Abstract
Study Objectives:
Structural and functional brain changes may contribute to neural dysfunction in patients with obstructive sleep apnea (OSA). However, the effect of OSA on resting-state brain activity has not been established. The objective of this study was to investigate alterations in resting-state functional connectivity (rsFC) of the common brain networks in patients with OSA and their relationships with changes in gray matter volume (GMV) in the corresponding brain regions.
Designs:
Resting-state functional and structural MRI data were acquired from patients with OSA and healthy controls. Seven brain networks were identified by independent component analysis. The rsFC in each network was compared between groups and the GMV of brain regions with significant differences in rsFC was also compared.
Setting:
University hospital.
Patients and Participants:
Twenty-four male patients with untreated OSA and 21 matched healthy controls.
Interventions:
N/A.
Measurements and Results:
OSA specifically affected the cognitive and sensorimotor-related brain networks but not the visual and auditory networks. The medial prefrontal cortex and left dorsolateral prefrontal cortex (DLPFC) showed decreased rsFC and GMV in patients with OSA, suggesting structural and functional deficits. The right DLPFC and left precentral gyrus showed decreased rsFC and unchanged GMV, suggesting a functional deficit. The right posterior cingulate cortex demonstrated increased rsFC and unchanged GMV, suggesting functional compensation. In patients with OSA, the rsFC of the right DLPFC was negatively correlated with the apnea-hypopnea index.
Conclusions:
OSA specifically affects resting-state functional connectivity in cognitive and sensorimotor-related brain networks, which may be related to the impaired cognitive and motor functions in these patients.
Citation:
Zhang Q; Wang D; Qin W; Li Q; Chen B; Zhang Y; Yu C. Altered resting-state brain activity in obstructive sleep apnea. SLEEP 2013;36(5):651-659.
Keywords: Brain networks, functional connectivity, independent component analysis, obstructive sleep apnea, voxel-based morphometry
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by repetitive episodes of partial or complete upper airway obstruction during sleep, resulting in fragmented sleep and chronically intermittent hypoxemia; it affects at least 2-4% of middle-aged adults.1,2 OSA is associated with cerebrovascular and cardiovascular morbidity, excessive daytime sleepiness, depression, anxiety, and increased risk for motor vehicle accidents.3,4
Patients with OSA exhibit impaired neural function and have memory, executive, attention, and motor function deficits.5 However, the underlying neural mechanisms remain largely unknown and have attracted much attention. Previous neuroimaging studies revealed that patients with OSA showed alterations in gray matter volume (GMV),6–8 white matter integrity,9 metabolism,7,10,11 regional cerebral blood flow (rCBF),12 activation,13–15 and deactivation16,17 in a variety of brain areas. Furthermore, several of these alterations were correlated with the severity of the disorder.8,10,12,14,17 However, it remains unclear whether the resting-state brain activity is altered in patients with OSA; such knowledge may improve understanding of the mechanisms of impaired neural functions in this disorder. Although resting-state brain activity can be investigated by several neuroimaging techniques, the resting-state functional MRI (rs-fMRI) is the most widely used. Among the methods for analyzing rs-fMRI data, the independent component analysis (ICA) is useful as a data-driven method that can automatically identify several meaningful brain networks.18,19 Each brain network is composed of several brain regions with similar activity patterns, whereas different networks show different activity patterns.20 The resting-state brain activity within these brain networks is changed in many diseases.21 Some of these diseases are similar to OSA, such as chronic hypoxia and sleep deprivation.22,23 These findings lead us to hypothesize that resting-state brain activity is changed in OSA, which may contribute to the deficits in neural functions in this disorder.
In the current study, we first compared the resting-state functional connectivity (rsFC, a measure of the resting-state brain activity) within each of the seven brain networks between patients with OSA and healthy controls to identify brain networks with altered rsFC in OSA. Next, we evaluated the relationships between the altered rsFC in a specific brain network and the severity of OSA. Then, we explored whether the brain regions with altered rsFC also had changed GMV to determine the implications of the altered rsFC on OSA. Finally, we also recruited 19 sleep-deprived subjects (who did not have OSA) to examine the effect of sleepiness on rsFC and to clarify which rsFC changes were specific to OSA.
METHODS
Participants
Twenty-four treatment-naïve male patients with moderate to severe OSA and 21 healthy controls matched for age, sex, years of education, and handedness were included in this study. Patients were enrolled from the Sleep Laboratory of the Respiratory Department of Tianjin Medical University General Hospital. The apnea-hypopnea index (AHI) was higher than 15 in the patients with OSA but lower than five in the control subjects. Each participant was assessed by a detailed clinical interview and physical examination. Exclusion criteria for both the patients and controls were as follows: (1) sleep disorders other than OSA; (2) history of a cardiovascular disease, hypertension, diabetes mellitus, or central nervous system disorders; (3) age younger than 30 years or older than 60 years; (4) left-handedness; (5) alcohol or illicit drug abuse, or current intake of psychoactive medications; (6) Mini Mental State Examination (MMSE) score < 24/30; (7) body weight > 125 kg; and (8) MRI contraindications, such as claustrophobia and metallic implants or devices in the body. The study was approved by the Medical Research Ethics Committee of Tianjin Medical University, and all participants provided written informed consent forms.
To examine whether functional brain changes were related to sleepiness in patients with OSA, 19 non-OSA sleep-deprived healthy male subjects with AHI < 5 were recruited. The subject exclusion criteria included a history of medical, neurologic, psychiatric, or sleep disorders (such as OSA), past history of alcohol or drug abuse, and current use of psychoactive medications. These individuals were scheduled for two rs-fMRI scans starting at 07:00. One scan was performed after a well-rested normal night following the individual's habitual sleep schedule, and the other scan was performed after 24 h of total sleep deprivation. Sleep deprivation was performed in a separate room where these individuals were continuously monitored and checked every 10 min. These individuals were instructed to refrain from smoking, caffeine and alcohol intake, and physical exercise in the 24 h before the second rs-fMRI scan. Caffeine-free meals were provided during the sleep-deprived period.
Polysomnography
Full nocturnal polysomnography (PSG) monitoring was performed on all patients with OSA and healthy controls using the Compumedics E-Series Sleep System (Compumedics Sleep, Abbotsford, Australia). Standard electroencephalogram (EEG), electrooculogram (EOG), chin electromyogram (EMG), electrocardiogram (ECG), oral and nasal airflow, thoracic and abdominal movements, oximetry, body position, and snoring were recorded. In accordance with the American Academy of Sleep Medicine guidelines, an obstructive apnea was defined as a reduction in airflow ≥ 90% lasting at least 10 sec and associated with persistent respiratory effort; a hypopnea was defined as a reduction in airflow ≥ 30% lasting at least 10 sec and accompanied by a 4% or greater oxygen desaturation, or with EEG arousal.24,25 The AHI was calculated as the average of the total number of apnea and hypopnea events experienced per hour of sleep. The percentage of time with saturation of oxygen (SaO2) below 90% during total sleep (% total sleep time [TST] < 90%) was also recorded. Excessive daytime sleepiness was evaluated by using the Epworth Sleepiness Scale (ESS).26
MRI Data Acquisition
Imaging was performed on a 3.0 Tesla MRI system (Signa HDx, General Electric, Milwaukee, WI, USA). Foam pads were used to minimize head movements, and earplugs were used to reduce scanner noise. The rs-fMRI scans were performed by an echo planar imaging (EPI) sequence with the following parameters: repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 × 240 mm2, matrix = 64 × 64, slice thickness = 3 mm, slice gap = 1 mm. Each brain volume comprised 38 axial slices, and each functional run consisted of 180 volumes. During fMRI scans, all patients were instructed to keep their eyes closed, to stay as motionless as possible, to think of nothing in particular, and not to fall asleep. High-resolution three-dimensional T1-weighted images were acquired by a brain volume (BRAVO) sequence (repetition time = 8.1 ms, echo time = 3.1 ms, inversion time = 450 ms, flip angle = 13°, field of view = 256 × 256 mm2, matrix = 256 × 256, slice thickness = 1 mm, no gap, 176 sagittal slices).
fMRI Data Preprocessing
The fMRI data were preprocessed with the Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). The first 10 volumes for each patient were discarded due to the signal reaching equilibrium and the participants adapting to the scanning noise. The remaining 170 volumes were corrected for acquisition time delay between different slices and corrected for geometric displacements according to the estimated head movement and realigned to the first volume. Head motion parameters were computed by estimating translation in each direction and the angular rotation on each axis for each volume. Each patient had maximum displacement in any of the cardinal directions (x, y, z) of less than 2 mm and a maximum spin (x, y, z) of less than 2°. Following this step, all functional data were spatially normalized to the Montreal Neurological Institute (MNI) EPI template, resampled to 3 mm3 voxels, and smoothed with a 6-mm full width at half maximum (FWHM).
Independent Component Analysis
A subject order-independent group ICA (SOI-GICA)27 was conducted for the 45 participants using the Infomax algorithm28 within the Stable and Consistent Group ICA Toolbox (http://www.nitrc.org/projects/cogicat/, MICA version beta 1.2). A three-step principal component analysis was used to decompose the dataset into 30 components. The GICA was run 100 times to obtain highly robust results. Seven meaningful components were identified via visual inspection, and these components are consistent with previous studies.19,20 The individual-level components were obtained by back-reconstruction and converted into calibrated rsFC maps.29 Here, the calibrated rsFC reflects the degree to which the time series of a given voxel correlates with the mean time series of its belonging component.
Statistical Analysis for fMRI Data
For a specific spatial component, the component maps for all of the participants were entered into a one-sample t-test (P < 0.05, after correcting for multiple comparisons using a family-wise error [FWE] method) to create a sample-specific component map. Each of these sample-specific component maps was used as a spatial mask for its corresponding brain network. For each brain network of interest, a two-sample t-test was used to identify group differences in the rsFC in a voxelwise manner within the spatial mask. Multiple comparisons were corrected using Monte Carlo simulations with a corrected threshold of P < 0.05 (AlphaSim program in AFNI software, http://afni. nimh.nih.gov/). The threshold of the cluster size was calculated by the following parameters: single voxel P = 0.01, 5,000 simulations, FWHM = 6 mm, cluster connection radius = 5 mm with each component mask (3 mm3 resolution). Paired sample t-test was used to compare the rsFC differences in the 19 subjects before and after sleep deprivation using the same statistical thresholds as comparisons between OSA patients and healthy controls.
In the brain networks showing significant group differences in rsFC, voxel-based partial correlation analyses controlling for age were performed to identify brain regions whose rsFC was correlated with clinical variables. Multiple comparisons were corrected using the same method as in the intergroup comparison of the rsFC.
For each subject, the mean rsFC of each cluster with signifi-cant group difference was extracted and used for region of interest (ROI)-based group comparison and correlation analysis with clinical variables. The ROI-based group comparisons were performed using a two-sample t-test (P < 0.05). The correlation analyses with clinical variables were performed using a partial correlation method (P < 0.05) with age as covariate of no interest.
VBM Analysis
The VBM analysis was performed using the SPM8 software. The detailed procedures have been described in our previous studies.30 After spatial preprocessing, the smoothed, modulated, normalized GMV maps were used for statistical analysis. For the whole-brain analysis, voxelwise GMV differences between the patients with OSA and healthy controls were examined by using a two-sample t-test with age as covariate of no interest (FWE correction, P < 0.05). Then, an ROI-based partial correlation analysis (controlling for age) was conducted to evaluate the relationship between GMV changes and clinical variables. Finally, the mean GMV of each cluster with significant group differences in the rsFC was calculated and compared between the two groups using a two-sample t-test (P < 0.05).
Statistical Analysis for Demographic and Clinical Variables
The demographic and clinical data were compared between the two groups using Statistical Package for the Social Sciences version 17.0 (SPSS, Chicago, IL, USA). Differences were considered significant when P was less than 0.05.
RESULTS
Demographic and Clinical Data
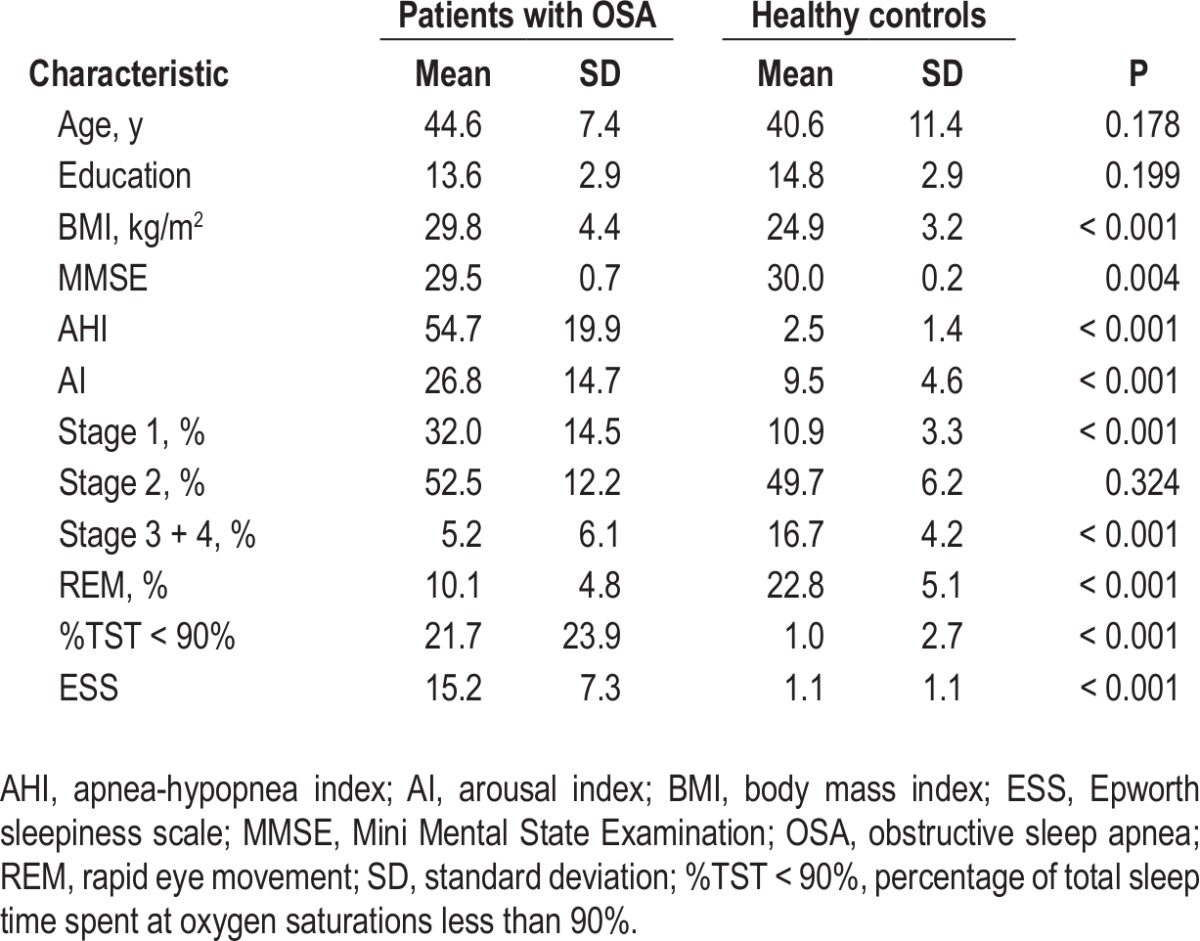
Demographic and clinical characteristics of each group are summarized in Table 1. All participants were male, right-handed, and free of any visible abnormalities on conventional MRI. There were no significant differences (P > 0.05) in age and years of education between the patients with OSA and healthy controls. As expected, the patients with OSA had significantly higher scores for body mass index (BMI) (t = 4.17, P < 0.001), AHI (t = 12.83, P < 0.001), %TST < 90% (t = 4.23, P < 0.001) and ESS (t = 9.38, P < 0.001) but a significant lower score for MMSE (t = -3.17, P = 0.004), compared to the controls. Sleep deprivation induced significant increases in ESS (3.3 ± 3.3 versus 20.5 ± 3.2, t = -16.89, P < 0.001, rested baseline versus sleep deprivation state) (Table S1).
Table 1.
Demographic and clinical characteristics of patients with OSA and healthy controls
Identification of the Resting-State Brain Networks
To visualize the main components of brain regions in a brain network, we adopted a strict statistical threshold of t > 7 and a cluster size threshold of > 200 voxels (Figure 1). The seven brain networks were as follows: (1) the anterior default-mode network (aDMN) included the medial prefrontal cortex (MPFC), anterior cingulate cortex, and superior frontal gyrus; (2) the posterior DMN (pDMN) consisted of the posterior cingulate cortex (PCC) and precuneus (Pcu); (3) the left frontoparietal network (LFP) was mainly composed of the left dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule; (4) the right frontoparietal network (RFP) was mainly composed of the right DLPFC and inferior parietal lobule; (5) the sensorimotor network (SMN) included the bilateral primary sensorimotor cortices and supplementary motor areas; (6) the auditory network (AN) consisted of the primary and secondary auditory cortices and the superior temporal gyrus; and (7) the visual network (VN) was composed of the primary, secondary, and high-level visual cortices.
Figure 1.
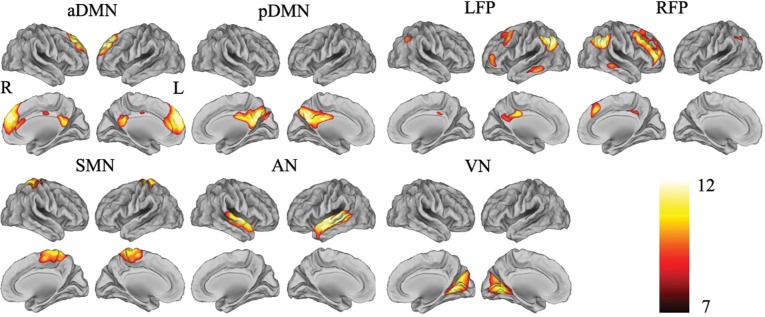
Visualization of meaningful resting-state brain networks. These seven brain networks are identified by independent component analysis (ICA) from the 45 subjects (t > 7 and cluster size > 200 voxels). aDMN, anterior default-mode network; AN, auditory network; L, left; LFP, left frontoparietal network; pDMN, posterior default-mode network; R, right; RFP, right frontoparietal network; SMN, sensorimotor network; VN, visual network.
The rsFC Differences between the Patients with OSA and Healthy Controls
In the voxel-based analysis, the rsFC differences between the patients with OSA and healthy controls are shown in Figure 2 and Table 2. Compared with the controls, the patients with OSA displayed decreased rsFC in the MPFC of the aDMN, the DLPFC of the LFP, the DLPFC of the RFP, and the left precentral gyrus of the SMN (P < 0.05, corrected). The significantly increased rsFC was only found in the right PCC of the pDMN. The rsFC in the AN and VN did not show any significant differences between the two groups.
Figure 2.
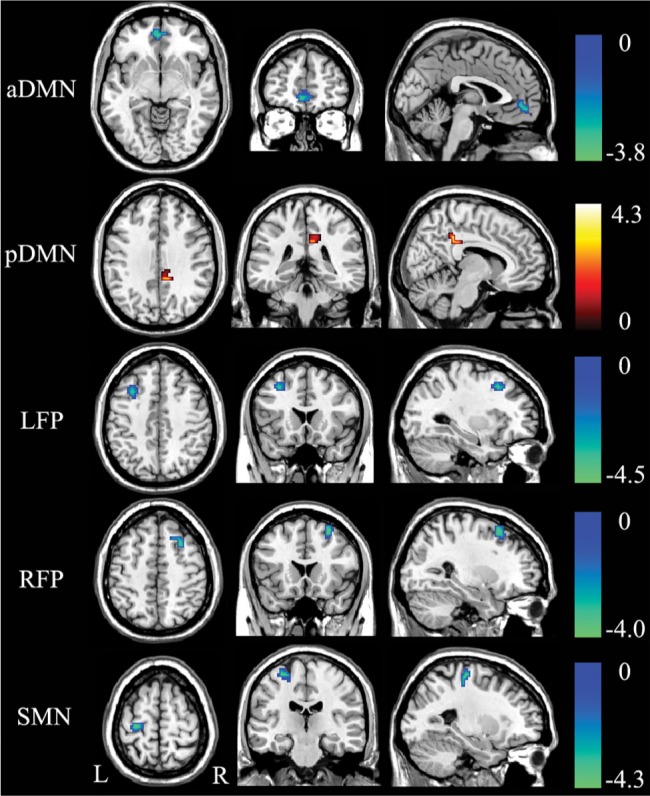
Significant (P < 0.05, corrected) increase (warm color) and decrease (cold color) in resting-state functional connectivity within each brain network in patients with OSA. aDMN, anterior default-mode network; L, left; LFP, left frontoparietal network; OSA, obstructive sleep apnea; pDMN, posterior default-mode network; R, right; RFP, right frontoparietal network; SMN, sensorimotor network.
Table 2.
Brain areas with significant differences in rsFC within each brain network between patients with OSA and healthy controls
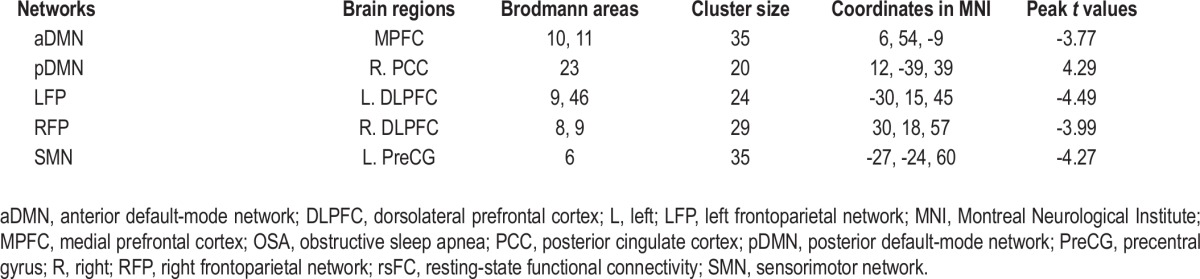
For the ROI-based analysis, the rsFC differences between the patients with OSA and healthy controls are shown in Figure 3 and Table S2. Compared with the controls, the patients with OSA showed significantly decreased rsFC in the MPFC of the aDMN (P = 0.001), the DLPFC of the LFP (P < 0.001), the DLPFC of the RFP (P < 0.001), and the left precentral gyrus of the SMN (P < 0.001). The right PCC of the pDMN showed significantly increased (P = 0.001) rsFC in the patients with OSA.
Figure 3.
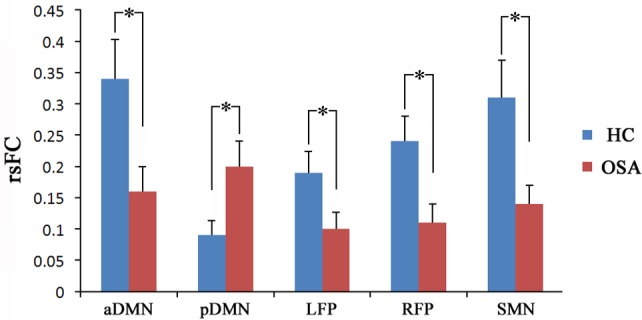
Region of interest (ROI) analyses revealed significant (P < 0.05) changes in rsFC in patients with OSA. An asterisk indicates significant intergroup difference. aDMN, anterior default-mode network; HC, healthy controls; LFP, left frontoparietal network; OSA, obstructive sleep apnea; pDMN, posterior default-mode network; RFP, right frontoparietal network; rsFC, resting-state functional connectivity; SMN, sensorimotor network.
Correlations between rsFC with Group Difference and Clinical Variables
In patients with OSA, voxel-based partial correlation analysis showed that the AHI scores were negatively correlated (P < 0.05, corrected) with the rsFC of the right DLPFC of the RFP (Figure 4), but they were not correlated with the rsFC of other brain regions of the RFP or the rsFC of any brain regions of other brain networks with significant group difference. There were no significant correlations (P > 0.05) between the %TST < 90% or ESS scores and the rsFC of brain regions of interest in patients with OSA. In the ROI-based correlation analysis, we only found significant correlation (r = -0.488, P = 0.018) between the rsFC of right DLPFC and the AHI in patients with OSA (Table S3).
Figure 4.
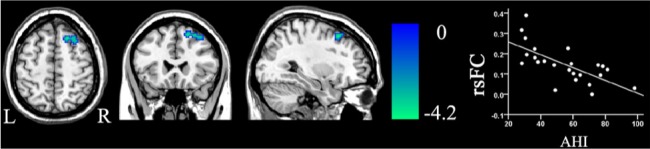
Correlation (P < 0.05, corrected) between the rsFC of the dorsolateral prefrontal cortex of the right frontoparietal network and the AHI scores in patients with OSA. AHI, apnea-hypopnea index; L, left; OSA, obstructive sleep apnea; R, right; rsFC, resting-state functional connectivity.
The GMV Differences between the Patients with OSA and Healthy Controls
For the whole-brain analysis, compared with healthy controls, the patients with OSA demonstrated reduced GMV in the left MPFC and left posterior inferior frontal gyrus (pIFG) (P < 0.05, FWE corrected) (Figure 5 and Table 3). With an uncorrected threshold of P < 0.0001, a reduction of GMV was also found in the bilateral hippocampi, the right cuneus, the right middle temporal gyrus, and the left DLPFC (Figure 6 and Table S4). Then we extracted the mean GMV of the left MPFC and left pIFG in the patients with OSA, and found that there were no significant correlations between the GMVs of the two ROIs and the AHI, %TST < 90% or ESS scores (P > 0.05) (Table S5).
Figure 5.
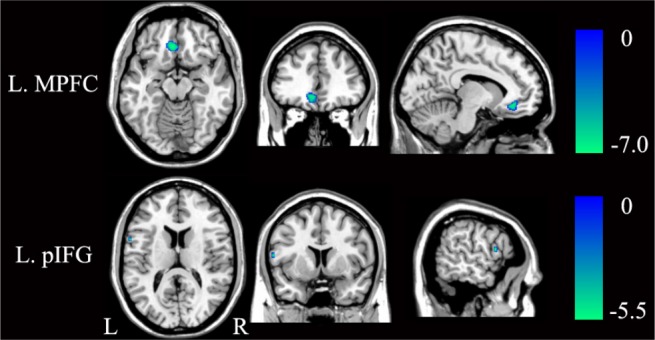
Significant (P < 0.05, FWE corrected) differences in GMV between patients with OSA and healthy controls. FWE, family-wise error; GMV, gray matter volume; L, left; MPFC, medial prefrontal cortex; OSA, obstructive sleep apnea; pIFG, posterior inferior frontal gyrus; R, right.
Table 3.
Brain areas with significant differences in GMV between patients with OSA and healthy controls (P < 0.05, FWE corrected)
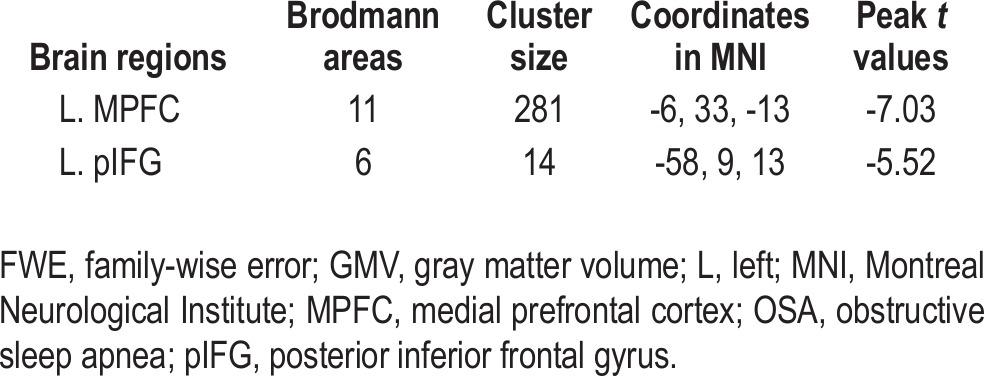
Figure 6.
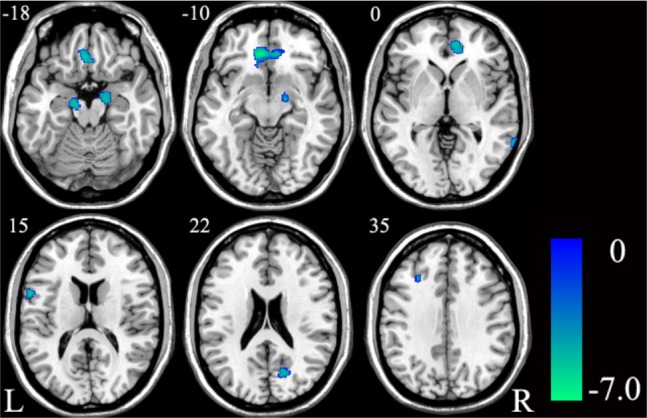
Significant (P < 0.0001, uncorrected) differences in GMV between patients with OSA and healthy controls. GMV, gray matter volume; L, left; OSA, obstructive sleep apnea; R, right.
GMV Differences in Brain Regions with Significant Group Difference in the rsFC
We calculated the mean GMV of brain regions with signifi-cant differences in the rsFC and compared them between the two groups using a two-sample t-test (Table S6). Compared with the controls, the patients with OSA showed significantly smaller GMV in the MPFC of the aDMN (P = 0.003) and in the left DLPFC of the LPF (P = 0.021) (Figure 7). Other brain regions of interest did not show any significant group differences in GMV.
Figure 7.
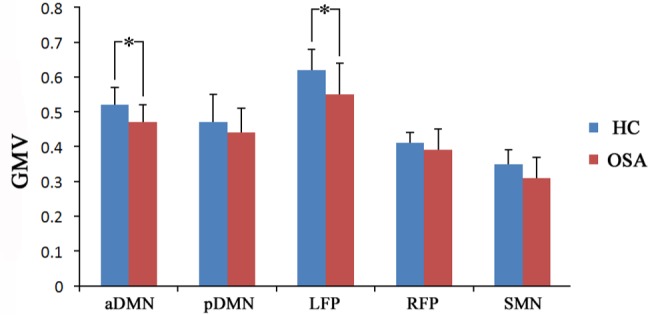
Significant (P < 0.05) GMV alterations in brain regions with significantly changed rsFC in patients with OSA. An asterisk indicates significant intergroup difference. aDMN, anterior default-mode network; GMV, gray matter volume; HC, healthy controls; LFP, left frontoparietal network; OSA, obstructive sleep apnea; pDMN, posterior default-mode network; RFP, right frontoparietal network; rsFC, resting-state functional connectivity; SMN, sensorimotor network.
The rsFC Differences before and after Sleep Deprivation
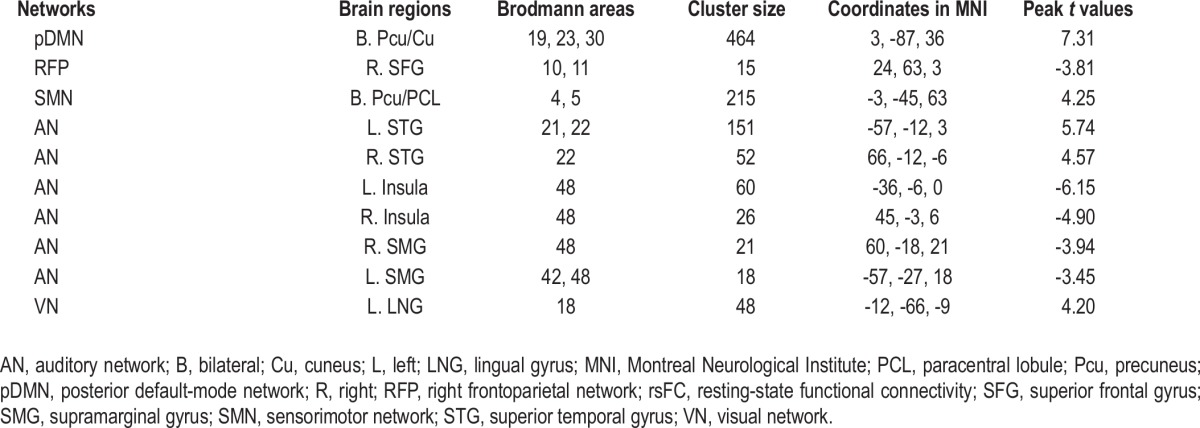
The MRI data acquisition and analysis for the sleep-deprived subjects were the same as those for the patients with OSA. In the sleep-deprived subjects, the rsFC differences before and after sleep deprivation are shown in Figures 8 and 9 and Table 4. Paired t-tests demonstrated increased rsFC in the bilateral Pcu and cuneus of the pDMN, bilateral Pcu, and paracentral lobule of the SMN, left superior temporal gyrus (STG) and right STG of the AN, left lingual gyrus of the VN in the sleep-deprived state (P < 0.05, corrected). The significantly decreased rsFC was only found in the right superior frontal gyrus of the RFP, bilateral insula, and supramarginal gyri of the AN (P < 0.05, corrected).
Figure 8.
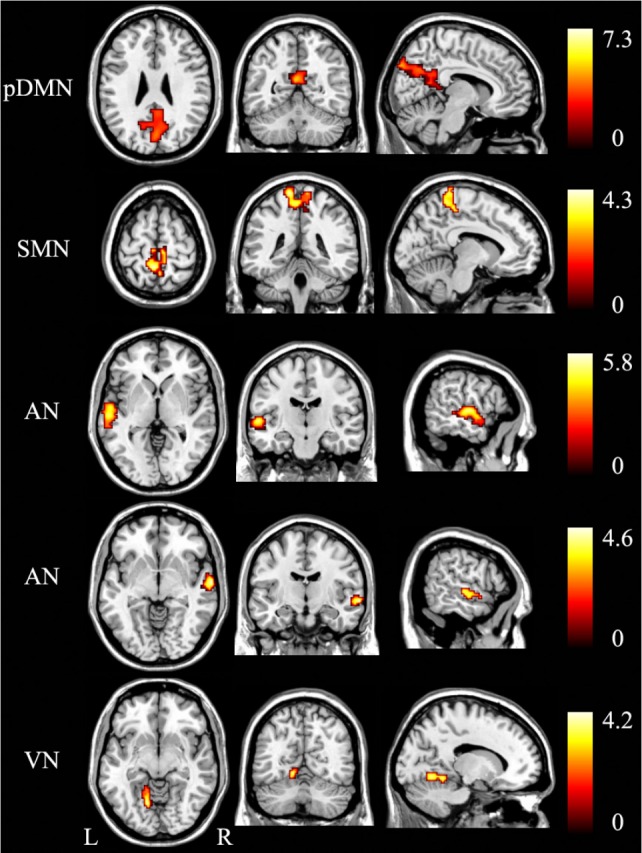
Significant (P < 0.05, corrected) increased rsFC (warm color) within each brain network after sleep deprivation. AN, auditory network; L, left; pDMN, posterior default-mode network; R, right; rsFC, resting-state functional connectivity; SMN, sensorimotor network; VN, visual network.
Figure 9.
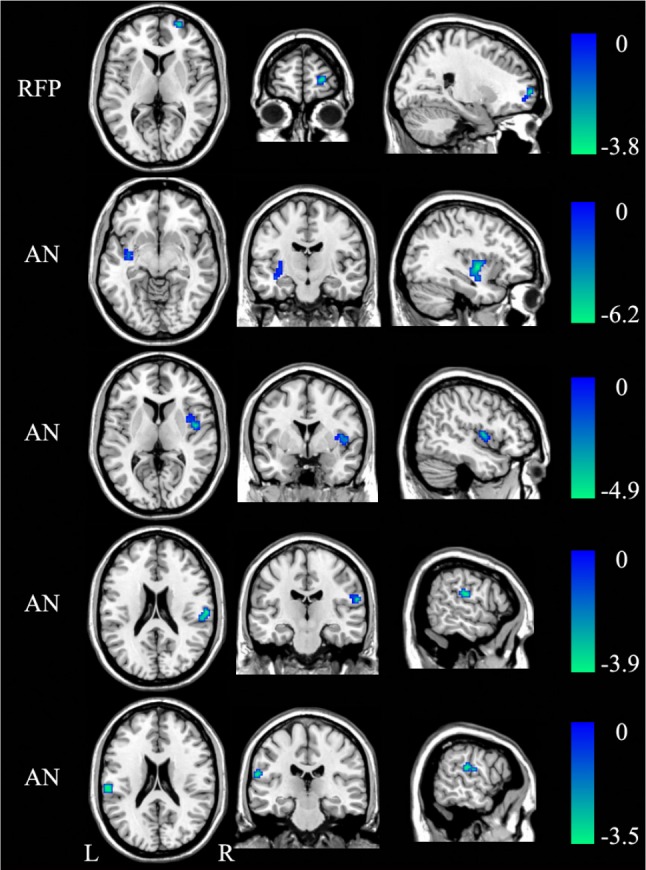
Significant (P < 0.05, corrected) decreased rsFC (cold color) within each brain network after sleep deprivation. AN, auditory network; L, left; R, right; RFP, right frontoparietal network; rsFC, resting-state functional connectivity.
Table 4.
Brain areas with significant differences in rsFC within each brain network before and after sleep deprivation
DISCUSSION
This study is, to the best of our knowledge, the first to investigate the effect of OSA on resting-state brain activity using rs-fMRI. Three main findings are prevalent: (1) OSA specifically affected the resting-state brain activity in cognitive and sensorimotor-related brain networks but not the auditory and visual networks, and these observations were consistent with the prominent cognitive and motor dysfunction observed in this disorder; (2) we identified three types of brain alterations in patients with OSA by combining the structural and functional analyses, i.e., structural and functional deficits in the aDMN and LFP, functional deficit in the RFP and SMN, and functional compensation in the pDMN; and (3) we also identified a link between the altered rsFC in the RFP and disease severity in patients with OSA.
Were the rsFC Changes Specific to OSA or Just Reflective of Sleepiness?
Although we instructed the patients with OSA not to fall asleep, we could not absolutely exclude the effect of sleepiness on the rsFC because the patients with OSA showed excessive daytime sleepiness and were prone to falling asleep, which might affect the rsFC.31–34 To clarify the specific rsFC changes in OSA, we compared rsFC changes resulting from simple sleep deprivation and OSA. We found four types of differences in rsFC changes between sleep-deprived subjects and OSA patients: (1) the aDMN and LFP showed decreased rsFC in patients with OSA but did not show any significant differences in sleep-deprived subjects, which suggested that the changes in these two networks were specific to OSA; (2) the AN and VN showed altered rsFC in sleep-deprived subjects, but did not show any significant changes in patients with OSA, which suggested that the changes in these two networks were specific to sleep deprivation; (3) the SMN showed increased rsFC in sleep-deprived subjects, but showed decreased rsFC in patients with OSA, suggesting the decreased rsFC in the SMN being specific to the OSA; and (4) although we found increased rsFC of the pDMN and decreased rsFC of the RFP in both patients with OSA and sleep-deprived subjects, the affected brain regions were completely different (Figure 10). The rsFC changes in patients with OSA mainly resulted from OSA itself rather than excessive sleepiness.
Figure 10.
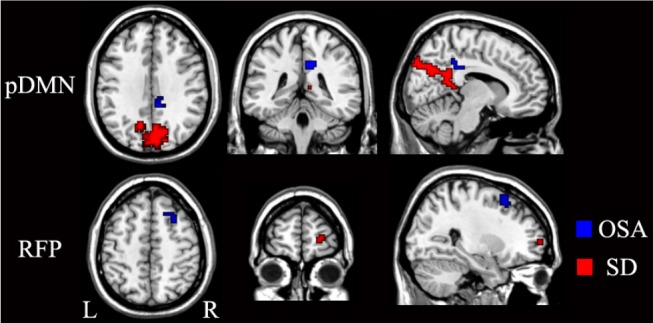
Brain regions with significantly differences in rsFC of the pDMN and RFP between the patients with OSA (blue) and the sleep-deprived subjects (red). L, left; OSA, obstructive sleep apnea; pDMN, posterior default-mode network; R, right; RFP, right frontoparietal network; rsFC, resting-state functional connectivity; SD, sleep deprivation.
Brain Networks with both Structural and Functional Deficits in Patients with OSA
OSA patients demonstrated decreased rsFC and reduced GMV in the MPFC of the aDMN, indicating structural and functional deficits. The DMN includes a set of brain regions that are more active during rest than during goal-directed tasks and is involved in a wide range of higher-order cognitive functions.35 Decreased rsFC in the DMN has been reported in Alzheimer disease and multiple sclerosis,36,37 both of which show impaired episodic memory. Similarly, patients with OSA also show abnormal deactivation in the DMN during working memory tasks17 and episodic memory impairment.5 As a core region of the aDMN, the MPFC mainly supports self-referential or introspectively oriented mental activity, such as autobiographical memory (AM) and theory of mind.38 It was reported that patients with OSA had impaired AM and improvement of AM recall could predict the recovery from depression after continuous positive airway pressure (CPAP) therapy.39 The prefrontal cortex was vulnerable to impairment by sleep disruption and intermittent hypoxia,40 and decreased task-related activation in the MPFC was frequently reported in this disorder.14 Additionally, the MPFC is also involved in emotional processing that exhibits deficit in patients with OSA despite adequate treatment and symptom improvement.41,42 The structural and functional impairments in the MPFC might be a candidate mechanism for the cognitive and emotional deficits observed in patients with OSA.
The frontoparietal network is also known as the frontoparietal control network, or executive attention network, and is involved in executive control processes.43–45 Executive dysfunction was frequently reported in patients with OSA and was not always reversed after CPAP treatment.3,4 In this study, patients with OSA exhibited both structural and functional deficits in the left DLPFC of the LFP. The DLPFC is critical for executive function and highly sensitive to sleep deprivation.46 Both the absence of the DLPFC activation and impaired executive function have been reported in patients with OSA.13 Moreover, both hypometabolism and decreased GMV have been found in the prefrontal cortex and inferior parietal lobule of the frontoparietal network in patients with OSA,7 which is consistent with our finding that the frontoparietal network showed structural and functional impairments.
Brain Networks with Functional Deficit in Patients with OSA
Although concurrent structural and functional deficits suggest a decreased possibility of functional recovery, functional impairment alone may indicate a higher possibility for functional recovery after adequate treatment. The right DLPFC of the RPF and the precentral gyrus of the SMN, both of which showed only functional deficit in patients with OSA, may be candidate targets for treatments to improve behavioral performance. As discussed previously, the functional impairment in the DLPFC of the RFP may contribute to the executive control deficits in patients with OSA. Moreover, it may also result in the attention deficits in these patients because the right frontoparietal network is critically important for processing spatial attention47 and is impaired in stroke patients with neglect.48 This hypothesis is supported by findings that patients with OSA commonly exhibit attention deficits and tend to remit with an appropriate treatment.4,5 We also found a negative correlation between the rsFC in the right DLPFC and the AHI scores in patients with OSA, which is consistent with the association between attention performance and the severity of OSA.49 This finding suggests that the rsFC in the right DLPFC could be used as a biomarker to monitor disease progress or treatment effects.
Reduced psychomotor speed and manual dexterity were commonly reported in patients with OSA4,5 who exhibited decreased activation and metabolism in the motor area.12,15 The decreased activation in the motor area was suggested to be associated with sleep fragmentation15 and the decreased rCBF in motor area was correlated with the AHI scores in patients with OSA.12 We found functional impairment in the left motor cortex, suggesting that the functional deficit might be reversed after adequate treatment.
Brain Network with Functional Compensation in Patients with OSA
The increased rsFC without significant GMV alternation in the PCC of the pDMN suggests functional compensation. The PCC has strong reciprocal connections with other memory-related structures, such as the hippocampus, and plays a critical role in episodic memory retrieval.50 Altered deactivation and metabolism in the PCC were observed in patients with OSA.7,16 The greater deactivation in the PCC in the treatment withdrawal condition has been ascribed to a compensatory mechanism.16 Similarly, increased rsFC in the PCC of the DMN was also found in patients with clinical isolated syndrome and mild cognitive impairment.51,52 Thus, we suggest that the increased rsFC in the PCC might reflect neural compensation for cognitive damage.
GMV Changes across the Whole Brain in Patients with OSA
Many brain regions with significant GMV changes in patients with OSA have been identified in previous studies, including frontal and parietal cortex, temporal lobe, hippocampus, and cerebellum.6–8 Similar to these studies, we also found reduced GMV in hippocampus, temporal lobe, and prefrontal cortex, which might partially explain the impairment in memory processes and executive functions in patients with OSA. In addition, we found reduced GMV in the right cuneus, which showed altered rCBF pattern in a previous study and might be associated with visual attention deficit in patients with OSA.12
Limitations
In addition to possible influence of excessive daytime sleepiness on resting-state brain activity, several limitations should be considered when interpreting our results. First, the current study only recruited male patients, which prevented us from generalizing the results to the total population. Second, higher BMI was found in the patients with OSA compared to healthy controls, and obesity likely influenced the resting-state brain activity. Thus, the role of obesity in the alteration of the rsFC in OSA should be further studied. Finally, only patients with moderate to severe OSA were included in the current study, which may lower the representation of the average patient who may come to the sleep clinic. Future studies are needed to test whether patients with mild OSA also demonstrate altered rsFCs as patients with moderate to severe OSA.
CONCLUSION
In this rs-fMRI study of patients with OSA, we identified several brain networks in which the resting-state brain activity was altered in these patients. We further investigated the association between the structural and functional alterations in OSA and categorized these alterations into three categories: structural and functional deficits, solely functional deficits, and functional compensation. Finally, we identified a correlation between the altered rsFC and the severity of the disease in patients with OSA. These results suggest that rs-fMRI is a promising tool for understanding the neural deficits in patients with OSA, monitoring the disease progression, and evaluating treatment effects.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest
ACKNOWLEDGMENTS
The authors acknowledge the help of Mr. Ning Zhou, Mrs. Meinan Guo, and Mrs. Yan Wang (Sleep Laboratory, Respiratory Department of Tianjin Medical University General Hospital, Tianjin, China) for polysomnographic recordings; and Mr. Jisheng Lv (Department of Radiology of Tianjin Medical University General Hospital, Tianjin, China) for the recruitment of healthy volunteers. This study was supported by the International Cooperation and Exchanges NSFC (No. 81061120533), the National Basic Research Program of China (973 program, No. 2011CB707801), and the Natural Science Foundation of Tianjin (No. 11JCZDJC19300).
Footnotes
A commentary on this article appears in this issue on page 631.
SUPPLEMENTAL MATERIAL
Demographic and clinical characteristics of sleep-deprived subjects
Comparison of rsFC in the ROIs of brain networks with significant group differences between the patients with OSA and healthy controls
Correlation between rsFC in the ROIs and disease severity of patients with OSA
Brain areas with significant differences in GMV between patients with OSA and control subjects (P < 0.0001, uncorrected)
Correlations between GMV of the ROIs with significant changes and disease severity of patients with OSA
Comparison of GMV in the ROIs of brain networks with significantly changed rsFC between the patients with OSA and the controls
REFERENCES
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549–54. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161–6. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 4.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 5.Décary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23:369–81. [PubMed] [Google Scholar]
- 6.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 7.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 8.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 9.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarchielli P, Presciutti O, Alberti A, et al. A 1H magnetic resonance spectroscopy study in patients with obstructive sleep apnea. Eur J Neurol. 2008;15:1058–64. doi: 10.1111/j.1468-1331.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 11.Algin O, Gokalp G, Ocakoglu G, Ursavas A, Taskapilioglu O, Hakyemez B. Neurochemical-structural changes evaluation of brain in patients with obstructive sleep apnea syndrome. Eur J Radiol. 2012;81:491–5. doi: 10.1016/j.ejrad.2010.12.092. [DOI] [PubMed] [Google Scholar]
- 12.Joo EY, Tae WS, Han SJ, Cho JW, Hong SB. Reduced cerebral blood flow during wakefulness in obstructive sleep apnea-hypopnea syndrome. Sleep. 2007;30:1515–20. doi: 10.1093/sleep/30.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas RJ, Rosen BR, Stern CE, Weiss JW, Kwong KK. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol. 2005;98:2226–34. doi: 10.1152/japplphysiol.01225.2004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Ma L, Li S, Wang Y, Wang L. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnea. Sleep Med. 2011;12:335–40. doi: 10.1016/j.sleep.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32:373–81. doi: 10.1093/sleep/32.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweet LH, Jerskey BA, Aloia MS. Default network response to a working memory challenge after withdrawal of continuous positive airway pressure treatment for obstructive sleep apnea. Brain Imaging Behav. 2010;4:155–63. doi: 10.1007/s11682-010-9095-y. [DOI] [PubMed] [Google Scholar]
- 17.Prilipko O, Huynh N, Schwartz S, et al. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. Sleep. 2011;34:293–301A. doi: 10.1093/sleep/34.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25:294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Zhang J, Shi J, Gong Q, Weng X. Cerebral and functional adaptation with chronic hypoxia exposure: a multi-modal MRI study. Brain Res. 2010;1348:21–9. doi: 10.1016/j.brainres.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 23.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 25.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 26.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Zuo XN, Ma SY, Zang YF, Milham MP, Zhu CZ. Subject order-independent group ICA (SOI-GICA) for functional MRI data analysis. Neuroimage. 2010;51:1414–24. doi: 10.1016/j.neuroimage.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 28.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 29.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Qin W, Zhang Y, Jiang T, Yu C. The neuronal correlates of digits backward are revealed by voxel-based morphometry and resting-state functional connectivity analyses. PLoS One. 2012;7:e31877. doi: 10.1371/journal.pone.0031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J Cogn Neurosci. 2010;22:1637–48. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sämann PG, Tully C, Spoormaker VI, et al. Increased sleep pressure reduces resting state functional connectivity. MAGMA. 2010;23:375–89. doi: 10.1007/s10334-010-0213-z. [DOI] [PubMed] [Google Scholar]
- 33.De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci U S A. 2009;106:4489–94. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 36.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonavita S, Gallo A, Sacco R, et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler. 2011;17:411–22. doi: 10.1177/1352458510394609. [DOI] [PubMed] [Google Scholar]
- 38.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svaldi JJ, Mackinger HF. Obstructive sleep apnea syndrome: autobiographical memory predicts the course of depressive affect after nCPAP therapy. Scand J Psychol. 2003;44:31–7. doi: 10.1111/1467-9450.00318. [DOI] [PubMed] [Google Scholar]
- 40.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11:1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26:480–91. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross RL, Kumar R, Macey PM, et al. Neural alterations and depressive symptoms in obstructive sleep apnea patients. Sleep. 2008;31:1103–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fair DA, Dosenbach NU, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–8. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 47.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163:1626–31. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 50.Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–72. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 51.Roosendaal SD, Schoonheim MM, Hulst HE, et al. Resting state networks change in clinically isolated syndrome. Brain. 2010;133:1612–21. doi: 10.1093/brain/awq058. [DOI] [PubMed] [Google Scholar]
- 52.Bai F, Watson DR, Shi Y, et al. Specifically progressive deficits of brain functional marker in amnestic type mild cognitive impairment. PLoS One. 2011;6:e24271. doi: 10.1371/journal.pone.0024271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and clinical characteristics of sleep-deprived subjects
Comparison of rsFC in the ROIs of brain networks with significant group differences between the patients with OSA and healthy controls
Correlation between rsFC in the ROIs and disease severity of patients with OSA
Brain areas with significant differences in GMV between patients with OSA and control subjects (P < 0.0001, uncorrected)
Correlations between GMV of the ROIs with significant changes and disease severity of patients with OSA
Comparison of GMV in the ROIs of brain networks with significantly changed rsFC between the patients with OSA and the controls


