Abstract
We evaluated the effect of repeated intraperitoneal administration of tribromoethanol on various parameters in C57BL/6NHsd mice. Mice (n = 68) were randomly assigned to 1 of 7 groups to receive tribromoethanol (500 mg/kg IP) on day 0 or days 0 and 8; vehicle (tert-amyl alcohol in sterile water) only on day 0 or days 0 and 8; sterile water injection on day 0 or days 0 and 8; or no treatment. A single dose of tribromoethanol failed to produce loss of pedal reflex and had no effect on median food and water consumption but altered median body weight on days 1 through 4 when compared with that in mice that received vehicle only or no treatment. Median body weight did not differ between mice that received a single dose of tribromoethanol and those that received an injection of water. Among mice given 2 doses of tribromoethanol, induction time, anesthetic duration, and recovery time varied widely. Repeated administration of tribromoethanol had no effect on median food and water consumption or body weight compared with those in controls. Median liver weight was significantly greater in mice that received 2 doses compared with a single dose of tribromoethanol. Median liver weight did not differ between untreated mice and those that received tribromoethanol. No significant organ or tissue pathology was observed in any study animal. Although tribromoethanol did not produce morbidity, mortality, or pathologic changes in treated animals, we urge caution in use of tribromoethanol in C57BL/6NHsd mice due to its variable anesthetic effectiveness.
Tribromoethanol, a nonpharmaceutical-grade anesthetic, has been used extensively for various manipulations in laboratory rodents due to its ready availability, lack of state and federal drug regulations associated with its use, and rapid anesthetic induction and recovery times.10 Tribromoethanol is commercially available as a white crystalline powder that, when reconstituted and administered, produces generalized CNS depression, including depression of respiratory and cardiovascular centers.11 Despite routine use, tribromoethanol use in rodents remains controversial due to contradictory reports regarding the compound's efficacy and associated pathology and mortality.8,13,14,15,16,20 Morbidities reported with tribromoethanol use in mice include intestinal ileus, peritonitis, muscle necrosis, serositis of abdominal organs, and death.8,16,20 In an attempt to balance animal welfare concerns and investigator needs, many IACUC have developed guidelines for tribromoethanol use, including prohibiting its repeated use in individual animals. A single study published in 1979 described high mortality after repeated tribromoethanol injection,5 and although no experimental details were provided, the report likely has influenced institutional policies.
In the current study, we determined the effect of repeated tribromoethanol administration on induction time, anesthetic duration, recovery time, food and water consumption, body weight, morbidity, mortality, and pathology in C57BL/6NHsd mice. To our knowledge, this study is the first to thoroughly evaluate the safety and efficacy of repeated tribromoethanol administration.
Materials and Methods
Animals.
Female C57BL/6NHsd mice (n = 68; age, 38 to 45 d) were obtained from Harlan Laboratories (Haslett, MI). Mice were singly housed in static, polysulfone, microisolation caging on corncob and cellulose bedding (Harlan Teklad, Madison, WI) and maintained on a 12:12-h light:dark cycle. Mice were provided tap water and standard, pelleted, rodent chow (Harlan Teklad) ad libitum. Caging, food, and water bottles were changed weekly by using aseptic technique within a laminar flow transfer station (Nuaire, Plymouth, MN). Mice were used pursuant to an IACUC-approved protocol and were housed and cared for in compliance with the Guide for the Care and Use of Laboratory Animals6 in an AAALAC-accredited program.
Group allocation.
Mice were acclimated for 5 d prior to experimental use. After acclimation, mice were randomly assigned to 1 of 7 groups to receive tribromoethanol (500 mg/kg IP) on day 0 or days 0 and 8; vehicle (tert-amyl alcohol in sterile water) intraperitoneally on day 0 or days 0 and 8; sterile water intraperitoneally on day 0 or days 0 and 8; or no treatment. All groups contained 10 mice each, except for the no-treatment group (n = 8).
Tribromoethanol.
The tribromoethanol dose (500 mg/kg IP) used in this study followed the preparation and dosing recommendations outlined by our IACUC.19 Briefly, a 1.61-g/mL stock solution was prepared by adding 6.2 mL tert-amyl alcohol (Sigma–Aldrich, St Louis, MO) to 10 g 2,2,2-tribromoethanol (99%; lot number A0278709, Acros Organics, Geel, Belgium). A 25-mg/mL working solution was prepared by adding 0.78 mL of the tribromoethanol stock solution to 49.2 mL tissue-grade double-distilled H20 (Fisher Scientific, Pittsburgh, PA); the working solution was filtered through a 0.2-mm syringe filter (Fisher Scientific) prior to injection in mice. The stock solution was made 1 d prior to injection and allowed to stir overnight at room temperature; the working solution was made immediately prior to injection. The pH of the working solution was not determined. Tribromoethanol powder from the same bottle and lot number was used throughout this study. The vehicle-only solution contained the same ratio of tert-amyl alcohol and sterile water as that in the tribromoethanol working solution.
Tribromoethanol efficacy and safety.
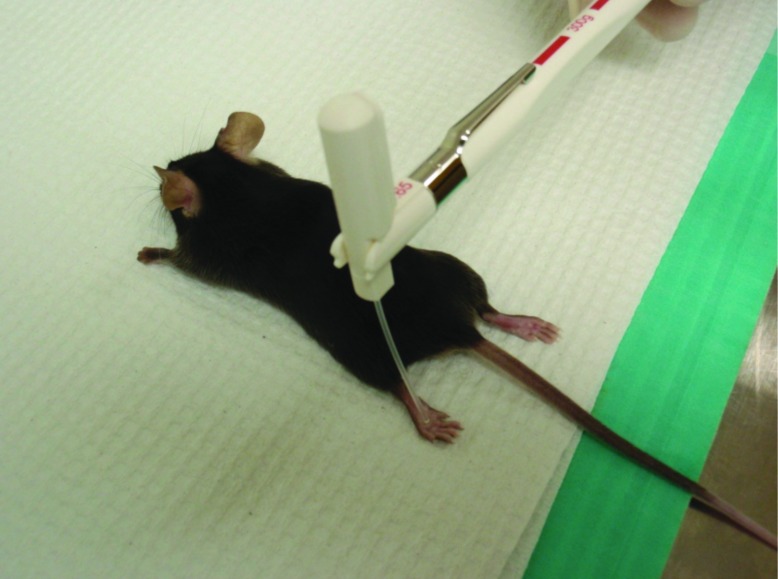
Intraperitoneal injections were performed according to group allocation and current body weight and by a single investigator (JT). All mice (except the untreated group) received injection volumes of 0.31 to 0.44 mL. After tribromoethanol injection, mice were maintained on a circulating-water heating pad (Gaymar Industries, Orchard Park, NY) and monitored for anesthetic induction time, duration of anesthesia, and recovery time. Induction time was defined as the time from tribromoethanol administration to loss of the pedal reflex. Anesthetic duration was defined as the time between the loss and return of pedal reflex. Recovery time was defined as the time between return of the pedal reflex to movement around the primary enclosure. According to a previously described procedure,8 we assessed the pedal reflex by using a Touch-Test Sensory Evaluator (North Coast Medical, Gilroy, CA) with a target force of 300 g (Figure 1); a single investigator (CC) performed all assessments. Presence of the pedal reflex was defined as withdrawal of the limb on contact by the sensory evaluator. Body weight and food and water intake were measured daily by using a digital laboratory scale (Denver Instrument, Arvada, CO). Daily intake was quantified by determining the remaining mass of offered food and water.
Figure 1.
Equipment used to evaluate the pedal reflex after tribromoethanol administration.
Pathologic evaluation.
Mice that received injections only on day 0 were euthanized on day 4; those injected on days 0 and 8 were euthanized on day 11. Untreated mice were euthanized on day 4 (n = 3) or 11 (n = 5). All mice were euthanized via cervical dislocation under CO2 anesthesia. After euthanasia, each mouse was necropsied, and gastrointestinal tract, spleen, and liver were harvested and immediately weighed. The stomach, small intestine, cecum, colon, liver, spleen, and a sample of the body wall were collected for histopathologic evaluation. Tissues were fixed in 10% formalin, stained with hematoxylin and eosin, and evaluated by a single veterinary pathologist (KN) blinded to treatment group. By using a previously published algorithm for evaluation of tribromoethanol-induced changes,8 histopathologic lesions were scored on a scale of 0 to 4 reflecting the severity of inflammation and percentage of organ affected.
Statistical analysis.
All statistical analyses were performed by using SAS version 9.2 (SAS Institute, Cary, NC). Mixed-model ANOVA (PROC MIXED) was used to evaluate food and water consumption and change in body weight over time until day 4 for all groups (that is, 1- and 2-injection mice were combined into a single group, thus yielding 4 groups for analysis) and during days 5 through 11 for untreated mice and those that received injections on days 0 and 8. The model included mouse ID, treatment, and day as class variables. Treatment, day, and the interaction of treatment and day were included as independent variables; day was treated as a repeated measure with mouse ID within treatment as subject. Mouse ID was treated as a random factor in the model. A second mixed-model ANOVA was used to assess the effect of treatment (1 or 2 doses) on liver and gastrointestinal tract weights at necropsy. In this model mouse ID and treatment were included as class variables, and treatment and mouse body weight at necropsy were included as independent variables. The fit of both models to the data was evaluated by comparing the residuals from the model to a normal distribution by using the test statistic of Shapiro–Wilk. When necessary, to meet the assumption of a normal distribution or residuals from the model, data were rank-transformed. The procedure of Bonferroni was used to adjust for multiple comparisons among treatment groups. Induction time, anesthetic duration, and recovery time were summarized for group 2 using the median and range. Data, whether transformation was necessary for the purposes of statistical analysis, are presented as raw means and range for each treatment group over the stated time period, for the purpose of consistency. A P value of less than or equal to 0.05 was used to determine statistical significance in all tests.
Results
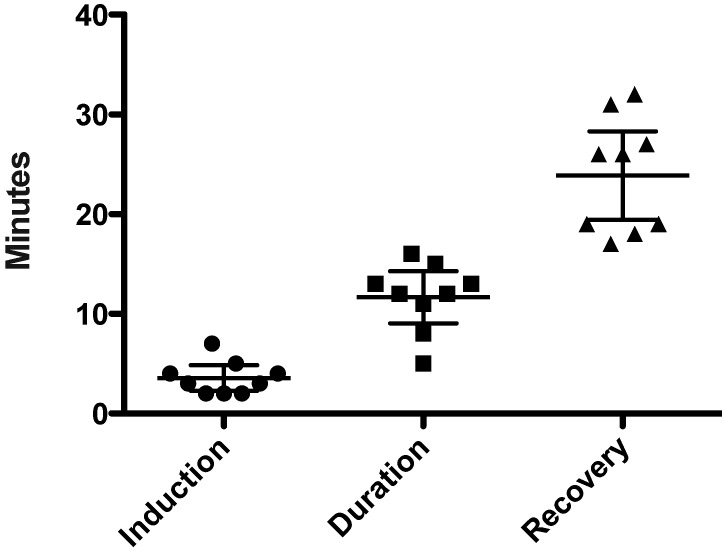
All mice (n = 20) that received tribromoethanol became recumbent yet retained pedal reflex after a single dose of tribromoethanol administration; 9 of the 10 mice that received a second dose 8 d after the first lost the pedal reflex after the repeated administration. In these 9 mice, induction time ranged from 2.0 to 7.0 min (median, 3.0 min), anesthetic duration ranged from 5.0 to 16.0 min (median, 12.0 min), and recovery time ranged from 17.0 to 32.0 min (median, 26.00 min; Figure 2). Tribromoethanol administration decreased (P < 0.05) median body weight on days 1 through 4 when compared with that of mice that received vehicle or no treatment but not sterile water (Table 1). Values for food consumption, water consumption, and body weight on days 5 through 11 are shown in Table 2. Median liver weight was significantly (P < 0.05) greater in mice that received tribromoethanol on days 0 and 8 than in those given tribromoethanol on day 0 only but did not differ between tribromoethanol-treated and untreated mice (Table 3). Splenic weights were 0.00 g for all mice. No gross or microscopic organ or tissue pathology was observed in any study animal, and a histopathologic score of 0 was assigned to all tissues analyzed.
Figure 2.
Anesthesia times (min) for 9 mice that reached a surgical anesthetic plane after repeated administration of tribromoethanol (500 mg/kg on days 0 and 8). Induction time was defined as the time between anesthetic administration to loss of pedal reflex. Anesthetic duration was defined as time between loss and return of pedal reflex. Recovery time was defined as time from return of pedal reflex to movement around the primary enclosure. Each symbol represents an individual mouse, and the horizontal bars represent the mean time ± 95% confidence interval.
Table 1.
Food and water consumption and body weight (g) in mice over days 1 through 4 after a single dose of tribromoethanol, vehicle, or sterile water
| Parameter | Treatment | Median (range) |
| Food intake | Tribromoethanol | 3.45 (2.40—6.60)a |
| Vehicle | 3.85 (2.40—7.00)a | |
| Water | 4.10 (2.80—6.30)a | |
| Untreated | 4.25 (0.00—7.60)a | |
| Water intake | Tribromoethanol | 9.45 (4.20—12.60)a,b |
| Vehicle | 10.10 (4.20—15.30)b | |
| Water | 9.60 (6.20—20.50)a | |
| Untreated | 8.55 (3.60—12.90)a,b | |
| Body weight | Tribromoethanol | 17.30 (15.30—19.70)c |
| Vehicle | 18.00 (16.10—19.80)a,b | |
| Water | 18.05 (15.40—21.70)b,c | |
| Untreated | 19.45 (17.50—21.80)a |
Treatment groups contained 20 mice each, except for the untreated controls (n = 8).
Within each parameter, values with different superscript letters are significantly (P < 0.05) different.
Table 2.
Food and water consumption and body weight (g) for mice over days 5 through 11 after repeated administration (that is, 2 doses) of tribromoethanol, vehicle, or sterile water
| Parameter | Treatment* | Median(range) |
| Food | Tribromoethanol | 4.45 (2.20—23.10)a,b |
| Vehicle | 3.85 (2.00—5.90)b | |
| Water | 4.55 (2.60—26.20)a | |
| Untreated | 4.60 (2.80—13.60)a,b | |
| Water | Tribromoethanol | 8.80 (4.10—12.30)a |
| Vehicle | 8.75 (5.80—13.30)a | |
| Water | 8.70 (6.30—38.00)a | |
| Untreated | 8.45 (6.40—12.40)a | |
| Body weight | Tribromoethanol | 19.40 (14.10—22.00)a |
| Vehicle | 20.40 (17.40—22.10)a | |
| Water | 18.70 (15.50—23.30)a | |
| Untreated | 20.35 (19.20—22.50)a |
Treatment groups contained 10 mice each, except for the untreated controls (n = 5).
Within each parameter, values with different superscript letters are significantly (P < 0.05) different.
Table 3.
Weights (g) of liver and gastrointestinal tract after 1 or 2 doses of tribromoethanol, vehicle, or sterile water
| Parameter | Treatment* | n | Median(range) |
| Liver | 1Tribromoethanol | 10 | 0.85 (0.70—1.00)b |
| 2Tribromoethanol | 10 | 1.10 (0.80—1.40)a | |
| 1Vehicle | 10 | 0.90 (0.90—1.20)a,b | |
| 2Vehicle | 10 | 1.15 (1.00—1.40)a,b | |
| 1Water | 10 | 1.05 (0.90—1.20)a | |
| 2Water | 10 | 1.20 (0.90—1.30)a | |
| 1Untreated | 3 | 1.20 (0.90—1.30)a,b | |
| 2Untreated | 5 | 1.20 (1.10—1.40)a,b | |
| Gastrointestinal tract | 1Tribromoethanol | 10 | 2.15 (2.00—2.60)c |
| 2Tribromoethanol | 10 | 2.60 (2.00—3.10)b,c | |
| 1Vehicle | 10 | 2.40 (2.20—2.70)b,c | |
| 2Vehicle | 10 | 2.75 (2.60—3.30)b,c | |
| 1Water | 10 | 2.75 (2.30—3.20)a,b | |
| 2Water | 10 | 3.00 (2.50—22.2)a | |
| 1Untreated | 3 | 2.80 (2.40—2.90)a,b,c | |
| 2Untreated | 5 | 3.00 (2.50—3.40)a,b,c |
Mice in that each received a single dose of tribromoethanol (or control) were euthanized on day 4; those that received 2 doses were euthanized on day 11.
Within each parameter, values with different superscript letters are significantly (P < 0.05) different.
Discussion
In veterinary practice, tribromoethanol has been used in cats, dogs, other mammals, reptiles, and birds.12 Although pharmaceutical-grade tribromoethanol is no longer commercially available, the anesthetic is still widely used for various manipulations in laboratory rodents including production of transgenic animals. The use of nonpharmaceutical-grade compounds has been discouraged by the USDA and Office of Laboratory Animal Welfare unless such use has been approved by the IACUC and is scientifically justified and when no acceptable veterinary or human pharmaceutical-grade compound exists.17,18 Given the extensive use of C57BL/6 mice in transgenic studies and the widespread use of tribromoethanol in the production of transgenic mice, this strain was ideal for the present study. The 500-mg/kg dose of tribromoethanol that was used in the current study followed the dosing recommendation of our IACUC.19 Although doses of 240 and 400 mg/kg have been reported to be effective,3 the 500-mg/kg dose has been used routinely by investigators at our institution without incidence of morbidity or mortality. The 8-d interval between tribromoethanol injections was selected because it is the shortest interval commonly used for repeated tribromoethanol injection in vaccine-challenge studies.
At the dose provided, a single injection of tribromoethanol did not reliably produce a surgical anesthetic plane in C57BL/6NHsd mice. All mice became recumbent yet retained the pedal reflex after tribromoethanol administration, and anesthetic times varied widely, as has been reported previously.1,4,7,8 All of the stock and working tribromoethanol solutions we used were prepared according to same method and by a single investigator (TS), and a single investigator (JT) administered all of the injections, thereby decreasing the likelihood of variability. The tribromoethanol powder we used was stored in the manufacturer's bottle at room temperature. Some researchers have hypothesized that changes may occur in tribromoethanol powder when the bottle is opened, exposed to air, and then stored.9 Such conditions existed between preparations of the 2 stock solutions used in our study and may be responsible for the observed differences. Otherwise, we are unable to explain why mice failed to lose pedal reflexes after a single tribromoethanol administration whereas 9 of 10 mice lost pedal reflexes after the second injection. Additional studies are warranted to determine whether these results are reproducible.
The tribromoethanol preparation used in this study appeared to be safe for use in C57BL/6NHsd mice, given that no morbidity, mortality, or pathologic changes were observed at the administered dose and frequencies. In a previous study, 10 of 47 female ICR mice were found dead or moribund after the administration of 400 mg/kg of freshly prepared tribromoethanol.8 The lethal dose of tribromoethanol may vary by mouse strain. Moreover, tribromoethanol purity may vary by supplier, lot number, and bottle and thus affect lethality. We noted no organ or tissue pathology in any study animal. Prior reports have described abdominal muscle necrosis, fluid distension of the stomach and small intestine, peritonitis, splenic serositis, and fibrinous visceral adhesions and tribromoethanol administration in ICR, CD-1, OF-1, NMRI, and (NCR) nu/nu mice.2,8,20 The tribromoethanol concentration and dose administered in these cited studies ranged from 12 to 25 mg/mL and 240 to 500 mg/kg, respectively, and included stored and freshly prepared preparations. Endpoints in the cited studies ranged from 24 h to 6 wk after tribromoethanol administration. In our current study, mice were euthanized 3 and 4 d after tribromoethanol administration. As a result, histopathologic changes associated with an acute inflammatory response would not have been observed. The absence of morbidity and mortality in the C57BL/6NHsd mice used in our study suggests that tribromoethanol toxicity may be strain-related.
On the basis of our current findings, tribromoethanol appears to be safe for repeated administration in C57BL/6NHsd mice. However we urge caution regarding the use of this anesthetic in this strain due to variable effectiveness.
Acknowledgments
This work was supported by a generous grant from the American College of Laboratory Animal Medicine Foundation and the Association for the Assessment and Accreditation of Laboratory Animal Care International.
References
- 1.Avila MY, Carre DA, Sone RA, Civan MM. 2001. Reliable measurement of mouse intraocular pressure by a Servo-Null micropipette system. Invest Ophthalmol Vis Sci 42:1841–1846 [PubMed] [Google Scholar]
- 2.Buetow BS, Ghen LI, Maggio-Price L, Swisshelm K. 1999. Peritonitis in nude mice in a xenograft study. Contemp Top Lab Anim Sci 38:47–49 [PubMed] [Google Scholar]
- 3.Cho YJ, Lee YA, Lee JW, Kim JI, Han JS. 2011. Kinetics of proinflammatory cytokines after intraperitoneal injection of tribromoethanol and tribromoethanol–xylazine combination in ICR mice. Lab Anim Res 27:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner DJ, Davis JA, Weina PJ, Theune B. 1995. Comparison of tribromoethanol, ketamine–acetylpromazine, Telazol–xylazine, pentobarbital, and methoxyflurane anesthesia in HSD:ICR mice. Lab Anim Sci 45:199–204 [PubMed] [Google Scholar]
- 5.Green CJ. 1979. Animal anesthesia. Laboratory handbook, no. 8. London (UK): Laboratory Animals. [Google Scholar]
- 6.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 7.Koizumi T, Maeda H, Hioki K. 2002. Sleep-time variation for ethanol and the hypnotic drugs tribromoethanol, urethane, pentobarbital, and propofol within outbred ICR mice. Exp Anim 51:119–124 [DOI] [PubMed] [Google Scholar]
- 8.Lieggi CC, Artwohl JE, Leszczynski JK, Rodriguez NA, Fickbohm BL, Fortman JD. 2005. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci 44:17–22 [PubMed] [Google Scholar]
- 9.Lieggi CC, Fortman JD, Kleps RA, Sethi V, Anderson JA, Brown CE, Artwohl JE. 2005. An evaluation of preparation methods and storage conditions of tribromoethanol. Contemp Top Lab Anim Sci 44:11–16 [PubMed] [Google Scholar]
- 10.Meyer RE, Fish RE. 2005. A review of tribromoethanol anesthesia for production of genetically engineered mice and rats. Lab Anim (NY) 34:47–52 [DOI] [PubMed] [Google Scholar]
- 11.Meyer RE, Fish RE. 2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p 27–82. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. Burlington (MA): Academic Press. [Google Scholar]
- 12.Mosby HS, Canter DE. 1956. The use of avertin in capturing wild turkeys and as an oral–basal anesthetic in other wild animals. Southwest Vet 9:132–136 [Google Scholar]
- 13.Norris ML, Turner WD. 1983. An evaluation of tribromoethanol (TBE) as an anaesthetic agent in the Mongolian gerbil (Meriones unguiculatus). Lab Anim 17:324–329 [DOI] [PubMed] [Google Scholar]
- 14.Papaioannou VE, Fox JG. 1993. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci 43:189–192 [PubMed] [Google Scholar]
- 15.Reid WC, Carmichael KP, Srinivas S, Bryant JL. 1999. Pathologic changes associated with use of tribromoethanol (avertin) in the Sprague–Dawley rat. Lab Anim Sci 49:665–667 [PubMed] [Google Scholar]
- 16.Tarin D, Sturdee A. 1972. Surgical anaesthesia of mice: evaluation of tribromoethanol, ether, halothane, and methoxyflurane and development of a reliable technique. Lab Anim 6:79–84 [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Agriculture. [Internet] 2011. Pharmaceutical-grade compounds in research. Animal care resources guide 3.2. [Cited 6 June 2012]. Available at: www.aphis.usda.gov
- 18.United States Department of Health and Human Services. [Internet] 2012. Position statement 3: nonpharmaceutical grade substances. [Cited 6 June 2012] Available at: http://grants.nih.gov/grants/olaw/olaw.htm
- 19.University of Tennessee, Knoxville. [Internet] 2009. Guidelines on preparation and use of avertin in mice. [Cited 20 April 2012]. Available at: http://iacuc.tennessee.edu/
- 20.Zeller W, Meier G, Bürki K, Panoussis B. 1998. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim 32:407–413 [DOI] [PubMed] [Google Scholar]