Abstract
Propionibacterium acnes is a key pathogen involved in the progression of acne inflammation. The development of a new agent possessing antimicrobial and anti-inflammatory activity against P. acnes is therefore of interest. In this study, we investigated the inhibitory effect of rosemary (Rosmarinus officinalis) extract on P. acnes–induced inflammation in vitro and in vivo. The results showed that ethanolic rosemary extract (ERE) significantly suppressed the secretion and mRNA expression of proinflammatory cytokines, including interleukin (IL)-8, IL-1β, and tumor necrosis factor-α in P. acnes–stimulated monocytic THP-1 cells. In an in vivo mouse model, concomitant intradermal injection of ERE attenuated the P. acnes–induced ear swelling and granulomatous inflammation. Since ERE suppressed the P. acnes–induced nuclear factor kappa-B (NF-κB) activation and mRNA expression of Toll-like receptor (TLR) 2, the suppressive effect of ERE might be due, at least partially, to diminished NF-κB activation and TLR2-mediated signaling pathways. Furthermore, three major constituents of ERE, carnosol, carnosic acid, and rosmarinic acid, exerted different immumodulatory activities in vitro. In brief, rosmarinic acid significantly suppressed IL-8 production, while the other two compounds inhibited IL-1β production. Further study is needed to explore the role of bioactive compounds of rosemary in mitigation of P. acnes–induced inflammation.
Key Words: anti-inflammation, NF-κB, Propionibacterium acnes, rosemary, TLR2
Introduction
Propionibacterium acnes, a Gram-positive anaerobic bacterium, is part of the normal human skin flora and is involved in inflammatory diseases, such as acne vulgaris,1 endocarditis,2 and granulomatous hepatitis.3,4 Several lines of evidence clearly reveal that P. acnes is responsible for the local inflammatory response of acne. After sebum production, P. acnes colonizes sebaceous follicles and releases lipase and proinflammatory mediators. P. acnes has been shown to induce immune cells to secrete several proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-8, and IL-1β, that are important for the development of skin inflammation.5 Previous studies demonstrated that monocytes, macrophages, sebocytes, and keratinocytes were stimulated by P. acnes via a Toll-like receptor (TLR)2–mediated nuclear factor kappa-B (NF-κB) signaling pathway.6–8 Since P. acnes is pivotal in the inflammatory process, suppression of P. acnes–induced inflammation is one of critical strategies for the treatment of acne vulgaris.
Traditional phytomedicine plays an adjuvant role in acne therapy. Oral and externally used dermatological preparations employing herbal extracts have been developed for the therapy of acne vulgaris.9 Oregon grape root, tea tree oil, and Saccharomyces may have potential as herbal therapeutics for acne.10 The methanolic extracts of duzhong (Eucommia ulmoides Oliv.) and yerba mate (Ilex paraguariensis) exhibited potent in vitro antimicrobial and anti-inflammatory activities against P. acnes.11 Recently, we reported that the ethyl acetate extract of wild bitter melon fruit attenuates P. acnes–induced inflammation.12
Rosemary (Rosmarinus officinalis L.), a well-known culinary spice, is an herbal remedy with demonstrated antioxidant, anti-inflammatory, anticarcinogenic, anti-antimicrobial, and various other health benefits.13–15 Several studies have demonstrated that rosemary extract or its components inhibit TLR4-mediated inflammatory responses stimulated by lipopolysaccharide (LPS).14,15 Therefore, we hypothesized that rosemary extract may inhibit P. acnes–induced inflammation through the modulation of TLR2-mediated signaling pathways. To test this hypothesis, we examined the inhibitory effect of ethanolic rosemary extract (ERE) on P. acnes–induced inflammation in vitro and in vivo, and explored the molecular mechanism of ERE in attenuating inflammatory responses. We also determined whether three major components in ERE, carnosol, carnosic acid, and rosmarinic acid, exerted the in vitro anti-inflammatory effect. Thus, this study provides new insights into the possible role of rosemary extract and its bioactive components in modulation of the inflammatory response induced by P. acnes.
Materials and Methods
Materials
The strain of P. acnes (BCRC10723, isolated from facial acne) was obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). P. acnes was cultured in brain heart infusion (BHI) broth (Difco, Detroit, MI, USA) with 1% glucose in an anaerobic atmosphere using the BBL GasPak system (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA). The human monocytic THP-1 cell line (BCRC 60430) was also obtained from the Bioresource Collection and Research Center. Cells were maintained in RPMI 1640 (Gibco, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a humidified atmosphere with 5% CO2.
The assay kits for TNF-α, IL-8, and IL-1β were purchased from Invitrogen (Carlsbad, CA, USA). An NF-κB/p65 ActivELISA kit was purchased from Imgenex (San Diego, CA, USA). Carnosol and carnosic acid were purchased from Sigma (St. Louis, MO, USA). Rosmarinic acid was purchased from Extrasynthese (Genay, France). All chemicals are of analytical-grade purity.
Preparation of ERE
Dried rosemary leaves were purchased from Tomax Enterprise Co. (Taipei, Taiwan). Ten grams of finely ground rosemary was extracted with 100 mL of ethanol at room temperature for 4 h. After extraction, the mixture was filtered, and the residue was re-extracted with 100 mL of fresh ethanol overnight. After centrifugation at 12,000 g for 10 min, the combined ethanol solution was collected and evaporated in a rotary evaporator. The ethanol extract was reconstituted in dimethyl sulfoxide (DMSO) to a concentration of 400 mg/mL for the subsequent experiments.
Determination of the viability of THP-1 cells
Alamar blue (Invitrogen) is designed to quantitatively measure the proliferation of various adherent or suspended cells. A THP-1 suspension of cells (1×106 cells/mL) was cultured in 96-well culture plates with various concentrations of ERE. After 24 h of incubation, 20 μL of alamar blue reagent was added to each well. After 2 h of incubation, the optical density of the resulting medium was measured, and the difference in the absorbance values at 570 and 600 nm was calculated using a Synergy HT multidetection microplate reader (Bio-Tek, Carson City, NV, USA).
Measurement of cytokine production in human monocytic THP-1 cells
To prepare the P. acnes suspension for the sequential stimulation of cells, the log-phase bacterial P. acnes culture was harvested, washed with phosphate-buffered saline (PBS), and then centrifuged at 10,000 g for 5 min. After two additional washes in PBS, the P. acnes pellet was resuspended in RPMI medium. Human monocytic THP-1 cells were seeded at 1×106 cells/mL in 24-well plates with serum-free medium, and were treated with tested samples alone or stimulated with live P. acnes (wet weight 200 μg/mL) alone or in combination with different concentrations of tested samples for 24-h incubation. Cell-free supernatants were collected, and concentrations of TNF-α, IL-1β, and IL-8 were analyzed with respective enzyme immunoassay kits.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated with the TRIzol reagent (Invitrogen), according to the manufacturer's instructions. Complementary DNA was generated from 2 μg of total RNA, with the oligo (dT) primer and 1 μL of reverse transcriptase (Promega, Madison, WI, USA). We used the 5′-AAG CTG AGG AAG ATG CTG-3′ and 5′-ATC TAC ACT CTC CAG CTG-3′ primers for IL-1β, the 5′-TGC CAA GGA GTG CTA AAG-3′ and 5′-CTC CAC AAC CCT CTG CAC-3′ primers for IL-8, the 5 ′-TCT TCT GCC TGC ACT TTG G-3′ and 5′-ATC TCT CAG CTC CAC GCC ATT G-3′ primers for TNF-α, the 5′-TCT CCC ATT TCC GTC TTT TT-3′ and 5′-GGT CTT GGT GTT CAT TAT CTT C-3′ primers for TLR2, and the 5′-GTG AAG GTC GGA GTC AAC G-3′ and 5′-TGA GGT CAA TGA AGG GGT C-3′ primers for GAPDH. The primers amplified a 157 bp fragment of the IL-8 cDNA, a 300 bp fragment of the IL-1β cDNA, a 224 bp fragment of the TNF-α cDNA, a 125 bp fragment of TLR2 cDNA, and a 113 bp fragment of the GAPDH cDNA. Real-time polymerase chain reactions (PCRs) were conducted in an iCycler iQ Real-Time detection system (Bio-Rad, Hercules, CA, USA) using iQ™ SYBR Green Supermix (Bio-Rad). Thermal cycling conditions for all assays were initial denaturation at 95°C for 3 min and 40 cycles at 95°C for 10 s and 55°C for 30 s. Melting analysis was performed by denaturing at 95°C for 1 min and cooling to 55°C for 1 min followed by heating at the rate of 0.5°C/s from 55°C to 95°C. The melting curve of each tube was examined to confirm the appearance of a single peak. The relative amounts of the PCR products were analyzed by iQ™5 optical system software, vers. 2.1. The messenger (m)RNA level of each sample for each gene was normalized to that of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Effect of ERE on P. acnes–induced inflammation in vivo
Eight-week-old male ICR mice were purchased from the Animal Center of College of Medicine, National Taiwan University, Taipei, Taiwan. All animal experiments were approved by the Animal Care Committee of the National Taiwan Normal University. Anti-inflammatory activity of ERE was then evaluated using the following procedure described by Nakatsuji et al.16 with a minor modification. In the preliminary test, intradermal injection of ERE (up to 1 mg/10 μL) did not cause any visible adverse reaction. Therefore, an administered dosage of 1 mg/10 μL was used for the following experiments. P. acnes (6×107 CFU per 10 μL in PBS) was intradermally injected into the right ear of ICR mice. Left ears received an equal amount (10 μL) of PBS (n=5). Ten microliters of ERE (1 mg) in 5% DMSO in PBS was injected into the same location of both ears right after P. acnes or PBS injection (n=5). Twenty-four hours after bacterial injection, the increase in ear thickness was measured using a microcaliper (Mitutoyo, Kanagawa, Japan). Mice were then sacrificed with carbon dioxide asphyxiation and ear disks of 4.0 mm diameter were punched out and weighed. The extent of edema was evaluated by the weight difference between the left and the right ear disc. The increase in ear thickness and weight of the P. acnes–injected ear was calculated and expressed as a percentage of the PBS-injected control. For histological observation, the paraffin embedded ears were vertically cut into cross-sections through ear with central cartilage. The cross-sections were stained with hematoxylin and eosin and then viewed on a microscope for the evaluation of cell-mediated inflammatory response.
NF-κB activation assay
NF-κB activation was analyzed using an NF-κB/p65 ActivELISA kit (Imgenex). The kit can detect and quantify the nuclear-translocated p65 subunit. To determine the effect of ERE on P. acnes–induced activation of NF-κB in THP-1 cells, human monocytic THP-1 cells (3×106 cells/mL) cultured in serum-free medium were stimulated with 200 μg/mL of P. acnes alone or in combination with the indicated concentrations of EREs (50, 100, and 200 μg/mL). The incubation times were 8 and 16 h, respectively. Cytoplasmic and nuclear extracts were prepared according to the manufacturer's instructions. Briefly, the cytoplasmic fraction was collected in the supernatant of whole-cell lysates after centrifugation at 12,000 g for 30 s at 4°C. The nuclear pellet was resuspended in 100 μL nuclear lysis buffer at 4°C for 30 min, and the suspension was centrifuged at 12,000 g for 10 min at 4°C. The supernatant containing the nuclear fraction was subjected to an enzyme-linked immunosorbent assay (ELISA) using specific anti-NF-κB antibodies, according to the manufacturer's instructions. The absorbance was read at 405 nm using a Synergy HT multidetection microplate reader.
Characterization of phenolic compounds in ERE
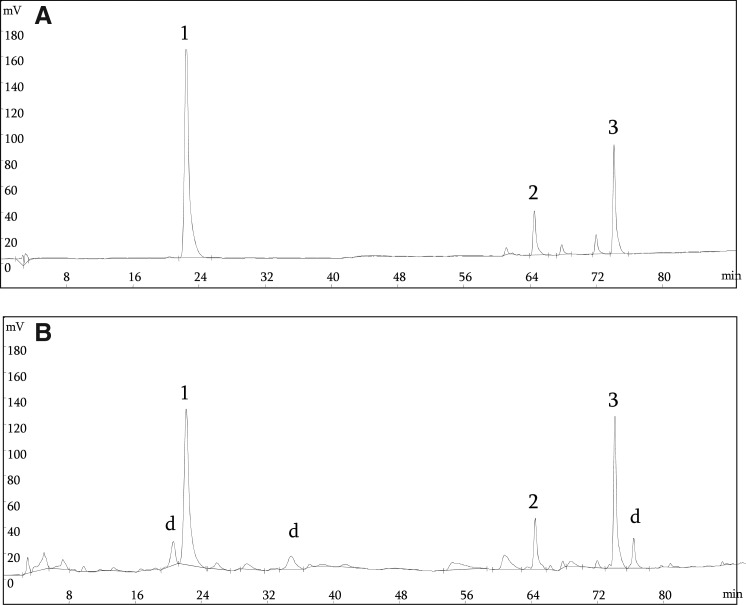
ERE (0.1 g) was dissolved in 10 mL of methanol, and passed through a 0.2-μm membrane filter (Millipore, Billerica, MA, USA). HPLC analysis was used to separate and determine individual phenolic compounds in ERE. HPLC analysis was performed using HPLC pumps (Ecom LCP 4100) equipped with a UV detector (Ecom LCD 2084) and chromatographic separations were performed on a C-18 reversed-phase silica Bondclone column (3.9 mm×300 mm, i.d. 10 μm; Phenomenex, Torrance, CA, USA). Chromatographic processing was done using the Peak-ABC Chromatography Data Handling System. The composition of solvents and used gradient elution conditions were described previously by Cuvelier et al.17 with some modifications. The mobile phase was a mixture of solvent A (acetonitrile/water/acetic acid, 15:80:0.85), and solvent B (methanol) according to a linear gradient elution from 0% B to 100% B during 90 min, at a flow-rate of 1 mL/min. The following gradient was used, 0% B for 30 min; 0–30% B for 10 min; 30–45% B for 10 min; 45–100% B for 40 min. The absorbance of elutes was measured at 284 nm, near the maximum absorption of most phenols. The injection volume was 20 μL and all samples were analyzed in triplicate. Identification of the individual compounds was based on the comparison of the retention times of unknown peaks to those of reference authentic standards.
Statistical analysis
All data are presented as the mean±standard deviation. Statistical analyses were performed using the SPSS 17.0 statistical package (Chicago, IL, USA). Student's t-test was used to compare differences between the DMSO vehicle and rosemary treatments. A P value of <.05 was considered statistically significant.
Results
Effects of ERE on inflammatory cytokine induction by P. acnes in vitro
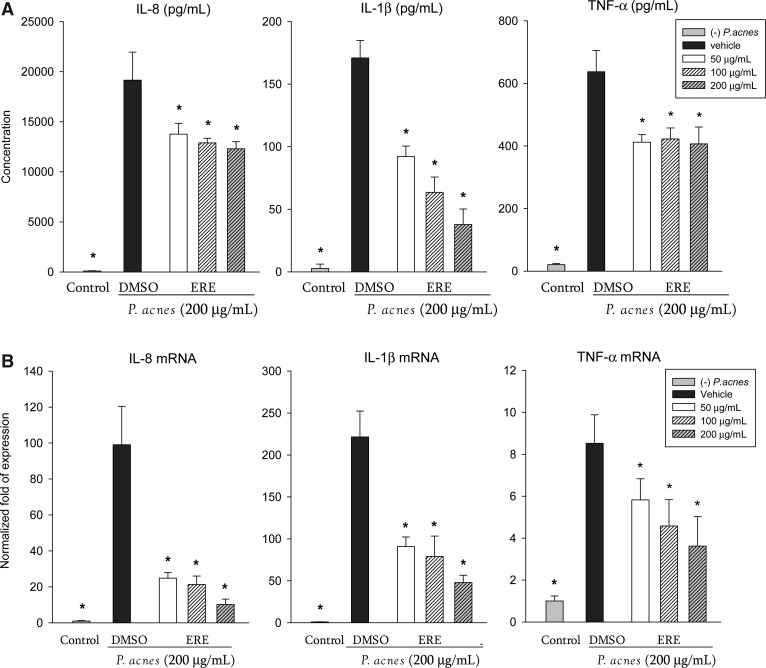
ERE (up to 200 μg/mL) exhibited no significant cytotoxicity in THP-1 cells, and did not affect the secretion of IL-8, IL-1β or TNF-α by THP-1 cells in the absence of P. acnes (data not shown). After treatment with P. acnes, the secretion of proinflammatory cytokines of THP-1 cells significantly increased (Fig. 1A). Treatment with ERE significantly suppressed the P. acnes–induced IL-8, IL-1β and TNF-α productions (Fig. 1A). We further analyzed the mRNA levels of proinflammatory cytokines by quantitative real-time PCR. ERE suppressed the gene expression of IL-8, IL-1β, and TNF-α in P. acnes–stimulated THP-1 cells (Fig. 1B). These data indicate that ERE attenuates the expression of these proinflammatory cytokines, at least partially, at the transcriptional level.
FIG. 1.
Effects of ethanolic rosemary extract (ERE) on production (A) and mRNA expression (B) of pro-inflammatory cytokines in Propionibacterium acnes–stimulated monocytic THP-1 cells. (A) Cells were co-incubated with dimethyl sulfoxide (DMSO; as vehicle) or the indicated concentration of ERE and viable P. acnes for 24 h. A control experiment without P. acnes treatment was done in parallel. The culture supernatants were subsequently isolated and analyzed for cytokine production. (B) The expression level of mRNA was determined using quantitative real-time polymerase chain reaction. The expression of cytokine mRNA was normalized to GAPDH mRNA and expressed as multiples of change with untreated THP-1 cells as the control. Each column shows the mean±standard deviation (SD) of three independent experiments with triplicate wells in each. *Significant difference from vehicle (P. acnes alone) at P<.05 by Student's t-test.
Effect of ERE on P. acnes–induced inflammation in vivo
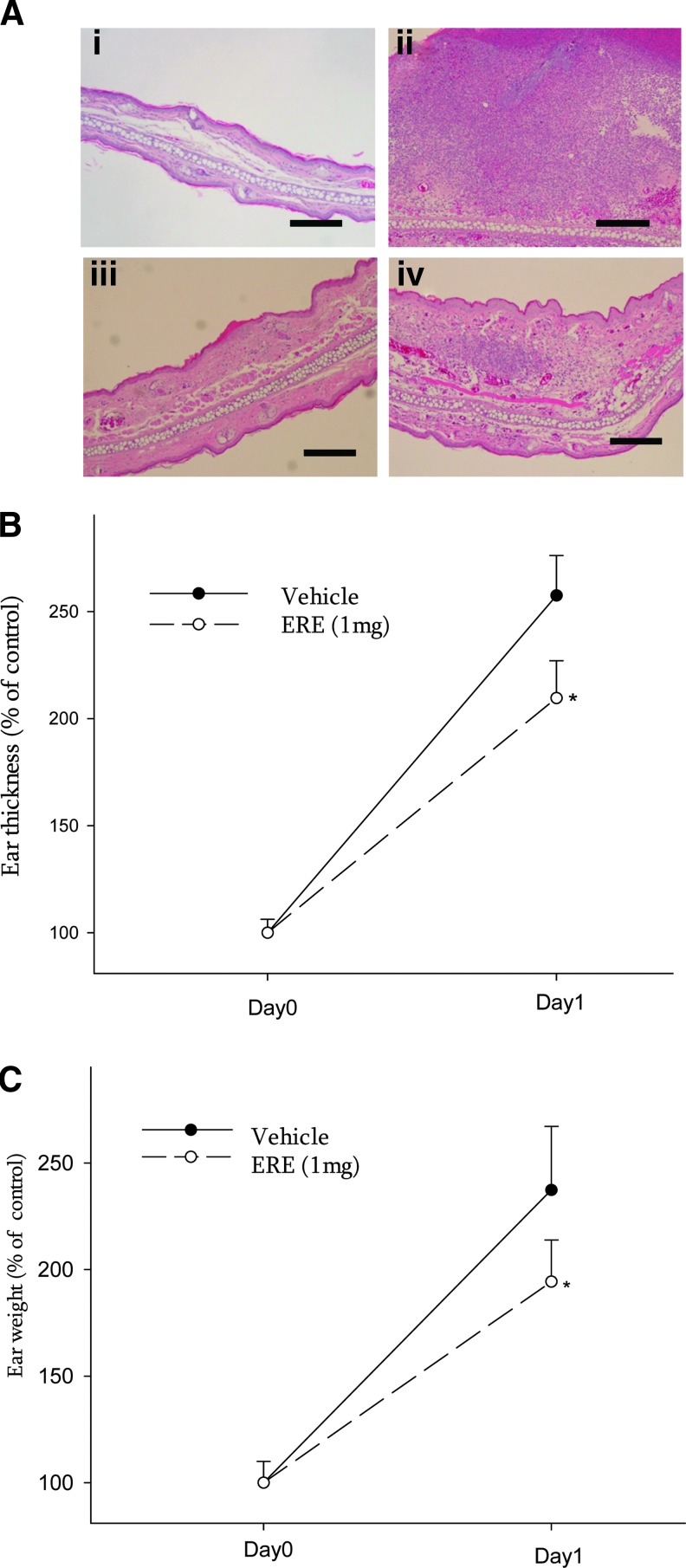
Before the determination of the anti-inflammatory effect of ERE in vivo, an intradermal injection test was performed to evaluate the skin irritation effect of ERE. Intradermal administration of ERE (1 mg/10 μL) alone showed no apparent irritation, such as ear swelling, redness, and cutaneous erythema (data not shown), and no increase in neutrophil infiltration (Fig. 2A-iii). To examine the in vivo anti-inflammatory effect of ERE, mouse ears were intradermally injected with viable P. acnes for 1 day. Coinjection of ERE (1 mg/10 μL) attenuated the granulomatous response to P. acnes as compared to coinjection with an equal amount of vehicle (Fig. 2A-ii, iv). In addition, coinjection of ERE significantly reduced P. acnes–induced ear swelling measured by ear thickness (Fig. 2B) and ear biopsy weight (Fig. 2C).
FIG. 2.
Inhibitory effect of ERE (1 mg) on P. acnes–induced inflammation. (A) Ear biopsies of ICR mice which were intradermally injected with PBS vehicle (i), P. acnes (ii), ERE (iii), or ERE+P. acnes (iv) were observed after hematoxylin and eosin staining. Scale bars represent 200 μm. The inhibitory effects of ERE on P. acnes–induced ear edema in mice were evaluated by measuring the ear thickness (B) and ear biopsy weight (C). *Significant difference from vehicle (P. acnes alone) at P<.05 by Student's t-test. Color images available online at www.liebertpub.com/jmf
ERE inhibited NF-κB activation in P. acnes–stimulated THP-1 cells
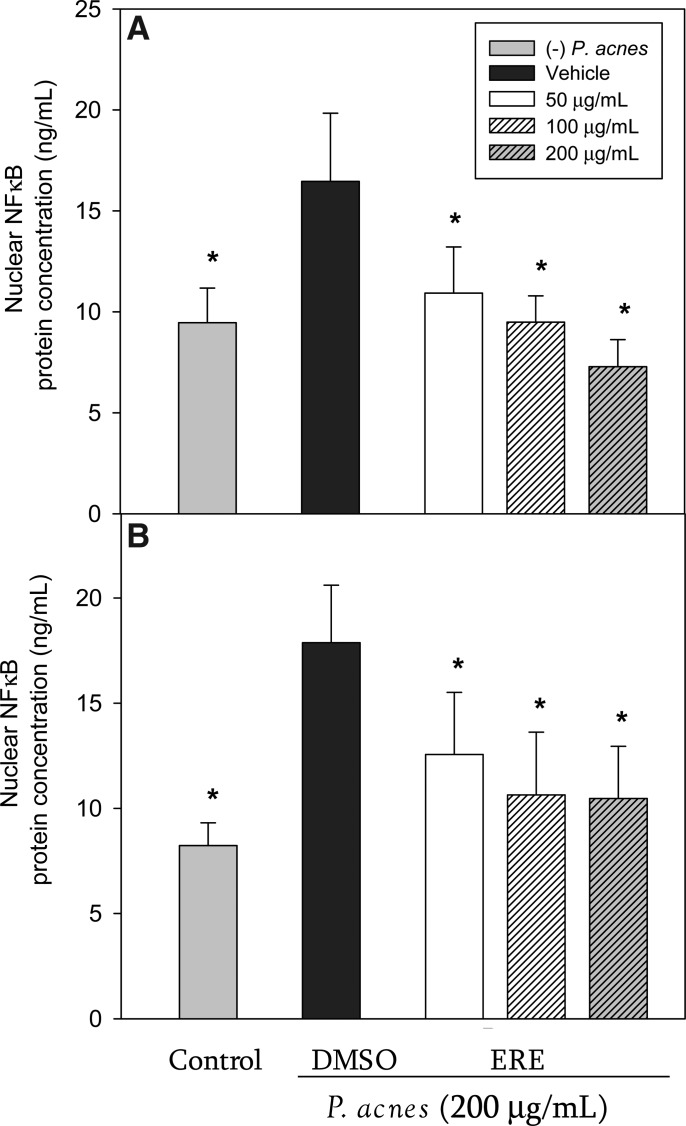
To explore the potential mechanism underlying the inhibitory effect of ERE on cytokine production, we investigated whether ERE reduced P. acnes–induced NF-κB activation. Stimulation of THP-1 cells with P. acnes resulted in a significant increase of NF-κB translocation after 8 h and 16 h of incubation (Fig. 3). However, treatment with ERE significantly attenuated the increased NF-κB translocation in P. acnes–stimulated THP-1 cells after 8 h (Fig. 3A) and 16 h (Fig. 3B) of incubation.
FIG. 3.
Effect of ERE on P. acnes–induced nuclear factor-κB (NF-κB) activation in THP-1 cells. THP-1 cells were incubated for 8 h (A) and 16 h (B) without P. acnes (control), with P. acnes alone (DMSO vehicle), and with P. acnes in the presence of the rosemary extract. Data are presented as the mean±SD. *Significant difference from vehicle (P. acnes alone) at P<.05 by Student's t-test.
ERE inhibited TLR2 mRNA overexpression in P. acnes–stimulated THP-1 cells
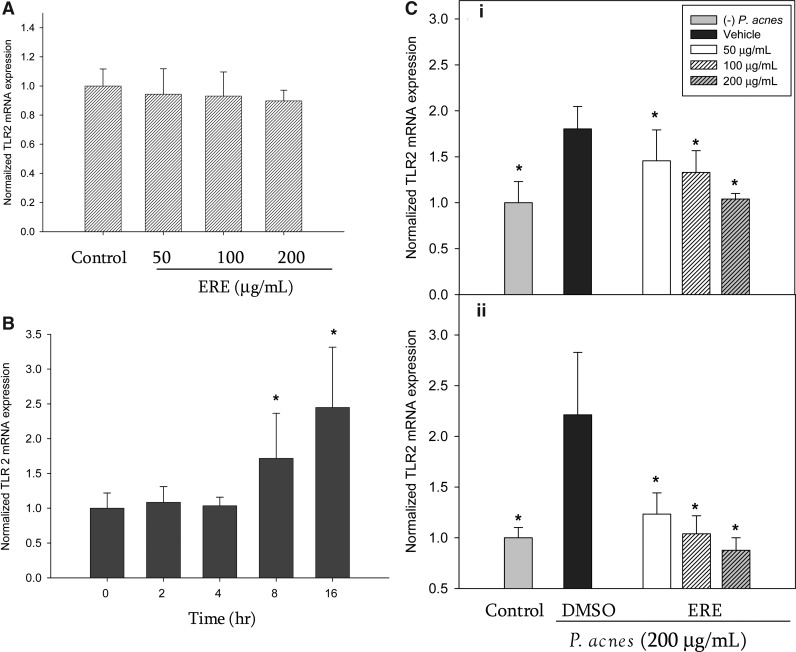
TLRs are important pattern recognition receptors for various pathogens, and are involved in the first step of the inflammatory response. We therefore, investigated TLR2 expression in THP-1 cells stimulated by P. acnes in the absence or presence of ERE. First, we examined the effect of ERE on constitutive TLR2 level of THP-1 cells. As shown in Figure 4A, in the absence of P. acnes, the ERE (up to 200 μg/mL) did not affect the constitutive gene expression of TLR2 in THP-1 cells. P. acnes caused a marked increase in the TLR2 mRNA level of THP-1 cells after either 8 or 16 h of incubation (Fig. 4B). In contrast, the ERE treatment significantly reduced the TLR2 expression in THP-1 cells stimulated with P. acnes for 8 h (Fig. 4C-i) and 16 h (Fig. 4C-ii).
FIG. 4.
Effect of ERE on toll-like receptor 2 (TLR2) mRNA expression. (A) ERE did not affect the constitutive TLR2 expression of THP-1 cells in the absence of P. acnes after 24h-incubation. (B) P. acnes induced TLR2 mRNA overexpression in THP-1 cells for 8 h and 16 h incubation. *P<.05 compared to time zero (baseline). (C) THP-1 cells were incubated for 8 h (i) and 16 h (ii) untreated with P. acnes (control), and treated with P. acnes alone (DMSO vehicle) and with P. acnes in the presence of the rosemary extract. The TLR2 mRNA expression was normalized to GAPDH mRNA and expressed as multiples of change with untreated THP-1 cells as the control. Data are presented as the mean±SD. *Significant difference from vehicle (P. acnes alone) at P<.05 by Student's t-test.
Characterization of phenolic compounds in ERE
The amounts of the three principal rosemary antioxidants found in ERE were analyzed by HPLC analyses (Fig. 5). The contents of carnosol, carnosic acid, and rosmarinic acid in ERE were 0.57, 1.98 and 0.43 mg/g dried leaves, respectively.
FIG. 5.
HPLC profiles of standard phenolic compounds (A) and phenolic compounds in ERE (B). Detection was at 284 nm. Peaks: 1, rosmarinic acid; 2, carnosol; 3, carnosic acid; d, unidentified.
Effects of ERE components on inflammatory cytokine induction by P. acnes in vitro
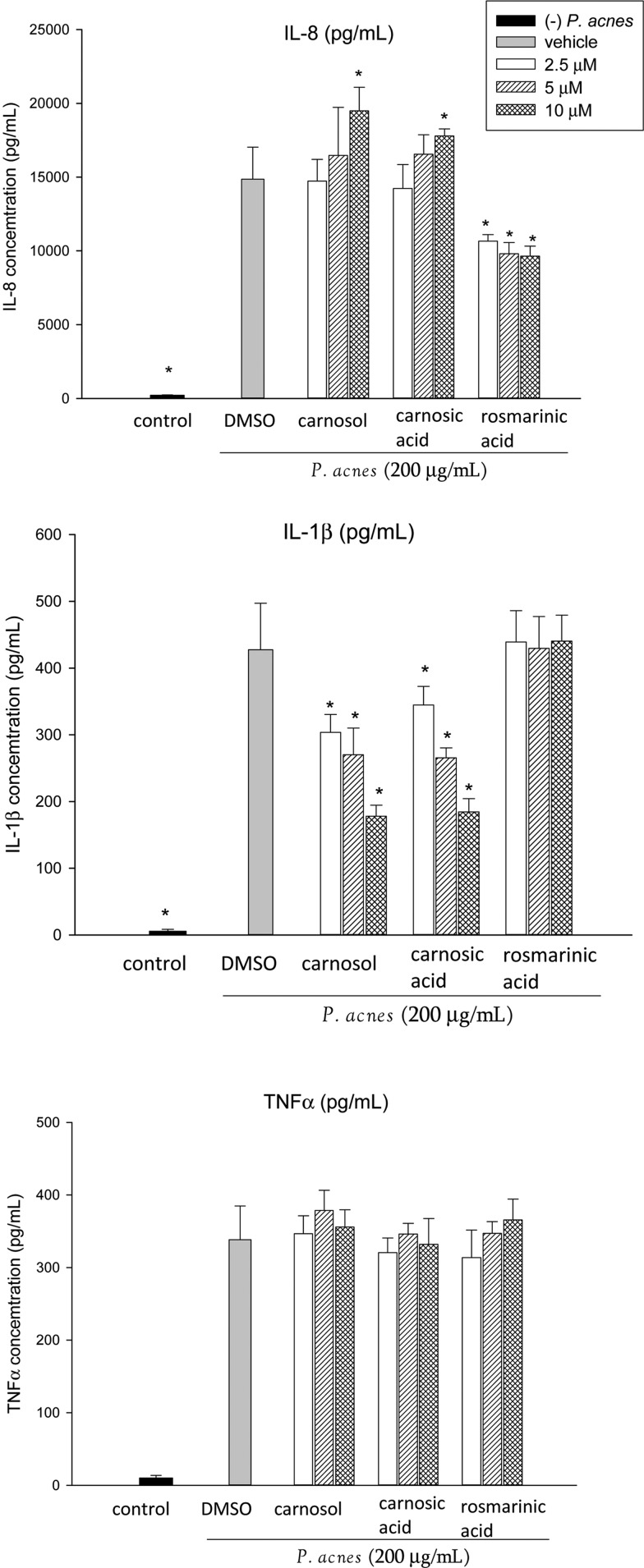
Carnosol, carnosic acid, and rosmarinic acid (up to 20 μM) exhibited no significant cytotoxicity toward THP-1 cells and did not affect the secretion of IL-8, IL-1β, or TNF-α by THP-1 cells in the absence of P. acnes (data not shown). As shown in Figure 6, rosmarinic acid significantly reduced the IL-8 production, but had no effect on the IL-1β level. Carnosol and carnosic acid significantly inhibited IL-1β levels at the concentration of 2.5 μM, but significantly increased IL-8 secretion at higher concentration (10 μM). In contrast, the three compounds (up to 10 μM) did not affect TNF-α level.
FIG. 6.
Effects of carnosol, carnosic acid, and rosmarinic acid on proinflammatory cytokine production in P. acnes–stimulated monocytic THP-1 cells. Cells were coincubated with DMSO (as vehicle) or the indicated concentration of samples and viable P. acnes for 24 h. A control experiment without P. acnes treatment was conducted in parallel. The culture supernatants were subsequently isolated and analyzed for cytokine production. Data are presented as the mean±SD. * Significant difference from vehicle (P. acnes alone) at P<.05 by Student's t-test.
Discussion
The most important findings of this study are that ERE inhibits P. acnes–stimulated production and mRNA expression of proinflammatory cytokines through the modulation of TLR2-mediated NF-κB signaling in THP-1 cells, and reduced ear edema in the mouse model, suggesting that ERE suppresses P. acnes–induced inflammation in vitro and in vivo.
Acne vulgaris is a chronic inflammatory disease involving colonization of P. acnes, and activation of immune cells with cytokines being released locally.5 In particular, IL-8 along with other P. acnes–induced chemotactic factors may play an important role in attracting neutrophils to the pilosebaceous unit.6 Recently, naturally occurring bioactive compounds in plant foods, such as carotenoids and polyphenols, have been shown to play important roles in modulating inflammatory and immunological processes. A variety of staple food plants, including cereals, fruits, vegetables, roots, and spices were used in personal care products/cosmetics.18 Among medicinal plants, rosemary extract and its components have been considered as potential therapeutic agents in cosmetic dermatology.13,19 For example, Newall and colleagues reported that a decoction of rosemary leaves has been used topically against eczema and other cutaneous diseases.20 Altinier et al.21 showed that topical treatment with methanolic rosemary extract (1 mg/cm2) modestly inhibited croton oil-induced ear edema in mice. Results depicted in Figure 2 show that the epicutaneous application of ERE (1 mg/10 μL) effectively suppressed the P. acnes–induced edematous response without significant skin irritant effect. Our results are consistent with findings that rosemary extract may be a useful therapy for cutaneous inflammatory disorders.20–22
NF-κB is a transcription factor critical for upregulation of the gene expressions of numerous cytokines.23 P. acnes induces inflammatory gene expression through induction of NF-κB transcriptional activity in human monocytes and keratinocytes.24,25 Numerous pharmacological agents used to suppress inflammation are known to inhibit NF-κB at one or multiple activation steps of the signaling pathways.23 Nicotinamide, an oral and topical anti-inflammatory agent, has been shown to downregulate the NF-κB pathway in P. acnes–stimulated keratinocytes.25 In this study, we determined whether the anti-inflammatory activity of ERE against P. acnes may be related to the modulation of NF-κB signaling and found that the ERE significantly inhibited P. acnes–induced NF-κB activation in THP-1 cells. The results presented in Figures 1 and 3 suggest that the anti-inflammatory effect of ERE may be partially attributed to the suppression of P. acnes–induced NF-κB activation.
TLR activation contributes to host inflammatory responses. TLRs are expressed by various cells of the innate immune system, such as monocytes, macrophages, and granulocytes.1 In acne, inflammation is due, in part, to the ability of P. acnes to activate TLR2 on monocytes leading to the production of inflammatory cytokines, including IL-8, IL-1β, and TNF-α.6 Hence, TLR2 may be a novel target for blocking inflammatory cytokine response in acne and other inflammatory conditions.1 Liu et al. showed that all-trans retinoic acid, a drug commonly used to treat acne vulgaris, downregulated TLR2 expression and inhibited cytokine production in P. acnes–stimulated human primary monocytes, indicating the agents inhibiting TLR expression and activation could be useful in the development of new therapeutic strategies for inflammatory diseases.26 Since the ERE caused a marked decrease in P. acnes–stimulated TLR2 mRNA expression (Fig. 4C-i, ii), the anti-inflammatory effect of ERE against P. acnes was thought to be at least partially a result of its inhibition of TLR2 expression.
Overproliferation of P. acnes is a major factor in the inflammatory reaction in acne vulgaris. P. acnes readily forms biofilms both in vitro and on medical devices in vivo.27–29 Biofilm formation of P. acnes is associated with antimicrobial agent tolerance and may be an important trigger for acne inflammation.27 Antibiotic-resistant P. acnes strains have been identified from acne patients who have undergone long-term antibiotic treatment.30 Therefore, nonantibiotic treatment should be developed as alternatives for mild to moderate cases of acne. We then examined the antimicrobial effectiveness of ERE using the broth dilution method previously described.11 We herein found that ERE exerted effective antimicrobial activity against P. acnes with a minimum inhibitory concentration of 4 mg/mL. Our results were consistent with previous reports that noted the antimicrobial activity of rosemary against P. acnes.31,32 Additionally, the inhibitory effect of ERE on preformed biofilm was also measured using a modified microtiter-plate test described by Stepanovic et al.33 The development of biofilm was determined using the crystal violet assay. Our data showed that ERE prevented cell adhesion on the polystyrene surface and inhibited preformed biofilm of P. acnes (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/jmf). Taken together, these data suggested that the antibacterial and antibiofilm activities of ERE may contribute to its anti-acne potential.
Previous studies demonstrated that the phenolic compounds found in rosemary, such as rosmarinic acid, carnosic acid, and carnosol, exhibit anti-inflammatory activities in LPS-stimulated macrophages.34–36 Rosmarinic acid has been demonstrated to inhibit LPS-induced upregulation of IL-1β, IL-6, TNF-α and suppresses expression of inducible nitric oxide synthase (iNOS).34 Carnosic acid, and carnosol also inhibit LPS-induced iNOS expression and suppress the formation of proinflammatory leukotrienes and 5-lipoxygenase.35,36 Oh et al.37 recently reported that carnosic acid strongly suppressed several skin inflammatory responses, such as the production of IL-6, IL-8, TNF-α and monocyte chemotactic protein-1 (MCP-1) managed by macrophages and keratinocytes. Topical application of methanolic rosemary extract, carnosol, or ursolic acid to mouse skin suppressed 2-O-tetra-decanoylphorbol-13-acetate (TPA)-induced inflammation and arachidonic acid-induced inflammation.22 Our results showed that ERE had a stronger inhibitory effect on cytokine production, particularly TNF-α, than its individual constituents alone. Carnosol, carnosic acid, and rosmarinic acid exerted differential modulatory effects on cytokine production. Carnosol and carnosic acid potently inhibited IL-1β secretion, and rosmarinic acid effectively suppressed IL-8 secretion in P. acnes–stimulated THP-1 cells. ERE with more potent inhibition of TNF-α could be attributed to another of rosemary's main components, caffeic acid,19 which showed potent inhibition of TNF-α production.38 Ursolic acid and luteolin, identified in rosemary,19 have been shown to block LPS-induced TNF-α release.39,40 However, a vast number of photochemicals have been identified in rosemary,19 it is difficult to examine each bioactive component of ERE in this study. Although we did not examine the in vivo anti-inflammatory effect of carnosol, carnosic acid, and rosmarinic acid in the present study, it is worthwhile to investigate bioactive constituents responsible for the suppressive effect of ERE against P. acnes–induced inflammation in vivo in the future.
The present study shows that the addition of ERE might contibute anti-inflammatory properties to cosmeceutical or dermatological products. Nevertheless, the safety of botanicals in personal care products/cosmetics is of concern.10,18 Our results indicated that a sole injection of ERE (l mg/10 μL) did not cause either skin irritation or inflammation. However, it is still obligatory to evaluate the safety and efficacy of rosemary extract and/or its bioactive ingredients when they are administered topically for acne therapy.
In conclusion, the present results provide evidence that ERE reduces P. acnes–induced inflammation in vitro and in vivo. Although the mechanism underlying the rosemary extract effect is not known in detail, it might involve the inhibition of NF-κB activation and TLR2 expression. Rosmarinic acid, carnosol, and carnosic acid; three bioactive compounds of rosemary, show differential modulatory effects on P. acnes–induced cytokine production. Further work is needed to understand the efficacy and mechanism of individual constituents of rosemary for P. acnes–induced inflammation.
Supplementary Material
Acknowledgment
This work was supported by research grants (NSC97-2320-B-003-005-MY3 and NSC100-2320-B-003-002) from the National Science Council, Taipei, Taiwan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–198. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- 2.Brook I. Endocarditis due to anaerobic bacteria. Cardiology. 2002;98:1–5. doi: 10.1159/000064684. [DOI] [PubMed] [Google Scholar]
- 3.Ferluga J. Allison AC. Role of mononuclear infiltrating cells in pathogenesis of hepatitis. Lancet. 1978;2:610–611. doi: 10.1016/s0140-6736(78)92828-3. [DOI] [PubMed] [Google Scholar]
- 4.Dong H. Toyoda N. Yoneyama H. Kurachi M. Kasahara T. Kobayashi Y. Inadera H. Hashimoto S. Matsushima K. Gene expression profile analysis of the mouse liver during bacteria-induced fulminant hepatitis by a cDNA microarray system. Biochem Biophys Res Commun. 2002;298:675–686. doi: 10.1016/s0006-291x(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 5.Vowels BR. Yang S. Leyden JJ. Induction of pro-inflammatory cytokines by a soluble factor of Propionibacterium acnes: implication for chronic inflammatory acne. Infect Immun. 1995;63:3158–3165. doi: 10.1128/iai.63.8.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J. Ochoa MT. Krutzik SR. Takeuchi O. Uematsu S. Legaspi AJ. Brightbill HD. Holland D. Cunliffe WJ. Akira S. Sieling PA. Godowski PJ. Modlin RL. Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köllisch G. Kalali BN. Voelcker V. Wallich R. Behrendt H. Ring J. Bauer S. Jakob T. Mempel M. Ollert M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurokawa I. Danby FW. Ju Q. Wang X. Xiang LF. Xia L. Chen W. Nagy I. Picardo M. Suh DH. Ganceviciene R. Schagen S. Tsatsou F. Zouboulis CC. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 9.Lalla JK. Nandedkar SY. Paranjape MH. Talreja NB. Clinical trials of ayurvedic formulations in the treatment of acne vulgaris. J Ethnopharmacol. 2001;78:99–102. doi: 10.1016/s0378-8741(01)00323-3. [DOI] [PubMed] [Google Scholar]
- 10.Reuter J. Merfort I. Schempp CM. Botanicals in dermatology: an evidence-based review. Am J Clin Dermatol. 2010;11:247–267. doi: 10.2165/11533220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Tsai TH. Tsai TH. Wu WH. Tseng TP. Tsai PJ. In vitro antimicrobial and anti-inflammatory effects of herbs against Propionibacterium acnes. Food Chem. 2010;119:964–968. [Google Scholar]
- 12.Hsu C. Tsai TH. Li YY. Wu WH. Huang CJ. Tsai PJ. Wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes–induced inflammation. Food Chem. 2012;135:976–984. doi: 10.1016/j.foodchem.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Baumann LS. Less-known botanical cosmeceuticals. Dermatol Ther. 2007;20:330–342. doi: 10.1111/j.1529-8019.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheung S. Tai J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep. 2007;17:1525–1531. [PubMed] [Google Scholar]
- 15.Peng CH. Su JD. Chyau CC. Sung TY. Ho SS. Peng CC. Peng RY. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci Biotechnol Biochem. 2007;71:2223–2232. doi: 10.1271/bbb.70199. [DOI] [PubMed] [Google Scholar]
- 16.Nakatsuji T. Kao MC. Fang JY. Zouboulis CC. Zhang L. Gallo RL. Huang CM. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuvelier ME. Richard H. Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chem Soc. 1996;73:645–652. [Google Scholar]
- 18.Antignac E. Nohynek GJ. Re T. Clouzeau J. Toutain H. Safety of botanical ingredients in personal care products/cosmetics. Food Chem Toxicol. 2011;49:324–341. doi: 10.1016/j.fct.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Offord E. Rosemary. In: Packer L, editor; Ong CN, editor; Halliwell B, editor. Herbal and Traditional Medicine: Molecular Aspects of Health. Marcel Dekker Publishing, Inc.; New York: 2004. pp. 457–469. [Google Scholar]
- 20.Newall CA. Anderson LA. Phillipson JD. Herbal Medicines, a Guide for Health-Care Professionals. Pharmaceutical Press; London, United Kingdom: 1996. [Google Scholar]
- 21.Altinier G. Sosa S. Aquino RP. Mencherini T. Loggia RD. Tubaro A. Characterization of topical antiinflammatory compounds in Rosmarinus officinalis L. J Agric Food Chem. 2007;55:1718–1723. doi: 10.1021/jf062610+. [DOI] [PubMed] [Google Scholar]
- 22.Huang MT. Ho CT. Wang ZY. Ferraro T. Lou YR. Stauber K. Ma W. Gerogiadis C. Laskin JD. Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents camosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 23.Kumar A. Takada Y. Boriek AM. Aggarwal BB. Nuclear factor-kappa B: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 24.Chen QJ. Koga T. Uchi H. Hara H. Terao H. Moroi Y. Urabe K. Furue M. Propionibacterium acnes–induced IL-8 production may be mediated by NF-κB activation in human monocytes. J Dermatol Sci. 2002;29:97–103. doi: 10.1016/s0923-1811(02)00013-0. [DOI] [PubMed] [Google Scholar]
- 25.Grange PA. Raingeaud J. Calvez V. Dupin N. Nicotinamide inhibits Propionibacterium acnes–induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci. 2009;56:106–112. doi: 10.1016/j.jdermsci.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Liu PT. Krutzik SR. Kim J. Modlin RL. Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005;174:2467–2470. doi: 10.4049/jimmunol.174.5.2467. [DOI] [PubMed] [Google Scholar]
- 27.Coenye T. Peeters E. Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol. 2007;158:386–392. doi: 10.1016/j.resmic.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Coenye T. Honraet K. Rossel B. Nelis HJ. Biofilms in skin infections: Propionibacterium acnes and acne vulgaris. Infect Disord Drug Targets. 2008;8:156–159. doi: 10.2174/1871526510808030156. [DOI] [PubMed] [Google Scholar]
- 29.Zedtwitz-Liebenstein K. Gabriel H. Graninger W. Pacemaker endocarditis due to Propionibacterium acnes. Infection. 2003;31:184–185. doi: 10.1007/s15010-002-2193-z. [DOI] [PubMed] [Google Scholar]
- 30.Nord CE. Oprica C. Antibiotic resistance in Propionibacterium acnes. microbiological and clinical aspects. Anaerobe. 2006;12:207–210. doi: 10.1016/j.anaerobe.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y. Zu Y. Chen L. Efferth T. Liang H. Liu Z. Liu W. Investigation of antibacterial activity of rosemary essential oil against Propionibacterium acnes with atomic force microscopy. Planta Med. 2007;73:1275–1280. doi: 10.1055/s-2007-981614. [DOI] [PubMed] [Google Scholar]
- 32.Tada M. Ohkanda T. Kurabe J. Syntheses of carnosic acid and carnosol, anti-oxidants in rosemary, from pisiferic acid, the major constituent of Sawara. Chem Pharm Bull (Tokyo) 2010;58:27–29. doi: 10.1248/cpb.58.27. [DOI] [PubMed] [Google Scholar]
- 33.Stepanovic S. Vukovic D. Dakic I. Savic B. Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 34.Zdařilová A. Svobodová A. Šimánek V. Ulrichová J. Prunella vulgaris extract and rosmarinic acid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicol in Vitro. 2009;23:386–392. doi: 10.1016/j.tiv.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Lo AH. Liang YC. Lin-Shiau SY. Ho CT. Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- 36.Poeckel D. Greiner C. Verhoff M. Rau O. Tausch L. Hörnig C. Dieter S. Manfred SS. Oliver W. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–97. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Oh J. Yu T. Choi SJ. Yang Y. Baek HS. An SA. Kwon LK. Kim J. Rho HS. Shin SS. Choi WS. Hong S. Cho JY. Syk/Src pathway-targeted inhibition of skin inflammatory responses by carnosic acid. Mediat Inflamm. 2012:2012. doi: 10.1155/2012/781375. article ID 781375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giovannini L. Migliori M. Filippi C. Origlia N. Panichi V. Falchi M. Bertelli AA. Bertelli A. Inhibitory activity of the white wine compounds, tyrosol and caffeic acid, on lipopolysaccharide-induced tumor necrosis factor-alpha release in human peripheral blood mononuclear cells. Int J Tissue React. 2002;24:53–56. [PubMed] [Google Scholar]
- 39.Checker R. Sandur SK. Sharma D. Patwardhan RS. Jayakumar S. Kohli V. Sethi G. Aggarwal BB. Sainis KB. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS One. 2012;7:e31318. doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xagorari A. Roussos C. Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br J Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.