Abstract
Interferon (IFN)-γ—like the well-known antitumor biotherapeutic IFN-α—is a powerful antiproliferative and immune modulatory cytokine, but mixed results from clinical trials, together with issues of systemic toxicity, have dampened enthusiasm for its use in the treatment of cancer. We suggest that at least 2 factors reduce the antitumor efficacy of IFN-γ: (1) poorly understood survival mechanisms that protect most tumor cells from IFN-γ-induced direct cytotoxicity, and (2) the short half-life of IFN-γ in serum. In this review, we outline avenues to overcome both these limitations. First, we have identified the transcription factor nuclear factor-kappa B (NF-κB) as a protective mechanism against IFN-γ-induced necrosis, and disabling NF-κB allows IFN-γ to trigger RIP1 kinase-dependent programmed necrosis (or necroptosis) in otherwise resistant cells. Second, we propose that fusing IFN-γ to tumor-specific antibodies will stabilize IFN-γ in serum and target this cytokine to tumor cells. We expect that such IFN-γ–antibody chimeras (called immunocytokines), when combined with agents that neutralize tumor-intrinsic survival signals such as NF-κB, will exert potent tumoricidal activity with minimized systemic side effects. Although this review will focus on exploiting IFN-γ-induced necrosis for treatment of renal cell carcinoma, these approaches are also directly applicable to several human cancers in which IFNs have shown therapeutic potential.
Introduction
The interferons (IFNs) are a family of cytokines classified into type I (predominantly α/β), type II (γ), and type III (λ), based primarily on the cell surface receptors they use, the stimuli that trigger their production, and the cells that produce them. All types of IFNs, however, activate very similar Jak-/STAT-dependent signaling cascades downstream of their structurally distinct receptors. Originally discovered as antiviral cytokines over 50 years ago, the IFNs also possess potent growth suppressive and immunomodulatory properties. The antitumor potential of these properties was recognized early in the history of IFN research, resulting in recombinant IFN-α becoming the first ever biological agent approved for the treatment of cancer (Borden and others 2007).
As IFN-γ—the lone type II IFN—exerts many of the same biological effects as IFN-α, several clinical trials with recombinant IFN-γ (mainly IFN-γ1b; Actimmune) were carried out in the mid-1980s to determine its potential against a variety of cancers and other diseases. As a consequence of these trials, Actimmune was approved by the Food and Drug Administration in 1991 for reducing the frequency and severity of recurrent microbial infections in chronic granulomatous disease, and (in 2000) for delaying progression of disease in patients with malignant osteopetrosis, an inherited disorder characterized by osteoclast defects and diminished phagocyte oxidative capacity. In both cases, the ability of IFN-γ to trigger superoxide generation via induction of a respiratory burst in phagocytes is thought to underlie its therapeutic effects [reviewed in (Miller and others 2009)].
The results from the use of IFN-γ in cancer trials were mixed. In many of these trials, IFN-γ showed significant clinical benefit, either as a monotherapy, or when combined with other agents. Other trials, however, showed no advantage to using IFN-γ, or had to be terminated prematurely because of toxicity arising from the combined use of IFN-γ with chemotherapeutic drugs [for examples, see (Foon and others 1985; Kurzrock and others 1985; Bennett and others 1986; Muss and others 1986; Vadhan-Raj and others 1986; D'Acquisto and others 1988; Lane and others 1989; Abbruzzese and others 1990; Yoshida and others 1990; Jett and others 1994); reviewed in (Miller and others 2009)]. The outcomes of clinical trials employing IFN-γ as an experimental therapy for advanced renal cell carcinoma (RCC) highlight the benefits and limitations of this cytokine as an anticancer agent, for which reason we will focus this review on IFN-γ and RCC. We will outline advances from our groups and from other laboratories that lend new insight into the mechanism of IFN-γ cytotoxic action and offer compelling next-generation possibilities for the revival of IFN-γ as a therapeutic approach for RCC and other cancers. In particular, we will describe (1) the identification of a novel necrotic cell death mechanism induced by IFN-γ; (2) a nuclear factor-kappa B (NF-κB)-dependent survival program that protects cells from IFN-γ; and (3) our ongoing efforts to induce tumor-selective necrosis by targeting IFN-γ to tumors.
IFN-γ and RCC
Kidney cancer is among the top 10 most-frequent cancers in Western countries, and the 13th most-common malignancy worldwide. Globally, about 270,000 cases of kidney cancer are diagnosed every year, with ∼116,000 annual deaths. Approximately 90% of all kidney cancers are RCCs (Ljungberg and others 2011). Although early-stage RCC can be effectively controlled by surgical and other interventions, RCC is largely asymptomatic, and 20%–30% of patients have metastatic disease at the time of presentation. Unlike early-stage disease, metastatic RCC is a chemotherapy-resistant cancer that is usually lethal (Chen and Uzzo 2011; Ljungberg and others 2011).
RCC comprises several distinct histological varieties, of which clear-cell (cc) RCC represents the dominant subtype and accounts for up to 85% of all RCC cases (Kovacs and others 1997; Storkel and others 1997). The best-recognized genetic hallmark of ccRCC is inactivation of the von Hippel Lindau (VHL) tumor suppressor gene (Linehan and others 2010). Somatic VHL gene mutations occur in ∼20%–70% of cases of sporadic ccRCC, with promoter hypermethylation of the VHL gene seen in up to 20% of patients (Sufan and others 2004; Nyhan and others 2008; Linehan and others 2010). The VHL gene product, pVHL, operates in a degradative E3 ubiquitin ligase complex necessary for controlling the protein levels of the alpha-subunits of hypoxia-inducible factor (HIF), a master regulator of the cellular response to hypoxia (Sufan and others 2004; Greer and others 2012). There are 3 HIF-α family members, HIF-1α, HIF-2α, and HIF-3α, of which HIF-2α appears to be especially important in VHL-defective renal carcinogenesis (Kondo and others 2003; Gordan and others 2008; Kaelin 2008). pVHL functions by directly interacting with HIF-α to mediate its degradation. The interaction between pVHL and HIF-α requires the hydroxylation of HIF-α on 2 conserved prolyl residues, an event that occurs in the presence of oxygen. The enzymes that mediate prolyl hydroxylation of HIF-α are inhibited under hypoxic conditions, resulting in loss of interaction between pVHL and HIF-α and consequent stabilization of HIF-α. Once stabilized, HIF-α interacts with its partner HIF-1β to form a heterodimer that binds specific DNA sequences and transactivates target genes involved in the cellular adaptation to low-oxygen conditions. When pVHL expression is lost, HIF-α accumulates even under normoxic conditions and inappropriately transactivates expression of its target genes. As the genes required for the hypoxic stress response (eg, angiogenic factors, growth factors, and glycolytic enzymes) are also potently tumorigenic, misexpressed HIF-α target genes are considered the primary orchestrators of VHL-deficient ccRCC tumor progression (Sufan and others 2004).
Significant improvements have been made in the treatment of advanced RCC over the last decade with the introduction of new small-molecule therapies that target angiogenesis and nutrient-sensing pathways (eg, the kinase inhibitor sunitinib and the rapamycin analog temsirolimus). Although most patients will temporarily benefit from these agents, such small-molecule-based approaches have 2 major shortcomings: (1) they require continuous administration, exposing patients to significant side effects, and (2) tumor cells rapidly become resistant to these agents: virtually all patients on small-molecule regimens will eventually relapse and succumb to metastatic disease within 2–5 years. Advanced RCC is therefore still in a desperate need of new therapeutic interventions [reviewed in (Hudes 2009; Jemal and others 2010; Pal and Figlin 2010; Pirrotta and others 2011)].
Before the introduction of these targeted pharmacological approaches, cytokine-based immunotherapy—interleukin-2 (IL-2) and IFN-α in particular—was the primary treatment modality for advanced RCC (Wirth 1993; MRCCC 1999; Motzer and others 2002; Coppin and others 2005). Based on its similarity to IFN-α, IFN-γ was subsequently evaluated in several clinical trials as a candidate immunotherapy for RCC [for examples, see (Rinehart and others 1986; Quesada and others 1987; Garnick and others 1988; Otto and others 1988)]. An important such phase I/II trial was conducted by Huber and colleagues (1989). This study took advantage of 2 observations: (1) that previous unsuccessful trials with IFN-γ in other cancers pursued a more-is-better ideal and used doses of IFN-γ close to the maximum tolerated dose for this cytokine, and (2) that immune modulation by IFN-γ in vivo followed a Gaussian (ie, bell-shaped) dose–response curve, such that low-to-moderate doses were more biologically active than higher doses. Based on these observations, an initial phase I dose-finding study on patients with metastatic RCC was performed to identify an optimal low-to-moderate dose of IFN-γ, and this dose [100 μg/patient, administered subcutaneously (s.c.) once weekly] was employed in a subsequent phase II efficacy trial. The results of this trial were very promising: IFN-γ was reasonably well tolerated, and produced overall response rates of 30% and complete response rates of 10% in patients with advanced RCC (Aulitzky and others 1989). A subsequent independent phase II trial with low-dose IFN-γ in metastatic RCC also yielded positive results (overall response rates of 15%) (Ellerhorst and others 1994). Based on these encouraging findings, a much larger randomized phase III trial employing low-dose IFN-γ (60 μg/m2, s.c. once-weekly) as a monotherapy for RCC was conducted (Gleave and others 1998). Disappointingly, this placebo-controlled multicenter trial did not find any significant effect of IFN-γ on overall response rates (4% for IFN-γ, compared to 7% with placebo alone), time to disease progression (1.9 months in both cases), or median survival (12.2 months with IFN-γ vs. 15.7 months with placebo). As a result, it was concluded that the successful outcomes seen in the earlier phase I/II trials may be attributable to biases inherent in uncontrolled studies, and consequently IFN-γ was not pursued much further as an anti-RCC therapy.
Resurrecting IFN-γ as an Antitumor Agent
Given new developments in our understanding of the biology of IFN-γ, we believe that this cytokine merits reconsideration as an antitumor agent for RCC and other cancers, and offer the following reasons for resurrecting IFN-γ. First, the prevalent view of IFN-γ as an immune modulator fails to take into consideration the direct tumoricidal properties of this cytokine. In particular, IFN-γ dosage parameters for the trials described above were calculated based on the conventional wisdom that IFN-γ operates primarily as an indirect immune modulatory cytokine; one that functions mainly to reactivate the antitumor immune response, rather than directly on the tumor (Gleave and others 1998; Younes and Amsden 2002; Miller and others 2009). Given the short half-life of IFN-γ, systemic administration of low doses may thus upregulate serum biomarkers without concomitant bioavailability of the cytokine at the tumor. We have identified a novel process of necrosis activated by IFN-γ—as well as a targetable mechanism of resistance in tumor cells—which now allows this cytokine's direct tumoricidal properties to be exploited (Thapa and others 2011).
Second, none of the current small-molecule agents currently used to treat metastatic RCC are curative, and only delay disease progression. In contrast IFN-γ—like other cytokine-based approaches—has the potential to provide lasting remission in occasional patients with metastatic RCC, an observation that cannot be easily ignored. In large part, the ability of IFN-γ to provide durable remission may stem from its pleiotropic nature: not only does IFN-γ activate a powerful immune response to the tumor, but it is also actively antiangiogenic, and directly tumoricidal to susceptible tumor cells (Borden and others 2007; Miller and others 2009; Thapa and others 2011). Concurrent deployment of this antineoplastic triad represents the primary advantage of IFN-γ over small-molecule approaches.
Third, the poor half-life of IFN-γ of ∼25–35 min in serum (Younes and Amsden 2002) can now potentially be overcome by fusing it to antibodies. This advance in itself will have the triple advantage of stabilizing IFN-γ in serum, targeting it to the tumor where its direct tumoricidal properties can be exploited, and greatly minimizing toxic side effects arising from system-wide activity of this cytokine. A number of groups have demonstrated that it is possible to stabilize and target cytokines to tumors or to tumor-associated vasculature by chemically conjugating the cytokine to an antibody or by constructing fusion proteins consisting of a cytokine and an antibody-based molecule [for a complete review, see (Pasche and Neri 2012)].
Mechanism of IFN-γ-Induced Necrosis
Virtually, all cells (including most tumor cell types) express the IFN-γ receptor and respond to this cytokine by activating the transcription of hundreds of genes, called IFN-stimulated genes (ISGs). The dominant signaling cascade downstream of the IFN-γ receptor requires the tyrosine kinases Jak1 and Jak2 and culminates in the activation and nuclear translocation of STAT1 homodimers. These STAT1 dimers associate with gamma-activated sequence elements in the promoters of target genes and drive the transcription of such genes. Although the Jak/STAT signaling axis is responsible for the expression of most ISGs, alternate STAT-independent signaling pathways (eg, NF-κB and PI-3 kinase/AKT) are also activated by IFN-γ and contribute to its downstream effects. In this manner, IFN-γ mediates its many antiviral, antiproliferative, and immune modulatory activities [reviewed in (Stark and others 1998; Taniguchi and Takaoka 2002; Platanias 2005; Pfeffer 2011)].
IFN-γ is generally nontoxic to cultured cells, and is often used by our laboratory to dissect innate immune signaling pathways (Balachandran and others 2004; Basagoudanavar and others 2011). During the course of such studies, we unexpectedly observed that dividing (ie, subconfluent and nonarrested) primary murine embryo fibroblasts (MEFs) lacking the NF-κB subunit RelA (rela−/− MEFs) succumbed to IFN-γ at doses that left control cells largely unharmed (Thapa and others 2011). The possible antitumor therapeutic ramifications of these observations were readily apparent to us, although why cells must be cycling for IFN-γ to fully exert its toxic effects is still unclear.
Loss of NF-κB revealed a novel, progressive form of caspase-independent cell death that was morphologically and molecularly distinct from apoptosis. Recently, Yuan and colleagues (2008) reported that IFN-γ, when combined with the synthetic dsRNA mimetic poly(I:C), induces a form of regulated necrotic death (sometimes termed necroptosis) that relies on the activity of the serine–threonine kinase RIP1. Given that aspects of IFN-γ-induced death in rela−/− MEFs were reminiscent of RIP1-dependent necrosis [eg, caspase independence and overproduction of reactive oxygen species (ROS), as described later], we tested if the allosteric RIP1 kinase inhibitor Necrostatin-1 (Nec-1) could inhibit IFN-γ-activated cell death in rela−/− MEFs. Indeed, we found that Nec-1 was able to rescue rela−/− MEFs from IFN-γ, demonstrating that IFN-γ induces RIP1-dependent necrosis when NF-κB survival signaling is lost (Thapa and others 2011).
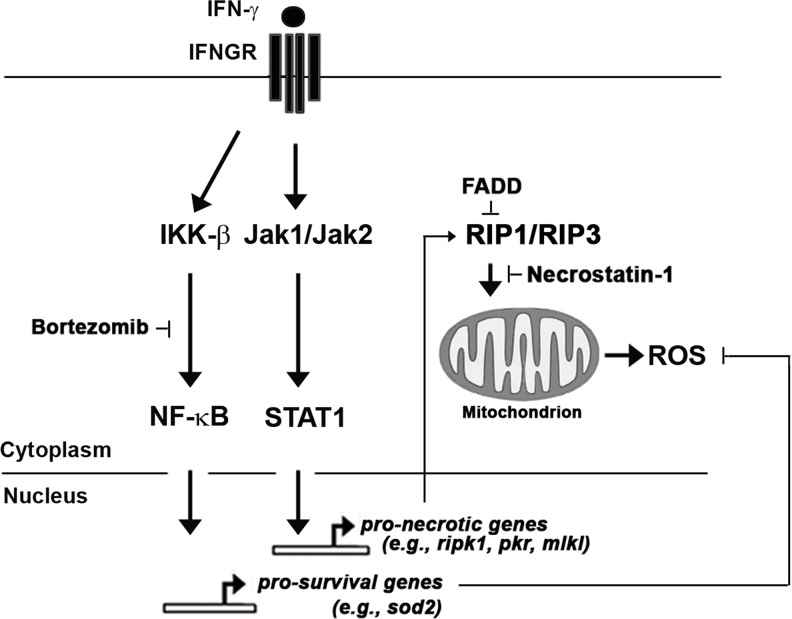
In subsequent experiments, we have determined that cells lacking the adaptor protein FADD are also susceptible to IFN-γ-induced RIP1-activated necrosis, and the use of fadd−/− and rela−/− MEFs has allowed us to unmask some of the molecular determinants of IFN-γ-induced necrotic death. In addition to RIP1, we have found that IFN-γ requires the kinase RIP3 to execute necrosis, but activates these kinases by mechanisms that appear to differ from the better-studied tumor necrosis factor-α (TNF-α)-activated necrosis pathway. During TNF-α-induced pronecrotic signaling, RIP1 and RIP3 are brought together by cytoplasmic (ie, nontranscriptional) signals into a kinase complex called the necrosome (Vandenabeele and others 2010). The necrosome then impinges on mitochondria by as-yet poorly defined mechanisms to alter their metabolic rates and trigger their dysfunction (Zhang and others 2009; Wang and others 2012). We find that IFN-γ also induces necrosome formation, but does so by mechanisms requiring Jak-/STAT-dependent transcription. Likely, IFN-γ induces the expression and/or activation of ISG-encoded signaling proteins that drive various aspects of RIP1–RIP3 assembly, activation, and consequent mitochondrial perturbation (Fig. 1). In this regard, it is noteworthy that several known effectors of necrosis and/or IFN-γ-induced cytotoxicity (such as RIP1 itself, the recently discovered necrosome adaptor molecule MLKL, and the kinases PKR and DAPK1) are encoded by ISGs (Deiss and others 1995; Balachandran and others 2000; Thapa and others 2011; Sun and others 2012; Zhao and others 2012).
FIG. 1.
Schematic of interferon (IFN)-γ-activated pronecrotic and prosurvival signaling pathways. IFN-γ utilizes classical Jak/STAT signaling downstream of the IFN-γ receptor (IFNGR) to induce expression of pronecrotic genes (such as those encoding RIP1, PKR, and MLKL proteins) and activate R1P1/RIP3 kinase-dependent necrosis. The IFN-γ-induced necrotic pathway is held in check by the adaptor protein FADD, and can be inhibited by the RIP1 kinase blocker Necrostatin-1. In parallel, IFN-γ also activates NF-κB via canonical IκB kinases (IKK)-β-dependent signaling to induce expression of prosurvival genes such as sod2. As NF-κB target genes protect against IFN-γ-activated necrosis, disabling NF-κB signaling (by bortezomib, for example) sensitizes cells to IFN-γ-induced necrotic death that proceeds via accumulation of mitochondrial reactive oxygen species (ROS).
FADD appears to function as an antinecrotic molecule by preventing IFN-driven association of RIP1 and RIP3 (manuscript in preparation). NF-κB, on the other hand, mediates cell survival by activating a transcriptional program that buffers mitochondria to prevent a late step of the necrotic program (Thapa and others 2011). How NF-κB mediates its cell survival effects during IFN-γ signaling are described next.
NF-κB as a Survival Factor Against IFN-γ
The NF-κB family of transcription factors is composed of dimeric combinations of 5 subunits: RelA, RelB, c-Rel, p50/NF-κB1, and p52/NF-κB2. All 5 subunits share a Rel homology domain in their N-termini, while RelA, RelB, and c-Rel additionally possess a C-terminal transactivation domain. In a simplified model, classical NF-κB signaling (the dominant mechanism in most cell types) proceeds mainly via activation of RelA:p50 heterodimers. Normally, RelA:p50 dimers are held inactive in the cytoplasm of unstimulated cells via binding to the IκB class of inhibitory proteins. In response to upstream stimuli, IκBs are phosphorylated on distinct serines by IκB kinases (IKKs), after which they are targeted for degradative ubiquitylation and rapidly processed by the proteasome. Degradation of IκB results in translocation of NF-κB to the nucleus, where it activates transcription of genes containing κB sites in their promoters [reviewed in (Hayden and Ghosh 2004; 2008; Gilmore 2006)].
NF-κB signaling as a mediator of cell survival is best described downstream of the TNF-α receptor. In that paradigm, the TNF-α signal bifurcates downstream of the receptor into at least 2 axes, one of which activates NF-κB to transactivate a potent cell survival response (Chen and Goeddel 2002). If this axis is inactivated (eg, in NF-κB-deficient cells or when transcription is inhibited), then a parallel proapoptotic axis triggers cell death (Beg and Baltimore 1996; Chen and Goeddel 2002). To test if IFN-γ, like TNF-α, stimulates an NF-κB dependent transcriptional response, we first confirmed that IFN-γ was capable of activating RelA:p50 dimers (although to a lesser degree than TNF-α) in a manner requiring IKK-β (Thapa and others 2011). How IKK-β is activated by the IFN-γ receptor is still unclear, but data suggest that the mechanism involved appears to be STAT1-independent and proceed via a direct (ie, transcription-independent) signaling intermediates such as the kinases Jak1 or PKR (Deb and others 2001).
We next employed whole-genome DNA microarray technology to identify (1) the contribution of NF-κB signaling to the overall IFN-γ transcriptional response, and (2) potential prosurvival genes among NF-κB targets. From this analysis (performed on IFN-γ-treated rela+/+ and rela−/− MEFs), we identified a total of ∼250 genes whose expression was induced at least 2-fold after 6 h of IFN-γ treatment. Most (∼85%) of these genes were induced to approximately the same extent in both rela+/+ and rela−/− MEFs, and are likely direct STAT1 targets. Of the remaining differentially induced genes, a total of 22 ISGs were found to be critically dependent on RelA for their expression. Among these obligate RelA-dependent targets was sod2, the gene encoding the mitochondrial antioxidant enzyme manganese superoxide dismutase (MnSOD). Subsequent chromatin immunoprecipitation analyses showed that RelA associated with an NF-κB-responsive element in the murine sod2 gene (Jones and others 1997) after IFN-γ treatment. IFN-γ also induced a 2–3-fold increase in MnSOD protein expression in wild-type, but not rela−/−, MEFs. These results identify sod2 as a bona fide NF-κB-dependent ISG (Thapa and others 2011).
As MnSOD is an established scavenger of mitochondrial ROS, we next examined if IFN-γ triggered RIP1-dependent ROS accumulation in rela−/− MEFs. We found that IFN-γ induced progressive ROS accrual in rela−/− (but not rela+/+) MEFs, leading to loss of mitochondrial membrane potential and eventual cell death. Overexpression of MnSOD (or incubation of cells with small-molecule antioxidants) was largely able to protect rela−/− MEFs from IFN-γ-induced necrosis, whereas acute RNAi-mediated ablation of sod2 expression rendered HeLa cells as susceptible to IFN-γ as loss of RelA itself. Collectively, these findings are consistent with a model in which IFN-γ activates an NF-κB-dependent transcriptional cell survival pathway that upregulates expression of antioxidant genes (such as sod2, but likely others as well) to quench mitochondrial ROS. When this pathway is disabled, exposure to IFN-γ results in necrotic death resulting from gradual accumulation of toxic ROS in mitochondria (depicted schematically in Fig. 1).
Based on these and other findings, we speculate that IFN-γ utilizes the kinases RIP1 and RIP3 to boost mitochondrial ATP output during host innate immune and inflammatory responses. How RIP kinases link to increased mitochondrial bioenergetics and increased ATP generation is currently unclear, but ATP produced in this way serves the purposes of (1) fueling antimicrobial enzymes such as NADPH oxidase, myeloperoxidase, and nitric oxide synthase (Bours and others 2006; Trautmann 2009), and (2) functioning as an inflammasome activator and alarmin to potentiate inflammatory cell recruitment when released from dying cells into surrounding tissue (Mariathasan and others 2006). The process of ATP biogenesis by oxidative phosphorylation however prematurely leaks electrons (mainly from complexes I and III of the electron transport chain) into the oxygen-rich mitochondrial intermembrane space, producing the highly reactive superoxide anion (Kowaltowski and others 2009). Normally, these unstable superoxide anions are rapidly eliminated by mitochondrial scavenging enzymes such as MnSOD, and cellular respiration rates can be increased without negative consequences. If, on the other hand, superoxide anions remain unquenched and instead accumulate in the intermembrane space, then they can oxidize and inactivate integral membrane enzyme systems, resulting in loss of mitochondrial membrane potential, termination of ATP biogenesis, and eventual cell death (Kowaltowski and others 2009).
NF-κB-mediated induction of sod2 thus ensures mitochondrial integrity by scavenging mitochondrial ROS to protect cells during inflammatory responses (Sakon and others 2003; Bubici and others 2006; Kowaltowski and others 2009). Notably, NF-κB is thought to protect cells against TNF-α-induced apoptosis by similar mechanisms (Sakon and others 2003). In addition to sod2, the gene encoding the antioxidant enzyme ferritin heavy chain (FHC), is an NF-κB target downstream of TNF-α (Pham and others 2004). FHC functions by oxidizing the cation Fe2+ to Fe3+, causing a reduction in the levels of free intracellular Fe2+ that can participate in the generation of free radicals through the Fenton reaction (Theil 1987). Loss of either MnSOD or FHC sensitizes cells to TNF-α-induced apoptosis, emphasizing the critical importance of preventing free-radical buildup during cytokine signaling (Sakon and others 2003; Pham and others 2004).
Exploiting NF-κB Survival Signaling in RCC
Although NF-κB-mediated survival signaling likely evolved to protect cells from mitochondrial flux inherent to normal physiological responses (eg, during cytokine-driven antimicrobial responses as described above), several observations make it plausible that the NF-κB prosurvival response has been usurped by tumor cells to promote their own viability. For example, (1) the founding member of the NF-κB family—the avian retroviral gene v-Rel—is a bona fide oncogene; (2) genes encoding NF-κB subunits and signaling components display activating mutations in several tumors; (3) certain tumors contain inactivating mutations in genes encoding negative regulators of NF-κB signaling; (4) tumor cells frequently show high levels of constitutive NF-κB activity; and (5) inhibiting NF-κB in tumor cells is often either directly cytotoxic (ie, the tumors are addicted to NF-κB survival signaling), or sensitizes these cells to chemotherapeutic, radiological, and other interventions [reviewed in (Baud and Karin 2009; Ben-Neriah and Karin 2011)]. Together, these observations suggest that tumor cells, despite their switch to aerobic glycolysis as a source of ATP (the Warburg Effect), are nevertheless reliant on free-radical-scavenging mechanisms to detoxify mitochondria during the process of oxidative phosphorylation. In agreement, oxidative phosphorylation is still thought to generate a significant fraction (from 25% to over 90%, depending on the type of malignancy) of the tumor cell's ATP needs (Moreno-Sanchez and others 2009; Mathupala and others 2010; Ramsay and others 2011; Rodriguez-Enriquez and others 2011). NF-κB prosurvival mitochondrial targets are not limited to antioxidant enzymes, and NF-κB-induced genes (such as the Bcl-2 family members Bcl-XL and Bfl-1) also actively prevent mitochondria from inducing cell death during genotoxic and metabolic stresses inherent to the process of tumorigenesis (Ben-Neriah and Karin 2011).
Several studies now suggest that constitutively elevated NF-κB signaling may be a widespread feature of RCC as well. Importantly, loss of pVHL has been causally linked to increased NF-κB activity, suggesting that activation of NF-κB may represent a common downstream consequence of VHL deficiency (An and others 2005; An and Rettig 2005; Yang and others 2007). Mechanistic understanding of how pVHL controls NF-κB was provided by Kaelin and colleagues (2007), who identified caspase recruitment domain-containing protein (CARD)9—an NF-κB adaptor protein—as a pVHL-interacting partner in a proteomic screen. The authors showed that pVHL, in a manner independent of HIF-α, promoted the inhibitory phosphorylation of CARD9 by casein kinase 2 to negatively regulate NF-κB signaling (Yang and others 2007). In a parallel study, Rettig and colleagues showed a role for the epidermal growth factor receptor (EGFR)-signaling pathway in maintaining elevated NF-κB function in RCC by demonstrating that (1) HIF-α accumulation activated EGFR signaling after pVHL loss, and (2) inhibiting EGFR signaling in RCC cells greatly reduced constitutive NF-κB activity in these cells (An and Rettig 2005, 2007). Although it is currently unclear if CARD9 functions in the same NF-κB-stimulatory signaling axis as that initiated by EGFR, these studies provide preliminary molecular insight into the mechanisms that cause elevated NF-κB activity in RCC.
As NF-κB has multiple functions in the body, agents that inhibit NF-κB have numerous toxic side effects, and (to the best of our knowledge) no dedicated NF-κB inhibitor has yet been approved for antitumor therapy [reviewed in (Baud and Karin 2009)]. Nevertheless, the proteasome inhibitor bortezomib has been shown to mediate its anticancer effects at least in part by inhibiting NF-κB [and ostensibly does so by preventing IκB degradation; reviewed in (Chen and others 2011; Cvek and Dvorak 2011)]. Preclinical studies in RCC cells show that inhibiting NF-κB signaling by bortezomib renders them susceptible to the direct cytotoxic action of multiple antineoplastic agents, including apoptosis by the cytokine TRAIL and oncolysis by encephalomyocarditis virus (An and others 2004; An and Rettig 2007; Shanker and others 2008; Brooks and others 2010; Roos and others 2010). We took advantage of this observation to test if bortezomib can sensitize cells to IFN-γ-activated necrosis. In preliminary experiments, we find that (1) bortezomib inhibits NF-κB activity in RCC cells, and (2) bortezomib renders a large panel of RCC cell lines susceptible to IFN-γ-induced necrosis at doses of each agent that are physiologically very achievable (manuscript in preparation).
These proof-of-principle experiments provide strong rationale for the combinatorial use of IFN-γ with inhibitors of NF-κB survival signaling (such as bortezomib) in RCC and other tumors, but the poor serum stability and significant toxicity of IFN-γ remain serious issues. To overcome these limitations, one approach currently being undertaken by our laboratories is the production of IFN-γ fusion antibodies (immunocytokines).
Immunocytokines
A potential solution to the problem of cytokine stability and toxicity can be found in the recently developed class of molecules known as immunocytokines, or antibody–cytokine fusions, in which a fully functional cytokine is chemically or genetically conjugated to an antibody molecule. Immunocytokines not only provide a mechanism for targeting the desired tissue of impact, but also enhance the cytokine's clinical utility by improving in vivo stability and prolonging residence time in the circulation. The prolonged half-life of intact antibodies—critical for exploitation of the direct antitumor properties of IFN-γ—is mediated by the interaction of their Fc domains with the immunoglobulin salvage receptor FcRn (Ghetie and Ward 2002).
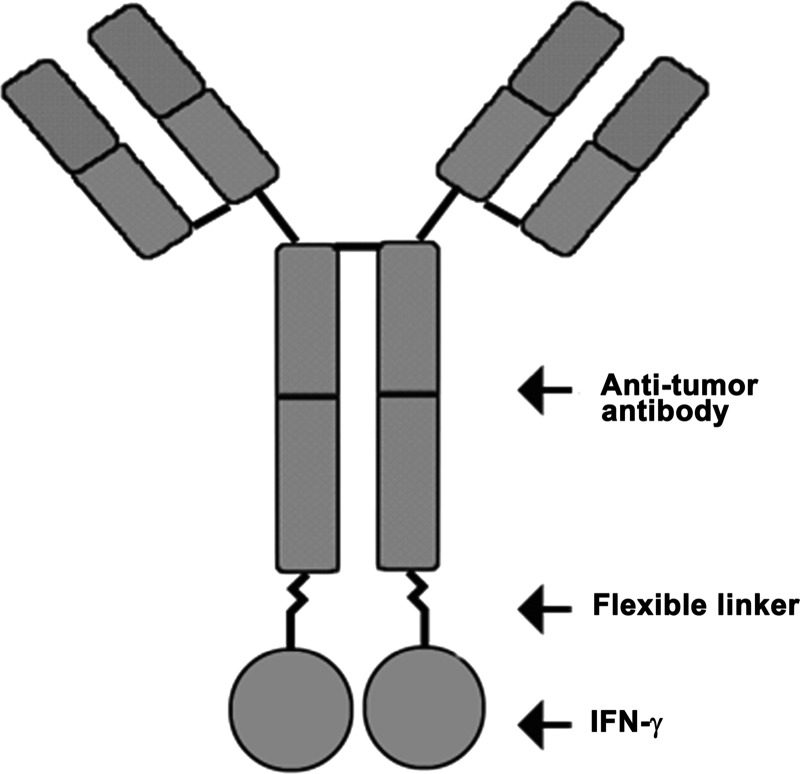
Most advanced immunocytokines have been developed by creating fusion proteins incorporating the desired cytokine into the carboxyl terminus of the heavy chain of an intact antibody molecule (see Fig. 2 for a schematic of a generic IFN-γ immunocytokine). The carboxyl terminus of the heavy chain is typically the preferred site, as this strategy spatially locates the cytokine at the opposite end of the antibody from the antigen-binding site, thus diminishing the likelihood of the cytokine sterically hindering the antibody–antigen interaction. As antibodies are composed of 2 identical light chains and 2 identical heavy chains, fusing cytokines to the heavy chain results in an agent with 2 cytokine moieties per antibody. The cytokine is typically separated from the antibody heavy chain by a polypeptide linker that is (1) flexible enough to allow engagement of the cytokine with its receptor, and (2) resistant to serum proteases that would otherwise release the cytokine before successful targeting. Nagamune and colleagues (2001) demonstrated that the distance between 2 domains of a fusion protein can be effectively controlled by the use of repetitive helical linkers, thereby maintaining the independent function of each domain (in this case, antibody and cytokine) and suggesting that linkers can be specifically designed to achieve these goals. Immunocytokines have been developed that incorporate IFN-α, IFN-γ, TNF-α, and several interleukins (eg, IL-2, IL-10, IL-12, and IL-15) for a variety of applications, including the treatment of cancer and autoimmune disorders. Of these, immunocytokines containing TNF-α, IL-2, IL-10, and IL-12 have been evaluated in early-phase clinical trials (Pasche and Neri 2012).
FIG. 2.
Schematic of IFN-γ fusion immunocytokine. IFN-γ is fused to the carboxyl terminus of the heavy chain of a tumor-targeting antibody with a flexible, protease-resistant linker. Fusion to an antibody not only targets IFN-γ to tumor cells, but also improves its stability in serum.
IFN-γ Immunocytokines
To date, a handful of IFN-γ immunocytokines have been developed. These constructs have included single-chain variable fragment (scFv)-based IFN-γ immunocytokines (Xiang and others 1993; Ebbinghaus and others 2005), dimerized antigen-binding fragment [F(ab′)2] IFN-γ immunocytokines (Xiang and others 1996), and intact antibody-based IFN-γ immunocytokines (Sharifi and others 2002; Mizokami and others 2003). While all these immunocytokines exhibited in vitro function, their ability to actively target tumor xenografts in vivo was not ubiquitous, likely due to a phenomenon equivalent to the tail wagging the dog. In other words, as both the antibody and the cytokine have an affinity for their targets, the antibody's in vivo targeting properties compete (and can be nullified) by interactions between the cytokine and its receptor on nonspecific tissues. This situation was encountered by Neri and colleagues (2005), with their IFN-γ-scFv molecule targeting fibronectin. In these studies, the ability of the immunocytokine to efficiently target tumor xenografts was diminished in wild-type mice, but significantly increased when the same studies were performed in IFN-γ receptor-deficient mice (Ebbinghaus and others 2005). This issue may be addressed in 2 ways: (1) by increasing the affinity of the antibody for the target antigen (eg, by altering valence from a single scFv binding arm to a divalent intact antibody), or (2) by decreasing the affinity of IFN-γ for its receptor via site-directed mutagenesis. Employing the former solution, Epstein and colleagues (2003) have successfully targeted IFN-γ immunocytokines to tumor xenografts growing in mice using a high-avidity intact antitumor antibody (TNT-3). The IFN-γ-TNT-3 fusion was well tolerated, had molar activity comparable to native IFN-γ, and manifested tumor-selective uptake and retention for up to 7 days postinjection (Sharifi and others 2002; Mizokami and others 2003). Encouragingly, our own unpublished results reveal that patient-derived RCC samples express higher levels of IFN-γ receptor mRNA than paired normal renal tissue controls, suggesting that IFN-γ immunocytokines targeting RCC will be retained once at the tumor.
While the in vivo results of Epstein and colleagues (2003) are promising, the TNT-3 antibody recognizes DNA released from dead cells, and as such is not ideal for the exploitation of the pronecrotic activity of IFN-γ on live tumor cells. To direct IFN-γ to RCC cells, we are currently developing IFN-γ immunocytokines targeting the membrane-bound TNF family member CD70. CD70 is expressed on ∼100% of metastatic ccRCC samples and RCC cell lines tested, but is mostly undetectable in normal kidney tissue and highly restricted in its expression in other normal tissues (Diegmann and others 2005; Junker and others 2005; Law and others 2006; Boursalian and others 2009). We expect such IFN-γ immunocytokines to provide selective accumulation of IFN-γ at RCC sites, a necessary step for effective pronecrotic interactions with bortezomib and other agents. Besides direct exploitation of the pronecrotic activity of IFN-γ, additional therapeutic benefits from the use of IFN-γ immunocytokines can be expected from the ability of these fusions to actively interact with the host immune system, both through the action of IFN-γ and from the intrinsic ability of the antibody molecule to direct the action of immune effector cells (such as Natural Killer cells), specifically to the tumor (Weiner and others 2010).
Concluding Remarks
Although the biotherapeutic approach described here is focused on the use of IFN-γ, the long-time RCC therapeutic IFN-α also induces cell death when survival signals—including NF-κB—are absent (Maher and others 2007; Pfeffer 2011). The biotherapeutic strategies outlined in this review therefore have ramifications beyond IFN-γ and RCC, and are broadly applicable to any of the aggressive cancers (eg, malignant melanoma and AIDS-associated lymphomas) in which IFNs have shown promise (Borden and others 2007). Key areas for future research include (1) determining how IFN-γ activates NF-κB to find new targets for combinatorial use with IFN-γ; (2) utilizing bioinformatics and whole-genome RNAi approaches to identify further molecular determinants of IFN-induced necrosis; and (3) employing similar screening strategies to delineate additional resistance pathways in RCC. We also suggest that determining how NF-κB specifically mediates cell survival (ie, identifying druggable prosurvival targets of NF-κB) is essential to the development of next-generation NF-κB-targeted antitumor agents. Such agents, by selectively blocking NF-κB prosurvival signaling without affecting other NF-κB functions, will be expected to minimize toxicity issues impacting current NF-κB inhibitors.
Acknowledgments
Work in S.B.'s laboratory is supported by an ACS Research Scholar Grant (RSG-09-195-01-MPC). G.P.A. is supported by NIH 1 R01 CA118159. Additional funds were provided by the Fox Chase Cancer Center via institutional support of the Kidney Cancer Keystone Program to S.B. and G.P.A. We thank Kerry Campbell, Christoph Seeger, and Amer Beg for critically reviewing this manuscript.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Abbruzzese JL. Levin B. Ajani JA. Faintuch JS. Pazdur R. Saks S. Edwards C. Gutterman JU. A phase II trial of recombinant human interferon-gamma and recombinant tumor necrosis factor in patients with advanced gastrointestinal malignancies: results of a trial terminated by excessive toxicity. J Biol Response Mod. 1990;9(5):522–527. [PubMed] [Google Scholar]
- An J. Fisher M. Rettig MB. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-kappaB-dependent mechanism. Oncogene. 2005;24(9):1563–1570. doi: 10.1038/sj.onc.1208348. [DOI] [PubMed] [Google Scholar]
- An J. Rettig MB. Mechanism of von Hippel-Lindau protein-mediated suppression of nuclear factor kappa B activity. Mol Cell Biol. 2005;25(17):7546–7556. doi: 10.1128/MCB.25.17.7546-7556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. Rettig MB. Epidermal growth factor receptor inhibition sensitizes renal cell carcinoma cells to the cytotoxic effects of bortezomib. Mol Cancer Ther. 2007;6(1):61–69. doi: 10.1158/1535-7163.MCT-06-0255. [DOI] [PubMed] [Google Scholar]
- An J. Sun Y. Fisher M. Rettig MB. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol Cancer Ther. 2004;3(6):727–736. [PubMed] [Google Scholar]
- Arai R. Ueda H. Kitayama A. Kamiya N. Nagamune T. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14(8):529–532. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- Aulitzky W. Gastl G. Aulitzky WE. Herold M. Kemmler J. Mull B. Frick J. Huber C. Successful treatment of metastatic renal cell carcinoma with a biologically active dose of recombinant interferon-gamma. J Clin Oncol. 1989;7(12):1875–1884. doi: 10.1200/JCO.1989.7.12.1875. [DOI] [PubMed] [Google Scholar]
- Balachandran S. Roberts PC. Kipperman T. Bhalla KN. Compans RW. Archer DR. Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74(3):1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S. Thomas E. Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432(7015):401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- Basagoudanavar SH. Thapa RJ. Nogusa S. Wang J. Beg AA. Balachandran S. Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. J Virol. 2011;85(6):2599–2610. doi: 10.1128/JVI.02213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V. Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA. Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y. Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Bennett CL. Vogelzang NJ. Ratain MJ. Reich SD. Hyponatremia and other toxic effects during a phase I trial of recombinant human gamma interferon and vinblastine. Cancer Treat Rep. 1986;70(9):1081–1084. [PubMed] [Google Scholar]
- Borden EC. Sen GC. Uze G. Silverman RH. Ransohoff RM. Foster GR. Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6(12):975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ. Swennen EL. Di Virgilio F. Cronstein BN. Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Boursalian TE. McEarchern JA. Law CL. Grewal IS. Targeting CD70 for human therapeutic use. Adv Exp Med Biol. 2009;647:108–119. doi: 10.1007/978-0-387-89520-8_7. [DOI] [PubMed] [Google Scholar]
- Brooks AD. Jacobsen KM. Li W. Shanker A. Sayers TJ. Bortezomib sensitizes human renal cell carcinomas to TRAIL apoptosis through increased activation of caspase-8 in the death-inducing signaling complex. Mol Cancer Res. 2010;8(5):729–738. doi: 10.1158/1541-7786.MCR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubici C. Papa S. Pham CG. Zazzeroni F. Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21(1):69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- Chen D. Frezza M. Schmitt S. Kanwar J. Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY. Uzzo RG. Evaluation and management of the renal mass. Med Clin North Am. 2011;95(1):179–189. doi: 10.1016/j.mcna.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Chen G. Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296(5573):1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Coppin C. Porzsolt F. Awa A. Kumpf J. Coldman A. Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;(1):CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- Cvek B. Dvorak Z. The ubiquitin-proteasome system (UPS) and the mechanism of action of bortezomib. Curr Pharm Des. 2011;17(15):1483–1499. doi: 10.2174/138161211796197124. [DOI] [PubMed] [Google Scholar]
- D'Acquisto R. Markman M. Hakes T. Rubin S. Hoskins W. Lewis JL., Jr A phase I trial of intraperitoneal recombinant gamma-interferon in advanced ovarian carcinoma. J Clin Oncol. 1988;6(4):689–695. doi: 10.1200/JCO.1988.6.4.689. [DOI] [PubMed] [Google Scholar]
- Deb A. Haque SJ. Mogensen T. Silverman RH. Williams BR. RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. J Immunol. 2001;166(10):6170–6180. doi: 10.4049/jimmunol.166.10.6170. [DOI] [PubMed] [Google Scholar]
- Deiss LP. Feinstein E. Berissi H. Cohen O. Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995;9(1):15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- Diegmann J. Junker K. Gerstmayer B. Bosio A. Hindermann W. Rosenhahn J. von Eggeling F. Identification of CD70 as a diagnostic biomarker for clear cell renal cell carcinoma by gene expression profiling, real-time RT-PCR and immunohistochemistry. Eur J Cancer. 2005;41(12):1794–1801. doi: 10.1016/j.ejca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus C. Ronca R. Kaspar M. Grabulovski D. Berndt A. Kosmehl H. Zardi L. Neri D. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int J Cancer. 2005;116(2):304–313. doi: 10.1002/ijc.20952. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA. Kilbourn RG. Amato RJ. Zukiwski AA. Jones E. Logothetis CJ. Phase II trial of low dose gamma-interferon in metastatic renal cell carcinoma. J Urol. 1994;152(3):841–845. doi: 10.1016/s0022-5347(17)32587-9. [DOI] [PubMed] [Google Scholar]
- Foon KA. Sherwin SA. Abrams PG. Stevenson HC. Holmes P. Maluish AE. Oldham RK. Herberman RB. A phase I trial of recombinant gamma interferon in patients with cancer. Cancer Immunol Immunother. 1985;20(3):193–197. doi: 10.1007/BF00205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnick MB. Reich SD. Maxwell B. Coval-Goldsmith S. Richie JP. Rudnick SA. Phase I/II study of recombinant interferon gamma in advanced renal cell carcinoma. J Urol. 1988;139(2):251–255. doi: 10.1016/s0022-5347(17)42379-2. [DOI] [PubMed] [Google Scholar]
- Ghetie V. Ward ES. Transcytosis and catabolism of antibody. Immunol Res. 2002;25(2):97–113. doi: 10.1385/IR:25:2:097. [DOI] [PubMed] [Google Scholar]
- Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- Gleave ME. Elhilali M. Fradet Y. Davis I. Venner P. Saad F. Klotz LH. Moore MJ. Paton V. Bajamonde A. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N Engl J Med. 1998;338(18):1265–1271. doi: 10.1056/NEJM199804303381804. [DOI] [PubMed] [Google Scholar]
- Gordan JD. Lal P. Dondeti VR. Letrero R. Parekh KN. Oquendo CE. Greenberg RA. Flaherty KT. Rathmell WK. Keith B. Simon MC. Nathanson KL. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14(6):435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SN. Metcalf JL. Wang Y. Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS. Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hayden MS. Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hitomi J. Christofferson DE. Ng A. Yao J. Degterev A. Xavier RJ. Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115(10 Suppl):2313–2320. doi: 10.1002/cncr.24239. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Xu J. Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Jett JR. Maksymiuk AW. Su JQ. Mailliard JA. Krook JE. Tschetter LK. Kardinal CG. Twito DI. Levitt R. Gerstner JB. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12(11):2321–2326. doi: 10.1200/JCO.1994.12.11.2321. [DOI] [PubMed] [Google Scholar]
- Jones PL. Ping D. Boss JM. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol Cell Biol. 1997;17(12):6970–6981. doi: 10.1128/mcb.17.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker K. Hindermann W. von Eggeling F. Diegmann J. Haessler K. Schubert J. CD70: a new tumor specific biomarker for renal cell carcinoma. J Urol. 2005;173(6):2150–2153. doi: 10.1097/01.ju.0000158121.49085.ba. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8(11):865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- Kondo K. Kim WY. Lechpammer M. Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1(3):E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G. Akhtar M. Beckwith BJ. Bugert P. Cooper CS. Delahunt B. Eble JN. Fleming S. Ljungberg B. Medeiros LJ. Moch H. Reuter VE. Ritz E. Roos G. Schmidt D. Srigley JR. Storkel S. van den Berg E. Zbar B. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183(2):131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ. de Souza-Pinto NC. Castilho RF. Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47(4):333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kurzrock R. Rosenblum MG. Sherwin SA. Rios A. Talpaz M. Quesada JR. Gutterman JU. Pharmacokinetics, single-dose tolerance, and biological activity of recombinant gamma-interferon in cancer patients. Cancer Res. 1985;45(6):2866–2872. [PubMed] [Google Scholar]
- Lane HC. Davey RT., Jr. Sherwin SA. Masur H. Rook AH. Manischewitz JF. Quinnan GV. Smith PD. Easter ME. Fauci AS. A phase I trial of recombinant human interferon-gamma in patients with Kaposi's sarcoma and the acquired immunodeficiency syndrome (AIDS) J Clin Immunol. 1989;9(4):351–361. doi: 10.1007/BF00918667. [DOI] [PubMed] [Google Scholar]
- Law CL. Gordon KA. Toki BE. Yamane AK. Hering MA. Cerveny CG. Petroziello JM. Ryan MC. Smith L. Simon R. Sauter G. Oflazoglu E. Doronina SO. Meyer DL. Francisco JA. Carter P. Senter PD. Copland JA. Wood CG. Wahl AF. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res. 2006;66(4):2328–2337. doi: 10.1158/0008-5472.CAN-05-2883. [DOI] [PubMed] [Google Scholar]
- Linehan WM. Srinivasan R. Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7(5):277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B. Campbell SC. Choi HY. Jacqmin D. Lee JE. Weikert S. Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Maher SG. Romero-Weaver AL. Scarzello AJ. Gamero AM. Interferon: cellular executioner or white knight? Curr Med Chem. 2007;14(12):1279–1289. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- Mariathasan S. Weiss DS. Newton K. McBride J. O'Rourke K. Roose-Girma M. Lee WP. Weinrauch Y. Monack DM. Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Mathupala SP. Ko YH. Pedersen PL. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim Biophys Acta. 2010;1797(6–7):1225–1230. doi: 10.1016/j.bbabio.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH. Maher SG. Young HA. Clinical use of interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami MM. Hu P. Khawli LA. Li J. Epstein AL. Chimeric TNT-3 antibody/murine interferon-gamma fusion protein for the immunotherapy of solid malignancies. Hybrid Hybridomics. 2003;22(4):197–207. doi: 10.1089/153685903322328929. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R. Rodriguez-Enriquez S. Saavedra E. Marin-Hernandez A. Gallardo-Perez JC. The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors. 2009;35(2):209–225. doi: 10.1002/biof.31. [DOI] [PubMed] [Google Scholar]
- Motzer RJ. Bacik J. Murphy BA. Russo P. Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- MRCCC. Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Medical Research Council Renal Cancer Collaborators. Lancet. 1999;353(9146):14–17. [PubMed] [Google Scholar]
- Muss HB. Caponera M. Zekan PJ. Jackson DV., Jr. Stuart JJ. Richards F. Cooper MR. Levin EA. Reich SD. Capizzi RL. Recombinant gamma interferon in advanced breast cancer: a phase II trial. Invest New Drugs. 1986;4(4):377–381. doi: 10.1007/BF00173511. [DOI] [PubMed] [Google Scholar]
- Nyhan MJ. O'Sullivan GC. McKenna SL. Role of the VHL (von Hippel-Lindau) gene in renal cancer: a multifunctional tumour suppressor. Biochem Soc Trans. 2008;36(Pt 3):472–478. doi: 10.1042/BST0360472. [DOI] [PubMed] [Google Scholar]
- Otto U. Conrad S. Schneider AW. Klosterhalfen H. Recombinant interferon gamma in the treatment of metastatic renal cell carcinoma. Results of a phase II trial. Arzneimittelforschung. 1988;38(11):1658–1660. [PubMed] [Google Scholar]
- Pal SK. Figlin RA. Targeted therapies for renal cell carcinoma: understanding their impact on survival. Target Oncol. 2010;5(2):131–138. doi: 10.1007/s11523-010-0145-6. [DOI] [PubMed] [Google Scholar]
- Pasche N. Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–590. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Pfeffer LM. The role of nuclear factor kappaB in the interferon response. J Interferon Cytokine Res. 2011;31(7):553–559. doi: 10.1089/jir.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CG. Bubici C. Zazzeroni F. Papa S. Jones J. Alvarez K. Jayawardena S. De Smaele E. Cong R. Beaumont C. Torti FM. Torti SV. Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119(4):529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Pirrotta MT. Bernardeschi P. Fiorentini G. Targeted-therapy in advanced renal cell carcinoma. Curr Med Chem. 2011;18(11):1651–1657. doi: 10.2174/092986711795471293. [DOI] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Quesada JR. Kurzrock R. Sherwin SA. Gutterman JU. Phase II studies of recombinant human interferon gamma in metastatic renal cell carcinoma. J Biol Response Mod. 1987;6(1):20–27. [PubMed] [Google Scholar]
- Ramsay EE. Hogg PJ. Dilda PJ. Mitochondrial metabolism inhibitors for cancer therapy. Pharm Res. 2011;28(11):2731–2744. doi: 10.1007/s11095-011-0584-5. [DOI] [PubMed] [Google Scholar]
- Rinehart JJ. Malspeis L. Young D. Neidhart JA. Phase I/II trial of human recombinant interferon gamma in renal cell carcinoma. J Biol Response Mod. 1986;5(4):300–308. [PubMed] [Google Scholar]
- Rodriguez-Enriquez S. Gallardo-Perez JC. Marin-Hernandez A. Aguilar-Ponce JL. Mandujano-Tinoco EA. Meneses A. Moreno-Sanchez R. Oxidative phosphorylation as a target to arrest malignant neoplasias. Curr Med Chem. 2011;18(21):3156–3167. doi: 10.2174/092986711796391561. [DOI] [PubMed] [Google Scholar]
- Roos FC. Roberts AM. Hwang II. Moriyama EH. Evans AJ. Sybingco S. Watson IR. Carneiro LA. Gedye C. Girardin SE. Ailles LE. Jewett MA. Milosevic M. Wilson BC. Bell JC. Der SD. Ohh M. Oncolytic targeting of renal cell carcinoma via encephalomyocarditis virus. EMBO Mol Med. 2010;2(7):275–288. doi: 10.1002/emmm.201000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon S. Xue X. Takekawa M. Sasazuki T. Okazaki T. Kojima Y. Piao JH. Yagita H. Okumura K. Doi T. Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22(15):3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker A. Brooks AD. Tristan CA. Wine JW. Elliott PJ. Yagita H. Takeda K. Smyth MJ. Murphy WJ. Sayers TJ. Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst. 2008;100(9):649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi J. Khawli LA. Hu P. Li J. Epstein AL. Generation of human interferon gamma and tumor Necrosis factor alpha chimeric TNT-3 fusion proteins. Hybrid Hybridomics. 2002;21(6):421–432. doi: 10.1089/153685902321043954. [DOI] [PubMed] [Google Scholar]
- Stark GR. Kerr IM. Williams BR. Silverman RH. Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Storkel S. Eble JN. Adlakha K. Amin M. Blute ML. Bostwick DG. Darson M. Delahunt B. Iczkowski K. Classification of renal cell carcinoma: Workgroup No 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80(5):987–989. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sufan RI. Jewett MA. Ohh M. The role of von Hippel-Lindau tumor suppressor protein and hypoxia in renal clear cell carcinoma. Am J Physiol Renal Physiol. 2004;287(1):F1–F6. doi: 10.1152/ajprenal.00424.2003. [DOI] [PubMed] [Google Scholar]
- Sun L. Wang H. Wang Z. He S. Chen S. Liao D. Wang L. Yan J. Liu W. Lei X. Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148(1–2):213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14(1):111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- Thapa RJ. Basagoudanavar SH. Nogusa S. Irrinki K. Mallilankaraman K. Slifker MJ. Beg AA. Madesh M. Balachandran S. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol. 2011;31(14):2934–2946. doi: 10.1128/MCB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2(56):pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- Vadhan-Raj S. Al-Katib A. Bhalla R. Pelus L. Nathan CF. Sherwin SA. Oettgen HF. Krown SE. Phase I trial of recombinant interferon gamma in cancer patients. J Clin Oncol. 1986;4(2):137–146. doi: 10.1200/JCO.1986.4.2.137. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P. Galluzzi L. Vanden Berghe T. Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Wang Z. Jiang H. Chen S. Du F. Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1–2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Weiner LM. Surana R. Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10(5):317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MP. Immunotherapy for metastatic renal cell carcinoma. Urol Clin North Am. 1993;20(2):283–295. [PubMed] [Google Scholar]
- Xiang J. Qi Y. Cook D. Moyana T. Targeting gamma interferon to tumor cells by a genetically engineered fusion protein secreted from myeloma cells. Hum Antibodies Hybridomas. 1996;7(1):2–10. [PubMed] [Google Scholar]
- Xiang J. Qi Y. Luo X. Liu E. Recombinant bifunctional molecule FV/IFN-gamma possesses the anti-tumor FV as well as the gamma interferon activities. Cancer Biother. 1993;8(4):327–337. doi: 10.1089/cbr.1993.8.327. [DOI] [PubMed] [Google Scholar]
- Yang H. Minamishima YA. Yan Q. Schlisio S. Ebert BL. Zhang X. Zhang L. Kim WY. Olumi AF. Kaelin WG., Jr pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28(1):15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T. Okazaki N. Yoshino M. Okhura H. Shimada Y. Phase II trial of high dose recombinant gamma-interferon in advanced hepatocellular carcinoma. Eur J Cancer. 1990;26(4):545–546. doi: 10.1016/0277-5379(90)90040-z. [DOI] [PubMed] [Google Scholar]
- Younes HM. Amsden BG. Interferon-gamma therapy: evaluation of routes of administration and delivery systems. J Pharm Sci. 2002;91(1):2–17. doi: 10.1002/jps.10007. [DOI] [PubMed] [Google Scholar]
- Zhang DW. Shao J. Lin J. Zhang N. Lu BJ. Lin SC. Dong MQ. Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhao J. Jitkaew S. Cai Z. Choksi S. Li Q. Luo J. Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]