Abstract
Aims
Functional tests have demonstrated minimal loss of vaginal closure force with age. So we tested the null hypotheses that age neither affects the maximum cross-sectional area (CSA) nor the volume of the levator muscle. Corresponding hypotheses were also tested in the adjacent obturator internus muscle, which served as a control for the effect of age on appendicular muscle in these women.
Methods
Magnetic resonance images of 15 healthy younger (aged 21 to 25 years) and 12 healthy older nulliparous women (aged > 63 years) were selected to avoid the confounding effect of childbirth. Models were created from tracing outlines of the levator ani muscle in the coronal plane, and obturator internus in the axial plane using 3D Slicer v. 3.4. Muscle volumes were calculated using Slicer, while CSA was measured using Imageware™ at nine locations. The hypotheses were tested using repeated measures analysis of variance with p < 0.05 being considered significant.
Results
The effect of age did not reach statistical significance for the decrease in levator ani muscle maximum CSA or the decrease in volume (4.3%, p=0.62 and 10.9%, 0.12, respectively). However, age did significantly adversely decrease obturator internus muscle maximum CSA and volume (24.5% and 28.2%, p<0.001, respectively). Significant local age-related changes were observed dorsally in both muscles.
Conclusions
Unlike the adjacent appendicular muscle, obturator internus, the levator ani muscle in healthy nullipara does not show evidence of significant age-related atrophy.
Keywords: pelvic floor, sarcopenia, aging, nullipara
Introduction
Pelvic floor disorders, including pelvic organ prolapse and incontinence, significantly affect women's daily activities and quality of life. Ageing is a known risk factor for these disorders: their incidence increases with each decade until eventually 11% of U.S. women require operative treatment (1). As the US population ages, the prevalence of pelvic floor disorders will increase substantially over the next decades (2) so there is a need to better understand how age is involved in the pathomechanics of these bothersome disorders.
The importance of the levator ani muscle in the role of pelvic floor function has been well established (for example, 3, 4, 5). Maternal birth-related levator ani injuries, observable on magnetic resonance images in affected women (6), are associated with a significantly increased risk of developing genital prolapse and a 40% decrease in pelvic floor muscle strength (7). Pelvic floor muscle isometric strength is usually measured using an instrumented speculum which can be used to measure maximum volitional vaginal closure force in the mid-sagittal plane (8). However, neither measurements of resting vaginal closure force, nor the maximum volitional augmentation of that force show a significant decline with increasing age (9). This is unexpected since a 30-40% loss of skeletal muscle volume and CSA is the usual finding in striated muscle with advancing age (10, 11). These reductions are responsible for the well researched age-related loss in isometric muscle strength and slower rate of developing axial or appendicular striated muscle strength in healthy older individuals (12).
Any functional muscle strength test can be limited by subject effort, especially in muscles like the levator ani whose activity is difficult to control volitionally. The significance of measuring muscle CSA is that it allows the maximal contractile force that can be developed in the direction of the fibers to be estimated, independent of effort level. This force is given by the product of the CSA and the maximum specific contractile force of striated muscle of about 2.8 Kgf/cm2(11,13). (Note – One Kilogram force (Kgf) is the magnitude of the force exerted on one kilogram of mass by a 9.81 m/s2 gravitational field; it is equal to 9.81 Newtons (N) of force). Muscle volume has also been found to be a good predictor of maximal muscle force (14), since the physiological CSA can be found by dividing the volume by the fiber length. It is presently not known whether age adversely affects the CSA of the levator ani muscle (15), or its volume. We reasoned that if there were no observational effects on levator volume or CSA, then this would help explain the apparent lack of an age effect on strength of the muscle described above.
The goal of this paper, therefore, was to test the primary null hypothesis that age neither affects the maximum CSA nor the volume of the levator ani muscle. We elected to select nulliparous women for this study to avoid the confounding effect of birth-induced levator ani muscle damage (16). Also, because levator muscle morphology varies between individuals (17) and age-related muscle atrophy can vary (18), we decided to measure nearby appendicular striated obturator internus muscle maximum CSA and volume as a control for the effect of age, in the same woman and in the same MR scan. A secondary hypothesis that there would be no regional effects of age in different parts of the levator muscle was also tested.
Methods
MR images of 12 healthy older nulliparous women without pelvic floor dysfunction (aged 63 years and over) were chosen from our prior work on age effects on pelvic floor function in nulliparous women (8). A sample of 15 healthy younger (aged between 21 and 25 years) was selected as a comparison group who served as nulliparous controls in a study of birth and pelvic floor injury (19). Written informed consent was provided for the use of these images as approved by the institutional review board. The sample size should provide 80% power for detecting an expected 30% age difference with an alpha of 0.05 in the volume and maximum CSA of levator ani, based on published studies of age-related changes in appendicular striated muscle (11).
Multi-planar, two dimensional, fast spin, proton density MR images we obtained using a 1.5 Tesla superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI). Turbo spin echo (TSE) techniques were used to image the sagittal, coronal, and axial planes. At rest, 30 images were obtained in each plane. Scan parameters were: repetition time (TR) range 2300-3000 ms, echo time (TE) 30 ms, 4 mm slice thickness, 1 mm gap, number of signal averages (NSA) 2, 256 × 255 voxels).
All of the women had full histories taken and a standardized physical examination including height, weight and pelvic organ prolapse quantification (POP-Q) as outlined in our earlier work (8,19). All subjects chosen had no known urogynaecological dysfunction according to history, physical examination and POP-Q examination.
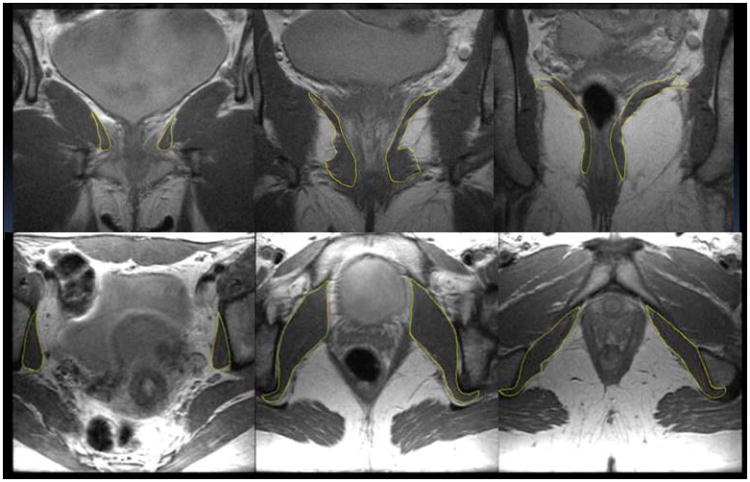
Virtual models of the muscles were made using 3D Slicer version 3.4 (Slicer.org). Outlines of the levator ani muscle were traced in the coronal plane (Fig 1a) as this showed the best view of the muscle (20). The obturator internus muscle models were generated from tracings made on axial plane images (Fig 1b). Obturator internus fibers converge towards the lesser sciatic foramen and then deviate back at a right angle over the ischium between its spine and tuberosity, join with the inferior and superior gemelli and insert onto the greater trochanter. Tracings were stopped at the level of the ischium due to the change in muscle direction and a primarily tendinous portion lateral to this point. Two of the 12 older women's MRI scans had incomplete levator ani images and could only be used for the obturator internus tracings. Models were then compared against the original scans with all three orthogonal image planes in view so as to detect artifacts from model creation. Errors were corrected iteratively by the blinded operator until each model and its source images matched.
Figure 1. a (above), sample traced outlines of levator ani (yellow) in coronal MR images from a young subject, and b (below), obturator internus outlined (yellow) in axial MR images.
Volumes were calculated within Slicer and then models were imported into Imageware™ for measurement of muscle CSA. In order to calculate muscle CSA, predominant fiber directions were determined. For the levator ani, the fiber direction and line-of-action were found by superimposing published fiber architecture (21) upon the model of the levator (Fig 2a). Because its architecture essentially consists of parallel muscle fibers from origin to insertion, the anatomic CSA was taken normal to the fiber direction. For the obturator internus, a fiber line was created from the midpoint of a line marking the ischium between its spine and tuberosity, to a midpoint at the dorsal aspect of the muscle. For each muscle, nine equidistant points were then marked along the line from an anterior to posterior position and the anatomical CSAs were calculated at those points in planes perpendicular to the fiber line (Fig 2b shows the levator ani).
Figure 2.
(Top) Ventrocaudal view looking down into a model of the pelvis showing the left levator ani (purple). The red line in the direction of the fibers is divided into eight segments by nine (yellow) equidistant lines. (Bottom) Strategy for assessing average levator ani line-of-action. The background colored lines are reproduced from Shobieri (11). Black dots indicate the upper, lower and median points along the mid-portion of the levator ani. A best fit line was constructed (black) to indicate the predominant fiber orientation. The nine perpendicular cross sections (red lines) were then constructed across the muscle to yield nine estimates of anatomic CSA (red dot “cloud”). PS, V, PP, PA, A, PR, PV, IC, C, CC, ATLA, and ATFP are usual abbreviations for pelvic floor structures (11).
To assess inter-rater reliability, outlines of levator ani were independently traced by two investigators on randomly selected scans of 3 young and 3 older women. Models were then created and volumes calculated. Inter-rater reliability was acceptable (r=0.9). Intra-rater reliability was also assessed using the obturator internus with acceptable repeatability (Investigator 1, r= 0.89; Investigator 2, r= 0.86).
The hypotheses involving muscle volume and maximal cross-sectional area were tested using repeated measures analysis of variance (mixed model procedures in SAS, v. 9.1; SAS, Inc. Cary, NC) and two-sided t-tests with p < 0.05 being considered significant. The Boneferoni correction was used for the CSA values at locations where the CSA was not maximal.
Results
The primary null hypothesis could not be rejected for the levator ani muscle. When the maximal CSA at any location was compared by group, the mean maximum CSA of the levator ani muscle for the older women was not significantly smaller in the older women than the younger women (406.7 [127.7] cm2 vs. 423.9 [106.8] cm2; group difference: 4.3%, p=0.62). Similarly, the mean [SD] levator muscle volume in the older women was also not significantly different in the older women than in the younger women (26.4 [5.3] cm3 vs. 29.6 [4.2] cm3, respectively; group difference: 10.9%, p=0.12).
The locations of the maximal levator CSA in both groups were recorded at either location 6 or 7 (young: 12 of 15 women; old: 9 of 10 women) but exceptions existed in both groups demonstrating anatomical variation. Figure 3 compares regional differences in levator ani CSA between young and old women, and the only location at which there was a significant age difference was at location 9, at the level of the iliococcygeus portion of the muscle (p= 0.006).
Figure 3.
The mean (SE, vertical bars) anatomical CSAs from young (n=15) and old (n=12) obturator internus. Location 1 is nearest the pubic symphysis and location 9 is closest to the ischial spine. In this and the following figure, * p<0.05.
The primary null hypothesis was rejected for the obturator internus muscle which, as an appendicular muscle was used as a control striated muscle for the levator muscle of the pelvic floor. There was a 24.5% decrease in greatest obturator internus CSA from young to old groups (p<0.0001). Differences existed at locations 2 and from 5 to 9 inclusive (Figure 4) as follows: location 2, 34.4% (p=0.03, non-significant (ns) after Boneferoni correction); location 5, 15.4% (p=0.02, ns); location 6, 21.1% (p=0.0006); location 7, 29% (p=0.001, ns), and location 8, 31.6% (p=0.01, ns), and 9, 29.1% (p=0.05. ns). Similarly, obturator internus muscle volume was significantly smaller in the older women than in the younger women (64.0 [10.3] cm3 vs. 89.1 [12.2] cm3); group difference: 28.2%, p<0.001).
Figure 4.
The mean (SE, vertical bars) anatomical CSAs of the levator muscle from young (n=15) and old (n=10) donors. Location 1 is close to the origin at the pubic symphysis and location 9 is close to the iliococcygeal/coccygeus insertion. [Note: the maximal values in the graph at location 7 are less than those reported for the mean maximal CSA because in some cases the maximal value occurred at a different location (usually 6 or 8).
Discussion
The results of this study indicate that the levator ani muscle does not undergo the same degree of age-related atrophy seen in adjacent appendicular muscle in healthy women. Aging causes the CSA of many striated muscles to decrease by approximately 25-40% between the ages of 20 and 60 years, the amount varying across different muscles (10, 11). Upon beginning this study we anticipated that the levator ani might undergo the same degree of age-related sarcopenic change as other muscles. We expected a significant decrease in CSA and volume, which could be invoked as a contributor to prolapse and its progression. We found, however, that the levator ani did not have the degree of change seen in the obturator internus muscle in our subjects.
The most likely explanation for the fact that obturator internus and levator ani muscle show different degrees of muscle loss may be that they have different fiber type compositions. The CSA of Type II fibers show a greater degree of atrophy with age than do Type I fibers, which are relatively spared (10). The levator ani muscle is comprised of between 66% and 90% Type I fibers (23-24), depending on biopsy site. This composition is consistent with levator postural function requiring fatigue-resistance in maintaining the closure of the urogenital hiatus in upright posture (5) that Type I fibers are well-suited to provide. This could explain why levator ani morphology and CSA may be less affected by age than the obturator muscle, which has a likely higher proportion of Type II fibers, given its activity and function in externally rotating the hip during gait.
One might argue that the instrumented speculum tests of levator isometric strength that failed to show an age effect (see Introduction) already have demonstrated that age does not affect the CSA or volume, since muscle contractile force is known to be proportional to CSA (13) and volume (14). This argument is weakened by two known confounding effects. Firstly, volitional motor control of the levator ani is not nearly as precise as that for appendicular or axial musculature (26), so tests of pelvic floor muscle strength do not necessarily give a good indication of levator CSA or volume unless tetanic electrical stimulation were to be used, and that can be painful. Secondly, when the levator muscle contracts, only the ventromedial portion acts to close the urogenital hiatus; the more dorsal portion acts like a shelf and contracting it elevate the posterior abdominal contents resting upon it (4). How much of the levator CSA acts to cause the vaginal closure force is unknown, so the latter is an unreliable measure of the total levator CSA or volume. However, now that the present study shows a lack of an age effect on levator CSA, this finding corroborates and extends the lack of an age effect on the vaginal closure force that has already been documented (8).
Other investigators have studied the relationship between levator ani muscle function and age. In a group of patients attending a tertiary urogynecology clinic a weak correlation (r= -0.25, p<0.01) was found between age and reduced levator strength on manual palpation after correcting for parity and levator defects (25). These findings reflect assessments of women with pelvic floor symptoms resulting in referral to the Urogynecology clinic and women who were primarily vaginally parous; factors that may help explain slight differences in findings from the current study. In a clinical population, most of the women will have given birth and so birth-associated injuries (6) will confound a study of age effects. The relationship between these findings among a group of individuals who had pelvic floor complaints and the present findings in normal volunteers requires further exploration.
When one considers the finding that increasing stages of prolapse are found with advancing age (27) it has tacitly been assumed that this finding is secondary to the adverse effects of age on pelvic floor support. If, as indicated by the present results, ageing has less effect on levator muscle size and strength than has been thought, then it remains to be elucidated how age influences other tissue properties and how these changes interact with processes of pregnancy and vaginal birth to result in the eventual failure of the pelvic floor support system.
It seems logical that the connective tissue elements undergo age-related deterioration and that they may be involved. Collagen components of connective tissue undergo remodeling in response to stress and injury. There is a reduction in collagen content in the genitourinary tissue of women with genital prolapse when compared with women without prolapse (28-29) and it is known that increasing age is associated with significant changes in collagen resulting in stiffening of the tissues (30). Ongoing research in this area should help to demonstrate which hypotheses are and are not supported.
A strength of the present study was the use of nullipara as the basis for comparison because it eliminated the major confounders of pregnancy and parity (see Introduction). A second strength was the use of published fiber directions (21) to improve the estimate of levator anatomic CSA because these are not always visible on MR scans. While we were able to demonstrate that the levator ani did not undergo the degree of age-related atrophy suggested in the literature, while the obturator exhibited the prototypical changes, the modest sample size limited the power available to demonstrate significant differences in levator CSA. A post-hoc analysis shows that with an effect size of 0.38 we would have needed to have studied over 46 women in each age group to be able to demonstrate a significant age effect in levator volume; with an effect size of only 0.15 for levator CSA, the group sizes would have had to have been two orders of magnitude larger. Clearly, the effect of age on levator morphology is so modest that it does not appear to be clinically significant.
As one check on external validity of the present results, we can compare the present findings with the Chen et al. study of levator CSA and volume results from the unaffected muscle in women with unilateral defects in the pubovisceral portion of their levator ani (31). In that study, the overall volume of the pubovisceral portion of levator ani without defects to average 20.8 cm3, which compares favorably with the values of 26.4 and 29.6 cm3 for the volumes of the entire levator ani found in the younger and older women respectively in the present study. The CSA of the pubovisceral muscle at the measurement location closest to the pubic bone was found to average 1.02 cm2 in the Chen et al. study, which compares with 0.2 cm2 and 0.7 cm2 found for the locations 1 and 2 in the present study. The difference can be partly explained by Chen et al. sampling the pubovisceral muscle at 5 locations, whereas 9 locations were used to sample the entire levator ani in this study, hence location 1 was closer to the pubic bone in the present study. In addition, by concentrating only on the pubovisceral portion, Chen et al. chose a fiber direction angled approximately 25 degrees more medial from the pubic bone than the most common levator fiber direction observed in the Shobeiri et al. data. This difference would account for another 8% (the reciprocal of the cosine of 25°) of the difference between the two studies. Hence, the results of the two studies are consistent.
As a second check on the external validity of the present CSA measurements, one can estimate how much of the CSA of the levator muscle would be needed to generate the mean maximal voluntary vaginal closure force of ∼10 N measured in healthy women (5). If we assume that the line-of-action of the pubovisceral portion of the levator muscle acts, bilaterally, at an angle of 30 degrees (estimated from 31) to the mid-sagittal plane in which the vaginal closure force is measured (see Introduction), the required tension in the pubovisceral muscle would have to be 10 N/[2 × cos(30°) ] or 5.8 N or 0.59 Kgf unilaterally. The CSA of muscle needed to develop this force can be calculated to be 0.59 Kgf divided by the maximum possible specific force of 2.8 Kgf/cm2 for striated muscle (see Introduction), giving 0.21 cm2. This corresponds almost exactly to the measured CSA (0.22 cm2) of the levator muscle at Location 1 (Fig. 4) which represents about 5% of the maximum levator CSA of muscle at Locations 6 or 7, depending on age. Hence, we can infer that only the most ventromedial five percent of the levator muscle contributes to the vaginal closure force measured during a volitional maximal contraction in young or older women. This novel insight requires corroboration by other investigators. But it is consistent with the observation in prolapse patients that when the muscle at Location 1, the region of levator most likely to be avulsed from the pubic bone during a difficult vaginal birth (6), exhibits a defect, then these patients develop ∼40% less vaginal closure forces than normal (7).
The conclusions of this study rest upon the assumption that the younger women are indeed representative controls. We believe this to be the case because BMI was matched in both groups, and the height and weight of the population of young nullipara from which this sample was drawn match U.S. norms. A methodological limitation was that we could not reliably identify fiber direction in obturator internus on these MR scans. Instead we relied on anatomic dissections to confirm that the obturator fibers arise from the inner surface of the superior ilium so as to converge, like a fan, onto a central tendon at the lesser sciatic notch, leading to the present method for measuring obturator internus physiological CSA. We do not believe that this latter limitation biased the main findings of the paper.
In conclusion, the levator muscle does not atrophy significantly with age in healthy nulliparae as limb muscles do. Though the pathophysiology of pelvic floor dysfunction is multifactorial, there is clearly an increase in prevalence with ageing. However, if other studies confirm our findings, then one can effectively rule out the effect of age alone on the levator ani as being a significant contributory factor to genital prolapse or incontinence (see Introduction). Clearly, establishing the manner in which genetic factors, muscle defects associated with vaginal childbirth, occupational factors, lifestyle variations, and other biological ageing processes interact to cause prolapse and incontinence requires a series of studies that clarify the individual effects of these interactions. Such studies will help inform improved treatment and prevention options.
Acknowledgments
Funding: We thank Julie Tumbarello and Quinn Hamilton at the University of Michigan for their assistance in recruiting and scheduling the women for the magnetic resonance images. Presentations based on certain aspects of the present data were presented at the 2010 Annual Meeting of the International Continence Society, Toronto, Canada and the 2010 Annual Meeting of the American Urogynecological Society, Long Beach, CA. This work was supported by the National Institute of Child Health and Human Development Grant R01
HD 38665, the Office for Research on Women's Health SCOR on Sex and Gender Factors Affecting Women's Health 1 P50 HD044406, and the University of Michigan Pepper Center P30 AG 024824-03.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114:1278–1283. doi: 10.1097/AOG.0b013e3181c2ce96. [DOI] [PubMed] [Google Scholar]
- 3.Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gynecol. 2004;191:856–861. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 4.Ashton-Miller JA, DeLancey JOL. On the Biomechanics of Vaginal Birth and Common Sequelae. Annual Review of Biomedical Engineering. 2009;11:163–176. doi: 10.1146/annurev-bioeng-061008-124823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan DM, Kaur G, Hsu Y, Fenner DE, Guire K, Miller J, Ashton-Miller JA, DeLancey JOL. Does Vaginal Closure Force Differ in the Supine and Standing Positions. Am J Obstetr Gynecol. 2005;192:1722–1728. doi: 10.1016/j.ajog.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 6.Kearney R, Miller JM, Ashton-Miller JA, Delancey JOL. Obstetric Factors Associated with Levator Ani Muscle Injury After Vaginal Birth. Obstet Gynecol. 2006;107(1):144–9. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLancey JOL, Miller JM, Morgan DM, Hussain H, Fenner DE, Umek W, Kearney R, Hsu Y, Guire K, Ashton-Miller JA. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 8.Ashton-Miller JA, DeLancey JOL, Warwick DN. US Patent # 6,468,232 B1. Method and Apparatus for Measuring Properties of the Pelvic Floor Muscles. 2002 Oct;
- 9.Trowbridge ER, Wei JT, Fenner DE, Ashton-Miller JA, Delancey JO. Effects of aging on lower urinary tract and pelvic floor function in nulliparous women. Obstet Gynecol. 2007;109:715–720. doi: 10.1097/01.AOG.0000257074.98122.69. [DOI] [PubMed] [Google Scholar]
- 10.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50A:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 11.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26:432–439. [PubMed] [Google Scholar]
- 12.Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of Age on Rapid Ankle Torque Development. J Gerontol A Biol Med Sci. 1996;51A:M226–232. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- 13.Ikai M, Fukunaga T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int J Angew Physiol Einschl Arbeitsphysiol, 1968. 1968;26:26–28. doi: 10.1007/BF00696087. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. AcIta Physiol Scand. 172:249–255. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 15.Hsu Y, Chen L, Heubner M, Ashton-Miller JA, DeLancey JOL. Quantification of Levator Ani Cross-Sectional Area Differences Between Women With and Those Without Prolapse. Obstet Gynecol. 2006;108:879–883. doi: 10.1097/01.AOG.0000233153.75175.34. [DOI] [PubMed] [Google Scholar]
- 16.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–712. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 17.Tunn R, Delancey JO, Howard D, Ashton-Miller JA, Quint LE. Anatomic variations in the levator ani muscle, endopelvic fascia, and urethra in nulliparas evaluated by magnetic resonance imaging. Am J Obstet Gynecol. 2003;188:116–121. doi: 10.1067/mob.2003.58. [DOI] [PubMed] [Google Scholar]
- 18.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLancey JO, Miller JM, Kearney R, Howard D, Reddy P, Umek W, Guire KE, Margulies RU, Ashton-Miller JA. Vaginal birth and de novo stress incontinence: relative contributions of urethral dysfunction and mobility. Obstet Gynecol. 2007;110:354–362. doi: 10.1097/01.AOG.0000270120.60522.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margulies RU, Hsu Y, Kearney R, Stein T, Umek WH, DeLancey JO. Appearance of the levator ani muscle subdivisions in magnetic resonance images. Obstet Gynecol. 2006;107:1064–1069. doi: 10.1097/01.AOG.0000214952.28605.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shobeiri SA, Chesson RR, Gasser RF. The internal innervation and morphology of the human female levator ani muscle. Am J Obstet Gynecol. 2008;199:686, e1–6. doi: 10.1016/j.ajog.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 22.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilpin SA, Gosling JA, Smith AR, Warrell DW. The pathogenesis of genitourinary prolapse and stress incontinence of urine A histological and histochemical study. Brit J Obstet Gyn. 1989;96:15–23. doi: 10.1111/j.1471-0528.1989.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 24.Helt M, Benson JT, Russell B, Brubaker L. Levator ani muscle in women with genitourinary prolapse: indirect assessment by muscle histopathology. Neurourol Urodyn. 1996;15:17–29. doi: 10.1002/(SICI)1520-6777(1996)15:1<17::AID-NAU2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Weemhoff M, Shek KL, Dietz HP. Effects of age on levator function and morphometry of the levator hiatus in women with pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21:1137–42. doi: 10.1007/s00192-010-1150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller JM, Ashton-Miller JA, Perruchini D, Delancey JO. Test-Retest Reliability of an Instrumented Speculum for Measuring Vaginal Closure Force. Neurourology and Urodynamics. 2007;26:858–863. doi: 10.1002/nau.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183:277–285. doi: 10.1067/mob.2000.107583. [DOI] [PubMed] [Google Scholar]
- 28.Wong MY, Harmanli OH, Agar M, Dandolu V, Grody MH. Collagen content of nonsupport tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol. 2003;189:1597–1599. doi: 10.1016/j.ajog.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 29.Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–1661. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 30.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735–55. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Hsu Y, Ashton-Miller JA, DeLancey JOL. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynec Obstet. 2006;90:234–241. doi: 10.1016/j.ijgo.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]