.2005.02291.x. [DOI] [PubMed] [Google Scholar]
- 10.Truilzi F, Scotti G, Di Natale B, Pellini C, Lukezic M, Swgnamiglio M, et al. Evidence of congenital midline anomaly in pituitary dwarfs: A magnetic resonance imaging study in 101 patients. Pediatrics. 1994;93:409–16. [PubMed] [Google Scholar]
- 11.Abrahams J, Trefelner E, Boulware AS. Idiopathic growth hormone deficiency; MR findings in 35 patients. Am J Radiol. 1991;12:155–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Ochi M, Morikawa M, Yoshimoto M, Kinoshita E, Hayashi K. Growth retardation due to idiopathic growth hormone deficiencies: MR findings in 24 patients. Pediatr Radiol. 1992;22:477–80. doi: 10.1007/BF02012987. [DOI] [PubMed] [Google Scholar]
- 13.Kornreich L, Horev G, Lazar L, Schwarz M, Sulkes J, Pertzelan A. MR findings in growth hormone deficiency: Correlation with severity of hypopituitarism. AJNR Am J Neuroradiol. 1998;19:1495–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Leger J, Garel C. Growth hormone deficiency with ectopic neurohypophysis: Anatomical variations and relationship between the visibility of the pituitary stalk asserted by magnetic resonance imaging and anterior pituitary function. J Clin Endocrinol Metab. 1999;84:2408–13. doi: 10.1210/jcem.84.7.5849. [DOI] [PubMed] [Google Scholar]
- 15.Maghnie M, Genovese E, Villa A, Spagnolo L, Campan R, Severi F. Dynamic MRI in the congenital agenesis of neural pituitary stalk syndrome: The role of the vascular pituitary stalk in predicting residual anterior pituitary function. Clin Endocrinol. 1996;45:281–90. doi: 10.1046/j.1365-2265.1996.00789.x. [DOI] [PubMed] [Google Scholar]
- 16.Genovese G, Maghnie M, Beluffi G, Villa A, Sammarchi L, Severi F, et al. Hypothalamic-pituitary vascularization in the pituitary stalk transection syndrome: Is the pituitary stalk really transected? Pediatr Radiol. 1997;24:48–53. doi: 10.1007/s002470050062. [DOI] [PubMed] [Google Scholar]
- 17.Maghnie M, Triulzi F, Larizza D, Preti P, Priora C, Scotti G, et al. Hypothalamic-pituitary dysfunction in growth hormone deficient patients with pituitary abnormalities. J Clin Endocrinol Metab. 1991;73:79–83. doi: 10.1210/jcem-73-1-79. [DOI] [PubMed] [Google Scholar]
- 18.Bozzola M, Adamsbaum C, Biscaldi I, Zecca M, Cisternino M, Genovese E, et al. Role of magnetic resonance imaging in the diagnosis and prognosis of growth hormone deficiency. Clin Endocrinol. 1996;45:21–6. [PubMed] [Google Scholar]
- 19.Shizume K, Harada Y, Ibavashi Y. Survey studies on pituitary disease in Japan. Endocrinol Jpn. 1977;24:139–46. doi: 10.1507/endocrj1954.24.139. [DOI] [PubMed] [Google Scholar]
- 20.Despert F, Guenault I, Bricaud P, Abury JC. Hypopituitarism caused by pituitary transection syndrome.Pathogenic hypotheses apropos of 7 cases. Pediatre. 1993;48:639–44. [PubMed] [Google Scholar]
- 21.Maghnie M, Larizza D, Triulzi F, Sampoalo P, Scotti G, Severi F. Hypopituitarism and stalk agenesis: A congenital syndrome worsened by breech delivery? Horm Res. 1991;35:104–8. doi: 10.1159/000181883. [DOI] [PubMed] [Google Scholar]


