Abstract
Cortisol measurements of hair are becoming a valuable tool in monitoring chronic stress. To further validate this approach in domestic dogs, we compared the variability of cortisol immunoreactivity in hair with that in saliva and feces of dogs housed under constant social and physical conditions. Fecal (n = 268), and hair (n = 21) samples were collected over 3 mo from 7 dogs housed in a kennel and kept for training veterinary students in minimally invasive procedures. Salivary samples (n = 181) were collected 3 times daily twice weekly during the last month of the study. Hair and salivary samples were analyzed by enzyme immunoassay and feces by radioimmunoassay. HPLC coupled with tandem mass spectrometry was used to confirm the presence of cortisol in 3 hair samples. Variability of cortisol was compared across sample types by using repeated-measures ANOVA followed by paired t tests. Within dogs, cortisol immunoreactivity was less variable in hair than in saliva or feces. Averaged over time, the variability of fecal samples approached that of hair when feces were collected at least 4 times monthly. As predicted, the stable social and environmental condition of the dogs maintained repeatability over time and supported the hypothesis that data from hair samples reflect baseline cortisol levels. These findings indicate that determining cortisol immunoreactivity in hair is a more practical approach than is using samples of saliva or feces in monitoring the effects of long-term stressors such as social or physical environments and disease progression.
Abbreviation: CV, coefficient of variability; LC-MS/MS, liquid chromatography–tandem mass spectrometry
Glucocorticoids are well-established biomarkers of stress in vertebrates, including birds,6 fish,3 and mammals.31 Stress—though adaptive in the short term—has been linked with impaired health and ultimately decreased fitness over prolonged periods.10,30-33 Consequently, measuring cortisol or other glucocorticoids over time can reveal how animals respond to prolonged stressors such as changes in their social or physical environments.8,12
Hair is now recognized as a valuable matrix for measuring cortisol in humans and other mammals.9,15,17,21,25,26 In contrast to biologic samples that reflect circulating steroid levels integrated over seconds to hours (for example, serum, saliva, urine, feces), hair integrates steroids over the entire period of hair growth (months to years).36 Additional advantages are that hair can be collected relatively noninvasively, is easy to store even for long periods, and is not affected by the short-term stress of handling.2 In a research setting, hair could be shaved at regular intervals to monitor chronic stress. Alternatively, hair could potentially be segmented to provide a retrospective record of cortisol concentrations;19 this approach would be valuable for documenting the progression of endocrine diseases such as Cushing syndrome37 in domestic animals presenting with symptoms for the first time and in studying wild animals that can be captured only once.
The currently accepted mechanism for steroid incorporation into growing hair is via the blood vessel that feeds the follicle and potentially from surrounding eccrine and sebaceous glands.26,28 Another possibility is that the follicle itself produces cortisol locally in coordination with a broader systemic stress response, in response to localized skin irritation or as part of normal hair follicle functioning.16 Once inside the follicle, binding of cortisol to the hair fiber is complex and likely involves both melanins and keratin.24,28 Regardless of the mechanism, cortisol concentrations in hair have been shown to reflect well-known endocrine patterns.13,19 One study in macaques showed a correlation between cortisol levels in hair and saliva.9 Two other studies have examined the relationship in dogs: one compared cortisol levels in hair and feces,1 and the other compared cortisol data from hair and saliva.4 In both of these studies,1,4 hair was collected only once from a single dog and compared with only one other tissue.
The primary objective of the current study was to build on these previous findings by evaluating the variability of hair-based measures of cortisol in a population of dogs with a common environment and diet. We hypothesized that cortisol immunoreactivity in hair would be less variable than that in saliva and feces. In addition, we designed the experiment to allow comparison of data from hair with that of both feces and saliva. We predicted that cortisol immunoreactivity in hair would correlate positively with that in saliva and feces averaged over a corresponding time period. Finally, we used HPLC coupled with tandem mass spectrometry (LC-MS/MS) as a ‘gold standard’ for confirming the presence of cortisol in dog hair.35
Materials and Methods
Animals.
Seven dogs were housed at the University of Saskatchewan Animal Resource Centre where they are kept for training of veterinary students. The dogs were all adult mixed-breed animals, which ranged in color from white to black, and 2 were female. Dogs were not regularly bathed and were kept out of the rain. At night, all were housed in individual kennels; during the day, they were kept together in an outdoor enclosure. These animals are used routinely for minimally invasive procedures that are part of the veterinary undergraduate training program. During the study, dogs were anesthetized once or twice, and a blood sample was taken. No more than 2 of these procedures were performed during the same month. This study was conducted under an approved animal care protocol (UCACS 20080087).
Collection of hair, feces, and saliva.
The sampling schedule and experimental design are summarized in Table 1. A patch of hair (10 × 10 cm) was collected once monthly from the right shoulder of each dog by using electric clippers. In addition, the left shoulder of each dog was shaved at time 0, and a 10×10-cm patch of hair was collected from the same place 3 mo later. The goal with shaving both shoulders was to see whether similar patterns of cortisol concentration were present in samples collected monthly compared with hair collected after 3 mo and segmented to correspond to a 1-mo growth period. Although steroids in hair are stable at room temperature,25 we stored samples in paper envelopes at −80 °C due to the long time between collection and analysis (more than 2 y).
Table 1.
Samples collected from 7 dogs
| Saliva | Feces | Hair (Right) | Hair (Left) | |
| No. of samples analyzed | 181 | 268 | 21 | 21 |
| Mean (range) no. of samples per dog | 26 (23–30) | 38 (33-41) | 3 | 1a |
| Month(s) sampledb | 3 | 1, 2, 3 | 1, 2, 3 | 3 |
| Sampling frequency | 3 times daily, 2 d each week | 3 times each week | once per month | once per study |
| Analysis | ELISA | Radioimmunoassay | ELISA | ELISA |
For each dog, these hair samples were divided into 3 segments corresponding to 1 mo of hair growth. Each segment was analyzed separately.
The study lasted 3 mo (month 1: Aug to Sep, month 2: Sep to Oct, month 3: Oct to Nov).
Feces were collected on 3 mornings each week over 3 mo from individual kennels or immediately after defecation in the outdoor enclosure. Most samples were collected within 2 h of defecation, and all samples were frozen immediately and kept at −20 °C until analysis.
Salivary samples were collected only during the third month of the study because of challenges in developing a feasible salivary collection protocol. The salivary samples were collected from each dog twice weekly, on the same days as fecal collections, 3 times daily (0800, 1130, and 1330). These times were set to minimize diurnal variations in salivary cortisol18 and were constrained by the daily schedule of the facility. Prior to collection of saliva, we swabbed the dog's mouth with a cotton ball infused with 5% citric acid solution. The citric acid stimulates saliva production but does not influence assay results.11 While gently restraining each dog, we placed a sterile nonwoven sponge (PrimeLine Medical Products, Edmonton, Alberta, Canada) attached to a string in the dog's mouth. The sponge was removed after 1 min and its contents squeezed into a 5-mL syringe. The entire procedure was completed within 4 min; this brief restraint likely would not have affected salivary cortisol measurements.20 This method yielded 100 to 1000 μL saliva; dogs received a small food reward after saliva collection. Samples were frozen immediately at −80 °C. To confirm that the oral sponge did not affect assay results, we squeezed 0.5 mL and 1 mL of assay diluent through the sponge. This test resulted in values of 0, as did assaying diluent alone.
Measurement of cortisol immunoreactivity in feces.
Thawed fecal samples were homogenized thoroughly; a subsample was lyophilized and powdered. Powdered feces (0.2 g) were weighed into a tube, and 5 mL of methanol was added. Fecal cortisol metabolites were extracted by inverting the tubes several times over 18 h. After extraction, samples were centrifuged for 20 min, and a 1-mL aliquot of the supernatant was dried under a stream of air. The dried extract was reconstituted by first adding 50 µL 100% ethanol and then 1 mL PBS. Samples were analyzed for cortisol immunoreactivity in duplicate by using a commercial radioimmunoassay kit (Coat-a-Count Cortisol, Siemens Medical Diagnostics, Los Angeles, CA). Previous research has shown that a cortisol immunoassay is effective in monitoring adrenocortical activity in dogs.34 The assay was reported by the manufacturer to crossreact with: aldosterone (0.03%), betamethasone (1.6%), corticosterone (0.94%), cortisone (0.98%), danazol (0.01%), 11-deoxycorticosterone (0.26%), 11-deoxycortisol (11.4%), dexamethasone (0.04%), estriol (0.01%), estrone (0.007%), flumethasone (0.017%), methotrexate (0.004%), methylprednisolone (12%), prednisolone (76%), prednisone (2.3%), pregnenolone (0.02%), progesterone (0.02%), tetrahydrocortisol (0.34%), and triamcinolone (0.13%). The sensitivity reported for the radioimmunoassay was 2 ng/mL. According to 3 assays, the mean intraassay coefficient of variation (CV) for the high (200 ng/mL) standard was 3.9% and that for the low (50 ng/mL) standard was 6.8%. The interassay CV were 4.9% and 7.2% for the high and low standards, respectively.
Measurement of cortisol immunoreactivity in saliva.
We used a commercial enzyme immunoassay kit (Salimetrics, Philadelphia, PA) that has been used previously to measure cortisol in saliva from dogs.4 Because the presence of blood can influence assay results, we used a commercial salivary blood contamination kit (Salimetrics) to confirm that no samples were contaminated with blood. The salivary cortisol assay was reported by the manufacturer to crossreact to the following: aldosterone (<0.004%), corticosterone (0.214%), cortisone (0.130%), 11-deoxycortisol (0.156%), 21-deoxycortisol (0.041%), dexamethasone (19.2%), dehydroepiandrosterone (<0.004%), 17β-estradiol (<0.004%), 17α-hydroxy-progesterone (<0.004%), prednisolone (0.568%), prednisone (<0.004), progesterone (0.015%), testosterone (0.006%), transferrin (<0.004%), and triamincinolone (0.086%). The sensitivity of the assay was reported to be 0.03 ng/mL. According to quality controls provided with the kit and assayed on 6 plates, the intraassay CV for the high standard (10.1 ng/mL) was 5.2% and that for the low standard (1.1 ng/mL) was 11.4%. Interassay CV for high and low standards were 7.3% and 16.5%, respectively. Where possible, all samples from the same dog were run on the same plate.
Measurement of cortisol immunoreactivity in hair.
Hair samples were processed similarly as described previously.9,21 To remove water-soluble substances such as feces or urine from the exterior of the hair, samples were washed twice with distilled water for 1 min per wash. After being thoroughly dried, the hair was washed twice (3 min each wash) with isopropanol to remove steroids that might contaminate the exterior of the hair. Dried hair was powdered in a ball mill (Mixer Mill 200, Retsch, Haan, Germany), and 25 mg of powder was weighed into a 20-mL glass scintillation vial. HPLC-grade methanol (2 mL) was added to each vial. Samples were sonicated for 30 min and extracted for 17 h at 45 °C while rotating at 130 rpm. The extract was transferred to a 2-mL microcentrifuge tube and centrifuged for 15 min at 14,000 × g. A 1.6-mL aliquot was removed and dried under a gentle stream of nitrogen. Samples were reconstituted in 170 µL of 5% methanol followed by 95% assay diluent. Each sample was assayed in duplicate according to instructions provided with the commercial kit. According to kit standards run on 3 plates, the intraassay CV were 2.0% and 5.7% for the high and low standards, respectively. The interassay CV were 2.4% and 11.5% for high and low standards. As an additional quality control, we assayed a pool of dog hair in duplicate 3 times on one plate and 4 times on a second plate; the intra- and interassay CV of these hair samples were 8.7% and 8.3%, respectively. We tested parallelism of the assay by comparing a serially diluted pool of dog hair with kit standards. Extraction efficiency was 95.5% according to 3 replicate hair samples spiked with 26 pg cortisol (0.2 µg/dL) prior to extraction and compared with 3 replicates spiked after extraction.
Analysis of hair by LC-MS/MS.
To confirm the presence of cortisol in dog hair, we used LC-MS/MS. Chromatographic conditions have been previously reported.22 Briefly, 100-mg samples of powdered hair were extracted with 20 µL of deuterated cortisol as an internal standard (800 pg per injection), as described earlier. Solid-phase extraction was performed as described previously.22 Samples were reconstituted in 100 µL 50:50 methanol:water and centrifuged for 10 min at 14,000 × g. A 40-µL aliquot was injected into the LC-MS/MS system.
Data analysis.
Because of the potential for crossreaction of antibodies with nontarget compounds or cortisol metabolites, immunoassays are less specific than is LC-MS/MS. For this reason, we report the results obtained by immunoassay as cortisol immunoreactivity. Parallelism of diluted hair extract and assay standards was tested by ANCOVA.
We obtained values for CV by dividing the standard deviation by the mean and multiplying by 100%. We compared the overall variability of cortisol measurements in hair (n = 3 per dog), saliva (n = 23 to 30 per dog) and feces (n = 33 to 41 per dog) by using repeated-measures ANOVA followed by paired t tests with an α level of 0.017 to account for the number of tests (that is, 3). We combined CV across the daily time points because repeated-measures ANOVA showed no difference among daily time points. We then investigated how many fecal samples would be required per dog to obtain a CV less than or equal to that of hair samples. For this analysis, we subsampled the fecal cortisol data to represent different frequencies of sample collection (1, 2, 4, 8, or 13 fecal collections per dog per month). Fecal cortisol was averaged within month of collection for each dog, and a CV was calculated from the 3-mo average. The mean CV fecal cortisol for each sampling scheme was compared with the mean CV of hair cortisol by using paired t tests with Bonferroni correction factor of α = 0.01 to account for the number of tests (that is, 5).
We used paired t tests to compare cortisol immunoreactivity in hair collected from the right and left shoulders and repeated-measures ANOVA to compare hair and fecal data collected in different months and salivary data collected at different times of the day. Fecal samples were averaged within months and salivary samples were averaged within time points for these analyses. Only hair from the right side of each dog was used in the repeated-measures model. The Mauchley test of sphericity was nonsignificant in all repeated-measures models. Relationships among cortisol immunoreactivity in hair, feces, and saliva were tested by using Spearman correlation tests (rs), which are based on ranks and therefore less influenced by outliers than are Pearson correlation tests.38 For comparisons of hair and feces, measurements were averaged over the entire 3-mo period and for correlations with saliva, only data from the third month were used. All tests were conducted by using SPSS statistical software (version 16.0; SPSS, Chicago, IL) with significance set at an α level of 0.05.
Results
Validation of hair cortisol assay.
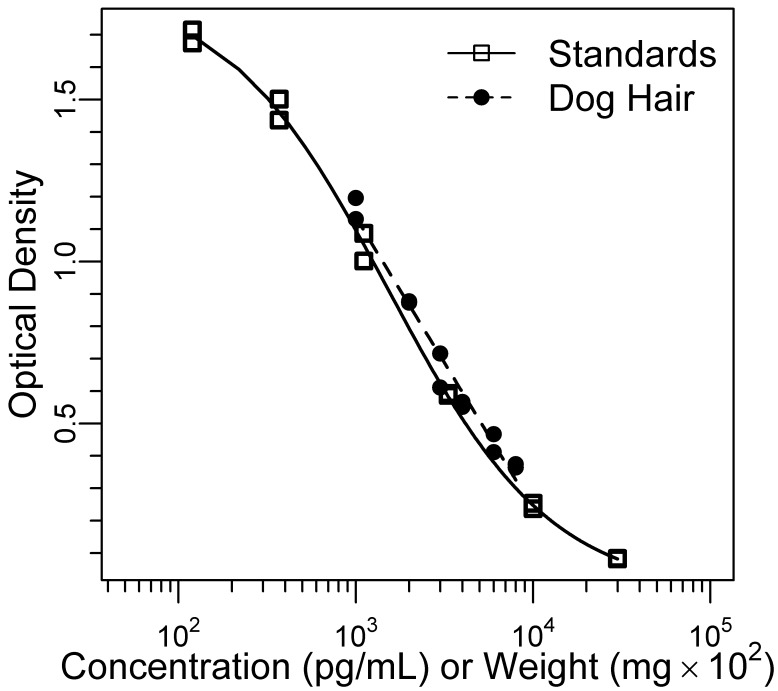
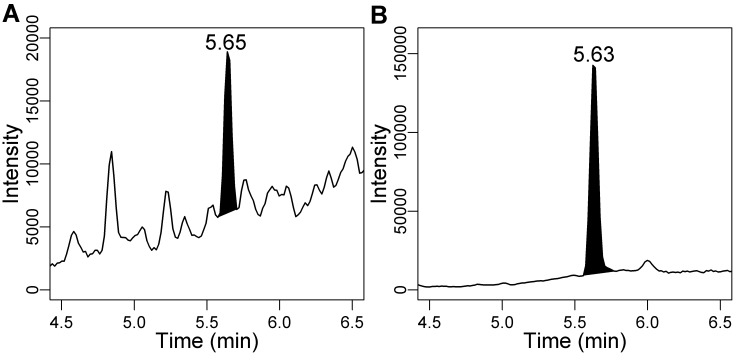
All measurements of cortisol immunoreactivity in hair were above the assay's limit of detection. Results from diluted standards were sufficiently parallel to those of diluted hair extract (F12,6 = 0.03, P = 0.87; Figure 1). Analysis of 3 dog hair samples by LC-MS/MS confirmed the presence of cortisol in dog hair (Figure 2).
Figure 1.
Parallelism of diluted dog hair extract (80-10 mg) and cortisol standards. The extract showed acceptable parallelism with cortisol standards (P = 0.87).
Figure 2.
Chromatograms showing the presence of (A) cortisol (76 pg per injection) and (B) a deuterated internal standard, cortisol-d4 (800 pg per injection), in a sample of dog hair analyzed by LC-MS/MS.
In addition to cortisol, the average concentrations of corticosterone, estradiol, and progesterone were measured in 3 dog hair samples by using LC-MS/MS. Based on these measurements and specificity information provided with the ELISA kits, crossreactivity with these steroids would amount to 0.13% of the crossreactivity to cortisol. The average cortisol concentration in 6 extracts from a pool of dog hair analyzed by ELISA before solid-phase extraction (9.6 pg/mg) was similar to that of 6 replicates analyzed after extraction (9.9 pg/mg).
Cortisol immunoreactivity in hair.
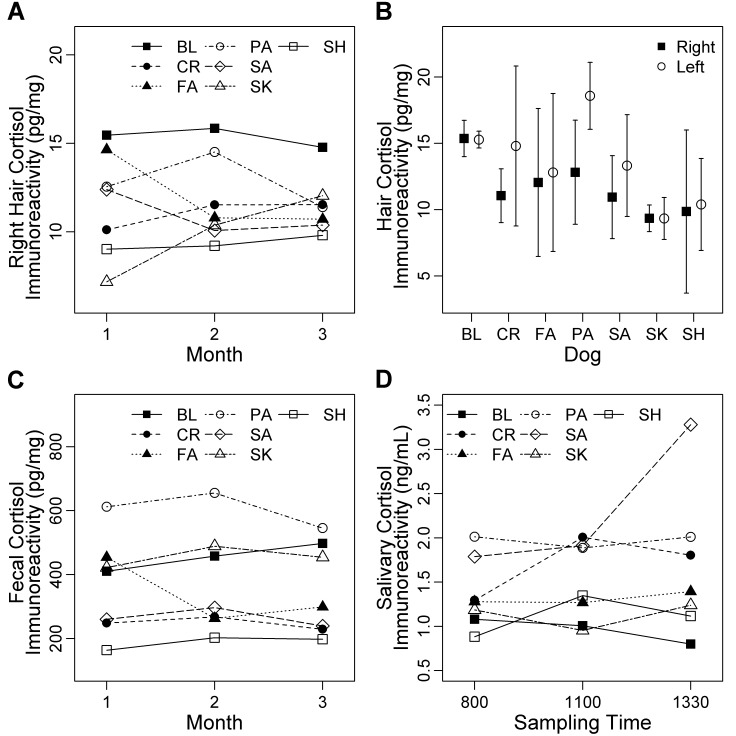
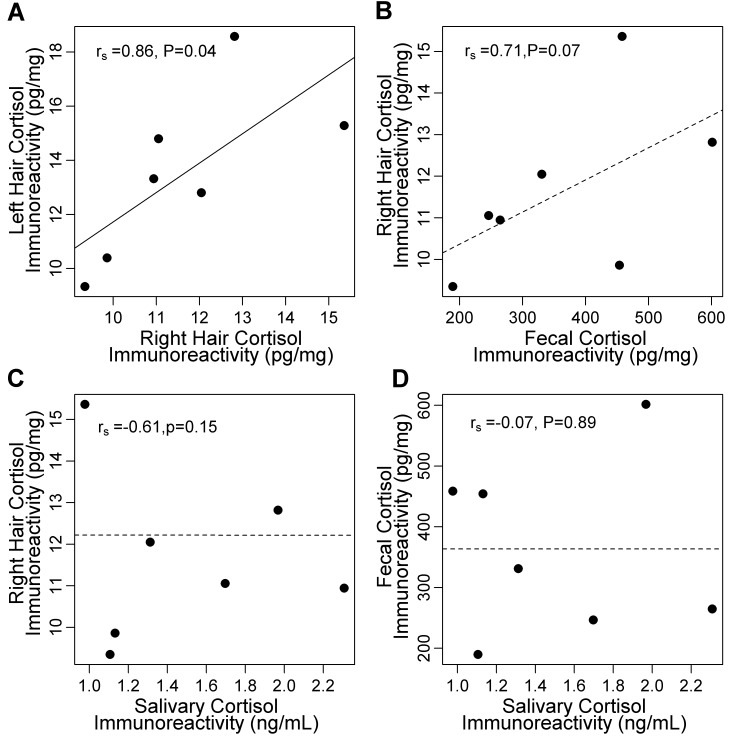
The cortisol immunoreactivity (mean ± 1 SD) in hair was 11.6 ± 0.8 pg/mg. According to repeated-measures ANOVA, cortisol immunoreactivity in hair changed minimally over time (F2,12 = 0.04, P = 0.96; Figure 3 A). For hair collected from the right shoulder, the overall CV was 19.8%. Within dogs, mean variability was 11.8% (range, 3.6% to 25.1%; Table 2). Overall, cortisol immunoreactivity in hair averaged over the 3-mo study period did not differ between the left and right shoulders of individual dogs (t = −2.24, df = 6, P = 0.07; Figure 3 B). Averaged across months, hair collected monthly from the right shoulder was correlated with segmented hair from the left shoulder (rs = 0.86, n = 7, P = 0.01; Figure 4 A).
Figure 3.
Cortisol immunoreactivity in hair, feces, and saliva collected from 7 adult dogs (BL, CR, FA, PA, SA, SK, SH) of mixed breed housed at the Animal Resource Centre (University of Saskatchewan). Hair samples were collected over 3 mo, and data (mean ± 95% confidence intervals) showed little variability (A) over time and (B) between the left and right shoulders. (C) Fecal samples, which were collected during the entire 3-mo period, showed little variation in cortisol levels over time. (D) Samples of saliva were collected during the third month at 3 time points on 2 d of each week. Most dogs showed low within-day variability, with the exception of dog SA.
Table 2.
Measurements of cortisol in hair, saliva, and feces from 7 dogs
| Hair (pg/mg) |
Saliva (ng/mL) |
Feces (pg/mg) |
||||||||||
| Dog | n | Mean | 1 SD | CV (%) | n | Mean | 1 SD | CV (%) | n | Mean | 1 SD | CV (%) |
| BL | 3 | 15.36 | 0.55 | 3.6 | 23 | 0.98 | 0.38 | 38.5 | 41 | 458 | 180 | 39.2 |
| CR | 3 | 11.05 | 0.82 | 7.4 | 26 | 1.70 | 0.77 | 45.1 | 38 | 246 | 143 | 58.1 |
| FA | 3 | 12.05 | 2.24 | 18.6 | 30 | 1.31 | 0.41 | 31.5 | 39 | 331 | 130 | 39.3 |
| PA | 3 | 12.82 | 1.58 | 12.3 | 26 | 1.97 | 0.55 | 27.8 | 40 | 601 | 204 | 34.0 |
| SA | 3 | 10.94 | 1.26 | 11.5 | 22 | 2.31 | 1.18 | 51.3 | 36 | 265 | 127 | 47.9 |
| SH | 3 | 9.35 | 0.41 | 4.3 | 25 | 1.11 | 0.54 | 48.8 | 41 | 190 | 86 | 45.4 |
| SK | 3 | 9.86 | 2.47 | 25.1 | 29 | 1.13 | 0.47 | 41.2 | 33 | 454 | 229 | 50.4 |
Measurements represent cortisol averaged over 3 mo (hair, feces) or 1 mo (saliva). Cortisol measurements in hair were less variable than were those in saliva (P = 0.001) or feces (P < 0.001).
Figure 4.
Relationships between measurements of cortisol immunoreactivity in hair, saliva, and feces collected from 7 adult mixed-breed dogs. (A) Measurements from hair samples collected from the left and right shoulders were correlated. There were no significant associations between cortisol immunoreactivity in (B) hair and feces, (C) saliva and hair, or (D) saliva and feces. Each data point represents cortisol immunoreactivity averaged over the 3-mo study period for comparisons of hair (n = 3 per dog) and feces (n = 33 to 41 per dog). For comparisons with saliva, data from samples (n = 23 to 30 per dog) were averaged over the third month only and compared with those of hair (n = 1 per dog) and feces (n = 14 or 15 per dog) collected during the same month.
Cortisol immunoreactivity in feces.
In feces, cortisol immunoreactivity was 363 ± 12.9 pg/mg, and the total variability was 58%. Within dogs, mean variability was 44.9% (range, 34.0% to 58.1%; Table 2). Cortisol immunoreactivity in feces was similar across months (F2,12 = 0.26, P = 0.63; Figure 3 C).
Cortisol immunoreactivity in saliva.
Cortisol immunoreactivity in saliva averaged 1.42 ± 0.06 ng/mL and had an overall CV of 60%. Within each dog, variability ranged from 27.8% to 51.3%, with a mean of 40.6% (Table 2). Across dogs, salivary cortisol immunoreactivity did not differ among daily time points (F2,12 = 1.32, P = 0.30; Figure 3 D). However, one dog (SA) showed high salivary cortisol immunoreactivity at the 1330 collection; this occurred the week after SA had minor ocular surgery and continued for 2 wk (until the end of the study). Cortisol immunoreactivity was not increased in feces or hair of dog SA.
Comparisons of cortisol immunoreactivity in hair, feces, and saliva.
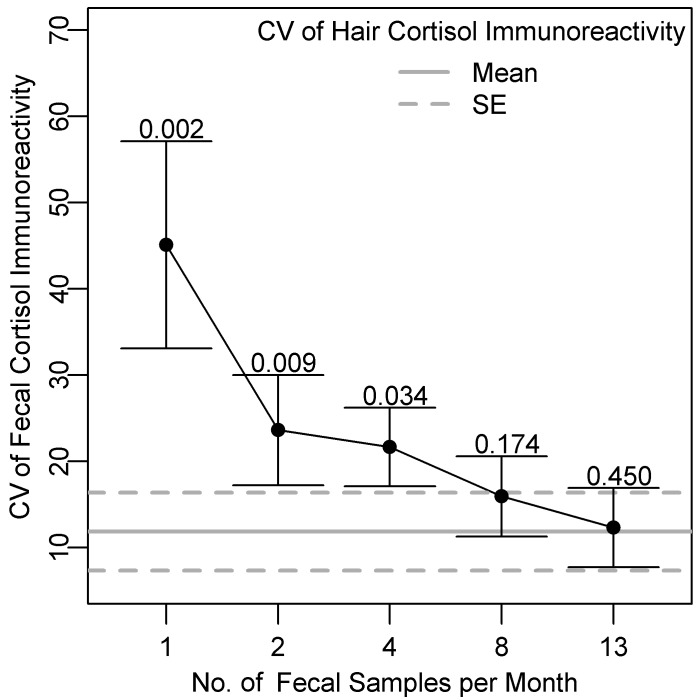
The CV differed among cortisol measurements from hair, feces, and saliva (F = 39.6, df = 2, P < 0.001; Table 2). Overall, cortisol measurements in hair were less variable than were those in feces (t = −7.92, df = 6, P < 0.001) or saliva (t = −5.69, df = 6, P = 0.001). Variability was similar between feces and saliva (t = −1.78, df = 6, P = 0.13). Averaged over time, the variability of cortisol immunoreactivity in feces decreased as the number of collections per month increased (Figure 5). The CV of cortisol immunoreactivity in feces were not significantly higher than those of hair when 4 samples were obtained per month (Bonferoni correction factor applied, P = 0.034; Figure 5). However the probability of obtaining a CV in feces less than or equal to that in hair was even lower with 8 (P = 0.17) or 13 (P = 0.45) fecal collections per month.
Figure 5.
Mean CV of cortisol immunoreactivity (bar, SE) according to the number of fecal samples collected per month compared with mean CV of cortisol immunoreactivity in hair collected once monthly from 7 dogs. To compare the CVs of different fecal sampling schemes, fecal cortisol data were subsampled from each dog corresponding to 1, 2, 4, 8, or 13 samples per month. For feces, the CV for each dog was calculated from cortisol immunoreactivity averaged within months (n = 3). For hair, CV for each dog were calculated from samples collected once per month (n = 3). The P values above the error bars are from paired comparisons of CVs for cortisol immunoreactivity in hair and feces. At least 4 fecal samples per month would need to be collected to obtain a CV less than or equal to that of hair with a Bonferroni correction factor of α = 0.01 to account for the number of comparisons (that is, 5).
There was a positive but nonsignificant correlation between cortisol immunoreactivity in hair and feces averaged over the 3-mo period (rs = 0.71; P = 0.07; n = 7; Figure 4 B). There was no evidence of a positive correlation between cortisol immunoreactivity in hair and saliva during the 1-mo sampling period (rs = −0.61, n = 7, P = 0.15; Figure 4 C), nor was there an overall correlation between saliva and feces (rs = −0.07, n = 7, P = 0.89; Figure 4 D). The relationships among saliva, feces, and hair did not improve after excluding data from dog SA, which had an exceptionally high cortisol level at the third salivary collection.
Discussion
Our findings indicate that cortisol immunoreactivity measurements in hair are less variable than are those in feces and saliva, adding to the growing evidence that hair is a valuable matrix for evaluating long-term cortisol levels in dogs. Moreover, at least 4 fecal samples per month were required to provide comparable repeatability to that of a single hair sample per month; this difference suggests that hair is more practical to sample than is feces. Lower variability in hair samples compared with saliva agrees with the data from a previous study,4 which examined overall variability in 94 hair samples from 48 dogs. Likewise, the mean and standard error of cortisol immunoreactivity in dog hair from the current study were similar to those reported previously (10.9 ± 0.6 pg/mg and 9.8 ± 11.4 pg/mg, respectively) in 2 studies that used comparable methods to ours.4,27 In contrast, another study1 reported a lower mean (2.1 ± 0.22 pg/mg) based on data obtained from dog hair by using a slightly different extraction procedure and radioimmunoassay.
Notably, the current study confirms that cortisol is present in dog hair. Previous studies have reported cortisol in human hair by using LC-MS/MS14 or LC-MS;5,7,29 however, we know of no previous studies that have used LC-MS/MS to measure cortisol in dog hair. Confirming the presence of cortisol in hair of different species by using LC-MS/MS—considered the most specific and sensitive analytical approach available for steroids35—is important due to potential interspecific differences in hair growth and composition. In addition, our LC-MS/MS analyses reveal that the immunoassay kit we used has negligible crossreactivity to several other steroids (corticosterone, estradiol, progesterone, and testosterone). Moreover, cortisol readings by ELISA were similar before and after solid-phase extraction, which would be expected to remove potentially crossreacting compounds such as polar cortisol conjugates (for example, cortisol sulfate). Further investigation using LC-MS/MS is required to detect other potentially crossreacting compounds.
We found that hair samples collected from the left and right sides of each dog were well correlated, as expected. Moreover, our results suggest that cutting full-length hair into segments corresponding to different periods of hair growth would be a helpful investigative tool. This approach likely would be particularly useful when repeated sampling is not practical or possible (for example, wildlife species) or in a clinical situation in which a patch of hair could be shaved and then resampled after regrowth, avoiding the short-term fluctuations intrinsic to serum cortisol concentrations. Depending on particular research applications, one limitation of this approach is the potential for cortisol degradation in older hair segments due to exposure to water or UV radiation.19,26
As expected, we found no evidence that cortisol immunoreactivity varied in hair or feces over the 3-mo study period. These findings reflect the stable physical and social environments of the dogs and clearly demonstrate that feces and especially hair are good indicators of long-term cortisol. Interestingly, we noted elevated cortisol in salivary samples from dog SA after minor ocular surgery. This finding could reflect oral contamination from grooming of the topical antiinflammatory ophthalmic medication with which this dog was probably treated, given that cortisol levels in fecal or hair samples from SA were not elevated.
Studies examining the relationship between cortisol in hair and sample matrices such as saliva have shown conflicting results.26 Contrary to our predictions, we found no evidence of correlations between hair and feces or hair and saliva, which contrasts with previous reports in dogs.1,4 This result was particularly surprising for saliva, because our sampling scheme (3 samples daily, 2 times each week) was designed to minimize confounding factors due to diurnal variations in salivary cortisol concentrations. In contrast, others found correlations when collecting saliva less frequently.4,9 Our strategy of multiple daily collections likely accurately reflects baseline salivary cortisol, which shows considerable variability from sample to sample; however, perhaps dogs, unlike humans, do not have a circadian rhythm in salivary cortisol.23 Indeed, the current results show no systematic circadian differences in salivary cortisol among the 3 daily collection times. Regardless of the mechanism, the discrepancy between our results and those of others may reflect the difficulty of using salivary cortisol as a measure of moderate to long-term cortisol concentrations.
Alternatively or concomitantly, another explanation for not detecting correlations among hair, saliva, and feces is lack of power due to the small sample size. In addition, the number of dogs available for the current study precluded examination of potential confounders such as sex, age, and (in particular) hair color. A previous study showed higher cortisol concentrations in white than black dog hair.4 In contrast, results from humans showed no effect of hair color on cortisol levels,29 perhaps because melanin would not preferentially bind neutral compounds such as cortisol.28,29 In the present study, hair color likely was not a confounding factor, because others authors found a significant correlation between cortisol immunoreactivity in hair and feces without accounting for hair color.1 Nonetheless, hair color and other potential confounders should be considered in future studies.
In conclusion, we show that cortisol is present in dog hair and that cortisol immunoreactivity in hair is less variable than that in either saliva or feces. Measuring hair cortisol over time may be particularly valuable in tracking gradual changes related to disease progression with conditions associated with adrenal hypo- or hyperfunction, such as Addison or Cushing disease, as well as responses to social or environmental stressors. For practitioners and researchers interested in long-term cortisol levels, our findings suggest that a single hair sample can be collected rather than multiple samples of saliva or feces.
Acknowledgments
We thank Susan Cook from Veterinary Biomedical Sciences, University of Saskatchewan, for assistance with fecal and salivary immunoassays. At the University of Calgary, we extend our gratitude to Lea Bond and Lee Koren for discussion and assistance with hair immunoassays and to Ella Ng for helping with LC-MS/MS. We are grateful to staff at the University of Saskatchewan Animal Resource Centre for their assistance with sample collections. This research was funded by the Western College of Veterinary Medicine, the University of Calgary Faculty of Veterinary Medicine, and National Science and Engineering Council (NSERC) grants to Dr Smits and Dr Wynne-Edwards. Ms Bryan was supported by an NSERC industrial postgraduate scholarship, Ms Adams by a University of Saskatchewan Undergraduate Student Experience Program, and Ms Invik in part by a stipend from the Raincoast Conservation Foundation. We appreciate the comments from several anonymous reviewers whose suggestions improved this manuscript.
References
- 1.Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Garnberoni M, Tarnanini C, Seren E. 2008. Cortisol determination in hair and faeces from domestic cats and dogs. Gen Comp Endocrinol 155:398–402 [DOI] [PubMed] [Google Scholar]
- 2.Ashley NT, Barboza PS, Macbeth BJ, Janz DM, Cattet MRL, Booth RK, Wasser SK. 2011. Glucocorticosteroid concentrations in feces and hair of captive caribou and reindeer following adrenocorticotropic hormone challenge. Gen Comp Endocrinol 172:382–391 [DOI] [PubMed] [Google Scholar]
- 3.Barton BA. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525 [DOI] [PubMed] [Google Scholar]
- 4.Bennett A, Hayssen V. 2010. Measuring cortisol in hair and saliva from dogs: coat color and pigment differences. Domest Anim Endocrinol 39:171–180 [DOI] [PubMed] [Google Scholar]
- 5.Bévalot F, Gaillard Y, Lhermitte MA, Pépin G. 2000. Analysis of corticosteroids in hair by liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B Biomed Life Sci 740:227–236 [DOI] [PubMed] [Google Scholar]
- 6.Bortolotti GR, Marchant TA, Blas J, German T. 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct Ecol 22:494–500 [Google Scholar]
- 7.Cirimele V, Kintz P, Dumestre V, Goulle JP, Ludes B. 2000. Identification of 10 corticosteroids in human hair by liquid chromatography–ionspray mass spectrometry. Forensic Sci Int 107:381–388 [DOI] [PubMed] [Google Scholar]
- 8.Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry 63:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261 [DOI] [PubMed] [Google Scholar]
- 10.Davidson RJ, McEwen BS. 2012. Social influences on neuroplasticity: stress and interventions to promote wellbeing. Nat Neurosci 15:689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreschel NA, Granger DA. 2009. Methods of collection for salivary cortisol measurement in dogs. Horm Behav 55:163–168 [DOI] [PubMed] [Google Scholar]
- 12.Fairhurst GD, Frey MD, Reichert JF, Szelest I, Kelly DM, Bortolotti GR. 2011. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 6:e17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourie NH, Bernstein RM. 2011. Hair cortisol levels track phylogenetic and age-related differences in hypothalamic–pituitary–adrenal (HPA) axis activity in nonhuman primates. Gen Comp Endocrinol 174:150–155 [DOI] [PubMed] [Google Scholar]
- 14.Gaillard Y, Vayssette F, Pepin G. 2000. Compared interest between hair analysis and urinalysis in doping controls. Results for amphetamines, corticosteroids, and anabolic steroids in racing cyclists. Forensic Sci Int 107:361–379 [DOI] [PubMed] [Google Scholar]
- 15.Gow R, Thomson S, Rieder M, Van Uum S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 196:32–37 [DOI] [PubMed] [Google Scholar]
- 16.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. 2005. Human hair follicles display a functional equivalent of the hypothalamic–pituitary–adrenal axis and synthesize cortisol. FASEB J 19:1332–1334 [DOI] [PubMed] [Google Scholar]
- 17.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. 2007. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med 30:E103–E107 [DOI] [PubMed] [Google Scholar]
- 18.Kirschbaum C, Hellhammer DH. 1989. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–169 [DOI] [PubMed] [Google Scholar]
- 19.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. 2009. Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–37 [DOI] [PubMed] [Google Scholar]
- 20.Kobelt AJ, Hemsworth PH, Barnett JL, Butler KL. 2003. Sources of sampling variation in saliva cortisol in dogs. Res Vet Sci 75:157–161 [DOI] [PubMed] [Google Scholar]
- 21.Koren L, Mokady O, Karaskov T, Klein J, Koren G, Geffen E. 2002. A novel method using hair for determining hormonal levels in wildlife. Anim Behav 63:403–406 [Google Scholar]
- 22.Koren L, Ng ES, Soma KK, Wynne-Edwards KE. 2012. Sample preparation and liquid chromatography–tandem mass spectrometry for multiple steroids in mammalian and avian circulation. PLoS ONE 7:e32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama T, Omata Y, Saito A. 2003. Changes in salivary cortisol concentrations during a 24-hour period in dogs. Horm Metab Res 35:355–357 [DOI] [PubMed] [Google Scholar]
- 24.Kronstrand R, Scott K. 2006. Drug incorporation into hair. In: Kintz P. Analytical and practical aspects of drug testing in hair. Boca Raton (FL): CRC Press. [Google Scholar]
- 25.Macbeth BJ, Cattet MRL, Stenhouse GB, Gibeau ML, Janz DM. 2010. Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): considerations with implications for other wildlife. Can J Zool 88:935–949 [Google Scholar]
- 26.Meyer JS, Novak MA. 2012. Minireview. Hair cortisol: a novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 153:4120–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piva E, Liverani V, Accorsi PA, Sarli G, Gandini G. 2008. Welfare in a shelter dog rehomed with Alzheimer patients. J Vet Behav 3:87–94 [Google Scholar]
- 28.Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370:17–49 [DOI] [PubMed] [Google Scholar]
- 29.Raul JS, Cirimele V, Ludes B, Kintz P. 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem 37:1105–1111 [DOI] [PubMed] [Google Scholar]
- 30.Reeder DM, Kramer KM. 2005. Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86:225–235 [Google Scholar]
- 31.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255 [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308:648–652 [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- 34.Schatz S, Palme R. 2001. Measurement of faecal cortisol metabolites in cats and dogs: a noninvasive method for evaluating adrenocortical function. Vet Res Commun 25:271–287 [DOI] [PubMed] [Google Scholar]
- 35.Shackleton C. 2010. Clinical steroid mass spectrometry: a 45-year history culminating in HPLC–MS–MS becoming an essential tool for patient diagnosis. J Steroid Biochem Mol Biol 121:481–490 [DOI] [PubMed] [Google Scholar]
- 36.Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. 2011. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887 [DOI] [PubMed] [Google Scholar]
- 37.Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SHM. 2010. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diabetes 118:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zar JH.1999. Biostatistical analysis. Uper Saddle River (NJ): Prentice Hall.