Abstract
Basic helix-loop-helix (bHLH) transcription factors (TFs) are crucial for inner ear neurosensory development. The proneural TF Atoh1 regulates the differentiation of hair cells (HCs) whereas Neurog1 and Neurod1 regulate specification and differentiation of neurons, respectively, but also affect HC development. Expression of Delta and Jagged ligands in nascent HCs and Notch receptors in supporting cells induce supporting cell differentiation through the regulation of neurogenic bHLH TFs (such as Hes1, Hes5) and suppression of limited Atoh1 expression. In sensorineural hearing loss, HCs are lost followed by supporting cells and progressive degeneration of neurons, at least in rodents. Regaining complete hearing may require reconstituting the organ of Corti (OC) from scratch, including the two types of HCs, inner (IHC) and outer (OHC) hair cells with the precise sorting of two types of afferent (type I and II) and efferent (lateral, LOC and medial, MOC olivo-cochlear) innervation. We review effects of bHLH TF dosage and their cross-regulation to differentiate HC types in the OC. We categorize findings of specific gene expressions in HCs: 1. as markers without meaning for the regeneration task, 2. as stabilizers who are needed to maintain or complete differentiation, and 3. as decision making genes, expressed and acting early enough to be useful in this process. Only one TF has been characterized that fits the last aspect: Atoh1. We propose that temporal and intensity variations of Atoh1 are naturally modulated to differentiate specific types of HCs. Importantly, the molecular means to modify the Atoh1 expression are at least partially understood and can be readily implemented in the attempts to regenerate specific types of HCs.
Keywords: Hair cell, organ of Corti, regeneration, innervation, Atoh1, Neurod1
1. Introduction
The mammalian auditory epithelium, the organ of Corti (OC) of the cochlear duct, is arguably the most precise cellular mosaic of the human body. The integrity and topology of each of the different cell types is relevant for the function to convert sound over a wide intensity and frequency range into electric signals. The OC is vulnerable leading to loss of hair cells (HCs) with time, causing deafness. The challenge of regenerative medicine is to functionally restore this cellular mosaic. Guiding regeneration requires understanding the cell fate decision making processes in this (or any) developing system. These processes involve: 1) establish the position of specific cell types through gradients of diffusible signals, 2) cross-regulation of transcription factors (TFs) for cell type specific differentiation and 3) cell-cell interactions to consolidate cell fate and to ensure a coordinated assembly of multiple cell types (Lander, 2011; Nahmad et al., 2011; Peter et al., 2011).
This general developmental principle, consolidated through studies in iPS cells (Buganim et al., 2012), also applies to the OC. The ancestral basilar papilla of tetrapods, similar to the vestibular epithelia out of which it evolved (Fritzsch et al., 2012), likely had only graded variations of one type of HC surrounded by one type of supporting cell (Manley et al., 1998). In contrast, mammals evolved two distinct types of HCs that are differentially distributed around the mammalian specific Pillar cells to ensure proper stiffness for sound processing. Developing these unique features is mediated by local interactions (Delta-Notch) and diffusible factors (Wnt’s, Fgf’s, Shh and Bmp’s) that form gradients which co-operate with regionally expressed TFs [Eya1, Six1, Gata3, Pax2, Sox2 (Ahmed et al., 2012; Bouchard et al., 2010; Duncan et al., 2010; Kiernan et al., 2005)] into novel patterns to define cell fate (Fritzsch et al., 2011; Groves et al., 2012). Cellular expression of proneural basic helix-loop-helix (bHLH) TFs (Atoh1, Neurog1, Neurod1) transforms the initial pre-patterning, likely generated by random expression of TF’s in a prosensory domain, comparable to fate determination in iPS cells (Buganim et al., 2012), into distinct HC types. This process ultimately involves restricting bHLH gene expression to HC precursors (Matei et al., 2005) and initiate supporting cell differentiation via the Delta/Notch system (Basch et al., 2011; Doetzlhofer et al., 2009; Fritzsch et al., 2011) and short range diffusible factors (Huh et al., 2012; Puligilla et al., 2007). While it is clear that in the ear the most crucial factor to differentiate HCs is Atoh1 (Bermingham et al., 1999; Pan et al., 2012b) it is unclear how topologically distinct HC types form [inner (IHCs) and outer (OHCs) HCs] through these patterning events and what unique mixture of TFs drives their specific differentiation. This lack of mechanistic understanding of decision making processes for HC subtype specification is in part due to the inability to segregate topologically restricted signals from the equally topologically restricted HC differentiation. In other words, positional signals cannot easily be segregated from cell type specific signals as they normally coincide.
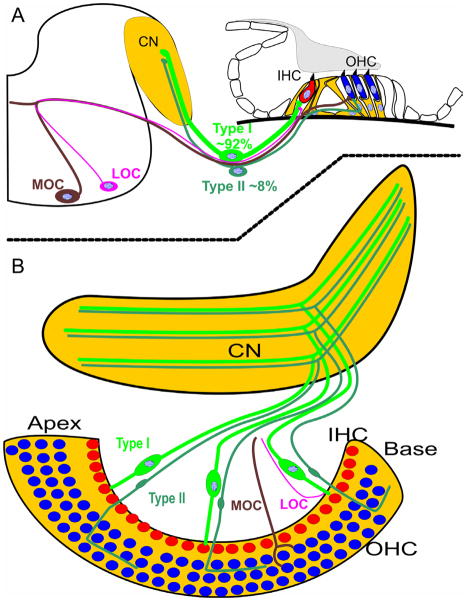
In most cases of sensorineural hearing loss, neuronal loss follows HC degeneration (Alam et al., 2007) but humans may differ in that respect (Linthicum et al., 2009). Given this apparent correlation, at least in rodents, it is important to understand how the two types of HCs become innervated during development by two distinct types of afferent (type I and II spiral ganglion neuron) and efferent fibers (lateral, LOC and medial, MOC olivo-cochlear efferents) to guide this process upon re-innervation (Chen et al., 2012). In essence, complete functional regeneration of the OC will require not only resolving the correct topological development of a given HC (IHCs modiolar to Pillar cells, OHCs lateral to Pillar cells) but also finding the molecular means to differentially affect the ingrowing afferents and efferents to re-establish the HC type specific distribution (Nayagam et al., 2011). Obviously, proper fiber sorting requires the IHC and OHC distribution to be established first (Fig. 1) thus putting the main emphasis on regenerating IHCs and OHCs precisely where they belong.
Figure 1. Scheme of two different types of afferent and efferent innervating the two types of HCs in the OC.
The OC of the inner ear consists of one row of IHCs and three rows of OHCs, except for the most apical and basal region. The overwhelming majority of the spiral ganglion neurons project as type I afferents to IHCs and only fewer than 10% of afferents end as type II fibers on OHCs. Among efferent fibers, the contralateral medial olivocochlear fibers (MOC) terminate on OHCs whereas the ipsilateral lateral olivocochlear efferents (LOC) terminate on the type I afferents at IHCs. During development these two afferent and efferent fiber types are sorted to the two different HC types and the accuracy of this sorting mechanism is crucial for the normal function of hearing. Drawing modified after (Bulankina et al., 2012; Rubel et al., 2002).
These considerations make it obvious that molecular cues to differentiate distinct HC types in specific locations are crucial for the complete functional restoration of the OC. No matter the entry point toward regeneration, be it gene therapy (Izumikawa et al., 2008) or stem cell therapy (Chen et al., 2012; Huisman et al., 2012; Kopecky et al., 2011; Oshima et al., 2010), even partial topologically correct HC type regeneration may ensure some functionality of whatever level of restoration of an OC can be achieved. To help focus on this general problem, this review will provide an overview of molecular understanding of HC development for reconstructing a functional OC, ‘the best hearing aid of the world’ (Puligilla et al., 2009), followed by insights gained thus far in afferent and efferent fiber sorting.
2. Structural and functional differences between IHC and OHC
Anatomical studies have long established morphological distinctions between IHCs and OHCs and their innervation and functional properties (Lim, 1980). In therian mammals, HCs in the OC are arranged in four rows, with one row of IHCs and three rows of OHCs, except for variations in the extreme apex and base. In human, there are approximately 3,500 IHCs and 12,000 OHCs (Lim, 1980). Although IHCs are the main sensory cells for converting sound into auditory information for the brain, OHCs play an important role to dynamically adjust the OC response to the sound level (Liberman et al., 2002). These two types of HCs are not only differentially distributed with respect to the Pillar cells but are also structurally very distinct and play vastly different functional roles in hearing.
IHCs are flask-shaped with thick shallow ‘C’ shaped stereociliary bundle protruding from its apical surface. In contrast, the OHCs are long, cylindrical with ‘W’ shaped hair bundles that consist of many more but much thinner stereocilia (0.2–0.3 μm compared to 0.6–0.8 μm) (Lim, 1986; Sziklai et al., 1996). In either HC type, the stereociliary bundles are orderly organized with distinct asymmetry: within each stereociliary bundle, the cilia are positioned according to height; the tallest row is in the periphery and the shortest row near the center of each cell. All bundles are pointed toward the lateral wall facing away from the modiolus. There is also a gradation of ciliary height along the length of OC, with the shortest found in the base and the longest in the apex (Lim, 1986; Sziklai et al., 1996). In addition to the differences in cell body shape, diameter and organization of stereocilia, there are consistent variations in OHC type along the OC. For example, the longest OHCs are in the apex whereas the basal OHCs are much shorter. OHCs length also varies radially. The outermost row of OHCs is the tallest whereas the innermost row of OHCs is the shortest. This is most pronounced in the apex, indicative of a longitudinal and radial positional cue of OHCs in the cochlea imposing a great physiological significance to low and high frequency hearing (Lim, 1986).
Another distinction between the IHCs and OHCs is that the top rows of stereocilia of OHCs are touching the tectorial membrane where they leave a permanent impression. In contrast, IHCs stereocilia are loosely coupled to the tectorial membrane (Lim, 1986; Sziklai et al., 1996). In addition, IHC are completely surrounded by the inner phalangeal cells, inner Pillar cells and outer Pillar cells whereas only the base of the OHCs are surrounded by Deiters’ cells, providing large ‘spaces of Nuel’ filled with perilymph.
IHCs and OHCs are also very distinct in their innervation. 92–95% of the afferent fibers are large, myelinated type I fibers that end on IHCs whereas only 5–8% are un-myelinated type II afferent fibers that reach OHCs (Bulankina et al., 2012; Romand et al., 1990; Yang et al., 2011). In contrast to the afferent innervation, the majority of the efferent fibers from the contra-lateral medial olivocochlear nucleus (MOC) terminate on the OHCs. Some ipsilateral MOCs but in particular the more numerous and smaller efferent fibers from the ipsilateral lateral olivocochlear nucleus (LOC) terminate on afferents innervating the IHCs (Brown et al., 1998) (Fig. 1). While IHC afferents and OHC efferents are well studied, very little is known about the function of efferents ending on IHC innervating afferents, or the type II fibers ending on OHCs. Likewise, how during development the OHCs are receiving only minor afferent and dominant efferent innervation while the inverse is true for IHC is still uncertain (Bulankina et al., 2012; Rontal et al., 2003).
Finally, only IHCs are responsible for transducing sound to the neural signal with high spatial and temporal resolution. In contrast, OHCs control the biomechanics of the OC by enhancing the sensitivity, tuning and the dynamic range without providing any auditory information to the brain. It is the active process of OHCs where apparently MOC efferent innervation plays a role for protecting the ear from overstimulation by hyperpolarization of the OHCs (Katz et al., 2011).
Therefore, all these striking distinctive features of the IHCs and OHCs make them unique with respect to their morphological and functional significance. As is obvious with the significant loss of sensitivity in mice without OHCs, without Pillar cell formation or without a tectorial membrane (Legan et al., 2000), the complexity of the OC is meaningful for the frequency sensitivity across a wide dynamic range of hearing. Fully functional regeneration would consequently need to re-establish essential components of this organ and proper connection to the tectorial membrane in order to have a complete hearing restoration. The task of hearing restoration thus should be to restore as much of these features as possible to achieve function closely comparable to the normal OC.
3. Molecular characterization of IHC and OHC
In addition to the long known morphological and functional differences, recent years have seen a growing set of molecular identifiers for IHC and OHC. Some of these proteins are now widely used as the molecular markers for the localization of specific cell types of the OC. For example, a large number of Ca2+ binding proteins (CaBPs) are differentially expressed in the different types of cells in the OC which vary in different developmental stage (Simmons et al., 2010). Such variability in the expression of these proteins indicates the onset of maturation of specific types of HC. For example, Oncomodulin is initially expressed in both IHCs and OHCs in early neonates but before the onset of hearing it is localized mostly in the OHCs (Sakaguchi et al., 1998; Simmons et al., 2010). Among other CaBPs, Calmodulin, Calbindin, Calretinin and α-Parvalbumin also show initial differential distribution followed by localization in distinct type of HC in the OC as demonstrated by immunofluorescence staining (Simmons et al., 2010) (Table. 1).
Table 1.
Protein markers of IHCs and OHCs.
| Marker protein | HC types | Properties [references] |
|---|---|---|
| Calretinin | IHC | Calcium binding proteins (Simmons et al., 2010) |
| Parvalbumin α | IHC | Calcium binding proteins (Sakaguchi et al., 1998) |
| Oncomodulin (Parvalbumin β) | OHC | Calcium binding protein (Simmons et al., 2010) |
| Prestin | OHC | Motor protein (Zheng et al., 2010) |
| Nesprin4 | OHC | Important for OHC maintenance (Lenz et al., 2012). |
| Alpha 9 and 10 nAChR | OHC | Nicotinic acetylcholine receptor subunit alpha 9 and10 (Katz et al., 2011; Simmons et al., 2011; Zuo et al., 1999) |
In addition to these CaBPs, there are several other proteins identified as being specifically localized either in IHCs or in OHCs (Table. 1). A membrane specific protein, Prestin which is characterized to be the cochlear amplifier (Zheng et al., 2010), has also differential distribution in OHCs in different stages of development. Prestin is localized in the cytoplasm in pre-hearing OHCs whereas restricted to the baso-lateral membrane in the post-hearing OHCs (Zheng et al., 2010). Nesprin-4, a member of Nesprin protein family found to interact with Kinesin-1, has recently been reported in the ear to be essential for the OHC maintenance; Nesprin-4 null mice have progressive high tone hearing impairment with loss of OHCs by P30 (Lenz et al., 2012). Few other genes that are expressed ubiquitously in developing HCs may eventually be expressed by only one cell type. Best known among those is the nicotinic acetylcholine receptors (nAchR) alpha 10, which is expressed progressively in all HCs, becomes later restricted to OHCs (Vetter et al., 2007). For many of these proteins it remains unclear when they are first upregulated in IHCs or OHCs, showing with quantitative polymerase chain reaction (qPCR) or other advanced techniques for the first appearance of mRNA consistent with onset of expression, and whether or not they play roles in the development and maintenance of these HCs or are simply convenient markers. Despite some of these open issues, these proteins are useful tools to identify type-specific HC loss in different genetic mutants.
4. Developmental expression of IHC/OHC specific molecules: molecular causes or consequences of cell type specific differentiation?
Obviously, when the OC starts to differentiate, HC precursors express molecules equally associated with vestibular HCs as well as some supporting cells. Such as Atoh1 is expressed not only in all HCs but also in Pillar cells (Matei et al., 2005; Yang et al., 2010) which is later suppressed functionally by neurogenic TFs (Doetzlhofer et al., 2009). The early expression recapitulates the clonal relationship of HCs and supporting cells (Groves et al., 2012). However, as development progresses, the OC HCs become more different from vestibular HCs and differentiate into the distinct IHC and OHC types of the OC.
What needs to be understood in order to guide specific and topological IHC and OHC regeneration (but not vestibular HCs) is the causality of molecular expressions with these cell decision events: We need to know whether genes expressed will initiate this differentiation or are expressed only after the fact, indicating that a critical decision step has been taken. Simply speaking, finding a marker that highlights that the decision is made without contributing to the decisions will be of no use for the attempt to drive OHC differentiation compared to IHC differentiation. One such early marker is Myo6 (Cotanche et al., 2010). This unusual myosin protein can first be detected in late embryos to be specifically associated with IHCs first before it also appears at lower levels in OHCs. Likewise, the neurotrophin factor Ntf3 (previously NT3) is first expressed in IHCs in late embryos (Pirvola et al., 1992) and upregulation of either alpha9 or alpha10 nAChR starts in IHCs followed by OHCs (Simmons et al., 2011; Vetter et al., 2007; Zuo et al., 1999). However, none of these proteins is a TF that can regulate gene expression changes through binding to promoter regions of genes. The expression of these genes and the distribution of the proteins in the cytoplasm or in the plasma membrane (but not in the nucleus) must therefore be regarded as a consequence, not a cause for the HC type specific differentiation. Consistent with this suggestion is that null mutants of these genes have either no effect on HC type specific differentiation (Vetter et al., 2007) or cause only morphologic alterations in HC stereocilia followed by the eventual loss of all HCs (Friedman et al., 1999).
More interesting are expressions of TFs, or diffusible substances such as Fgf8, selectively in one specific HC type. However, while Fgf8 is clearly one of the most clear-cut markers of IHCs starting in late development (Pirvola et al., 2000), deleting Fgf8 has no effect on IHC differentiation consistent with the fact that the preferred receptor of Fgf8, Fgfr3, is expressed in supporting cells but not in HCs (Jacques et al., 2007; Puligilla et al., 2007).
Thus, unique expressions of a gene, such as Fgf8, even though it may start early and is needed for overall development of the OC, may not regulate how IHCs (or OHCs) become molecularly specified and when that happens during development. Obviously, in null mutants for Myo6 or Fgf8, there is no specific loss of a given type of HCs, thus indicating without doubt that they are not playing a role in the cell fate decision process. In contrast to this lack of effect on cell type specific differentiation, one would expect that loss of a molecule involved in decision making would have effects on HC type development. In other words, it is essential to know the molecular cues needed that can be used to manipulate the cell fate decision process to form a OC specific HC, either a IHC or a OHC. Molecules that provide a convenient indicator of such commitment are useful post-hoc tools, but not in generating this distinction, and are thus not useful for regeneration attempts to make specific hair cell types. We will provide below some insights into such a potentially useful molecule that, as it turns out, is a modifier of the general HC differentiation program through differential expression levels, an emerging issue in cell fate decision making (Purvis et al., 2012).
5. Insights from loss and gain of function studies in mouse mutants
The two types of HCs of the OC are molecularly distinct in addition to their structural, distribution and functional differences as described above. This is particularly obvious in mutants that lose either IHCs or OHCs (Ahmed et al., 2012; Brooker et al., 2006; Deol, 1981; Holley et al., 2010; Huh et al., 2012; Kiernan et al., 2006; Pan et al., 2012b). In addition to these differential loss, there can be an expansion of rows of remaining HC types (Brooker et al., 2006; Doetzlhofer et al., 2009; Holley et al., 2010; Kiernan et al., 2006) or specific loss of OHCs (Huh et al., 2012; Pirvola et al., 2002; Pirvola et al., 2000) or partial loss of one HC type (Deol, 1981; Nakano et al., 2012; Pan et al., 2012b). We will discuss some of these mutant lines and propose possible underlying causes.
Null mutants of the homeobox gene Emx2, develop only two rows of IHCs in the OC, but also have alterations in vestibular HC types (Holley et al., 2010). There is no formation of OHCs, as demonstrated with Fgf8 positive IHCs only and reduction of Fgfr1 expression (Holley et al., 2010). This finding is consistent with the data that show particular sensitivity of OHC formation on low levels of Fgfr1 (Pirvola et al., 2002) and dependence on Fgf20, which signals through the Fgfr1 (Huh et al., 2012). Although Emx2, Fgfr1 or Fgf20 seem not to regulate Notch signaling, Jag1 null mutant mice show phenotypic similarity with the Emx2 or Fgfr1 null mice (Brooker et al., 2006; Kiernan et al., 2006). Jag1 null mice also show multiple rows of patchy IHCs with loss of OHCs in the cochlea (Brooker et al., 2006; Kiernan et al., 2006). It would be important to understand the apparent similarities in the phenotypes at the molecular level by establishing causality.
In contrast to the above mutants, other mutant mice illustrate specific loss of IHCs such as the spontaneous mutant, Bronx-Waltzer (bv) mice (Deol, 1981; Nakano et al., 2012) or a ‘self-terminating’ Atoh1 conditional null mice using Atoh1-cre (Pan et al., 2012b). While the latter retains only two rows of patchy OHCs with almost complete loss of IHCs (Pan et al., 2012b), the former loses large patches of IHCs but retains all rows of OHCs. Recent work indicates how the selective loss of bv mutants might work. The mutation alters a serine arginine repetitive matrix (Srrm4), a protein that regulates differential splicing in many cells. How Srrm4 causes near complete and selective IHC death is not yet clear. However, Srrm4 is identified as a downstream target of Atoh1 in the cerebellum (Klisch et al., 2011) which also critically depends on Atoh1 for development (Klisch et al., 2011; Pan et al., 2009). This raises the possibility that Srrm4 might be a part of a feedback loop that maintains high level of Atoh1 expression which is particularly important for IHCs development (Pan et al., 2012b). It is essential to investigate this possible cross-regulation of Atoh1 and Srrm4. If true, the reduction of Atoh1 and/or negative effect of Srrm4 on Atoh1 signaling could be directly compared with the transient expression of Atoh1 in the ‘self-terminating’ Atoh1 mice (Pan et al., 2012b). Combined, these data suggest that IHC require a more profound signaling of Atoh1 to maintain the differentiation of IHCs compared to OHCs. We therefore will explore this preposition in more detail below.
5.1. Atoh1 levels correlate with and regulate HC types
Atoh1 is necessary for any hair cell differentiation. Regeneration of the chick cochlea demonstrates that after HC loss, Atoh1 can directly transdifferentiate some supporting cells into HCs up to a definite threshold of Atoh1 for committing this transdifferentiation before the supporting cells re-enter the mitotic proliferation (Cotanche et al., 2010). This indicates that, at least in chicken, the supporting cells can function as bi-potential state where a defined dose of Atoh1 can convert them into HCs. Systemic loss of Atoh1 in mice results in lack of any HCs differentiation, but non-specialized ‘epithelial cells’, or even supporting cells or neurosensory precursor cells (Bermingham et al., 1999; Fritzsch et al., 2005) remain, indicating that at least some unspecified precursor cells form in the absence of Atoh1 protein, but are unable to differentiate. It also indicates that Atoh1 is not selecting HC precursors but is expressed as a consequence of this selection process. Indeed, in the absence of Atoh1 protein formation, Atoh1 mediated expression of the reporter LacZ is enhanced in the undifferentiated apical epithelium (Fritzsch et al., 2005). This may indicate that Atoh1 protein is not only activating a positive feedback loop by binding to its enhancers (Kim et al., 1997). Atoh1 may also activate a negative feed-back loop inside precursor cells, possible mediated by Neurod1 that requires Atoh1 protein for its expression (Pan et al., 2012b) and clearly suppresses Atoh1 (Jahan et al., 2010a). In addition, formation of few Myo7a and Fgf8 positive cells in Atoh1 conditional null suggests that expression of these ‘HC markers’ can be regulated in some extent by unknown factors (Jahan et al., 2012; Pan et al., 2011). The existence of these Myo7a positive cells may indicate the ability of partial differentiation of HC-like cells without Atoh1 or, alternatively, upregulation of Myo7a and Fgf8 is possible in partial or undifferentiated HCs (Cotanche et al., 2010; Self et al., 1998) as much as some cerebellar granule neurons can express specific genes even in Atoh1 null mice (Klisch et al., 2011). It has been also shown that few other genes like Prox1, Gata3, Sox2 are variably regulated in the absence of Atoh1 (Pan et al., 2011) and play a role in hair cell development (Duncan et al., 2010; Fritzsch et al., 2010b; Kiernan et al., 2005). In absence of Atoh1 protein, the OC ultimately transforms into a ‘flat epithelium’ in neonates (Pan et al., 2011) once the undifferentiated precursors degenerate.
More revealing for the problem of HC type specification are emerging concepts in developmental cell fate decision making (Purvis et al., 2012) that imply random stochastic expression as a first step followed by deterministic expression to consolidate the fate. Recent data generated in a delayed deletion of Atoh1, a novel ‘self-terminating’ conditional null mouse model with temporal and intensity alteration of Atoh1, are relevant here. These mice show a differential survival of HCs in an Atoh1 dose dependent manner where IHCs are the most susceptible (Pan et al., 2012b). This mutant has initially a normal expression of Atoh1 followed by a rapid variable deletion of Atoh1, progressing from base to apex of the cochlea with limited expression of Atoh1 in the apex remaining at postnatal day 4 (Pan et al., 2012b). This ‘self-terminating’ Atoh1 mutant mice have preferential base to apex susceptibility of HCs, where the outermost row of OHC never form and the IHC degenerate first followed by the other two rows of OHCs in 3 weeks followed by an eventual transformation into a near ‘flat epithelium’. This mutant demonstrates that a stable and precise Atoh1 level is needed for the development and maintenance of different HC types (Pan et al., 2012b) possibly through regulation of many downstream genes that are essential for HC differentiation (Klisch et al., 2011) and are conserved across phyla (Senthilan et al., 2012).
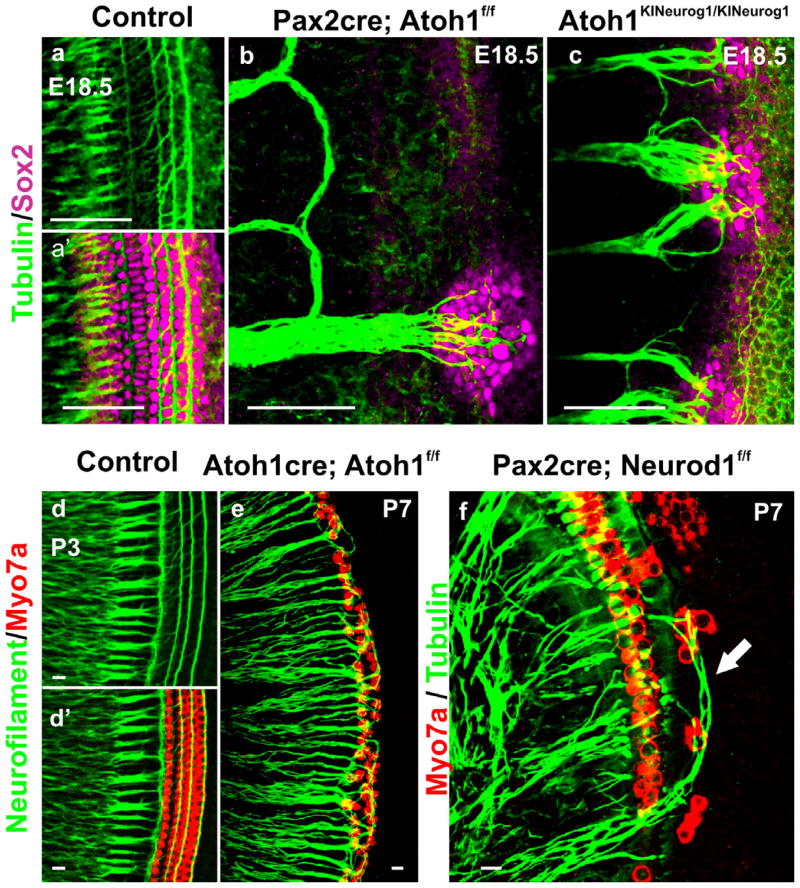
In many developing systems other than the ear, targeted misexpression of genes or replacement of genes by a closely related genes has helped to test the functional redundancy as well as to provide information of the normal function of a gene beyond the scope of simple knockout studies (Conway et al., 2010; Gehring, 2011). In mice, the replacement of mammalian Atoh1 by the fly ortholog, atonal, shows that a fly gene can replace a mammalian gene (Wang et al., 2002). However, it could be that the rescue is not a specific function of atonal but rather a general function of any bHLH gene. To test this possibility, we recently generated a novel mutant mice where we replace Atoh1 with Neurog1 (Atoh1KINeurog1/KINeurog1) to determine if a closely related bHLH gene can substitute the function of Atoh1 and differentiate HCs (Jahan et al., 2012). Surprisingly, Neurog1 misexpression can rescue only some partially differentiated patches of cells with formation of cells carrying some rudimentary microvilli on their apical surface in the OC (Jahan et al., 2012). However, this misexpression of a closely related bHLH TF does not result in complete differentiated HCs or fate change of HCs to neuron as Atoh1 misexpression can do in inner ear ganglia (Jahan et al., 2010b). This verifies that fly atonal does have signaling capacity in the mammalian HC that is not shared by even a closely related bHLH family member, possibly because of unique binding properties to E-boxes (Fritzsch et al., 2010a). However, Neurog1 knockin heterozygous mice (Atoh1+/KINeurog1) show subtle but progressive morphological aberration in both HCs and supporting cells (Jahan et al., 2012). In contrast to the Atoh1 haploinsufficient mice which do not have any defect on HCs, co-expression of Neurog1 and Atoh1 shows reduction of Fgf8 expression in some IHCs and forms atypical patches of stereocilia in IHCs (Fig. 2) (Jahan et al., 2012). More recent work showed that forced expression of Atoh1 in older OCs can regenerate stereocilia in a noise-deafened cochlea (Yang et al., 2012). Likewise, specific microRNA (miR)-96 is essential for stereocilia bundle maturity (Kuhn et al., 2011; Soukup et al., 2009). We recently showed that miR-96 expression is regulated by Atoh1 in HCs (Jahan et al., 2012). Therefore, this sophisticated alteration in Atoh1 dosage by co-expression of Atoh1 and Neurog1 in a given HC affects both intra- and inter-cellular signaling and modulates genes like Fgf8 or miR-96 and thereby alters specific types of HC (IHC) morphology (Jahan et al., 2012).
Figure 2. Co-expression of Atoh1 and Neurog1 in HCs modifies HC specification.
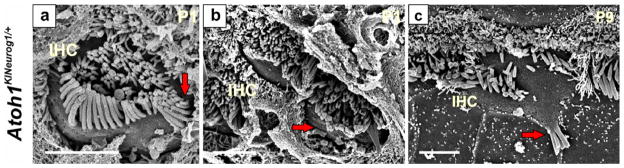
Scanning electron microscopy shows that heterozygous knockin mice (Atoh1KINeurog1/+) with one allele of Atoh1 replaced by Neurog1 reveal HC stereocilia defects (a–c). IHCs show partial duplication of stereocilia bundles (arrow in a), paired IHCs without any supporting cells separating them (arrow in b) or patchy distribution of stereocilia (arrow in c) This indicates that even slight aberration of Atoh1 signaling through heterozygosity of Atoh1 combined with Neurog1 expression can modify the normal HC differentiation, in particular of IHCs. Modified after (Jahan et al., 2012). Bar indicates 2 μm.
In contrast to the preferred loss or defect of IHCs in reduced or altered Atoh1 signal, Neurod1 loss results in a premature overexpression of Atoh1 (Fig. 3) resulting in ectopic IHCs formation by partial transformation of OHCs (Jahan et al., 2010a). In the absence of Neurod1, Atoh1 is not only prematurely upregulated but progresses from apex to base instead of the normal base to apex progression of control littermates (Fig. 3; Table 2). Neurod1 suppresses Atoh1 (Jahan et al., 2010a) whereas Atoh1 protein is needed for Neurod1 expression in HCs (Pan et al., 2012b). In contrast to the Neurog1 null where Neurog1 loss results in altered Atoh1 and Neurod1 upregulation as well as premature cell cycle exit (Matei et al., 2005), absence of Neurod1 does not affect the proliferation of the OC cells (Fig. 3a, b). Generally the post-mitotic OC cells in the apex require at least 6–7 days before they fully differentiate and upregulate Atoh1 [Tables 2, 3; (Lanford et al., 2000; Matei et al., 2005)]. Our data on Neurod1 mutants (Jahan et al., 2010a) suggest that this delay in Atoh1 upregulation in the apex somehow provides enough time for proper specification of HC types in the apex. Neurod1 loss disrupts this waiting period where abrupt Atoh1 upregulation immediately after cell cycle exit accelerate the HC differentiation resulting in altered HC fate specification in the apex of the cochlea. Atoh1 expression is in Neurod1 null mice not only changed in a longitudinal gradient but also radially: it is upregulated first in the OHCs and later in IHCs in comparison to IHCs to OHCs progression in control cochlea (Fig. 3). In summary, Neurod1 loss results in premature overexpression of Atoh1 in the apex which alters the specification of some OHCs into IHCs-like cell fate (Fig. 4, 5).
Figure 3. Deletion of Neurod1 results in alteration of Atoh1 and Fgf8 expression without altering the proliferation.
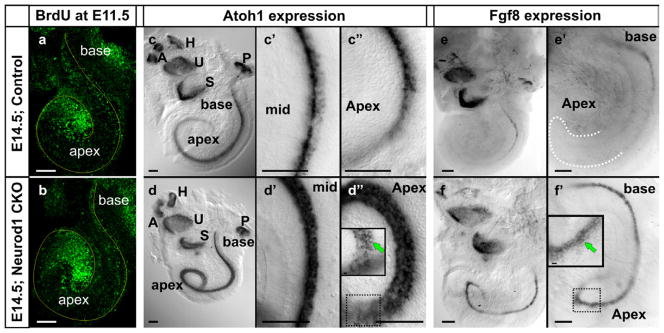
E14.5 control and Neurod1 conditional null (CKO) mice demonstrate a similar pattern of cell cycle exit only near the apex, three days after BrdU injection at E11.5 (a,b). In situ hybridization of Atoh1 (c-d″) and Fgf8 (e-f′) is shown in the E14.5 Neurod1 conditional null and control littermate mice. Atoh1 is prematurely expressed in the apex of the Neurod1 mutant cochlea and is expressed strongly in all the HCs with higher expression in the OHCs than IHCs (d–d″, insert in d″). In control mice, Atoh1 is barely expressed in the apex and restricted mainly in the IHCs (c–c″). Fgf8 is restricted in the IHCs of the base of the E14.5 control cochlea (e, e′), whereas it is ectopically expressed in the apex and expanded radially to OHCs in the Neurod1 CKO littermate (f, f′, insert in f′). Note the overall similar expression of Fgf8 in the vestibular organs (S, saccule; U, utricle) indicating that these HCs are less affected by the loss of Neurod1. Modified after (Jahan et al., 2010a). Bar indicates 100 μm.
Table 2.
Atoh1 expression window in mouse cochlea in comparison to cell cycle exit (CCE). Data are from (Jahan et al., 2010b; Lanford et al., 2000; Matei et al., 2005).
| CCE | Atoh1 ↑ in control | Atoh1 ↑ in Neurod1 CKO | |
|---|---|---|---|
| Apex | E11.5 | P0 | E13.5 |
| Middle | E13.5 | E14.5 | E14 |
| Base | E14.5 | E15 | E14.5 |
Table 3.
Atoh1 expression in mouse cochlea relative to cell cycle exit (CCE). Data are from (Jahan et al., 2010b; Lanford et al., 2000; Matei et al., 2005).
| Time delay in CCE vs Atoh1 ↑ in control. | Time delay in CCE vs Atoh1 ↑ in Neurod1 CKO | |
|---|---|---|
| Apex | ~ 7 days | ~ 1 days |
| Middle | ~ 1days | ~ 0.5 days |
| Base | ~ 0.5 days | ~ 0 days |
Figure 4. Absence of Neurod1 leads to ectopic IHCs formation in the OHC region.
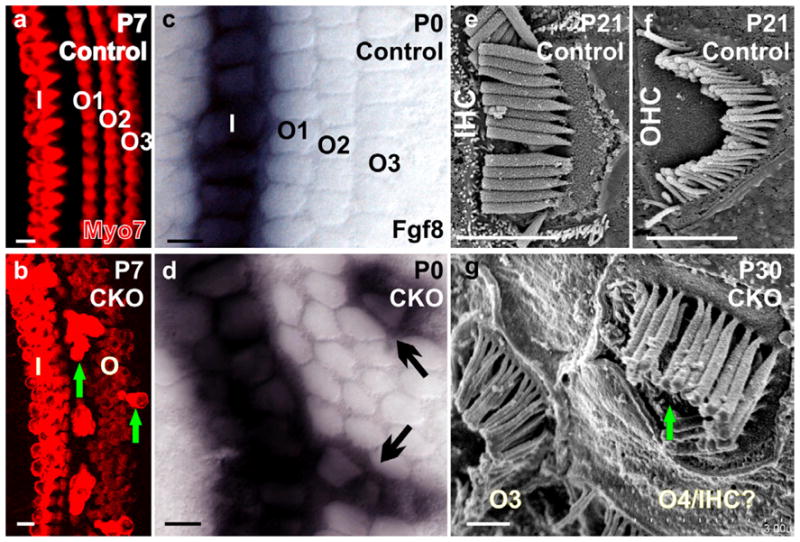
Myo7a immunocytochemistry reveals one row of IHCs and 3 rows of OHCs in the postnatal day (P) 7 control mice (a). Neurod1 conditional null (CKO) mice show 2 rows of IHCs with multiple rows of OHCs as well as some extensively stained Myo7a positive ectopic IHCs in the position of OHCs (b, arrows in b). Fgf8 is expressed only in the IHCs in control mice (c) but is expressed in some OHCs (arrows in d) in Neurod1 CKO mice. Scanning electron microscopy confirms that ectopic IHCs have the thick stereocilia typical of IHCs but in the configuration of OHC stereocilia (e–g, arrow in g). I, IHCs; O1-O3, OHC row 1–3. Modified after (Jahan et al., 2010a). Bar indicates 100 μm except e–g, where it indicates 1 μm.
Figure 5. Hypothetical correlation of time and magnitude of Atoh1 expression on HC type specification.
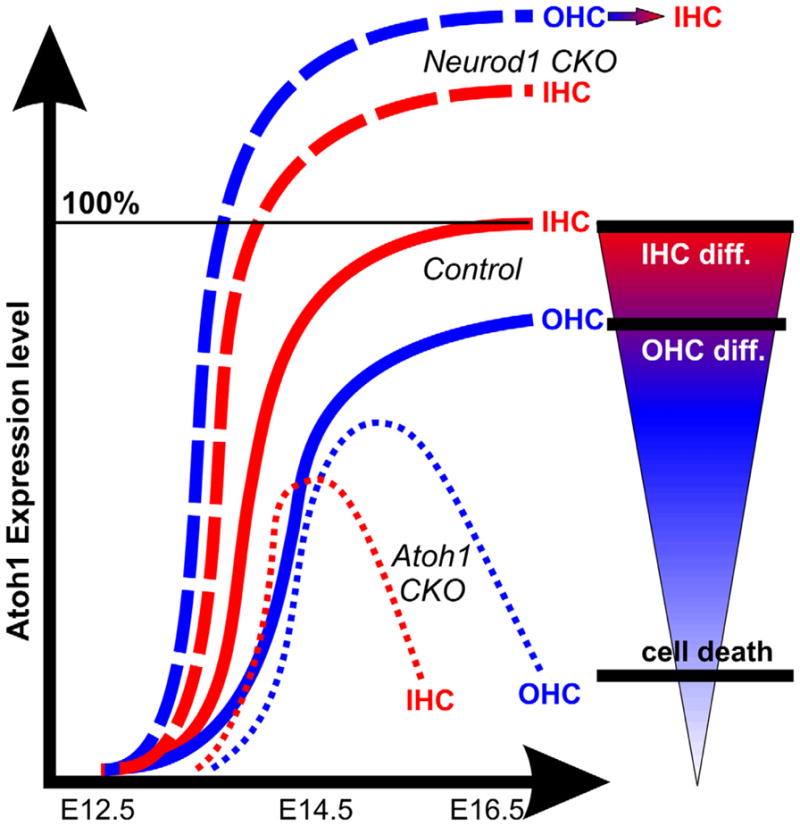
In control animals, Atoh1 is progressing from IHCs to OHCs, starting around E13.5 near the base (continuous thick lines). Neurod1 loss mediates premature and elevated expression of Atoh1 and converts some OHCs into IHCs fate in Neurod1 conditional null (CKO) mouse (thick dotted lines). Thus, alteration of radial Atoh1 expression timing and level can disrupt the HC type specification, whereas reduction of Atoh1 affects HC survival. Thus, in ‘self-terminating’ Atoh1 CKO mouse, Atoh1 is lost longitudinally (from base to apex) and radially (from IHCs to OHCs) that results in differential loss of IHCs (thin dotted lines). This ‘self-terminating’ Atoh1 CKO mouse never forms the outermost row of OHCs, possibly due to insufficient levels of Atoh1. Furthermore, IHCs die first due to earlier downregulation of Atoh1 with patchy survival of inner two rows of OHCs for 2–3 weeks. Combined these data suggest that the precise Atoh1 level and length of expression is crucial for each type of HCs development and viability. Combined after (Jahan et al., 2010a; Pan et al., 2012b).
This negative regulation of Atoh1 by Neurod1 is also known for the cerebellum, where Neurod1 deletion mediated aberrant expression of Atoh1 can cause premature migration and death of cerebellar granule neuron in certain lobules (Pan et al., 2009). Neurod1 loss de-represses Atoh1 in inner ear neurons and convert some of these cells into HCs (Jahan et al., 2010a). However, it is not clear if this bi-potent cells derive from common hair cell/neuron precursors which give rise to both neurons and HCs (Fritzsch et al., 2006; Sapede et al., 2012). Nevertheless, removal of Neurod1 suppression of Atoh1 switches these bi-potent cells to adopt an alternate hair cell fate defined by Atoh1 expression. This suggest a minimal, stochastic expression of Atoh1 in these precursors cells which can be de-repressed by the alteration of yet to be fully defined feedback loops (Fritzsch et al., 2010a; Matei et al., 2005; Pan et al., 2012a). In support to that, Sox2 was recently identified as a potent regulator of Atoh1 which also activates negative regulators of Atoh1 like Neurod1, Neurog1 and thereby suppress premature HC differentiation (Neves et al., 2012). Indeed, cellular reprogramming studies indicate that stochastic gene expression may be common in early stage prior to definitive cellular determination and deregulation of gene during this stochastic phase may allow alternate cell fates (Buganim et al., 2012).
Elimination of Neurod1 also affects upregulation of Myo7a showing higher intensity in the ectopic IHCs in Neurod1 null cochlea (Fig. 4) compared to the lower expression in the surrounding typical OHCs. These ectopic IHC develop the thick stereocilia of typical IHCs (Fig. 4). In addition Fgf8 is prematurely upregulated in the apex of the cochlea and ectopically expanded to the OHCs rows in both embryos and newborn Neurod1 null (Fig. 3, 4). Neurod1 loss thus alters the pattern of Atoh1 expression in the HCs and the expression of Fgf8 or Myo7a and few other downstream target genes and thereby alter the HC types (Jahan et al., 2010a). Moreover, absence of Neurod1 converts some Deiters’ cells into Pillar cells around these ectopic IHCs. This may happen because of the ectopic expression of Fgf8 in the ectopic IHCs that can signal through Fgfr3 and modify Deiters’ cell differentiation into Pillar cell-like differentiation (Jahan et al., 2010a; Puligilla et al., 2007). Thus, Neurod1 plays a major role in differentiation of OHCs as well as Pillar cells in topologically correct position in correct numbers by regulating level of Atoh1 and Fgf8 expression.
This loss-of-function combined with gain-of-function (Neurod1 loss mediated Atoh1 overexpression) effects of Neurod1 mutant mice allow to critically assess the role of various intra- and intercellular signals related to the stereotyped patterning of the OC. Such analyses are until now hampered by the inability to discriminate between correlation and causality of HC type and topology, which is usually closely interwoven. These mutants (delayed Atoh1 knockout or Neurod1 null) show that either too little or too much Atoh1 can alter the stereotypic organization of the OC, most likely by disrupting the Atoh1 mediated Delta-Notch and diffusible factor signaling.
This information could be valuable for future attempts to induce specific HC differentiation using established protocols for adenoviral transfection (Izumikawa et al., 2008; Izumikawa et al., 2005) or enhance hair cell differentiation out of bi-potent progenitors (Chen et al., 2012; Oshima et al., 2010). Knocking down Neurod1 while expressing simultaneously Atoh1 should preferentially induce formation of IHCs in the cochlea, the cells that are the mechano-electric transducers of the OC.
5.2. Regulation of diffusible factors in HCs type differentiation
Fgfs are essential for development of the inner ear (Groves et al., 2012; Huh et al., 2012; Pauley et al., 2003; Pirvola et al., 2002). Recently it was shown that absence of Fgf20 results in loss of the OHCs in addition to the loss of lateral supporting cells (Huh et al., 2012). In contrast, Fgf8 expressed only in the IHCs regulates the development of Pillar cells, signaling through Fgfr3 (Jacques et al., 2007; Puligilla et al., 2007). Variation of dosage of TFs also modulates Fgf signaling and alters the differentiation of HCs and supporting cells (Jahan et al., 2012; Pan et al., 2012b). How the interaction of diffusible factors (Huh et al., 2012) and TFs (Doetzlhofer et al., 2009; Jahan et al., 2012; Pan et al., 2012b) secure specific topologically correct cellular differentiation requires further investigation. Most important are the apparent similarities of Fgf20 null and ‘self-terminating’ Atoh1 mice with respect to outer row of OHC loss and formation of patches separated by gaps as in Fgfr1 null mice (Pirvola et al., 2002). A possible interaction could relate to the antagonistic role of Fgf’s and Bmp’s in general neuronal development (Fritzsch et al., 2006).
It is well known that Fgf signaling interact with the Bmps to form the non-sensory compartment to flank the OC (Ohyama et al., 2010; Pan et al., 2011). Specifically, Fgf10 and Bmp4 flank the neural side (GER, greater epithelial ridge) and abneural side of the OC, respectively (Pan et al., 2011). Atoh1 mutants with the differential defect of OC demonstrate that Fgf10 and Bmp4 expression domains are expanded to the abneural and neural side, respectively (Jahan et al., 2012; Pan et al., 2012b). The expression changes of bHLH TFs signaling from the OC modulate the morphogen gradient from the surrounding non-sensory regions and modify ultimately the patterning of the OC, possibly by setting the stage for more epithelial or more neurosensory development through intracellular interactions (Fritzsch et al., 2006). Further molecular analysis of the developmental expression of downstream genes of this signaling cascade is needed to reveal how this cellular patterning develops over time to drive specific HC type development.
5.3. How can the generalized Delta-Notch signal influence the highly patterned HC type specification
It has been reported that the generation of IHCs and OHCs is differentially dependent on different Notch ligands. For example, deletion of Jagged1 results in loss of almost all OHCs whereas the IHCs remain intact or are even formed in excess (Brooker et al., 2006; Kiernan et al., 2006). The expression of Atoh1 in designated HCs drives the coordinated development of HCs and of supporting cells via the lateral inhibition of the Delta-Notch system (Daudet et al., 2005). Whereas the role of Notch in pro-sensory induction is controversial (Basch et al., 2011; Daudet et al., 2007), there is no differentiation of OC without that signal, despite the fact that a transient upregulation of Atoh1 expression is possible in the absence of Notch signaling. In addition, the upregulation of Atoh1 is aberrant in this RBPjk conditional null mutant mouse, showing premature radial expansion along the cochlea (Basch et al., 2011). The wider Atoh1 expression is consistent with previous data in various Notch inactivation mutants, which results in premature and overproduction of HCs that ultimately fail to survive (Doetzlhofer et al., 2009; Haddon et al., 1998) and the transient expression of Atoh1 in some supporting cells (Matei et al., 2005; Yang et al., 2010). Therefore, the role of Notch stabilizes sensory cell selection but does not initiate their differentiation.
The deletion of Atoh1 or misexpression of Neurog1 in Atoh1 locus massively reduces the expression of Notch ligands (such as Jag1) or the effector target genes, like Hes5 (Jahan et al., 2012). Replacing Atoh1 by Neurog1 disrupts the activation of the Delta-Notch system in the adjacent cells, which are consequently unable to mediate cell-cell interaction for proper supporting cell differentiation. In contrast, overexpression of Notch signaling or gain of function of Jag1 shows ectopic HC and supporting cell generation in the non-sensory region of the cochlea (Pan et al., 2010), indicating that misexpression of this ligand could recruit all relevant expression for sensory epithelia differentiation. However, the exact mechanism for this transformation is not yet clear, in particular how this relates to other essential TFs apparently needed for OC development such as Eya1, Pax2, Sox2 and Gata3 (Ahmed et al., 2012; Bouchard et al., 2010; Duncan et al., 2010; Kiernan et al., 2005), which may be regulated by LIM homeodomain genes (Deng et al.; Koo et al., 2009; Nichols et al., 2008). Ectopic HC formation in the non-sensory region has also been reported in the Neurog1 null mice (Fritzsch et al., 2011; Ma et al., 2000), a TF that appears to initiate activation of Notch signaling (Ma et al., 1998). This transformation of non-sensory cells into HCs seems to be regulated by the cross-regulation of Atoh1 and Neurog1 and by the fate switch of some common progenitors of sensory/non-sensory lineage (Fritzsch et al., 2006; Raft et al., 2007). This ability to induce sensory epithelia formation is consistent with the widespread expression of multiple factors throughout the otic placode or other placodes (O’Neill et al., 2012; Schlosser, 2010) that are later on restricted to sensory epithelia such as Eya1, Pax2 and Gata3. Overall, while a number of factors have been identified to play more or less crucial roles in OC formation and cell type specification, the hierarchy of their interactions is not well understood, in large measure due to the fairly crude approach of knocking genes out or over-expressing them. Consistent with more subtle effects on cell fate decision achieved with level and temporal expression changes in specific genes (Purvis et al., 2012), more recent data indicate that level and temporal patterns of Atoh1 expression play important roles, possible with more subtle variations in level of expression of other already well known factors to ultimately drive expression of Atoh1 in the right cells at the right time for the right amount (Ahmed et al., 2012). Translating these insights would be possible with a simultaneous knockdown of Neurod1 while overexpressing Atoh1 to drive primarily IHC development in the OC.
6. Molecular cues for sorting type specific innervation to two types of HCs
We have outlined above how HC type development in the OC depends on Atoh1 and its modification by other factors such as Neurod1. Likewise, inner ear neuronal development primarily depends on Neurog1 and Neurod1 for neuronal specification and differentiation, respectively (Kim et al., 2001; Ma et al., 1998). OC is an excellent model for studying the neuronal connection formation where only two types of HCs are innervated by just two different types of afferents (type I and II) and efferent fibers (MOCs and LOCs). Particularly the timing of spiral ganglion neuron survival is variable in relation to the degree and types of HC or supporting cell defects in different mouse models (Alam et al., 2007) and efferents have been related to OHC differentiation and viability (Walsh et al., 1998). In this section, we will review how different types of afferent and efferent innervation can be modified by the variety of defects of OC in different genetic mouse mutants.
It has been suggested that HCs in the OC are the main attractors for growth of the afferent innervation by releasing the neurotrophins such as Bdnf. However, in the absence of differentiated HCs, Atoh1 null mice can release Bdnf from the HC precursors and can initially attract afferent and efferent fibers to the OC (Fritzsch et al., 2005; Pan et al., 2011). Nevertheless, the density of radial fibers is severely reduced and most of the fibers form loops instead of extending into the OC. Only some fibers reach the Sox2 positive OC precursors cells [Fig. 6; (Pan et al., 2011)]. In contrast to this loop-like innervation, Neurog1 misexpression in Atoh1 locus, dramatically enhance the spiral ganglia survival and overall radial fiber density in contrast to Atoh1 null {Fig. 6; (Jahan et al., 2012)}. Interestingly, the Neurog1 knockin mice maintain a widespread Ntf3 expression from the patchy undifferentiated OC cells. In contrast, Bdnf seems not to be influenced by Neurog1 misexpression and rather shows the expression only in the apex of the cochlea comparable to Atoh1 null (Fritzsch et al., 2005; Jahan et al., 2012). Apparently, the Neurog1 misexpression influences some degree of differentiation of supporting cells which express more profoundly Sox2 and Ntf3, markers for embryonic supporting cells, sufficient to rescue more neurons and induce the fiber projections into the undifferentiated OC. Even after complete and very delayed loss of HCs, residual differentiation of supporting cells with minimum Ntf3 expression can preserve some spiral ganglion neurons in Brn3c null (Xiang et al., 2003).
Figure 6. HC defects affect differentially the innervation pattern.
Tubulin and Sox2 immunochemistry at E18.5 control mice show the projection of type I fibers to IHCs and the type II to OHCs (a, a′). Conditional deletion of Atoh1 with Pax2-cre results in the absence of HCs differentiation with atypical looping fibers modiolar to the OC, except for some fibers reaching to the Sox2 positive patches of undifferentiated OC cells (b). Homozygotic Neurog1 misexpression in Atoh1 knockin mice (Atoh1KINeurog1/KINeurog1) results in increased density of innervation as well as projection of radial fibers to Sox2 positive OC cells (c) indicating that Neurog1 can partially compensate for the lack of Atoh1. Neurofilament and Myo7a co-labeling displays type I and type II fiber projection to the IHCs and OHCs, respectively in the P3 control mice (d, d′). Delayed deletion of Atoh1 using Atoh1-cre reveals formation of only two rows of OHCs, resulting in aberration of innervation (e): all fibers, possibly including type I fibers, project to the remaining OHCs in the absence of their target cell, the IHCs, in this mutant (e). Neurod1 loss results in ectopic formation of IHCs in the region of OHCs, in addition to the formation of multiple rows of IHCs and OHCs (f). Tubulin co-labeling with Myo7a demonstrates the atypical overshooting of the type I fibers to the ectopic IHCs in the region of OHCs as well as disorganization of overall fiber projection (f). These mutants indicate that the variable alterations in the OC HCs can differentially modify the type-specific innervation pattern. Modified after (Jahan et al., 2010b; Jahan et al., 2012; Pan et al., 2012b). Bar indicates 100 μm in a–c and 10 μm in d–f.
However, in contrast to the apparent dense innervation in the undifferentiated HCs in mutant models described above, the detailed pattern of innervation with respect to the two types of afferent and efferent segregation demonstrate different events. For example, in delayed Atoh1 conditional null mice, where only the inner two rows of OHCs form with predominant IHCs loss, moderately dense innervation forms to the remaining HCs. However, both afferent and efferent fiber outgrowth to OHCs fail to organize properly with no indication of type I or type II afferents, also some short spiraling of some fibers develops (Pan et al., 2012b). Closer examination elucidates the unusual branching of what appear to be type I fibers, which cross the tunnel of Corti and project to the remaining OHCs [Fig. 6; (Pan et al., 2012b)]. Similarly, Bronx Waltzer mutant mice reveal a comparable expansion of what appear to be type I fibers, which project to the OHCs where there are no IHCs (Whitlon et al., 1991). Complete absence or patchy loss of IHCs seems not to eliminate completely the type I fibers but rather to modify their final destination and redirect them to the remaining HCs. This data suggest that fibers will innervate the ‘wrong’ HC if forced to do so by the loss of the ‘right’ HC. A more detailed study of the developmental progression of this sorting process is needed as well as a more detailed analysis of the long term effect of the apparent type I fibers redirected to OHCs in the absence of IHCs.
Interestingly, formation of ectopic IHCs in the region of OHCs in the Neuord1 null mutant can attract what appear to be type I fibers, which overshoot to innervate selectively these ectopic IHCs. We interpret these fibers to be type I as they end selectively on ectopic IHCs without contributing to the inner spiral bundles under the OHCs {Fig. 6; (Jahan et al., 2010b)}. In addition, these fibers are thicker and more like type I fibers (Pan et al., 2012a). We suggest that in the presence of ectopic IHCs in the region of OHCs, type I fibers can cross the tunnel of Corti and project to the ectopic IHCs where they branch to end at multiple ectopic IHCs in Neurod1 null apex (Jahan et al., 2010b). The source of the attraction of these fibers might be the expression of Ntf3 from these ectopic IHCs in the region of OHCs as IHCs, not OHCs, express Ntf3 (Farinas et al., 2001).
Consistent with our data is that HC are not required for the long-term survival of spiral ganglion neurons, which will be supported by the supporting cell expressed Ntf3 in both adult (Zilberstein et al., 2012) and embryonic (Farinas et al., 2001) OC. Another factor that is relevant for supporting cell differentiation, Prox1, deletion of which, results in aberrant projections of type II fiber to OHCs despite apparently normal differentiation of the OC (Fritzsch et al., 2010b). In fact, conditional deletion of Prox1 in neurons results in a nearly identical phenotype despite a completely normal OC development. Moreover, this phenotype is more profound than in Fgfr3 null mice, in which the phenotype directly correlates to the degree of disorientation of the OC (Puligilla et al., 2007), suggesting that Prox1 may regulate the molecular means of type II fibers to interact with supporting cells.
In summary, initial neuron formation and afferent fiber projection may initiate independent of HC formation but cannot maintain a type-specific arrangement to the specific types of HCs with distinct organization of afferents if those HC types do not develop normally or show altered distribution. Further evaluation is necessary to distinguish type I and type II fibers using specific labeling such as Peripherin in these mutants as well as to identify the differential dependency of efferents on afferents. Interactions between afferents and efferents have been proposed during normal development (Rontal et al., 2003). Our innervation defective mutants, briefly outlined above, should provide an excellent model to test these suggestions currently hinging on descriptive studies.
7. Conclusion
Studies on fruit fly ‘bristle’ and ‘hair’ development in the specific configuration on the thorax suggests that the developing sensory organs can be viewed as a landscape of TFs acting in a combinatorial manner to confer molecular identity to each region (Garcia-Bellido et al., 2009; Powell et al., 2008a; Powell et al., 2008b). In other words, within a complex genetic regulatory process, a precisely regulated level of expression of a gene ultimately will specify an individual cell in a specific position and number in a context dependent manor.
Atoh1 is crucial for formation of all inner ear HCs (Bermingham et al., 1999; Fritzsch et al., 2005) but its role in defining vestibular HCs as compared to OC HCs as well as in generating subtypes of OC HCs is not yet fully explored. For example, it is not yet established how much or how long Atoh1 is needed for efficient specification of each type of HCs and their long term maintenance. Comparable to other developing systems in which the role of intensity and duration of gene expression for cell specification and differentiation is well known (Conway et al., 2010; Menshykau et al., 2012; Purvis et al., 2012), we propose that specific dosages of Atoh1 may define HC types. Clearly, the critical concentrations of Atoh1 dosage and dynamics in combination with interacting partner TFs needs to be established for translation into attempts to regenerate an OC.
Currently the best available treatment for the severe-to-profound deaf patients is the cochlear implant. However, a fully functional OC would be a better solution. Novel approaches may soon allow regenerating HCs via stem cells and/or gene therapy (Chen et al., 2012). However, transformation of various multipotent stem cells in vitro into HC-like cells (Oshima et al., 2010), or conversion of supporting cells into HCs in vivo by transfection of genes with viral vector (Batts et al., 2009), does not yet amount to the proper reconstitution of a functional OC. This would require precise restoration of all different cell types in precise positions on the basilar membrane to convert sound induced vibration to near normal hearing, including normal pattern of afferent and efferent innervation. Regulating time and intensity of Atoh1 including crucial co-factors such as Neurod1 or neurogenic genes may be one way to improve the outcome of these approaches. Given the current limited insights into afferent and efferent fiber sorting mechanisms during development (Bulankina et al., 2012; Echteler, 1992; Fritzsch et al., 2004; Rontal et al., 2003), it is possible that generating the right type of OC HC in the right position will suffice for a crude afferent and efferent fiber sorting in such a regenerated OC.
Since Atoh1 was discovered as a main player to differentiate HCs in conjunction with multiple other, partially known TFs, using the approach of misexpressing or overexpressing Atoh1 to replace HCs has run its course: What was already known since 1997 (Kim et al., 1997), namely that Atoh1 alone cannot convert ectoderm into HCs, has become clear for the attempts to make new adult HCs using Atoh1 expression in the ear (Kelly et al., 2012; Liu et al., 2012). Indeed, we need to embrace the complexity of the emerging molecular interactome that guides HC development outlined here and move forward by defining the minimal size of partition of this interactome (Koch, 2012) needed to guide not only regeneration of short lived formation of partially functioning HCs but long lived specific types of HCs in the correct position. This review might help focusing the attention on this basic problem, fostering the relevant understanding to mediate ultimately the successful regeneration of an OC, possibly through re-initiating the ‘stemness’ of the OC with appropriate molecular therapy (Waldhaus et al., 2012).
Highlights.
Atoh1 is essential for hair cells differentiation but can by itself not specify them.
Precise dose and duration of Atoh1 generates distinct hair cell types.
Neurod1 limits Atoh1 expression to permit outer hair cells differentiation.
Proper sorting of afferents and efferents follows topology of hair cell types.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders (NIDCD) (R01 DC 005590 to BF) and by Hearing Health Foundation (HHF), 2012 Emerging Research Grant to Israt Jahan. We thank the Office of the Vice President for Research (OVPR), University of Iowa College of Liberal Arts and Sciences (CLAS), and the P30 core grant for support (DC 010362). We express our gratitude to Dr. H. Zoghbi for providing the floxed Atoh1 mice and Drs. T. Ohyama and A. Groves for the Tg(Pax2-cre) line used for this study and to Drs. JM Saalbaum and K. Beisel for their expert assistance with the generation of the Neurog1 knockin mice at the University of Nebraska medical Center (UNMC) mouse transgenic center. We also thank Dr. H. Zoghbi (Atoh1) and Dr. U. Pirvola (Fgf8) for providing the plasmids for in situ hybridization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Ahmed M, Wong, Elaine YM, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 Interaction Is Sufficient to Induce Hair Cell Fate in the Cochlea by Activating Atoh1 Expression in Cooperation with Sox2. Developmental Cell. 2012;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–52. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Basch ML, Ohyama T, Segil N, Groves AK. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci. 2011;31:8046–58. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts SA, Shoemaker CR, Raphael Y. Notch signaling and Hes labeling in the normal and drug damaged organ of Corti. Hear Res. 2009;249:15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Busslinger M, Xu P, De Caprona D, Fritzsch B. PAX2 and PAX8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol. 2010;10:89. doi: 10.1186/1471-213X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–86. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brown MC, Kujawa SG, Duca ML. Single olivocochlear neurons in the guinea pig. I. Binaural facilitation of responses to high-level noise. J Neurophysiol. 1998;79:3077–87. doi: 10.1152/jn.1998.79.6.3077. [DOI] [PubMed] [Google Scholar]
- Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, van Oudenaarden A, Jaenisch R. Single-Cell Expression Analyses during Cellular Reprogramming Reveal an Early Stochastic and a Late Hierarchic Phase. Cell. 2012;150:1209–22. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulankina AV, Moser T. Neural circuit development in the mammalian cochlea. Physiology (Bethesda) 2012;27:100–12. doi: 10.1152/physiol.00036.2011. [DOI] [PubMed] [Google Scholar]
- Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–82. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SJ, Firulli B, Firulli AB. A bHLH code for cardiac morphogenesis. Pediatric cardiology. 2010;31:318–24. doi: 10.1007/s00246-009-9608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hear Res. 2010;266:18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–51. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–78. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Deng M, Pan L, Xie X, Gan L. Requirement for Lmo4 in the vestibular morphogenesis of mouse inner ear. Developmental biology. 338:38–49. doi: 10.1016/j.ydbio.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol MS. The inner ear in Bronx waltzer mice. Acta Otolaryngol. 1981;92:331–6. doi: 10.3109/00016488109133269. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Lim K-C, Engle JD, Fritzsch B. GATA-3 is required for Morphogenesis and Histogenesis of the Inner Ear. Int J Dev Biol. 2010;55:297–303. doi: 10.1387/ijdb.103178jd. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to 739 mammalian auditory hair cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6324–7. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TB, Sellers JR, Avraham KB. Unconventional myosins and the genetics of hearing loss. Am J Med Genet. 1999;89:147–57. doi: 10.1002/(sici)1096-8628(19990924)89:3<147::aid-ajmg5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? Bioessays. 2006;28:1181–93. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010a;67:3089–99. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–78. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Dillard M, Lavado A, Harvey NL. Canal cristae growth and fiber extension to the outer hair cells require Prox1 activity. PLoS One. 2010b;5:1–12. doi: 10.1371/journal.pone.0009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hear Res. 2011;276:16–26. doi: 10.1016/j.heares.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233:570–83. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T. Evolution and Development of the Tetrapod Auditory System: an Organ of Corti-Centric Perspective. Evolution & Development. 2012 doi: 10.1111/ede.12015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, de Celis JF. The complex tale of the achaete-scute complex: a paradigmatic case in the analysis of gene organization and function during development. Genetics. 2009;182:631–9. doi: 10.1534/genetics.109.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ. Chance and necessity in eye evolution. Genome Biol Evol. 2011;3:1053–66. doi: 10.1093/gbe/evr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–57. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–44. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Holley M, Rhodes C, Kneebone A, Herde MK, Fleming M, Steel KP. Emx2 and early hair cell development in the mouse inner ear. Developmental biology. 2010;340:547–56. doi: 10.1016/j.ydbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SH, Jones J, Warchol ME, Ornitz DM. Differentiation of the Lateral Compartment of the Cochlea Requires a Temporally Restricted FGF20 Signal. PLoS Biol. 2012;10:e1001231. doi: 10.1371/journal.pbio.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman MA, Rivolta MN. Neural crest stem cells and their potential application in a therapy for deafness. Front Biosci (Schol Ed) 2012;4:121–32. doi: 10.2741/s255. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240:52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–9. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010a;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010b;341:95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Calisto LE, Morris KA, Kopecky B, Duncan JS, Beisel KW, Fritzsch B. Expression of neurog1 instead of atoh1 can partially rescue organ of corti cell survival. PLoS One. 2012;7:e30853. doi: 10.1371/journal.pone.0030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Fuchs PA. Cholinergic inhibition in hair cells. In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and vestibular efferents. Springer; New York: 2011. pp. 103–134. [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699–710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–5. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kim P, Helms AW, Johnson JE, Zimmerman K. XATH-1, a vertebrate homolog of Drosophila atonal, induces a neuronal differentiation within ectodermal progenitors. Developmental biology. 1997;187:1–12. doi: 10.1006/dbio.1997.8572. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–26. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A. 2011;108:3288–93. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Systems biology. Modular biological complexity. Science. 2012;337:531–2. doi: 10.1126/science.1218616. [DOI] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Developmental biology. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Fritzsch B. Regeneration of Hair Cells: Making Sense of All the Noise. Pharmaceuticals (Basel) 2011;4:848–879. doi: 10.3390/ph4060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Johnson SL, Furness DN, Chen J, Ingham N, Hilton JM, Steffes G, Lewis MA, Zampini V, Hackney CM, Masetto S, Holley MC, Steel KP, Marcotti W. miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2355–60. doi: 10.1073/pnas.1016646108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Pattern, growth, and control. Cell. 2011;144:955–69. doi: 10.1016/j.cell.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. Journal of the Association for Research in Otolaryngology: JARO. 2000;1:161–71. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan PK, Lukashkina VA, Goodyear RJ, Kossi M, Russell IJ, Richardson GP. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 2000;28:273–85. doi: 10.1016/s0896-6273(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Lenz DR, Horn HF, Dror AA, Brownstein Z, Shivatzki S, Roux KJ, Kozlov S, Burke B, Stewart CL, Avraham KB. Nesprin4 Knock-Out Mice Suffer from Progressive Hearing Loss Due to Outer Hair Cell Degeneration. Abstract # 1007. 35th Annual MidWinter Meeting; February 25 – 29, 2012; Association for Research in Otolaryngology; 2012. [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–4. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am. 1980;67:1686–95. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Functional structure of the organ of Corti: a review. Hear Res. 1986;22:117–46. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32:6600–10. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–43. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–82. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Manley GA, Koppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8:468–74. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshykau D, Kraemer C, Iber D. Branch mode selection during early lung development. PLoS computational biology. 2012;8:e1002377. doi: 10.1371/journal.pcbi.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmad M, Lander AD. Spatiotemporal mechanisms of morphogen gradient interpretation. Curr Opin Genet Dev. 2011;21:726–31. doi: 10.1016/j.gde.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Jahan I, Bonde G, Sun X, Hildebrand MS, Engelhardt JF, Smith RJ, Cornell RA, Fritzsch B, Banfi B. A mutation in the srrm4 gene causes alternative splicing defects and deafness in the bronx waltzer mouse. PLoS Genet. 2012;8:e1002966. doi: 10.1371/journal.pgen.1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hearing research. 2011;278:2–20. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–58. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill P, Mak SS, Fritzsch B, Ladher RK, Baker CV. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat Commun. 2012;3:1041. doi: 10.1038/ncomms2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–51. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Lee JE, Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009;337:407–28. doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Kopecky B, Jahan I, Fritzsch B. Understanding the evolution and development of neurosensory transcription factors of the ear to enhance therapeutic translation. Cell Tissue Res. 2012a;349:415–32. doi: 10.1007/s00441-012-1454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B. A novel atoh1 “self-terminating” mouse model reveals the necessity of proper atoh1 level and duration for hair cell differentiation and viability. PLoS One. 2012b;7:e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15798–803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–15. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH. Evolution of gene regulatory networks controlling body plan development. Cell. 2011;144:970–85. doi: 10.1016/j.cell.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9915–9. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–80. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20:6125–34. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Jarman AP. Context dependence of proneural bHLH proteins. Curr Opin Genet Dev. 2008a;18:411–7. doi: 10.1016/j.gde.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Deaton AM, Wear MA, Jarman AP. Specificity of Atonal and Scute bHLH factors: analysis of cognate E box binding sites and the influence of Senseless. Genes Cells. 2008b;13:915–29. doi: 10.1111/j.1365-2443.2008.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Kelley MW. Building the world’s best hearing aid; regulation of cell fate in the cochlea. Curr Opin Genet Dev. 2009;19:368–73. doi: 10.1016/j.gde.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–17. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–4. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–15. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Romand MR, Romand R. Development of spiral ganglion cells in mammalian cochlea. J Electron Microsc Tech. 1990;15:144–54. doi: 10.1002/jemt.1060150206. [DOI] [PubMed] [Google Scholar]
- Rontal DA, Echteler SM. Developmental segregation in the efferent projections to auditory hair cells in the gerbil. J Comp Neurol. 2003;467:509–20. doi: 10.1002/cne.10931. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. J Histochem Cytochem. 1998;46:29–40. doi: 10.1177/002215549804600105. [DOI] [PubMed] [Google Scholar]
- Sapede D, Pujades C. Neurod1 regulates hair cell and neuron development in the zebrafish ear. J Neurosci. 2012 in press. [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int Rev Cell Mol Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–66. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, Winkler M, Möbius W, Howard J, Göpfert MC. Drosophila Auditory Organ Genes and Genetic Hearing Defects. Cell. 2012;150:1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Simmons D, Duncan J, Crapon de Caprona D, Fritzsch B. Development of the inner ear efferent system. In: Ryugo DK, Fay RR, Popper AN, editors. Auditory and vestibular efferents. Springer Verlag; New York: 2011. pp. 187–216. [Google Scholar]
- Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol. 2010;518:3785–802. doi: 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, Harfe BD. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Developmental biology. 2009;328:328–41. doi: 10.1016/j.ydbio.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziklai I, He DZ, Dallos P. Effect of acetylcholine and GABA on the transfer function of electromotility in isolated outer hair cells. Hear Res. 1996;95:87–99. doi: 10.1016/0378-5955(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J. The alpha10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc Natl Acad Sci U S A. 2007;104:20594–9. doi: 10.1073/pnas.0708545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhaus J, Cimerman J, Gohlke H, Ehrich M, Muller M, Lowenheim H. Stemness of the organ of Corti relates to the epigenetic status of Sox2 enhancers. PLoS One. 2012;7:e36066. doi: 10.1371/journal.pone.0036066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, McFadden SL, Liberman MC. Long-term effects of sectioning the olivocochlear bundle in neonatal cats. J Neurosci. 1998;18:3859–69. doi: 10.1523/JNEUROSCI.18-10-03859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol. 2002;12:1611–6. doi: 10.1016/s0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Sobkowicz HM. Patterns of hair cell survival and innervation in the cochlea of the bronx waltzer mouse. J Neurocytol. 1991;20:886–901. doi: 10.1007/BF01190467. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010;48:407–13. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SM, Chen W, Guo WW, Jia S, Sun JH, Liu HZ, Young WY, He DZ. Regeneration of stereocilia of hair cells by forced atoh1 expression in the adult Mammalian cochlea. PLoS One. 2012;7:e46355. doi: 10.1371/journal.pone.0046355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res. 2011;278:21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Zheng J, Whitlon DS, Garcia-Anoveros J, Bartles JR. Targeting of the hair cell proteins cadherin 23, harmonin, myosin XVa, espin, and prestin in an epithelial cell model. J Neurosci. 2010;30:7187–201. doi: 10.1523/JNEUROSCI.0852-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32:405–10. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Treadaway J, Buckner TW, Fritzsch B. Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A. 1999;96:14100–5. doi: 10.1073/pnas.96.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]