Abstract
Rabbits are widely used as an animal model for urologic research studies in which urinary bladder catheterization is required. However, standard manual retrograde urinary catheterization proved to be difficult to perform on anesthetized male rabbits in a research study, with frequent misplacement of the catheter into the vesicular gland. Attempts to reposition the catheter into the bladder after initial entry into the vesicular gland frequently failed and resulted in exclusion of the animal from the study. We assessed the normal anatomy of the lower urinary tract of male rabbits to determine the cause of catheterization misdirection into the vesicular gland and to develop a more reliable technique for urinary bladder catheterization. A modified ‘digital (finger) pressure’ catheterization technique was developed for successful urinary catheterization of male rabbits. Retrospective statistical analysis of 45 rabbits used for urinary catheterization studies showed improvement in the success rate of catheterization by using the digital pressure technique over the standard method of retrograde urinary catheter insertion. In addition, we here review the relevant gross and histologic anatomy of the urogenital tract of male rabbits.
Abbreviation: VG, vesicular gland
Rabbits are commonly used as an animal model of urinary tract infection and other urologic studies that require urinary catheterization.3-5,12,16-25 Correct urinary catheterization is crucially important in studies requiring evaluation of urine parameters, such as diagnostic or research investigations in preclinical drug development and discovery studies, medical device investigations, and veterinary clinical practice.
Simple manual retrograde insertion of a urinary catheter is the standard technique described in textbooks for the urinary catheterization of male rabbits.6-8,11,15 This insertion technique is used in various other laboratory animal species with high success rates, including mice, rats, dogs, and primates. However, in our hands, using the traditional manual technique in studies requiring urinary catheterization of male New Zealand white rabbits resulted in a catheterization failure rate of as high as 40%. We examined the normal anatomy of the male rabbit lower urinary tract to determine the cause of catheterization failure and realized that manual urinary catheterization frequently failed due to misplacement of the catheter into the vesicular gland (VG). Attempts to reposition the catheter into the bladder after initial entry into the VG by using the standard manual retrograde technique frequently failed and resulted in exclusion of the animal from the study. The opening of the VG gland lies on the dorsal aspect of the urethral lumen close to the neck of the urinary bladder. Our examination of rabbit anatomy led to our hypothesis that redirection of the catheter tip by using external counter pressure over the caudal urethra would decrease the frequency of catheter entry into the VG opening and increase rates of successful urinary bladder catheterization. Counter pressure applied externally over the caudal urethra during catheterization caused ventral deflection and repositioning of the catheter tip from the VG into the urinary bladder; this placement was demonstrated by using CT imaging. Here, in a retrospective study, we compared the reliability of the ‘digital pressure’ technique for urinary catheter placement in male rabbits with that of the standard manual catheterization technique. In addition, we used computed tomography and gross and microscopic examination to demonstrate the unique anatomy of the urogenital tract of the male rabbit.
Materials and Methods
All procedures were performed in rabbits enrolled in research protocols approved by the IACUC of The University of Texas MD Anderson Cancer Center (Houston, TX) and conducted in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals10 and the Animal Welfare Act.2 Male New Zealand White rabbits (Oryctolagus cuniculus; n = 48) were obtained from a commercial vendor (Myrtle's Rabbitry, Thompson's Station, TN). The rabbits were tested at the vendor by using ELISA, microbiologic culture, PCR, and pathology and were negative for Treponema cuniculi, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, Pasteurella multocida, Clostridium piliformis, Salmonella spp., and endo- and ectoparasites. The animals were housed individually in stainless steel cages (24 in. × 14 in. × 16 in.) in an environmentally controlled room (68 ± 2 °F [20.0 ± 1.1 °C], 30% to 70% relative humidity, 12:12-h light:dark cycle, 10 to 15 air changes per hour). Rabbits were fed a commercial pelleted diet (Laboratory Rabbit Diet 5321, Lab Diet, PMI Nutrition International, St Louis, MO) and tap water supplied ad libitum by an automated watering system. Enrichment was provided by dietary supplementation with alfalfa cubes and cage enhancements (stainless steel rattle, perforated jingle ball). Rabbits were acclimated for a minimum of 7 d after arrival before any research manipulations were performed.
This study was conducted in conjunction with an approved animal research protocol to examine the efficacy of antimicrobial urinary catheter coating on the incidence of bacterial urinary tract infection. For this study, rabbits received buprenorphine (0.05 mg/kg IM; Buprenex, Reckitt and Colman, Kingston-upon-Hull, England) every 12 h for 48 h, with the first dose administered immediately prior to surgery, and were anesthetized by using 5% isoflurane (IsoThesia Isoflurane, Butler Animal Health Supply, Dublin, OH) in oxygen by face mask, followed by endotracheal intubation and maintenance on 2% to 3% isoflurane in oxygen. All rabbits in the study then underwent surgery for insertion of a jugular vein catheter and manual insertion of a urinary bladder catheter and urine collection system.
In the first cohort of rabbits, we noted that attempts to manually catheterize the urinary bladder of anesthetized male rabbits by using a retrograde, blind, manual technique frequently were unsuccessful. To diagnose the problem, 2 rabbits in which urinary catheterization was unsuccessful after multiple attempts were euthanized (Beuthanasia-D Special, Schering Plough Animal Health, Quebec, Canada) while under isoflurane anesthesia. Euthanasia was performed in the same manner for all rabbits in these studies at experimental endpoints or when a rabbit was removed from study because of urinary catheterization failure. In these 2 rabbits, gross necropsy was performed, and abdominal and pelvic structures were examined. The catheter failed to enter the bladder during catheterization due to misplacement of the catheter into the VG. The movement of the catheter tip was monitored as it was withdrawn from the VG and introduced into the urinary bladder while assessing various external manipulations. During these manipulations, the direction of catheter tip was corrected by using digital pressure to allow the catheter tip to bypass the VG entrance and directly enter into the urinary bladder.
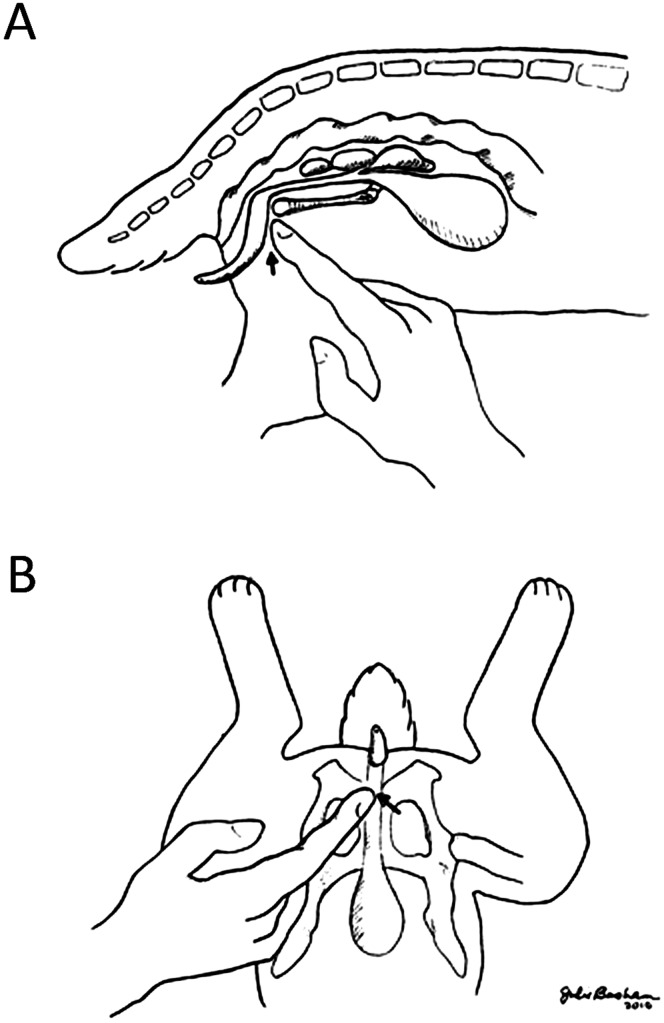
Therefore, using these observations, we developed a new ‘digital pressure’ technique to increase the frequency of transurethral urinary catheter placement into the bladder of anesthetized male rabbits. In this technique, the male rabbit was placed in dorsal recumbency and under isoflurane anesthesia. By using sterile techniques, the tip of a lubricated 10-French catheter was introduced into the penile urethra. The index finger tip of one hand was placed immediately caudal to the pubic symphysis, while the catheter was threaded slowly into the urethra by using the opposite hand. During advancement of the catheter, the operator's index finger was used to depress a point immediately caudal to pubic symphysis (Figure 1 A and B). The advancing tip of the catheter could be felt at the fingertip during this step. Once the catheter was advanced into the bladder lumen, placement was confirmed by aspirating urine. This technique diverted the catheter tip from entering into the VG, allowing successful entrance into the urinary bladder and urine collection.
Figure 1.
Schematic demonstration of the digital (finger) pressure technique of urinary catheterization of male rabbits. Arrows indicate the point of pressure application. (A) Lateral view. (B) Ventral view.
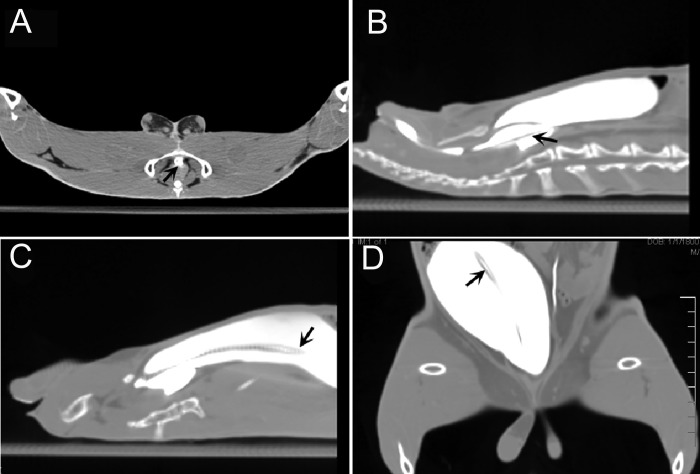
To confirm these findings, the misplacement of a catheter as well as successful catheter advancement by using the digital pressure technique was demonstrated during CT imaging of a third rabbit (Figure 2). After induction and maintenance of general anesthesia with isoflurane, a lubricated 10-French catheter was inserted into the urethral meatus and slowly advanced retrograde through the urethra toward the urinary bladder by using the standard blind catheterization technique. However, no urine could be obtained by light suction from a syringe. To identify the location of the catheter tip, 30 mL of iothalamate meglumine contrast agent (Conray, Mallinckrodt, St Louis, MO) was infused into the urinary tract through the catheter. After infusion of contrast agent, the catheter was advanced slowly, and a CT image of the pelvis and lower abdomen obtained to visualize the tip of the catheter (Figure 2 B). This image confirmed the misplacement of the catheter tip into the VG instead of the urinary bladder. The catheter then was withdrawn completely, reinserted into the urethra, and advanced toward the urinary bladder by using the digital pressure technique. This technique achieved correct placement of the catheter into the urinary bladder. The location of the contrast-filled catheter within the urethra and below the pubic symphysis is shown in Figure 2 A. Confirmatory CT images of the pelvis and abdomen during the failed attempt at catheterization with displacement of the catheter tip into the VG is shown in Figure 2 B and subsequent successful placement into the urinary bladder by using the digital pressure technique are provided in Figure 2 C and D.
Figure 2.
CT images demonstrating misplacement of the catheter into the VG and corrected placement by using digital pressure technique. (A) Axial CT section showing the position of the urethra under the pubic symphysis (arrow). (B) Sagittal section demonstrating misplacement of the catheter tip into the VG (arrow). (C) Sagittal and (D) coronal images demonstrating correct placement of the catheter tip within the urinary bladder of the same rabbit by using digital pressure technique (arrows).
Once we had demonstrated that the digital pressure technique allowed the catheter to bypass the VG and successfully enter the urinary bladder in male rabbits, we assessed the technique in additional cohorts of rabbits enrolled in the same research study.5 A total of 45 additional animals were catheterized by using 1 of the 2 techniques, either the traditional blind technique or digital pressure technique. Collection of urine from these rabbits was recorded as a success in these data. Data from 23 rabbits catheterized by using the digital pressure technique were compared with those from 22 rabbits catheterized by using the traditional blind technique.
Cases were defined as either a ‘catheterization failure’ or a ‘catheterization success.’ Catheterization failure included rabbits from which no urine could be collected after repeated attempts at urinary catheterization and those in which rupture of the urinary tract resulted in the entrance of the catheter tip into the abdominal cavity. Catheterization success comprised rabbits in which the catheter tip was introduced into the urinary bladder, as confirmed by the ability to collect urine samples.
Univariate analysis was used to calculate the odds ratio and 95% confidence interval for the effect of the digital pressure technique in urinary catheterization. The Fisher exact test was used to determine significance. Results with a 2-sided α level of 0.05 (that is, a P value less than 0.05) were considered significant. All statistical analyses were performed by using STATA statistical software (Stata, College Station, TX). In addition, we describe the anatomy of the urogenital tract of the male rabbit. A male rabbit was necropsied and the urogenital tract examined grossly and microscopically. Sequential cross sections of formalin-fixed tissues throughout the urogenital tract with the urinary catheter in place were examined grossly and histologically.
Results
The digital pressure technique resulted in successful urinary catheterization in greater than 95% of rabbits and proved to be superior to the blind catheterization technique. Among rabbits catheterized by using the blind technique, catheterization failure occurred in 8 of the 22 (36.4%) rabbits, whereas only 1 of 23 (4.35%) rabbits had catheterization failure when the digital pressure technique was used. Among rabbits in which catheterization failed, 8 of 9 failures (88.9%) occurred when using the traditional method compared with 1 failure (11.1%) when using the digital pressure method. These results indicated a significant (P = 0.01) protective odds ratio (0.079; 95% confidence interval, 0.002 to 0.74), showing a 92% reduction in the risk of urinary catheterization failure when the digital pressure technique was used. Therefore, use of the digital pressure technique prevented the failure of catheter placement and the associated inability to collect necessary urine samples that we had encountered frequently during catheterization of male rabbits by using the traditional method.
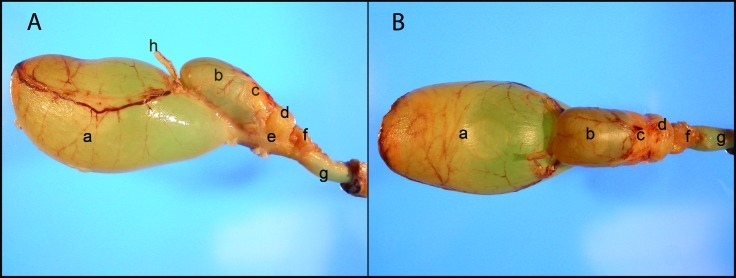
Macroscopic examination of the urogenital tract anatomy of a male rabbit demonstrated the presence of following sexual accessory glands on the dorsal aspect of the urethra within the pelvic cavity: ampulla ductus deferentis, VG, proprostate, prostate, paraprostate, and bulbourethral gland (Figure 3 A and B). The VG is located on the dorsal surface of the urethra and neck of urinary bladder. Bilaterally on the ventral aspect of the VG and dorsal aspect of the urethra and the neck of urinary bladder are the 2 ampullae of the deferent ducts. These ampullae enter into the ejaculatory duct through small orifices. Dorsal and caudal to the VG are the proprostate, prostate, and paraprostate. The VG is a saccular structure that is composed of an expandable thin wall and a lumen. The lumen can dilate greatly with the accumulation of clear viscous or gelatinous fluid secretion from the glands within the vesicular wall. The lumen of the VG narrows caudally, forming a short ejaculatory duct. This ejaculatory duct communicates with urethra through a crested cleft orifice at the apex of colliculus seminalis, near the neck of the urinary bladder (Figure 4 A and B). The colliculus seminalis is a protuberance on the dorsomedial aspect of urethral wall. The colliculus seminalis controls the release of sperm and seminal vesicle secretions into the urethra, while preventing urine from entering into the reproductive organs.
Figure 3.
Photographs of male rabbit urogenital tract. (A) Lateral view. (B) Dorsal view. Anatomic structures: a, urinary bladder; b, vesicular gland; c, proprostate; d, prostate; e, paraprostate; f, bulbourethral gland; g, urethra; h, deferent duct.
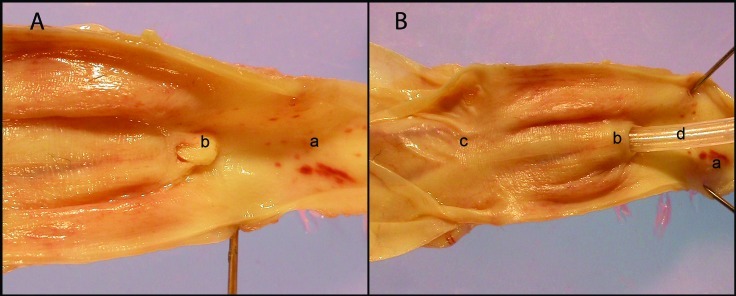
Figure 4.
(A) Urethra, colliculus seminalis, and the neck of urinary bladder. (B) Misplacement of catheter into the Vesicular gland. a, urethral lumen; b, colliculus seminalis and opening of ejaculator duct; c, the neck of urinary bladder; d, catheter.
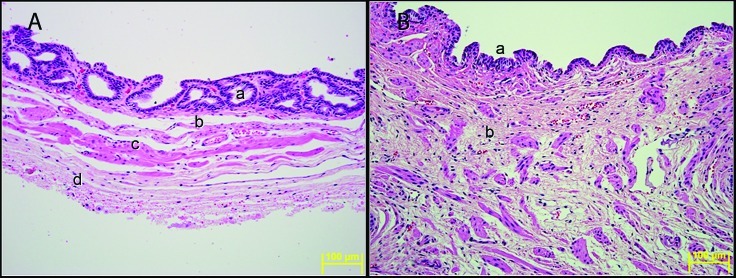
Histologic examination revealed glandular mucosa lining the lumen of VG, with submucosa, tunica muscularis, and adventitia on the external surface (Figure 5 A). The mucosa of the VG is composed of superficial simple tall cuboidal or columnar epithelia and subepithelial lamina propria with simple or branched tubular glands lined by simple tall cuboidal or columnar epithelia. The lamina propria blends with the underlying submucosa, which is composed of a thin layer of fibrovascular connective tissue that separates the mucosa from the underlying tunica muscularis. The tunica muscularis is composed of interwoven bundles of smooth muscle fibers. External to tunica muscularis is a layer of loose connective tissue that forms the adventitia. The mucosa in the caudal region of the VG contains fewer glands than does the cranial region. Near the ejaculatory duct, the mucosa lacks glands and consists of a single layer of simple columnar to tall cuboidal epithelium lined by a thick layer of abundant loose connective tissue intermixed with numerous irregularly oriented bundles of smooth muscle fibers (Figure 5 B).
Figure 5.
(A) Vesicular gland. a, mucosa with glands; b, submucosa; c, tunica muscularis; d, adventitia. (B) Vesicular gland near the ejaculator duct. a, mucosal epithelium; b, thick wall of loose connective tissue with bundles of smooth muscle fibers. Hematoxylin and eosin stain. Magnification, 200×.
Discussion
To our knowledge, this report is the first to describe a digital pressure technique to improve transurethral urinary catheterization of male rabbits. The primary obstacle to successful urinary catheterization of anesthetized male rabbits was demonstrated as inadvertent entry of the catheter tip into the ejaculatory canal and VG. We developed the digital pressure technique to correct the orientation of catheter tip, redirecting it ventrally within the urethra, thereby avoiding inadvertent placement of catheters into the VG. A retrospective study confirmed the efficacy of this new technique, demonstrating greater than 95% success in catheter placement within the bladder by using the digital pressure technique during retrograde transurethral urinary catheterization, compared with a 40% success rate by using the standard placement technique.
Rabbits are commonly used in research studies requiring catheterization of the urinary bladder for multiple, timed urine sample collection or continuous urine collection. For example, urine collection is integral in studies of urinary tract infection,5,17-21,23 physiology of the urinary system,14 treatment of urologic diseases,25 pharmacologic and toxicologic studies,26 and urologic oncology studies.1 As the most commonly used large animal tumor model in which a well-characterized, transplantable tumor is available, rabbits are important in urologic oncology research.13
Our conversations with 2 research groups in 2 countries revealed a shared experience of the difficulty of urinary catheterization of anesthetized male rabbits, but this problem has not been identified in the literature. Most rabbit clinical medicine textbooks recommend the blind technique of urinary catheterization of male rabbit without any reference to the difficulties that might arise.6-8,11,15,18 A literature search on methods for urinary catheterization in rabbits did not yield any publications describing alternative techniques. Using the blind technique routinely described in current text books,6-8,11,15 our group was frequently unable to successfully catheterize the urinary bladder in male rabbits for urine sampling studies, due to misplacement of catheter tip into the VG. Once the VG was entered, subsequent attempts at retrograde catheter placement were usually unsuccessful and resulted in trauma to the urinary tract and penis, even with careful and gentle handling. The reason for this apparent contradiction between descriptions in clinical textbooks and our experience in research setting could be due to the relatively low frequency of urinary catheterization of rabbits in clinical practice or to differences in the techniques used to perform catheterization, including the types of catheters and anesthesia used.
The unique anatomy of the urogenital tract in male rabbits as well as the anesthesia technique used during catheterization may play a role in misplacement of catheters into the VG. The colliculus seminalis, a valvelike structure at the entry of VG from the urethra, controls the release of sperm and VG secretion in rabbits. The caudal narrow lumen of VG, the lumen of ejaculator duct, and its urethral opening can expand greatly during ejaculation.9 Therefore, one of the reasons for inadvertent insertion of catheter tip into the VG could be relaxation of VG opening to the urethra caused by an ejaculatory response originating from penile manipulation for catheterization in an anesthetized rabbit. Another possible factor could be the type of anesthesia that was used in the rabbits in this study. Anesthesia might have led to relaxation of ejaculatory duct as well as dysfunction of the colliculus seminalis, thus increasing the likelihood of the catheter entering the VG opening by using standard technique. All catheter placement attempts in our study were conducted in rabbits under isoflurane general anesthesia. Various regimens are available for producing sedation or anesthesia in rabbits, but we did not compare the effect of the anesthetic agent on the difficulty of urinary catheterization.
A limitation of this study might be the size of the urinary catheters used. The size of urinary catheter that is used for most clinical applications in rabbits is 9 French.6 The research study in which the catheterization failure was identified was designed to evaluate urinary catheter-related infection and required the use of 10- to 12-French catheters.5 The possibility of larger catheters causing aberrant entry of the catheter tip to VG compared with that of smaller catheters seems to be low. However, we did not assess the role of catheter size in ease of urinary catheterization.
We here have demonstrated limitations in successful urinary catheter placement in male rabbits by using the blind techniques described in standard textbooks. We developed a new digital pressure technique for successful blind transurethral urinary catheter placement in male rabbits. In a retrospective study, we demonstrated that the new technique significantly improved successful bladder catheterization using large-size catheters (10 to 12 French) by preventing inadvertent placement of the catheter tip into the VG. This information is relevant in research and clinical practice to educate personnel performing urinary catheterization in rabbits about potential causes of catheterization failure and describes a technical refinement that improves the success rate of manual urinary catheterization in male rabbits.
Acknowledgments
We thank Julie Basham for her contribution in creating the figures and Katherine Dixon for support with CT imaging of the rabbits. This research was supported in part by the NIH through the Cancer Center Support Grant CA016672.
References
- 1.Alexiou C, Jurgons R, Schmid RJ, Bergemann C, Henke J, Erhardt W, Huenges E, Parak F. 2003. Magnetic drug targeting—biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J Drug Target 11:139–149 [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2008. 7 USC §2131−2156.
- 3.Barlas M. 2002. The effects of piroxicam to the muscosal barrier of the bladder after overdistension—an experimental study in rabbits. Int Urol Nephrol 34:321–324 [DOI] [PubMed] [Google Scholar]
- 4.Csanaky I, Gregus Z. 2002. Species variations in the biliary and urinary excretion of arsenate, arsenite, and their metabolites. Comp Biochem Physiol C Toxicol Pharmacol 131:355–365 [DOI] [PubMed] [Google Scholar]
- 5.Hachem R, Reitzel R, Borne A, Jiang Y, Tinkey P, Uthamanthil R, Chandra J, Ghannoum M, Raad I. 2009. Novel antiseptic urinary catheters for prevention of urinary tract infections: correlation of in vivo and in vitro test results. Antimicrob Agents Chemother 53:5145–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harkness JE, Wagner JE. 1995. The biology and medicine of rabbits and rodents, 4th ed. Baltimore (MD): Williams and Wilkins. [Google Scholar]
- 7.Hau J, Van Hoosier GL. 2003. Handbook of laboratory animal science: essential principles and practices, 2 ed. Boca Raton (FL): CRC Press. [Google Scholar]
- 8.Hillyer EV, Queensberry K. 1997. Ferrets, rabbits, and rodents clinical medicine and surgery. Philadelphia (PA): WB Saunders. [Google Scholar]
- 9.Holtz W, Foote RH. 1978. The anatomy of the reproductive system in male Dutch rabbits (Oryctolagus cuniculus) with special emphasis on the accessory sex glands. J Morphol 158:1–20 [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals, 7th ed. Washington (DC): National Academy Press.
- 11.Kaplan HM, Timmons EH. 1979. The rabbit: a model for the principles of mammalia physiology and surgery. Burlington (MA): Academic Press. [Google Scholar]
- 12.Khoury AE, Olson ME, Lam K, Nickel JC, Costerton JW. 1989. Evaluation of the retrograde contamination guard in a bacteriologically challenged rabbit model. Br J Urol 63:384–388 [DOI] [PubMed] [Google Scholar]
- 13.Lee JM, Kim SW, Chung GH, Lee SY, Han YM, Kim CS. 2003. Open radiofrequency thermal ablation of renal VX2 tumors in a rabbit model using a cooled-tip electrode: feasibility, safety, and effectiveness. Eur Radiol 13:1324–1332 [DOI] [PubMed] [Google Scholar]
- 14.Levin RM, Monson FC, Longhurst PA, Wein AJ. 1994. Rabbit as a model of urinary bladder function. Neurourol Urodyn 13:119–135 [DOI] [PubMed] [Google Scholar]
- 15.Manning PJ, Ringler DH, Newcomer CE. 1994. The biology of the laboratory rabbit. New York (NY): Academic Press. [Google Scholar]
- 16.Morck DW, Lam K, McKay SG, Olson ME, Prosser B, Ellis BD, Cleeland R, Costerton JW. 1994. Comparative evaluation of fleroxacin, ampicillin, trimethoprimsulfamethoxazole, and gentamicin as treatments of catheter-associated urinary tract infection in a rabbit model. Int J Antimicrob Agents 4 Suppl 2:S21–S27 [DOI] [PubMed] [Google Scholar]
- 17.Morck DW, Olson ME, McKay SG, Lam K, Prosser B, Cleeland R, Costerton JW. 1993. Therapeutic efficacy of fleroxacin for eliminating catheter-associated urinary tract infection in a rabbit model. Am J Med 94:23S–30S [PubMed] [Google Scholar]
- 18.Morck DW, Olson ME, Read RR, Buret AG, Ceri H. 1999. The rabbit model of catheter-associated urinary tract infection, p 453–462. In: Zak O, Sande MA. Handbook of animal models of infection. Boston (MA): Academic Press. [Google Scholar]
- 19.Nickel JC, Grant SK, Costerton JW. 1985. Catheter-associated bacteriuria. An experimental study. Urology 26:369–375 [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC, Grant SK, Lam K, Olson ME, Costerton JW. 1991. Bacteriologically stressed animal model of new closed catheter drainage system with microbicidal outlet tube. Urology 38:280–289 [DOI] [PubMed] [Google Scholar]
- 21.Nickel JC, Olson ME, Costerton JW. 1987. In vivo coefficient of kinetic friction: study of urinary catheter biocompatibility. Urology 29:501–503 [DOI] [PubMed] [Google Scholar]
- 22.Papavramidis TS, Lazou TP, Cheva A, Gamvros OJ. 2010. Chronically increased intraabdominal pressure: validating a model. Obes Surg 20: 900–905 [DOI] [PubMed] [Google Scholar]
- 23.Pugach JL, DiTizio V, Mittelman MW, Bruce AW, DiCosmo F, Khoury AE. 1999. Antibiotic hydrogel-coated Foley catheters for prevention of urinary tract infection in a rabbit model. J Urol 162:883–887 [DOI] [PubMed] [Google Scholar]
- 24.Richards CL, Hoffman KC, Bernhard JM, Winslow SD, Norman JC, Whalen RL. 2003. Development and characterization of an infection-inhibiting urinary catheter. ASAIO J 49:449–453 [PubMed] [Google Scholar]
- 25.Schroder A, Kogan BA, Lieb J, Levin RM. 2001. Increased blood flow after catheterization and drainage in the chronically obstructed rabbit urinary bladder. Urology 58:295–300 [DOI] [PubMed] [Google Scholar]
- 26.Schuster P, Bertermann R, Rusch GM, Dekant W. 2010. Biotransformation of 2,3,3,3-tetrafluoropropene (HFO-1234yf) in rabbits. Toxicol Appl Pharmacol 244:247–253 [DOI] [PubMed] [Google Scholar]