Abstract
The lowest observed adverse effect level for bisphenol A (BPA) in mice and rats is currently poorly defined due to inconsistent study designs and results in published studies. The objectives of the current study were to (1) compare the estrogenic content of rodent diets, bedding, cages, and water bottles to evaluate their impact on the estrogenic activity of BPA and (2) review the literature on BPA to determine the most frequently reported diets, beddings, cages, and water bottles used in animal studies. Our literature review indicated that low-dose BPA animal studies have inconsistent results and that factors contributing to this inconsistency are the uses of high-phytoestrogen diets and the different routes of exposure. In 44% (76 of 172) of all reports, rodents were exposed to BPA via the subcutaneous route. Our literature review further indicated that the type of diet, bedding, caging, and water bottles used in BPA studies were not always reported. Only 37% (64 of 172) of the reports described the diet used. In light of these findings, we recommend the use of a diet containing low levels of phytoestrogen (less than 20 µg/g diet) and metabolizable energy (approximately 3.1 kcal/g diet) and estrogen-free bedding, cages, and water bottles for studies evaluating the estrogenic activity of endocrine-disrupting compounds such as BPA. The oral route of BPA exposure should be used when results are to be extrapolated to humans.
Abbreviation: BPA, bisphenol A; EDC, endocrine-disrupting chemical; EPA, Environmental Protection Agency; LOAEL, lowest observed adverse effect level; NIEHS, National Institute of Environmental Health Sciences; VO, vaginal opening
The potential adverse health effects of endocrine-disrupting compounds (EDC) are of great concern globally.25,33,85,92,99 Therefore, in 1996, Congress enacted laws that authorized the US Environmental Protection Agency (EPA), in conjunction with the Organization for Economic Cooperative Development, to develop and validate a screening program to identify and test approximately 80,000 suspected EDC. The objective of this program was to evaluate their potential to disrupt normal endocrine functions and cause adverse health effects in humans, fish and wildlife.33,45-46,85-87 EDC are exogenous chemicals, including pesticides and herbicides that may interfere with the body's endocrine system and produce adverse developmental, reproductive, neurologic, and immune effects in both humans and wildlife.33,85 The EPA's working definition states that endocrine disruptors “interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis (normal cell metabolism), reproduction, development, and/or behavior.”33,85 Currently, the EDC of greatest global attention for humans and wildlife and the most studied is bisphenol A (BPA; 2, 2-bis [4-hydroxyphenyl] propane; CASRN 80-05-7). BPA is an estrogenic compound found widely in the environment as a component of plastic cages, plastic bottles, food-can liners, and dental sealants. BPA has been detected in water, human sera, tissues, and urine and has been linked to reproductive and neurobehavioral disorders, heart disease, type 2 diabetes, prostate and breast cancers, and many other diseases.37,88,89,91,92 Currently, there is no consensus regarding the lowest observed adverse effect levels (LOAEL) for BPA because rodent studies have yielded inconsistent data regarding its toxicity.6,20,24,25,55,81,89,91,93-95 Several studies have shown that low doses of BPA (less than 50 µg/kg body weight daily) can adversely affect rats and mice; consequently exposure to low doses of BPA have been suggested to have similar effects in humans.29,36,51,55,88,89,92,96,97 However, low doses of BPA also have been reported to have minimal or no adverse effects in mice and rats.1,2,8,9,23,24,27,43,44,54,64,65,80-83 Therefore, the LOAEL for BPA in rodents and the mechanism by which BPA exerts its biologic actions are unclear.92 Consequently, the EPA, NIH, and National Institute of Environmental Health Sciences (NIEHS) recently have increased funding for these areas of research. Factors that may influence the assessment of BPA's estrogenicity in animal studies include the species or strain, genetic background, age, sex, dose and bioavailability of the chemical compound, developmental stage (gestation, weanlings, or adults), duration of exposure, and route of administration.
There are more than 5000 reports on BPA.25 Several extensive literature reviews on BPA have been published within the last 3 y, with one review containing 845 references.89 These literature reviews have focused on the potential adverse effects of BPA in rats and mice,25,43,44,51 biomonitoring of human88-91 or wildlife89 exposure, and the importance of biomonitoring studies and adverse effects on human health and future research objectives.88-90 The conclusions from these reviews are inconsistent.25,89,91 Early reviews focused on the LOAEL of BPA in humans or rodents. In contrast, the specific objectives of the current report are to briefly review the literature on rodent experiments with BPA, focusing on the maternal diet; the content of exogenous estrogens in the experimental diet, bedding, cages, and water bottles; and their effects on accurately determining the estrogenic activity of EDC.
Literature Review: Sources of Exogenous Estrogens in Rodent Studies
Rodent diets.
The primary source of exogenous estrogenic substances for laboratory animals comes from the animals’ diet.76,79 Phytoestrogens are defined as plant compounds that exert estrogenic effects on the central nervous system, induce estrus, and stimulate growth of the genital tract of female animals. More than 300 plants and plant products, including some used in laboratory animal diets, contain phytoestrogens.5,21 Natural dietary ingredients, including soybeans, wheat, barley, corn, alfalfa, and oats, are the major dietary ingredients that have been reported to contain phytoestrogens.21 The phytoestrogen content in the natural dietary ingredients used in rodent diets has been reported.76 Soybean meal is the main source of the phytoestrogens daidzein, genistein, and glycitein, and their concentrations were directly correlated with the percentage of soybean meal in the diet.59,60,76 The phytoestrogens daidzin, genistin, and glycitin are present as glucosides in soybeans and after ingestion are converted to their bioactive forms of daidzein, genistein, and glycitein by intestinal bacteria.57-60 Wheat and oats contained only trace levels of daidzein and genistein.76 Two other phytoestrogens are coumestrol (normally found in alfalfa) and biochanin A (found in clover).21
Commercial rodent diets are available in many different formulations designed to meet the needs of animal production (breeder colonies), growth, and maintenance. In addition, various special diets are available for specific research needs.3,7,79 The 2 main types of diet are (1) natural-ingredient diets consisting of milled grains such as soybean meal, alfalfa meal, corn, wheat, and oats with added vitamins and minerals and (2) purified diets consisting primarily of sucrose, dextrose, corn starch, casein, and cellulose with added vitamins and minerals.3,79 Diets that contain high contents of soymeal and alfalfa have high and variable levels of phytoestrogens.7,67,71,73,74,76-79 By contrast, soy- and alfalfa-free natural-ingredient diets and purified diets that contain casein as the protein source have trace or undetectable levels of phytoestrogens.3,66,67,73,76-79
Rodent diets also are classified as open-formula or closed-formula diets. In open-formula diets, the ingredients and their concentrations are disclosed, whereas closed-formula diets report only the list of ingredients. Most breeder diets used during gestation and lactation are closed-formula diets that are high in protein (18% to 24%) and total metabolizable energy, and some have high and variable levels of phytoestrogens.67,73,76-79
In past years, most studies were conducted with little consideration given to the effect of the maternal or the experimental diet on study outcomes. The maternal diet used during gestation and weaning and the selection of the experimental diet used for determining the estrogenic activity of EDC may determine the outcome of a study. For example, maternal diets supplemented with high levels of DNA methyl donors such as folic acid have been shown to alter the epigenetic genome in agouti mouse pups.12,98 In addition, maternal diets containing 250 to 300 µg genistein/g diet can alter gene expression and potentially negate the epigenetic effects of coadministered BPA (50 µg/g diet).18,19
Selection of the optimal diet(s) must be given serious consideration in experiments with EDC. There are large differences in the estrogenic activity, nutrient composition, and metabolizable energy of animal breeder diets, soy- and alfalfa-free phytoestrogen-reduced diets, and purified diets that contain casein or soy protein as their source of protein.67,70,74,78 Therefore, marked differences in study outcomes can be expected with diets containing high phytoestrogen content when compared with those containing very low levels of phytoestrogens.66,78,79 The effect of dietary phytoestrogens in animal models is well-documented.3,6,7,10,18,41,47,59,60,66,76,78,79 Some studies show that a low phytoestrogen level in the diet increases fetal serum estradiol concentrations resulting in the ‘fetal estrogenization syndrome’ and obesity in CD1 mice.52 In addition, the choice of animal feed can alter fetal steroid levels and may mask developmental effects of EDC.53
Rodent bedding.
Corncob bedding can be a source of exogenous estrogens.38,68 In 2006, the NIEHS Quality Assurance Laboratory surveyed 18 types of rodent bedding for estrogenic activity.68 The bedding types included hardwood, softwood (cedar and pine shavings), various types of corncob, paper, and blends. All bedding types were negative for daidzein, genistein, and coumesterol and, except for corncob bedding, failed to advance the time of vaginal opening (VO) in immature CD1 mice.68 However the time of VO was slightly advanced in mice exposed to corncob bedding containing low levels of zearalenone.68 Zearalenone is an estrogenic mycotoxin produced by a variety of fungi belonging to the genus Fusarium that is found in food and feeds. Zearalenone is ubiquitous in corncob bedding and primarily is isolated from moldy corn and corn byproducts and to a lesser extent from oats, wheat, and barley infected with Fusarium graminearum, F. roseum, F. culmorum, F. cerealis, and common soil fungi that grow in temperate and warm climates.75,84 In addition, previous reports showed that 4 to 6 ppm zearalenone in the diet significantly increased the uterine weights of mice during a 7-d bioassay.75,84
Cages and water bottles.
Additional sources of exogenous substances to consider include animal cages and water bottles, which can be sources of exogenous estrogenic-like compounds that may alter the outcome of studies determining the estrogenic activity of BPA and other EDC.28,37,69,92 Polycarbonate cages and water bottles have been reported to leach BPA.28,37 In addition, BPA-free plastic cages made from polyethylene terephthalate were reported to leach other unidentified estrogenic monomers, which have been detected by using in vitro assays.100 Further studies are needed to identify these chemical monomers and to determine whether these estrogenic monomers are estrogenic in uterotropic or VO bioassays.
Route, time, and bioavailability of BPA exposure.
The developmental stage and route of exposure are key factors to consider when conducting toxicologic or estrogenic studies with BPA. Animals exposed to chemicals during gestation or neonatally are more sensitive to toxic or exogenous estrogenic compounds than are older animals.4,14-18,22,25,43,48,51,63,89,92,99
In addition, route of exposure has a significant effect on BPA bioavailability.14-17 Aglycone BPA has weak estrogen agonist activity (that is, affinity for and transactivation of estrogen receptors α and β in the micromolar range) in vitro, whereas its glucuronide conjugate is inactive.40 In recent reports,14,15 the serum pharmacokinetics of aglycone (active) and conjugated (inactive) BPA were compared by using identical doses (100 µg/kg body weight) in rats, mice, and monkeys after oral and subcutaneous administration. Deuterated BPA was administered to avoid contamination with ubiquitous background BPA. The serum concentration of aglycone BPA was substantially lower when it was given orally compared with subcutaneous injection. After ingestion of BPA, conjugation occurs during first-pass metabolism in the gastrointestinal tract and liver, so that most of the BPA detected in the serum, urine, and tissues is in the conjugated (that is, inactive) form of BPA.11,14-17,48-50
Original Data: Zearalenone and BPA in Rodent Housing Components
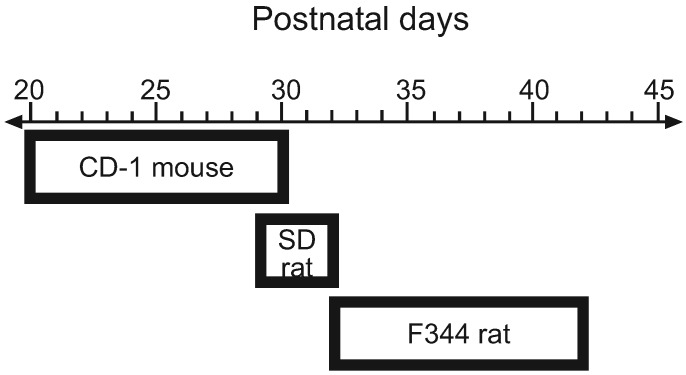
This section presents original data generated in the NIEHS Quality Assurance Laboratory to determine the content of zearalenone in corncob bedding and rodent diets as well as original data on the amount of BPA that leaches from various types of animal cages and water bottles. We also summarize previously published data regarding the effects of rodent diets on the time of VO in immature CD1 mice, F344, and CD Sprague–Dawley rats (Figure 1).
Figure 1.
Range of the mean time of VO in CD1 mice, Sprague–Dawley, and F344 rats fed various diets containing high or low levels of phytoestrogens and metabolizable energy. These data indicate that the mean time of VO varies approximately 3 d in Sprague–Dawley rats, compared with 10 d in CD1 mice and F344 rats, indicating that Sprague–Dawley rats are not the most sensitive model for evaluating the mean time of VO in rodents fed diets containing variable levels of phytoestrogens or metabolizable energy for evaluating the estrogenic activity of EDC on VO bioassays.
Rodent diets.
For more than 25 y, the NIEHS Quality Assurance Laboratory has analyzed natural-ingredient, purified, and custom-formulated rodent diets used by investigators at NIEHS. These assays were conducted to determine the source and effect of dietary phytoestrogens and zearalenone on study outcomes. The diets were assayed for the phytoestrogens daidzein, genistein, formononetin, biochanin A, and coumestrol. Soy flakes were assayed as a positive control. Dietary phytoestrogens were assayed by using a previously described HPLC procedure.7,58-60 Briefly, dietary estrogens were extracted from 5-g duplicate samples. The diets were analyzed in a blinded fashion. The concentration of each phytoestrogen was determined from calibration standards and was expressed in micrograms per gram (ppm) of diet. In addition, diets containing high or unexpected levels of phytoestrogens were submitted to an independent laboratory (Covance Laboratories, Madison, WI) for confirmatory assays. Assays for zearalenone were conducted by an independent laboratory (Romer Labs, Union, MO). Samples (25 g) of each diet were analyzed in duplicate in a blinded manner by using HPLC procedures. The limit of detection was 100 ng/g.
The phytoestrogen content of various representative natural-ingredient and purified rodent diets are shown in Table 1. Some diets contain greater than 350 total genistein equivalents, a sufficiently high level to potentially affect the outcome of studies designed to evaluate the estrogenic activity of EDC.67,74,79 In addition, diets containing variable levels of daidzein, genistein, and total metabolizable energy67,74,79 can alter the mean time of VO in immature CD1 mice and F344 rats (Figure 1). These data indicate that rodent diets can alter hormonal endpoints in studies evaluating the estrogenic activity of EDC.
Table 1.
The content of daidzein, genistein and metabolizable energy of various rodent diets used in estrogenic studies to determine the potential adverse effects of BPA
| Diet | No. of batches analyzed | Metabolizable energy (kcal/g) | Daidzein | Genistein | Total daidzein + genistein | Estimated total genistein equivalents | Approximate diethylstilbestrol equivalentsa |
| Purina 5001b | 8 | 3.04 | 228 ± 52 | 257 ± 61 | 484 ± 113 | 439 ± 102 | 4.7 |
| Purina 5002b | 14 | 3.10 | 141 ± 51 | 167 ± 63 | 308 ± 113 | 180 ± 103 | 3.9 |
| Purina 5008b | 19 | 3.31 | 242 ± 66 | 277 ± 78 | 520 ± 143 | 471 ± 130 | 5.0 |
| Purina 5010b | 6 | 3.08 | 281 ± 62 | 321 ± 65 | 602 | 546 | 5.2 |
| Phytoestrogen-reduced natural-ingredient dietsc | 20 | 2.87–3.20 | <10 | <10 | <10 | <10 | not done |
| Phytoestrogen-free purified dietsd | 15 | 3.83 | <10 | <10 | <10 | <10 | not done |
All contents and equivalents (mean ± 1 SD) are given as micrograms per gram of diet. Data for metabolizable energy were obtained from the vendor websites.
From reference 5.
Data for Purina 5001, 5002, and 5008 diets were obtained from phytoestrogen assays conducted by the Quality Assurance Laboratory at NIEHS; values for Purina 5010 diet are those from reference 38.
Purina 5K96, Harlan 2016, Zeigler Brothers Phyto-Reduced I and II diets were evaluated by the Quality Assurance Laboratory at NIEHS.
Casein-based diets AIN76A, AIN93G, and AIN93M were evaluated by the Quality Assurance Laboratory at NIEHS.
We assayed the experimental diets that we used in our previous publications for zearalenone. All 11 mill dates of the NIH31 diet (Zeigler Bros, Gardner, PA) milled between 2007 and 2010 had nondetectable levels of zearalenone. In addition, 8 of 10 Purina diets milled during the last 2 y had nondetectable levels of zearalenone; the remaining 2 Purina diets contained 101 and 107 ng/g zearalenone of diet. In addition, 20 of 25 diets from Harlan Teklad (Madison, WI) milled between 2009 and 2012 had undetectable levels of zearalenone. The range of zearalenone detected in the remaining 5 Harlan Teklad diets was 114 to 223 ng/g (mean, 152 ng/g) of diet. These results indicate that low levels of zearalenone are present in natural-ingredient animal diets containing corn. When used in studies to evaluate the estrogenic activity of EDC, these diets should be tested for zearalenone to minimize exposure to exogenous estrogens.
Assay and effects of zearalenone in corncob bedding.
The NIEHS Quality Assurance Laboratory purchased 189 samples of corncob bedding from all bedding vendors to determine the zearalenone content of each sample. Zearalenone assays were conducted on 25-g samples by independent laboratories using HPLC and mass spectrometric procedures (Romer Labs and North Carolina State University [Raleigh, NC]). The limit of detection was 100 ng/g.
Zearalenone has greater estrogenic potency than does BPA in uterotrophic bioassays and VO assays.5,75,84 Corncob bedding is a widely used, highly absorbent bedding, and zearalenone is ubiquitous in this type of bedding.38 We previously reported75 that commercially available corncob bedding that was naturally contaminated with zearalenone significantly advanced the time of VO in immature CD1 mice. In the current study, 158 of the 189 samples (84%) of corncob bedding tested were positive for zearalenone, with levels as high as 7000 ng/g (mean, 600 ng/g) of bedding. Approximately equal numbers of the different bedding types were purchased from each bedding vendor; the percentage of samples positive for zearalenone was similar for each bedding vendor. Because corncob bedding is an exogenous source of estrogen-like compounds, it should not be used in studies evaluating the estrogenic activity of BPA or other EDC.75
Assay and effects of BPA leached from rodent cages and water bottles.
Several studies28,37 have addressed the potential effect of BPA leached from new and used polycarbonate cages and water bottles.37 To examine this potential effect further, we determined the concentration of BPA that leached into water from new and used polysulfone, polycarbonate, polystyrene, and polyethylene terephthalate cages and from glass and used polycarbonate water bottles. Reverse-osmosis, deionized ‘pico pure’ water was poured into 6 different types of new or used cages (polypropylene, polycarbonate, polystyrene, polyethylene terephthalate, and polysulfone) and 3 types of water bottles (glass and 2 different types of polycarbonate bottles). These bottles were filled with reverse-osmosis, deionized water and left at room temperature for 14 d. In addition, reverse osmosis, deionized water was boiled in polycarbonate bottles for 1 and 2 h to determine the concentration of BPA leached as a result of boiling the water. Samples (50 mL) of water were collected at the time of filling and at weekly intervals and were assayed for BPA. BPA concentrations were determined by HPLC–tandem mass spectrometry. Chromatography was performed by using a C18 column and a water–methanol gradient. The mass spectrometer was operated in negative ion electrospray ionization mode with multiple-reaction monitoring. The parent–fragment ion transition m/z 227.1 > 211.8 was used for quantification, and m/z 227.1 > 132.6 was used for confirmation. BPA labeled with 6 deuterium atoms was used as an internal standard.
BPA was not detected in water stored in glass bottles or in polyethylene terephthalate cages for as long 2 wk. In addition, the amount of BPA that leached from other cages or polycarbonate water bottles at room temperature was less than the limit of detection (1.0 ppb). Therefore, a negligible amount of BPA leaches from new or used polycarbonate cages maintained at room temperature. Although accurate assessment of exposure to BPA from drinking water bottles is relatively easy, such assessments are more difficult from cages because exposure may occur through skin and inhalation.38
Boiling water in polycarbonate water bottles has been shown to accelerate the leaching of BPA into the water.37 We confirmed this observation by boiling BPA-free reverse-osmosis deionized water in used polycarbonate bottles for 1 or 2 h. The BPA content of the water was 1.67 after 1 h of boiling and 3.16 ng/mL after 2 h. Therefore, heating or autoclaving water in polycarbonate water bottles should be avoided. In most animal facilities, water bottles are sanitized and dried before being filled with water.
Despite concerns about potential leaching of BPA from polycarbonate water bottles, our current results suggest that rodent diets and corncob bedding are more important contributors of unwanted estrogen exposure. Our results confirm previous reports24,54 that BPA leaching from polycarbonate cages or water bottles does not adversely affect endocrine disruptor studies. In addition, we have reported that drinking water spiked with doses of 5 to 500 µg/mL BPA do not significantly advance the time of VO in CD1 mice.77 To minimize exogenous inadvertent exposure of rodents to BPA in low-dose EDC studies, we recommend using BPA-free cages and glass water bottles.
These data highlight the importance of critically considering the type of diet, bedding, cages, and water bottles as sources of exogenous estrogenic compounds and reemphasizes the need to consider the diet when the effects of BPA or other potential EDC, especially at low levels of exposure, are studied. Ideally, all nonspecific sources of exogenous estrogenic compounds should be eliminated.
Literature Review: Potential Estrogenic Effects of Rodent Diets, Bedding, Caging, and Water Bottles
To highlight the extent to which the potential estrogenic effects of rodent diets, bedding, caging, and water bottles are considered in studies evaluating the estrogenic activity of BPA, we conducted 2 separate literature reviews that included a total of 172 published studies dated between 1997 and 2010 that used rats or mice. In our review, we noted the developmental stage of BPA exposure (during gestation, weanlings, adults), and the route of administration (oral via feed, water, or gavage; nonoral via subcutaneous injections, osmotic pumps, or implants). We also noted whether the diet was identified accurately, that is, with the manufacturer's name and product code or catalog number (for example, no. 5008, Purina Mills, Richmond, IN).
The first review included 69 publications (40 on rats and 29 on mice) from 1997 to 2004, with most publications coming from review articles.92,96,97 The second review covered 103 publications (51 on rats and 52 on mice) from 2005 to 2010. In particular, we wanted to determine whether the use of phytoestrogen-reduced diets had increased, given that these natural-ingredient diets were first reported in 1998.66
The effects of dietary phytoestrogens and metabolizable energy on uterotrophic assays and VO endpoints.
For more than 25 y, a key goal of the NIEHS Quality Assurance Laboratory has been to determine the concentration of exogenous estrogenic substances in animal diets,70,72-74,76,78,79 bedding, and cages and to evaluate the effects of these substances on experimental outcomes.38,68,75 Our recent bioassays have focused on VO as an endpoint to determine the estrogenic activity in rodent diets because VO is an estrogen-sensitive biomarker of sexual maturation in rodents.39,67,78 VO can be accelerated by endogenous estrogens, total metabolizable energy,70,72,73 and exogenous estrogens.67,75 The VO assay does not require the euthanasia of test subjects. The extreme baseline dynamic response ranges in the mean time of VO in CD1 mice, Sprague–Dawley rats, and F344 rats fed a variety of diets containing variable, high, or reduced levels of phytoestrogens and metabolizable energy are shown in Figure 1. The difference in the baseline dynamic response ranges in the mean time of VO was generated over 3 to 4 y and may differ for each species and strain when fed different diets. For example, the Harlan 2014 or 2016 diets are low in total metabolizable energy (approximately 3.0 kcal/g) and low in phytoestrogen content and delay the mean time of VO in rodents. In contrast, the Purina diets 5001, 5002, 5008, and 5010, which contain high levels of phytoestrogens and moderate levels of metabolizable energy (3.1 to 3.3 kcal/g), advance the time of VO and puberty in rodents. In addition, the purified soy-based AIN76A diet and the purified casein-based AIN93G diet, both of which have a high total metabolizable energy (approximately 3.8 kcal/g), significantly increase uterine weights and advance the time of VO in CD1 mice when compared with those in mice fed a soy- and alfalfa-free diet containing a low level of metabolizable energy (approximately 3.0 kcal/g). In 1987, we reported that rodent diets differ significantly in estrogenic activity and suggested that diets free of or reduced in phytoestrogen should be used for animal studies having estrogen-sensitive endpoints that might be altered by dietary estrogens.72-74 In addition, we recommended66 the use of a natural ingredient phytoestrogen-free diet with a low level of metabolizable energy, specifically formulated for uterotrophic and VO endpoints. Commercial diets are known to contain sufficient levels of phytoestrogens to advance the time of VO and increase uterine weights in immature CD1 mice and F344 rats.78,79 The batch-to-batch variation in phytoestrogen content can vary as much as 3-fold between different mill dates of the same diet.78,79 This variability makes it difficult to reproduce results within or between laboratories over time. However, the batch-to-batch variability in phytoestrogen content can be eliminated by using a soy- and alfalfa-free natural-ingredient diet66,67,76-79 or a casein-based purified diet (Table 1).
The effects of dietary daidzein and genistein on EDC studies.
The EPA's Endocrine Disruptor Screening Program has published a Standard Evaluation Procedure for conducting the uterotropic assay.87 This guideline recommends the use of diets containing less than 350 µg total genistein equivalents per gram of diet for immature female Sprague–Dawley and Wistar rats and containing less than 175 µg/g diet for ovariectomized adult mice.87 The guideline recommends that the phytoestrogen levels in the diet should be reported, because dietary phytoestrogens can increase uterine weights in rodents and may interfere with the uterotrophic assay. In addition, the guideline states that several semisynthetic casein-based diets that are free of soy, alfalfa, and phytoestrogens are available.87
This recommendation87 is problematic for several reasons. First, implementing the recommendation is complicated by the large variation in the isoflavone content of commercial diets and the inability to control for these levels. Second, the possibility that dietary isoflavones could interact with the target EDC must be considered. For example, previous studies18,19 have shown that, when given individually, both genistein and BPA alter DNA methylation and gene expression, resulting in changes in coat color in agouti mice, but that when coadministered, genistein negates the effect of BPA.18 We suggest that investigators consider using the most estrogen-sensitive rodents and hormonal endpoints consistent with their experimental goals. For example, immature CD Sprague–Dawley rats are relatively insensitive to dietary estrogens as determined by using VO bioassays.70,77 However, different strains of the Sprague–Dawley rats may be more sensitive to other estrogenic and molecular endpoints.70
We reported67,76-79 that dietary phytoestrogens at less than 350 µg/g diet significantly altered the results of uterotrophic bioassays in CD1 mice and VO endpoints in CD1 mice and F344 rats. Comparing the estrogenic potentials of different compounds can be prohibitively difficult because of the complexity of steroid action, but the effects of exposures of different compounds can be compared by using the same specific endpoint.5 For example, in the uterotrophic bioassay using CD1 mice, a diet containing 500 to 600 µg daidzein plus genistein per gram of diet has a similar effect on uterine weight as does a diet containing 4 to 5 ppb of the potent estrogen diethylstilbestrol (Table 1). This estrogenic effect of the diet exceeds that of low doses of BPA reported in many studies (Table 2). The rationale for using ovariectomized or immature mice or rats in the uterotrophic bioassay is to minimize the circulating levels of endogenous estrogens, thus enhancing the sensitivity of the assay. Similarly, minimizing exposure to dietary phytoestrogens by mandating the use of a standardized phytoestrogen-free (that is, soy- and alfalfa-free) diet when evaluating the estrogenic activity of potential EDC will increase the sensitivity and reproducibility of the assay and the ability to obtain accurate and meaningful results that can be compared within and between laboratories.78,79 The diet selected should reduce rather than add variability to studies evaluating the estrogenic activity of EDC.32,34,66,67,73,76,78,79 Only then can the estrogenicity and true LOAEL for EDC, such as BPA, be determined with a high degree of certainty.
Table 2.
Example comparison of the daily dose of BPA administered via drinking water with the daily ingestions of dietary daidzein and genistein in mice
| BPA dose administered in water (μg/mL) |
||||||||||
| Mice | 0 | 5 | 25 | 50 | 100 | 250 | 500 | 1000 | 2000 | |
| Daily BPA intake (µg) via drinking water | ||||||||||
| Postnatal days 15–20 | 0 | 25 | 125 | 250 | 500 | 1250 | 2500 | 5000 | 10000 | |
| Adult female | 0 | 35 | 175 | 350 | 700 | 1750 | 3500 | 7000 | 14000 | |
| Pregnant female | 0 | 50 | 250 | 500 | 1000 | 2500 | 5000 | 10000 | 20000 | |
| %Daidzein + genistein of total daily intake | ||||||||||
| Adult female | 100 | 99 | 95 | 91 | 84 | 68 | 51 | 34 | 21 | |
| Pregnant female | 100 | 99 | 94 | 89 | 81 | 62 | 45 | 29 | 17 | |
| %BPA of total daily intake | ||||||||||
| Adult female | 0 | 1 | 5 | 9 | 16 | 32 | 49 | 66 | 79 | |
| Pregnant female | 0 | 1 | 6 | 11 | 19 | 38 | 55 | 71 | 83 | |
Data are based on mean daily food intakes of 5, 7, and 8 g; mean daily water intakes of 5, 7, and 10 mL; and total daily daidzein and genistein intakes from Purina 5008 of 2600, 3640, and 4160 µg for mice at postnatal days 15 through 20 (mean weight, 10 g), adult female mice (mean weight, 30 g), and pregnant female mice (mean weight, 50 g), respectively.
The BPA obtained from drinking water at a BPA dose of 500 µg /mL is approximately equal to the daily intake of daidzein and genistein from the Purina 5008 diet (see Table 1).
Problems might occur if exogenous estrogens in the diet, bedding, caging, and other sources supplement or negate the effects of the test estrogen (for example, BPA), subsequently yielding results that are difficult to interpret or reproduce. This possibility is considerable, given that phytoestrogen levels vary from batch to batch within the same diet.78 Furthermore, early exposure to phytoestrogens or to DNA methyl donor supplements (for example, folic acid) from the maternal diet can alter epigenetic and gene expression in the offspring, thereby influencing subsequent animal studies.4,12,13,18,19,21,26,30,35,47,63,89,98
Effects of dietary phytoestrogens and BPA on the development of mouse oocytes.
Studies29,36,41 evaluating the effects of BPA on oocyte development in mice fed Purina 5010 diet produced inconsistent results that appear to correlate with changes in mill dates of the diet. This observation, coupled with other reports6,66,67,78,79 suggested that dietary phytoestrogens are a confounding variable in studies of EDC, prompted a study41 to evaluate the effects of diet and BPA on oocyte development. Prior to mating, C57BL/6J female mice were placed on a high-phytoestrogen diet (Harlan Teklad 8656 diet) or a low-phytoestrogen diet (AIN93G) containing different levels of BPA. Oocytes were collected 7 d later, and meiotic spindle and chromosome characteristics of oocytes were compared between control and BPA-treated mice. Significant diet-related variations in the frequency of oocyte abnormalities occurred in untreated female mice and those exposed to BPA at doses as high as 500 µg/kg daily. The authors concluded that both dietary phytoestrogens and low doses of BPA can adversely influence mouse oocyte development. Furthermore, these data suggested that inconsistent findings from BPA studies probably reflect differences in the diets and methodologies used and that high-phytoestrogen diets (for example, Purina 5010 and Harlan Teklad 8656 diets) are not suitable for EDC studies.41 In the cited study,41 the levels (mean ± 1SD) of genistein and daidzein in the Purina 5010 diet were 321.0 ± 65.5 and 281.8 ± 62.5 µg/g diet, respectively, and the total isoflavone (genistein + daidzein + glycitein) content was 602 ± 127.5 µg/g diet (Table 1).
The daidzein and genistein contents in the Purina 5010 and Harlan Teklad 8656 diets are comparable to those we reported for the Purina 5008 breeder diet that is often used during gestation and lactation and the Purina 5001 maintenance diet used for postweaning studies (Table 1). Therefore, diets high in phytoestrogens may influence the results of EDC studies, including those from BPA exposure (Tables 1 and 2). It is also notable that the Purina 5008, 5010, 5001, and 5002 diets were the diets used most frequently in the reviewed published studies on BPA (Tables 1 and 4).
Table 4.
Review of BPA studies to determine the types of rodent diet, bedding, caging, and water bottle reported most frequently
| Publication date |
|||
| 1997–2004 (n = 69)a no. (%) | 2005–2010 (n = 103)ano. (%) | Change from 1997–2004 to 2005–2010 (%) | |
| Diet reported | 40 (58) | 69 (67) | +9.0 |
| Diet reported but described incompletely | 19 of 40 (48) | 26 of 69 (38) | −10.0 |
| Diet not reported | 29 (42) | 34 (33) | −9.0 |
| Diet reported and described sufficiently | 21 (30) | 43 (42) | +12.0 |
| Phytoestrogen-reduced diet reported | 2 (3) | 20 (19) | +16.0 |
| Daidzein and genistein contents reported | 2 (3) | 3 (3) | 0 |
| Bedding reported | 17 (25) | 19 (18) | −6.0 |
| Corncob bedding used | 5 of 17 (29) | 5 of 19 (26) | −3.0 |
| Caging reported | 26( 38) | 43 (42) | +3.7 |
| Polycarbonate caging used | 7 of 26 (27) | 10 of 43 (23) | −4.0 |
| Water bottles reported | 12 (17) | 33 (32) | +15.0 |
| Glass water bottles used | 9 of 12 (75) | 26 of 33 (79) | −3.8 |
Fewer than 5% of all reports performed estrogenic assays on the bedding, caging, or drinking water.
Percentages based on 69 or 103 reports reviewed (depending on publication date), except where noted.
Effects of phytoestrogens and DNA methyl donor supplements in the maternal diet on epigenetic and gene expression studies.
Recent reports have emphasized the importance of DNA methyl donors and phytoestrogens in the maternal diet of studies on epigenetic and gene expression.4,30,35,63 These reports indicate that exposure during early development may cause adverse effects in adulthood.4,21,47 The major phytoestrogens in soybeans have been shown to possess estrogenic activity as determined in uterotrophic bioassays.5,13,67,76-79 In addition, when present in sufficient levels, these phytoestrogens can alter estrogen-sensitive gene expression both in vitro31,56,61 and in vivo.12,13,42,62,98
In one study,42 daily subcutaneous administration of 17α-ethynyl estradiol (10 µg/kg), BPA (400 mg/kg), or genistein (100 mg/kg) to Sprague–Dawley rats during gestation (days 11 to 20) resulted in dose-dependent transcript profiles in which these 3 chemicals significantly modified a common set of genes and in which each chemical also modified a unique set of genes. The authors concluded that all estrogenic chemicals do not act alike and that different chemicals with estrogenic activity may change different expression profiles of estrogen-sensitive genes in tissues.42
Methyl-supplemented diets containing betaine, folic acid, vitamin B12, choline, and L-methionine can modulate epigenetic mechanisms, especially DNA methylation, in developing embryos, thereby altering gene expression in the offspring of Avy/a agouti mice.12,98 The dietary methyl supplements in various commercial rodent diets compared with the contents in diets fortified to alter gene expression in Avy agouti mice offspring are shown in Table 3. The levels of folic acid in some commercially available diets (Table 3) may be high enough to alter epigenetic mechanisms on gene expression. In these studies,12,18,98 the NIH31control diet was fortified with DNA methyl donor supplements at 2 different levels and fed to the agouti mice. The offspring were evaluated to determine the degree of haircoat variation. Whether these DNA methyl donors act independently or collectively to alter gene expression is unclear. In addition, the minimal levels required for each of these DNA methyl donors to alter epigenetic effects and gene expression in the offspring is unknown and requires research.12,98 Recent studies using the AIN93G phytoestrogen-free diet have shown that BPA (50 µg per gram of diet), genistein (250 µg per gram of diet), and dietary methyl donors (folic acid, 4.3 mg per kilogram of diet; vitamin B12, 0.53 mg per kilogram of diet; betaine, 5.9 g per kilogram of diet; and choline chloride, 7.9 g per kilogram of diet) cause DNA methylation and alter gene expression.18,19 However, when BPA and genistein are coadministered in the same diet, genistein blocks or negates the epigenetic effects of BPA.18 Daidzein and genistein are the major isoflavones in soybean meal, and both are present in approximately equal content in breeder diets (for example, Purina 5002, 5008, and 5010 diets) commonly used during gestation and weaning and in the Purina 5001 maintenance diet (Table 1). Due to batch-to-batch variation in the phytoestrogen content between different mill dates of the same diet, there may be enough genistein (at least 250 µg per gram of diet) to negate the epigenetic effects of BPA (dose, 50 µg per gram of diet) when it is administered in the diet18 (Table 1). For example, in the current study, our laboratory analyzed 19 batches of the Purina 5008 diet, in which the genistein content (mean ± SEM) was 277 ± 78 µg per gram of diet, the daidzein content was 243 ± 66 µg per gram of diet, and the total daidzein + genistein content in these 19 batches was approximately 520 ± 143 µg per gram of diet. The phytoestrogen daidzein is estrogenic in rodent uterotrophic5 and VO assays,5,13 but whether dietary daidzein blocks or negates the epigenetic effects of BPA is unknown. It has been reported that levels of daidzein and BPA that fail to increase uterine weight can modulate the expression of estrogen-sensitive genes in the rat uterus.13
Table 3.
Comparison of dietary methyl donors in various commercial rodent diets and in diets fortified to alter gene expression in Avyagouti offspring
| NIH31 |
AIN93G |
Purina |
|||||||
| Methyl donor | Normal | Fortifieda | Normal | Fortifiedb | 5001c | 5002c | 5008c | 5010c | 5K96c |
| Folic acid (d) (mg/kg) | 2 | 5 | 2.1 | 4.3 | 7.1 | 3.1 | 3.0 | 6.1 | 2.7 |
| Vitamin B12 (µg/kg) | 60 | 500 | 29 | 530 | 50 | 51 | 20 | 50 | 43 |
| Choline chloride (g/kg) | 1.89 | 5.00 | 1.25 | 7.97 | 2.25 | 2.00 | 2.00 | 2.20 | 1.80 |
| L-methionine (%) | 0.4 | 0 | 0.52 | 0 | 0.67 | 0.43 | 0.43 | 0.66 | 0.45 |
| Betaine (g/kg) | 0 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
The levels of folic acid in the Purina 5001 and 5010 diets are similar to those in the NIH31 and AIN93G fortified diets.
From reference 98.
From reference 18.
Data were obtained from the vendor websites.
The 3 major dietary phytoestrogens in soybeans are genistein (approximately 50% of total phytoestrogen content), daidzein (approximately 40%), and glycitein (approximately 10%).31,59,60 Daidzein and glycitein may have similar effects to estradiol on some estrogen-responsive genes.31 One previous study31 evaluated 172 estrogen-responsive genes by using a customized DNA microarray, and the expression profiles of 13 pure phytoestrogens including genistein, daidzein, and glycitein were compared with that of 17β-estradiol. In addition, extracts from 2 varieties of soybeans were compared. With regard to changes in gene expression, genistein (at 10 µM) showed higher correlation with 17β-estradiol (R = 0.93) than did daidzein (R = 0.74) or glycitein (R = 0.59). The gene expression profiles from both soybean extracts were more highly correlated with daidzein (R = 0.75) than with genistein (R = 0.62). Additional reports of microarray gene expression profiles among 17β-estradiol, 17α-ethynyl estradiol, genistein, daidzein, BPA, and other xenoestrogens indicate that daidzein, genistein, and BPA can alter the expression of estrogen-responsive genes.31 The daidzein content of the diet may be even more important than the genistein content because daidzein is efficiently converted to S-(–)equol in the rodent intestine, and the estrogenic activity of equol is about one order of magnitude higher than that of daidzein in vitro.60 These results indicate that further research is needed to determine the potential effect of daidzein on gene expression and on studies of BPA in rodents.
We compared the daily intake of dietary phytoestrogen in mice fed the Purina 5008 diet, which contains genistein (277 µg/g) and daidzein (243 µg/g) with the daily BPA intake resulting from their consumption of drinking water containing 0, 5, 25, 50, 100, 250, 500, 1000, or 2000 µg BPA/mL (Table 2). For example, a 30-g mouse dam provided Purina 5008 diet and drinking water containing 500 µg BPA/mL typically consumes 7 g of food and 7 mL of water daily and consequently will be exposed to nearly equivalent levels of BPA in water and isoflavones in the diet (Table 2). In addition, these dietary estrogens may be more potent than BPA and thus have a greater effect on some hormonal endpoints. The presence of extraneous estrogenic compounds from dietary sources complicates the assessment of the estrogenic effects of BPA.
Studies on BPA published between 1997 and 2004.
Of the 69 studies reviewed, only 40 of 69 (58%) reported or attempted to identify the type of diet used, and only 17 of 69 (25%) reported the type of bedding used (Table 4). The diet was identified appropriately (that is, product number, mill date, lot number, and name of manufacturer) in only 21 (30%) of the 69 reports. The most frequently used misidentification phrase was ‘food and water were given ad libitum.’ Some examples of insufficiently identified diets included the use of terms such as standard diet, standard rodent diet, standard rodent chow, soy-based diet, pelleted diet, solid diet, standard mouse pellets, autoclaved diet, powdered diet, standard mouse chow, and regular rat chow; these descriptors make it impossible to know whether the diets were an inadvertent source of estrogen. Only 2 reports (3%) stated that a phytoestrogen-reduced diet was used. The most frequently reported diets used in BPA studies were the Purina 5008, 5001, 5010, and 5002 diets, and the most commonly reported rodent beddings were hardwood and corncob. The type of cages and water bottles used was reported in only 38% and 17%, respectively, of published studies on BPA (Table 4).
Studies on BPA published between 2005 and 2011.
Reviewing 103 publications from 2005 to 2011 (Table 4) revealed that only 69 of 103 (67%) reports provided information on the diet used. Furthermore, 20 of the 103 (19%) studies used phytoestrogen-reduced diets, 10 were identified as phytoestrogen-free purified diets (that is, AIN93G or AIN93M), and 10 were natural-ingredient, soy- and alfalfa-free, phytoestrogen-reduced diets. Although the use of these phytoestrogen-free diets was substantially increased over the previous period (19% compared with 3%), the data nevertheless imply that approximately 80% of studies may have used diets containing sufficient phytoestrogens to produce estrogenic responses. Furthermore, fewer than 5% of all reports provided the isoflavone content of the diets (Table 4). Despite recent increased awareness that diets containing high levels of phytoestrogens can significantly alter the results of estrogenic studies, it is surprising that the diet was identified appropriately in only 43 (42%) of the 103 BPA publications and was not reported or was misidentified in the remaining 60 (58%) publications (Table 4).
Route and time of BPA exposure.
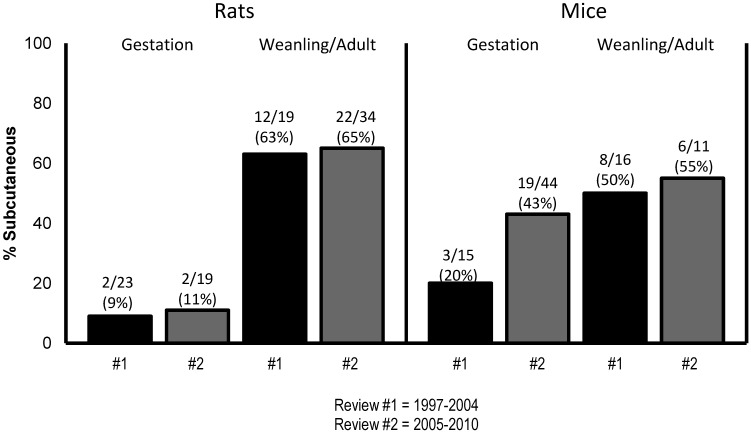
The findings regarding the route and age of exposure to BPA from both reviews (1997–2004, 2005–2010) are summarized in Table 5 and Figure 2; percentages do not always total 100% because multiple routes were used in some studies.
Table 5.
Summary of both literature reviews in regard to route and developmental stage for BPA exposure
| Developmental stage at initial exposure |
||||||||||
| Overall |
Gestation |
Weanling or adult |
||||||||
| Route of exposure (no. [%]) |
Route of exposure (no. [%]) |
Route of exposure (no. [%]) |
||||||||
| Publication date | No. of reports | Oral | SC | No. of reports | Oral | SC | No. of reports | Oral | SC | |
| Rats | ||||||||||
| 1997–2004 | 40 | 28 (70) | 14 (35) | 23 (58) | 21 (95) | 2 (9) | 19 (48) | 7 (37) | 12 (63) | |
| 2005–2010 | 51 | 27 (53) | 24 (47) | 19 (37) | 17 (89) | 2 (11) | 34 (67) | 12 (35) | 22 (65) | |
| Total | 91 | 55 (60) | 38 (42) | 42 (46) | 38 (90) | 4 (10) | 53 (58) | 19 (36) | 34 (64) | |
| Mice | ||||||||||
| 1997–2004 | 29 | 19 (66) | 12 (41) | 15 (52) | 12 (80) | 3 (20) | 16 (55) | 8 (50) | 8 (50) | |
| 2005–2010 | 52 | 30 (58) | 26 (50) | 44 (85) | 25 (57) | 19(43) | 11 (21) | 5 (45) | 6 (55) | |
| Total | 81 | 49 (60) | 38 (47) | 59 (73) | 37 (63) | 22 (37) | 27 (33) | 13 (48) | 14 (52) | |
‘Oral’ indicates exposure via feed, water, or gavage; ‘subcutaneous’ (SC) indicates through injection or osmotic pump. Numbers and percentages don't always agree because some studies used multiple species, routes, or both.
The percentage of mice dosed nonorally during gestation increased 23% between 1997–2004 and 2005–2010.
Figure 2.
Trends in the subcutaneous route of exposure to BPA in rats and mice during 1997–2004 and 2005–2010. These data indicate that the number of reports where mice were exposed to BPA via the SC route during gestation increased from 20% (1997–2004) to 43% (2005–2010). In 76 of 172 (44%) reports, rodents were exposed to BPA via the subcutaneous route.
In 60% of the studies using rats, animals were exposed to BPA orally (in feed or water, by gavage); in 42% of rat studies, animals were exposed via subcutaneous injections or osmotic pumps. In 46% of the rat studies, animals were exposed to BPA during gestation, and in 58% of studies, rats were exposed as weanlings or adults.
In 60% of the studies involving mice, animals were exposed orally (in feed or water, by gavage), and in 47%, mice were exposed via the other routes of administration (Table 5, Figure 2). In 73% of mouse studies, animals were exposed to BPA during gestation, and in 33% of the studies, mice were exposed as weanlings or adults.
Use of the oral compared with subcutaneous route of BPA exposure in published studies.
Trends in the use of subcutaneous BPA exposure in rats and mice from both reviews between 1997 to 2004 and 2005 to 2010 are shown in Figure 2 and Table 5. Overall, subcutaneous administration was used in approximately 10% of the gestational exposure studies in rats. By contrast, the subcutaneous route was used in more than 60% of studies involving weanling and adult rats. For mice, subcutaneous administration was used in approximately 20% of the gestational exposure studies in the early reports (1997 to 2004); this proportion increased to 43% in the later studies (2005 to 2010). Overall, weanling and adult mice were exposed to BPA subcutaneously in approximately 50% of studies in both reviews (Figure 2, Table 5). Most human BPA exposure occurs via oral ingestion;25,99 because the route of administration used in animal studies for human risk assessment should be the same as that of human exposure when conducting BPA studies in rodents for the purpose of extrapolating data to humans for evaluation of potential adverse health effects, the oral route of administration should be used.25 Exceptions to oral exposure can be exposure by the dermal route from topical administration.25 In addition, useful comparative data can be gained from studies using both subcutaneous and oral routes of exposure.
Effect of the bioavailability of BPA on study outcomes.
A recent review article25 about the effects of human exposure to BPA concluded that the current tolerable daily intake of BPA (50 µg/kg) is adequately justified and that the available evidence indicates that BPA exposure represents no noteworthy risk to human health, including that of newborns and infants. Factors considered in coming to these conclusions were: 1) most human BPA exposure occurs via oral ingestion; 2) only animal studies using the oral route have relevance to human risk assessment;25 3) many of the studies showing adverse effects at low doses of BPA used subcutaneous injection or delivered BPA by osmotic pumps;25 4) and the adverse effects observed after subcutaneous administration of BPA may be due to the lack of first-pass metabolism and to the slow release of BPA from the oil suspensions injected.
Conclusions from Our Studies and Literature Reviews
Our review of the literature indicates that the reporting of the types of diet, bedding, water bottles, and cages used in rodent studies of BPA are inconsistent among studies (Table 4). In 76 of the 172 (44%) reports evaluated, animals were exposed to BPA via the subcutaneous route. In addition, only 64 of the 172 reports (37%) accurately described the rodent diet. Furthermore, none of the reports indicated whether the maternal diet was assayed for BPA.
Animal studies designed to determine the adverse effects of EDC should carefully consider the choice of the maternal and experimental diets, bedding, cages, and water bottles used, to minimize the potential effect of exogenous estrogenic substances on study outcomes. In the future, investigators should consider the potential effects of dietary phytoestrogens and the estrogenic mycotoxin zearalenone in corncob bedding.38,75 Of the studies reporting low-dose effects of BPA, the most frequently used diets were the Purina 5008, 5010, 5002, and 5001 diets and Harlan 8656 breeder diets, which contain high variable levels of phytoestrogens (Tables 1 and 4); these choices inevitably will yield to inconsistent results regarding the estrogenicity of BPA. The estrogenic potential of isoflavones in these diets far exceeds that of the low levels of BPA being tested, making it difficult to accurately estimate a LOAEL for BPA from these studies. We conclude that diet, bedding, animal species, strain, timing, and route of administration are crucial factors that influence the effects of the compound being tested, including BPA.
Final Recommendations
In summary, we offer the following recommendations regarding the design and conduct of rodent studies evaluating the various effects of EDC. First, we suggest that increased consideration should be given to the content of phytoestrogens and methyl donors in the maternal rodent diet when performing studies evaluating the estrogenic activity of EDC such as BPA. Second, experimental diets for studies of potential EDC should contain low or no phytoestrogens, to reduce extraneous variability in estrogen-responsive endpoints. Ideally, an estrogen-free diet, bedding, caging, and water bottles should be used for studies where the primary goal is to determine the estrogenic activity of BPA or other EDC. Diet and housing materials should be tested for BPA to help characterize the background levels to the test compound. Third, if rodents are purchased from a supplier that uses a high-phytoestrogen diet or corncob bedding, the F0 generation should be placed on a low-phytoestrogen diet, with paper or hardwood bedding as soon as they arrive at the research facility (ideally, beginning at weaning) and then bred inhouse to ensure that maternal exposure to exogenous estrogens is minimized. Maternal diets should be tested for BPA, phytoestrogens, and mycoestrogens, to ensure that rodents are exposed to as few exogenous estrogens as possible. Fourth, for animal studies that will provide data for human risk assessment, the route of exposure used in the study should be the same as that for human exposure to the EDC of interest. Fifth, journals and reviewers should require investigators to sufficiently describe the diet, bedding, caging, and water bottles used and, when possible, the phytoestrogen content (daidzein and genistein) of the diet and bedding (zearalenone) should be determined for all EDC studies, including BPA. Finally, both the aglycone (active) and conjugated (inactive) BPA contents in serum, urine, feces, and any other relevant tissues should be measured when assessing the effects of BPA in rodent or human studies.
Acknowledgments
We thank Drs John D Roberts, Diane Forsythe, Suzanne R Fenton, and Humphrey Hung-Chang Yao for their excellent suggestions during their internal review of the manuscript. We thank Drs Daniel R Doerge, K Barry Delclos, and Frederick A Beland as outside reviewers from the US Food and Drug Administration at the National Center for Toxicology Research (Jefferson, AR) for their excellent suggestions. In addition, we thank Dr Janos Zempleni for his helpful suggestions and comments on the effect of DNA methyl donors in the maternal diet on epigenetic gene expression. We also thank Mrs Meg Fender, Mrs Vigdis Engebretsen, and Mrs Jessica Uhlir for their excellent suggestions and typing of the manuscript. The opinions expressed in this article do not necessarily represent those of the US Food and Drug Administration.
References
- 1.Ashby J, Tinwell H. 1998. Uterotrophic activity of bisphenol A in the immature rat. Environ Health Perspect 106:719–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashby J, Tinwell H, Hasman J. 1999. Lack of effects for low dose levels of bisphenol A and diethylstilbestrol on the prostate gland of CF1 mice exposed in utero. Regul Toxicol Pharmacol 30:156–166 [DOI] [PubMed] [Google Scholar]
- 3.Barnard DE, Lewis SM, Teter BB, Thigpen JE. 2009. Open and closed formula laboratory animal diets and their importance to research. J Am Assoc Lab Anim Sci 48:709–711 [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal AJ, Jirtle RL. 2010. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol 88:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickoff EM, Livingston AL, Henderson AP, Booth AN. 1962. Forage estrogen: relative potencies of several estrogen-like compounds in forages. J Agric Food Chem 10:410–412 [Google Scholar]
- 6.Borrell B. 2010. Toxicology: the big test for bisphenol A. Nature 464:1122–1124 [DOI] [PubMed] [Google Scholar]
- 7.Brown NM, Setchell KD. 2001. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 81:735–747 [DOI] [PubMed] [Google Scholar]
- 8.Cagen SZ, Waechter JM, Jr, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. 1999. Normal reproductive organ development in CD1 mice following prenatal exposure to bisphenol A. Toxicol Sci 50:36–44 [DOI] [PubMed] [Google Scholar]
- 9.Cagen SZ, Waechter JM, Jr, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. 1999. Normal reproductive organ development in Wistar rats exposed to bisphenol A in the drinking water. Regul Toxicol Pharmacol 30:130–139 [DOI] [PubMed] [Google Scholar]
- 10.Cederroth CR, Vinciguerra M, Kühne F, Madani R, Doerge DR, Visser TJ, Foti M, Rohner-Jeanrenaud F, Vassalli JD, Nef S. 2007. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ Health Perspect 115:1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. 2008. NTP–CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 83:157–395 [DOI] [PubMed] [Google Scholar]
- 12.Cooney CA, Dave AA, Wolff GL. 2002. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132 Suppl:2393S–2400S [DOI] [PubMed] [Google Scholar]
- 13.Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H. 2000. Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol 73:1–10 [DOI] [PubMed] [Google Scholar]
- 14.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. 2010. Pharmacokinetics of bisphenol A in neonatal and adult Sprague–Dawley rats. Toxicol Appl Pharmacol 247:158–165 [DOI] [PubMed] [Google Scholar]
- 15.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. 2011. Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice; inter-species comparisons with Sprague–Dawley rats and rhesus monkeys. Toxicol Lett 207:298–305 [DOI] [PubMed] [Google Scholar]
- 16.Doerge DR, Twaddle NC, Woodling KA, Fisher JW. 2010. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicol Appl Pharmacol 248(1):1–11 [DOI] [PubMed] [Google Scholar]
- 17.Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. 2010. Lactational transfer of bisphenol A in Sprague–Dawley rats. Toxicol Lett 199:372–376 [DOI] [PubMed] [Google Scholar]
- 18.Dolinoy D, Huang D, Jirtle R. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 104:13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolinoy D, Weidman JR, Waterland RA, Jirtle RL. 2006. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 114:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Entine J. [Internet]. End game on bisphenol A? Have we reached a tipping point on the science of this ubiquitous chemical? Huffington Post, 17 October 2011. [Cited September 2012. Available at: http://www.huffingtonpost.com/jon-entine/end-game-on-bisphenol-a-h_b_1014730.html.
- 21.Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HH. 1975. Potential value of plants as sources of new antifertility agents I. J Pharm Sci 64:535–598 [PubMed] [Google Scholar]
- 22.Fenton SE, Reed C, Newbold RR. 2012. Perinatal environmental exposures affect mammary development, function, and cancer risk in adulthood. Annu Rev Pharmacol Toxicol 52:455–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SA, Law CD, Jr, Abshire JS. 2011. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol Sci 124:149–160 [DOI] [PubMed] [Google Scholar]
- 24.Gray LE, Ryan B, Hotchkiss AK, Crofton KM. 2010. Rebuttal of “Flawed Experimental Design Reveals the Need for Guidelines Requiring Appropriate Positive Controls in Endocrine Disruption Research.”. Toxicol Sci 115:614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U. 2011. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol 41:263–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr 2008. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male Long–Evans hooded rat. Toxicol Sci 102:371–382 [DOI] [PubMed] [Google Scholar]
- 28.Howdeshell KL, Peterman PH, Judy GM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV. 2003. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect 111:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. 2003. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13:546–553 [DOI] [PubMed] [Google Scholar]
- 30.Ichi S, Costa FF, Bischof JM, Nakazaki H, Shen YW, Boshnjaku V, Sharma S, Mania-Farnell B, McLone DG, Tomita T, Soares MB, Mayanil CS. 2010. Folic acid remodels chromatin on Hes1 and Neurog2 promoters during caudal neural tube development. J Biol Chem 285:36922–36932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, Kiyama R. 2005. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett 579:1732–1740 [DOI] [PubMed] [Google Scholar]
- 32.Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. 2003. The OECD program to validate the rat uterotrophic bioassay. Phase 2: coded single-dose studies. Environ Health Perspect 114:1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavlock RT, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. 1996. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the US EPA-sponsored workshop. Environ Health Perspect 104 suppl 4:715–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendziorski JA, Kendig EL, Gear RL, Belcher SM. 2012. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17α-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol 34:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YI. 2004. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen 44:10–25 [DOI] [PubMed] [Google Scholar]
- 36.Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt P. 2010. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod 84:79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le HH, Carlson EM, Chua JP, Belcher SM. 2008. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locklear J, Thigpen J, Glassbrook N, Caviness GF, Kissling G, Hackney S, Rooney M, Grant MG, Forsythe DB. 2010. Corncob bedding spiked with zearalenone significantly advances the timing of vaginal opening in immature CD1 haired and SKH1 hairless mice. J Am Assoc Lab Anim Sci 49:743 [Google Scholar]
- 39.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. 2001. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect 109:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews JB, Twomey K, Zacharewski TR. 2001. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol 14:149–157 [DOI] [PubMed] [Google Scholar]
- 41.Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, Hunt PA. 2009. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod 80:1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, Daston GP. 2002. Gene expression profile induced by 17α-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci 68:184–199 [DOI] [PubMed] [Google Scholar]
- 43.National Toxicology Program 2008. NTP–CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NIH publication no. 08-5994. Bethesda (MD): NIH [Google Scholar]
- 44.National Toxicology Program. [Internet]. Final report of the endocrine disruptors low-dose peer review panel. Raleigh, NC. [Cited 15 November 2011]. Available at: http://ntp.niehs.nih.gov/index.cfm?objectid=06F5CE98-E82F-8182-7FA81C02D3690D47.
- 45.Organization for Economic Cooperation and Development. [Internet]. 2006. Draft test guideline on the uterotrophic bioassay in rodents: a short-term screening test for oestrogenic properties. [Cited 2 November 2007]. Available at: http://oecd.org/dataoecd/38/15/37773938.pdf.
- 46.Owens W, Ashby J, Onyon L. 2003. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dietary phytoestrogen analyses. Environ Health Perspect 111:1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padilla-Banks E, Jefferson WN, Myers PH, Goulding DR, Williams CJ. 2012. Neonatal phytoestrogen exposure causes hypospadias in female mice. Mol Reprod Dev 79:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patisaul HB, Fortino AE, Polston EK. 2006. Neonatal genistein or bisphenol A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol 28:111–118 [DOI] [PubMed] [Google Scholar]
- 49.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM., Jr 2000. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci 54:3–18 [DOI] [PubMed] [Google Scholar]
- 50.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. 2011. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague–Dawley rats. Reprod Toxicol 31:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, Vom Saal FS. 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS. 2008. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the fetal estrogenization syndrome and obesity in CD1 mice. Environ Health Perspect 116:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruhlen RL, Taylor JA, Maoa J, Kirkpatrick J, Welshons WV, vom Saal FS. 2011. Choice of animal feed can alter fetal steroid levels and mask developmental effects of endocrine disrupting chemicals. J Dev Orig Health Dis 23:36–48 [Google Scholar]
- 54.Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr 2010. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci 114:133–148 [DOI] [PubMed] [Google Scholar]
- 55.Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. 2010. Perinatal exposure to bisphenol A and the development of metabolic syndrome in CD1 mice. Endocrinology 151:2603–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satih S, Chalabi N, Rabiau N, Bosviel R, Fontana L, Bignon YJ, Bernard-Gallon DJ. 2010. Gene expression profiling of breast cancer cell lines in response to soy isoflavones using a pangenomic microarray approach. OMICS 14:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Setchell KDR.1985. Naturally occurring nonsteroidal estrogens of dietary origin, p 69–85. In: McLachlan JA. Estrogens in the environment. New York (NY): Elsevier Science Publishing.
- 58.Setchell KDR, Adlercreutz H.1988. Mammalian lignans and phytoestrogens. Recent studies on their formation, metabolism, and biological role in health and disease, p 315–345. In: Rowland I. Role of the gut flora in toxicity and cancer. London (UK): Academic Press.
- 59.Setchell KDR, Brown NM, Zhao X, Lindley SL, Heubi JE, King EC, Messina MJ. 2011. Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr 94:1284–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setchell KDR, Clerici C. 2010. Equol: history, chemistry, and formation. J Nutr 140:1355S–1362S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shioda T, Chesnes J, Coser KR, Zou L, Hur J, Dean KL, Sonnenschein C, Soto AM, Isselbacher KJ. 2006. Importance of dosage standardization for interpreting transcriptomal signature profiles: evidence from studies of xenoestrogens. Proc Natl Acad Sci USA 103:12033–12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. 2010. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharm Res 27:1115–1127 [DOI] [PubMed] [Google Scholar]
- 63.Stein RA. 2012. Epigenetics and environmental exposures. J Epidemiol Community Health 66:8–13 [DOI] [PubMed] [Google Scholar]
- 64.Stump DG, Beck MJ, Radovsky A, Garman RH, Freshwater LL, Sheets LP, Marty MS, Waechter JM, Jr, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Chappell AH, Hentges SG. 2010. Developmental neurotoxicity study of dietary bisphenol A in Sprague–Dawley rats. Toxicol Sci 115:167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK. 2011. Twenty-four–hour human urine and serum profiles of bisphenol A during high-dietary exposure. Toxicol Sci 123:48–57 [DOI] [PubMed] [Google Scholar]
- 66.Thigpen JE, Haseman J, Locklear J, Ahlmark K, Caviness GF, Willamson RL, Goelz MF, Forsythe D. 1998. Comparative estrogenic activity of 3 new closed-formula natural ingredient diets formulated to reduce the concentration of phytoestrogens. Contemp Top Lab Anim Sci 37:10312456145 [Google Scholar]
- 67.Thigpen JE, Haseman JK, Saunders H, Whiteside T, Locklear J, Caviness GF, Grant MG, Forsythe DB. 2003. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD1 mice. Comp Med 53:607–615 [PubMed] [Google Scholar]
- 68.Thigpen JE, Joshi S, Caviness GF, Whiteside TE, Locklear J, Grant MG, Forsythe DB. 2006. Survey of various types of rodent bedding for estrogenic activity. J Am Assoc Lab Anim Sci 45:87 [Google Scholar]
- 69.Thigpen JE, Kissling G, Collins B, Setchell KDR, Adsit F, Caviness GF, Locklear J, Whiteside T, Grant MG, Forsythe DB. 2010. The impact of rodent diets and bedding in studies evaluating the estrogenic activity of endocrine disruptor compounds (EDCs). J Am Assoc Lab Anim Sci 49:748 [Google Scholar]
- 70.Thigpen JE, Kissling G, Locklear J, Caviness G, Whiteside T, Setchell K, Brown N, Padilla-Banks E, Hackney S, Grant M, Forsythe DB. 2010. Effects of rodent diets containing high or low levels of phytoestrogens on time of vaginal opening in 4 rat strains. J Am Assoc Lab Anim Sci 49:741 [Google Scholar]
- 71.Thigpen JE, Lebetkin EH, Dawes ML, Richter CB, Crawford D. 1987. The mouse bioassay for the detection of estrogenic activity in rodent diets. III. Stimulation of uterine weight by dextrose, sucrose and corn starch. Lab Anim Sci 37:606–609 [PubMed] [Google Scholar]
- 72.Thigpen JE, Li LA, Richter CB, Lebetkin EH, Jameson CW. 1987. The mouse bioassay for the detection of estrogenic activity in rodent diets I. A standardized method for conducting the mouse bioassay. Lab Anim Sci 37:596–601 [PubMed] [Google Scholar]
- 73.Thigpen JE, Li LA, Richter CB, Lebetkin EH, Jameson CW. 1987. The mouse bioassay for the detection of estrogenic activity in rodent diets II. Comparative estrogenic activity of purified certified and standard open- and closed-formula rodent diets. Lab Anim Sci 37:602–605 [PubMed] [Google Scholar]
- 74.Thigpen JE, Locklear J, Haseman JK, Saunders HE, Caviness G, Grant MF, Forsythe DB. 2002. Dietary factors affecting uterine weights of immature CD1 mice used in uterotrophic bioassays. Cancer Detect Prev 26:381–393 [DOI] [PubMed] [Google Scholar]
- 75.Thigpen JE, Padilla-Banks E, Kissling G, Caviness GF, Whiteside T, Locklear J, Grant MG, Forsythe DB. 2008. Commercially available corncob bedding naturally contaminated with zearalenone significantly advances the time of vaginal opening in immature CD1 mice. J Am Assoc Lab Anim Sci 47:159 [Google Scholar]
- 76.Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. 1999. Phytoestrogen content of purified open- and closed-formula laboratory animal diets. Lab Anim Sci 49:530–536 [PubMed] [Google Scholar]
- 77.Thigpen JE, Setchell KDR, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. 2007. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD1 mice and F344 rats but not in CD Sprague–Dawley rats. Environ Health Perspect 115:1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thigpen JE, Setchell KDR, Locklear J, Kissling G, Caviness GF, Whiteside T, Belcher S, Brown N, Collins B, Lih F, Tomer K, Grant M, Forsythe D. 2011. The effects of bisphenol A on the timing of vaginal opening in CD1 mice fed a high- or low- phytoestrogen diet. J Am Assoc Lab Anim Sci 50:744 [Google Scholar]
- 79.Thigpen JE, Setchell KDR, Saunders HE, Haseman JK, Grant MG, Forsythe DB. 2004. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J 45:401–416 [DOI] [PubMed] [Google Scholar]
- 80.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J. 2002. Normal sexual development of 2 strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci 68:339–348 [DOI] [PubMed] [Google Scholar]
- 81.Tyl R. 2010. Good laboratory practices are not synonymous with good scientific practices, accurate reporting, or valid data: Tyl responds. Environ Health Perspect 118:A60–A61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter JM., Jr 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD1 (Swiss) mice. Toxicol Sci 104:362–384 [DOI] [PubMed] [Google Scholar]
- 83.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM. 2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague–Dawley rats. Toxicol Sci 68:121–146 [DOI] [PubMed] [Google Scholar]
- 84.Underhill KL, Rotter BA, Thompson BK, Prelusky DB, Trenholm HL. 1995. Effectiveness of cholestyramine in the detoxification of zearalenone as determined in mice. Bull Environ Contam Toxicol 54:128–134 [DOI] [PubMed] [Google Scholar]
- 85.US Environmental Protection Agency. [Internet]. 1996. Guidelines for reproductive toxicity risk assessment. EPA/630/R-96/009, Oct 1996. [Cited September 2012]. Available at: http://www.epa.gov/raf/publications/guidelines-reproductive-tox-riskassessment.htm.
- 86.US Environmental Protection Agency. 2009. OPPTS 890.1600. Uterotrophic assay: endocrine disruptor screening program test guidelines. Washington (DC): Environmental Protection Agency. [PubMed]
- 87.US Environmental Protection Agency. 2011. OPPTS 890.1600. Uterotrophic assay: endocrine disruptor screening program test guidelines. Washington (DC): Environmental Protection Agency. [PubMed]
- 88.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee D, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33: 378–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. 2007. Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177 [DOI] [PubMed] [Google Scholar]
- 91.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum L, Crain A, Eriksen M, Farabollini F, Guillette L, Hauser R, Heindel J, Ho S, Hunt P, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. 2007. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals, and potential to impact human health at current levels of exposure. Reprod Toxicol 24:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vom Saal FS, Akingbemi BT, Belcher SM, Crain DA, Crews D, Guidice LC, Hunt PA, Leranth C, Myers JP, Nadal A, Olea N, Padmanabhan V, Rosenfeld CS, Schneyer A, Schoenfelder G, Sonnenschein C, Soto AM, Stahlhut RW, Swan SH, Vandenberg LN, Wang HS, Watson CS, Welshons WV, Zoeller RT. 2010. Flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research. Toxicol Sci 115:612–613 [DOI] [PubMed] [Google Scholar]
- 94.Vom Saal FS, Myers JP. 2010. Good laboratory practices are not synonymous with good scientific practices, accurate reporting, or valid data. Environ Health Perspect 118:A60–A61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vom Saal FS, Prins GS, Welshons WV. 2012. Report of very low, real-world exposure to bisphenol A is unwarranted based on a lack of data and flawed assumptions. Toxicol Sci 125:318–320 [DOI] [PubMed] [Google Scholar]
- 96.Vom Saal FS, Welshons WV. 2005. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res 100:50–76 [DOI] [PubMed] [Google Scholar]
- 97.Welshons W, Thayer K, Judy B, Taylor J, Curran E, Vom Saal F. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolff GL, Kodell RL, Mooree SR, Cooney CA. 1998. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12:949–957 [PubMed] [Google Scholar]
- 99.World Health Organization, Food and Agriculture Organization. [Internet]. 2010. Summary report of the joint FAO–WHO expert meeting to review toxicological and health aspects of bisphenol A. [CitedSept. 2012. Available at: http://www.who.int/foodsafety/chem/chemicals/BPA_Summary2010.pdf.
- 100.Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. 2011. Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect 119:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]