Abstract
Study Objectives:
Recent studies have suggested that nonrestorative sleep (NRS) symptoms may be distinct from nocturnal insomnia symptoms (NIS). However, there is limited information on the demographic, medical, and biologic correlates of NRS independent from NIS in the general population. This report presents the sociodemographic correlates, patterns of comorbidity with other sleep and physical disorders, C-reactive protein (CRP) levels, and general productivity associated with NIS and NRS in a nationally representative sample of US adults.
Design:
National Health and Nutrition Examination Survey (NHANES).
Setting:
The 2005-2008 surveys of the general population in the United States.
Participants:
There were 10,908 individuals (20 years or older)
Interventions:
N/A.
Measurements and Results:
Respondents were classified by the presence or absence of NIS and NRS. Compared with those without insomnia symptoms, respondents with NIS were older and had lower family income and educational levels than those with NRS. In addition, there was a significant association between NIS and cardiovascular disease, whereas NRS was associated with other primary sleep disorders (including habitual snoring, sleep apnea, and restless legs syndrome), respiratory diseases (emphysema and chronic bronchitis), thyroid disease, and cancer as well as increased CRP levels. In addition, the study participants with NRS only reported poorer scores on the Functional Outcomes of Sleep Questionnaire (FOSQ) than those without insomnia symptoms or those with NIS only.
Conclusions:
These findings suggest that there are substantial differences between NIS and NRS in terms of sociodemographic factors, comorbidity with other sleep and physical disorders, increased CRP level, and functional impairment. An inflammatory response might play a unique role in the pathogenesis of NRS.
Citation:
Zhang J; Lamers F; Hickie IB; He JP; Feig E; Merikangas KR. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. SLEEP 2013;36(5):671-679.
Keywords: C-reactive protein, functional impairment, medical condition, nocturnal insomnia symptoms, nonrestorative sleep.
INTRODUCTION
Nationally representative surveys have demonstrated that insomnia symptoms are common in the US general population, with 1-year or 1-month prevalence ranging from 17.4% to 36.3%.1–4 However, insomnia symptoms are highly heterogeneous in terms of causes and manifestations. The three major diagnostic systems for sleep disorders are divided into three subtypes of insomnia symptoms including difficulty initiating sleep (DIS), difficulty maintaining sleep (DMS) and/or nonrestorative sleep (NRS).5–7 Although far less is known about the epidemiology and medical correlates of NRS than those of nocturnal insomnia symptoms (NIS), recent studies have documented that there may be distinct risk factors and correlates for these two types of insomnia symptoms. For example, although the sociodemographic characteristics of NIS and NRS, such as female sex, separated/divorced status, and unemployment, are similar,8,9 there is a reverse association between age and these two sleep conditions. Specifically, NRS is more common among young adults, whereas NIS is more frequent in older people.10 Furthermore, sleep laboratory evidence suggests that NRS can occur without NIS and hence may comprise a distinct component of insomnia.11 Moreover, people with NRS only (without NIS) have a stable course over 3 months and comparable impairment to those with NRS + NIS (DIS and/or DMS). In addition, NRS has been reported to have more effect on daytime functioning when compared with other insomnia symptom subtypes in the National Comorbidity Survey-Replication.1
Although this emerging evidence suggests the importance of distinguishing NRS from NIS, most of the previous epidemiologic studies have not investigated NRS separately from NIS.1,4,12–14 Therefore, the differences between NRS and NIS in medical comorbidity and associations with other sleep problems warrant further investigation.15 In particular, because NRS is a core symptom of several disorders associated with increased inflammatory responses, distinguishing patterns of medical comorbidity between NIS and NRS might inform interventions and etiology.16 This study aimed to examine the sociodemo-graphic correlates, patterns of medical and sleep comorbidity, C-reactive protein (CRP; as a biomarker for inflammation), and functional impairment of NRS and NIS in a nationally representative US sample.
METHODS
The sample is composed of participants in the National Health and Nutrition Examination Surveys (NHANES) from 2005-2008. The NHANES is a nationally representative probability sample of noninstitutionalized US citizens. The participants were selected using a complex, stratified, and multistage probability cluster design. The current study focuses on self-reported insomnia symptoms among subjects 20 years or older (n = 10,908). Detailed survey material of the NHANES 2005-2008 is available on the NHANES Web site.17 The questions on demographics, sleep disorders, and medical conditions were administered by a computer-assisted personal interview (CAPI), which is programmed with built-in consistency checks to reduce data entry errors. CAPI also uses online help screens to assist interviewers in defining key terms used in the questionnaire.
Analyses
Covariates
Sex, age, and race/ethnicity were obtained by self-report. Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, and other race including multiple races. Socioeconomic status was assessed by the poverty income ratio (PIR), which is calculated as the ratio of self-reported family income to the family's poverty threshold level, based on family size, according to US Census Bureau poverty guidelines. PIR was defined as three groups; < 1, 1-3, and ≥ 3. Higher PIR indicates less poverty. The Patient Health Questionnaire (PHQ-9) was used to assess depressive symptoms.18 Subjects with a score of ≥ 10 were considered to have clinically relevant symptoms of depression.18 Sleep duration was collected by the following question in the NHANES interview: “How much sleep do you usually get at night on weekdays or workdays?”19 Response categories were 1-12 h; those participants with a sleep duration of < 6 h were considered to be short sleepers.
Exposures: NIS and NRS
Three questions regarding the frequency of NIS during the past month were measured in the NHANES study: (1) “Do you: have trouble falling asleep (DIS)? (2) wake up during the night and have trouble getting back to sleep (DMS)? (3) wake up too early in the morning and be unable to get back to sleep (Early Morning Awakening - EMA)?” Another question on NRS was also included in the sleep disorders section: “In the past month, how often did you feel unrested during the day, no matter how many hours of sleep you have had?”19 All of the questions were rated on a five-point Likert scale (never, rarely [once a month], sometimes [2-4 times a month], often [5-15 times a month]), almost always [16-30 times a month]). A response of “almost always” was defined as abnormal.19,20 For the current analyses, the participants were divided into four groups according to their condition of NIS and NRS: no insomnia; NIS only; NRS only; NIS + NRS. The NRS only, NRS only, and NIS + NRS groups were combined to create an ‘any insomnia symptoms’ group.
Outcome Measures
Medical conditions: Participants were asked whether they had ever been told by a doctor or other health professional that they had the following conditions: congestive heart failure, coronary heart disease, angina, heart attack, stroke, arthritis, emphysema, thyroid problems, chronic bronchitis, liver problems, diabetes, cancer, and asthma. Systolic and diastolic blood pressures (average of three recordings) in mm Hg were also measured during the interview. Those participants with average systolic blood pressure ≥ 140 mm Hg, with diastolic blood pressure ≥ 90 mm Hg, and/or a history of antihypertensive medications during the past month were considered hypertensive. Persons with an interviewer-measured body mass index (BMI) ≥ 30 kg/m2 were considered to be obese. In total, there were 15 medical conditions measured in the current study.
Inflammatory marker (CRP): Serum CRP was measured by latex-enhanced nephelometry. A total of 9,830 participants had a valid CRP measure. Because CRP data were abnormally distributed, they were natural-log transformed and tested by the General Linear Model (GLM) procedure. The CRP values were transformed back to the original scale for easier interpretation (Figure 1). In addition, we also examined the association of risk quintile of CRP with different groups, and the upper quintile (> 0.53 mg/dL) was used as risk quintile in the current study as done in a previous study.21
Figure 1.
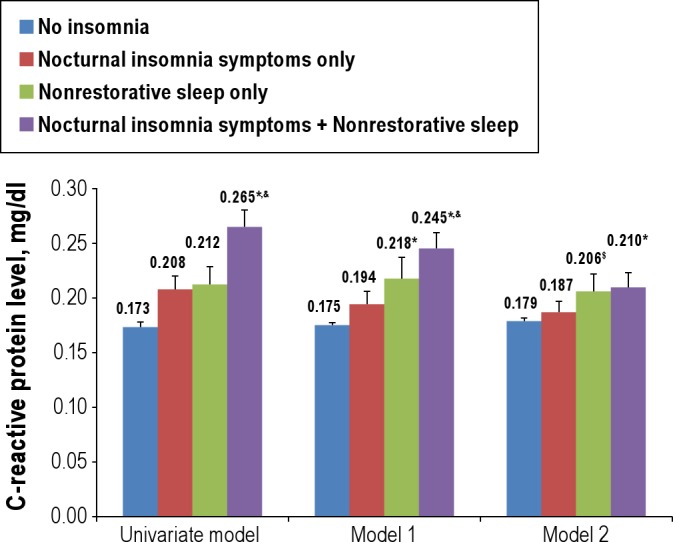
Comparison of mean C-reactive protein levels (mg/dL) across groups. Nocturnal insomnia symptoms include difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening. *P < 0.05 significant difference when compared with normal sleep. $P < 0.10 when compared with normal sleep. &Significant difference between NIS-only and NIS + NRS. Model 1 adjusted for age, sex, poverty income ratio, ethnicity, educational level, and marital status. Model 2 adjusted for age, sex, poverty income ratio, ethnicity, educational level, marital status, Patient Health Questionnaire (PHQ-9) total score, body mass index, sleep duration, any cardiovascular disease, arthritis, lifetime smoking, clinician-diagnosed sleep apnea, habitual sleeping pill use, habitual snoring, and restless legs syndrome. Error bars = SEM.
Sleep problems: Participants were asked whether they had ever been told by a doctor or other health professional that they had a sleep disorder.19 If the answer was yes, they were asked to specify among the following options: sleep apnea, insomnia, restless legs, or other. Subjects with multiple sleep disorders were allowed to answer yes to all that applied. Habitual snoring was defined as snoring at least 5 nights/week. Those participants taking pills to help sleep at a frequency of at least five times/month were considered to have habitual sleeping pill use.
General productivity assessments: The general productivity in the current study was assessed using the general productivity subscale of the Functional Outcomes of Sleep Questionnaire (FOSQ). The FOSQ is a 30-item, disease-specific, self-reported measure designed to assess the daily functioning impairment of excessive daytime sleepiness.22 The general productivity sub-scale is an eight-item subscale with a total score from one to four. A lower score indicates greater functioning impairment. A total of 10,877 participants with valid responses on FOSQ general productivity subscale were included in this analysis.
Statistical Analyses
Statistical Package for the Social Sciences (SPSS) 19.0 for Windows (SPSS Inc, Chicago, IL) was used for statistical analyses. All procedures accounted for clustering, weighting, and stratification of complex sampling design features to accurately calculate point estimates and standard errors. Descriptive statistics were given as means ± standard errors and frequencies (percentages). The associations across groups and sociodemographics were tested using the chi-square test. Logistic regression was used to determine the odds ratios (ORs) and 95% confidence intervals (CIs) for the associations of the four groups (no insomnia, NIS only, NRS only, NIS + NRS) with medical conditions and other sleep problems as assessed after controlling for demographic characteristics, sleep duration, and PHQ-9 total score, which set no insomnia as a reference (Table 1). In addition, the differences in the strength of associations with medical and sleep problems between NIS-only and NRS-only groups were further tested in the logistic regression models. As medical conditions or sleep problems may confound the associations between insomnia symptoms and increased CRP level, we adjusted for these variables. In these logistic models, grouping based on NIS or NRS status was treated as an independent variable whereas the medical conditions, other sleep problems, or CRP level were treated as dependent variables. Mean differences in natural-log transformed CRP level and FOSQ general productivity subscale score across groups were examined by the GLM. Two-sided P values less than 0.05 were considered statistically significant.
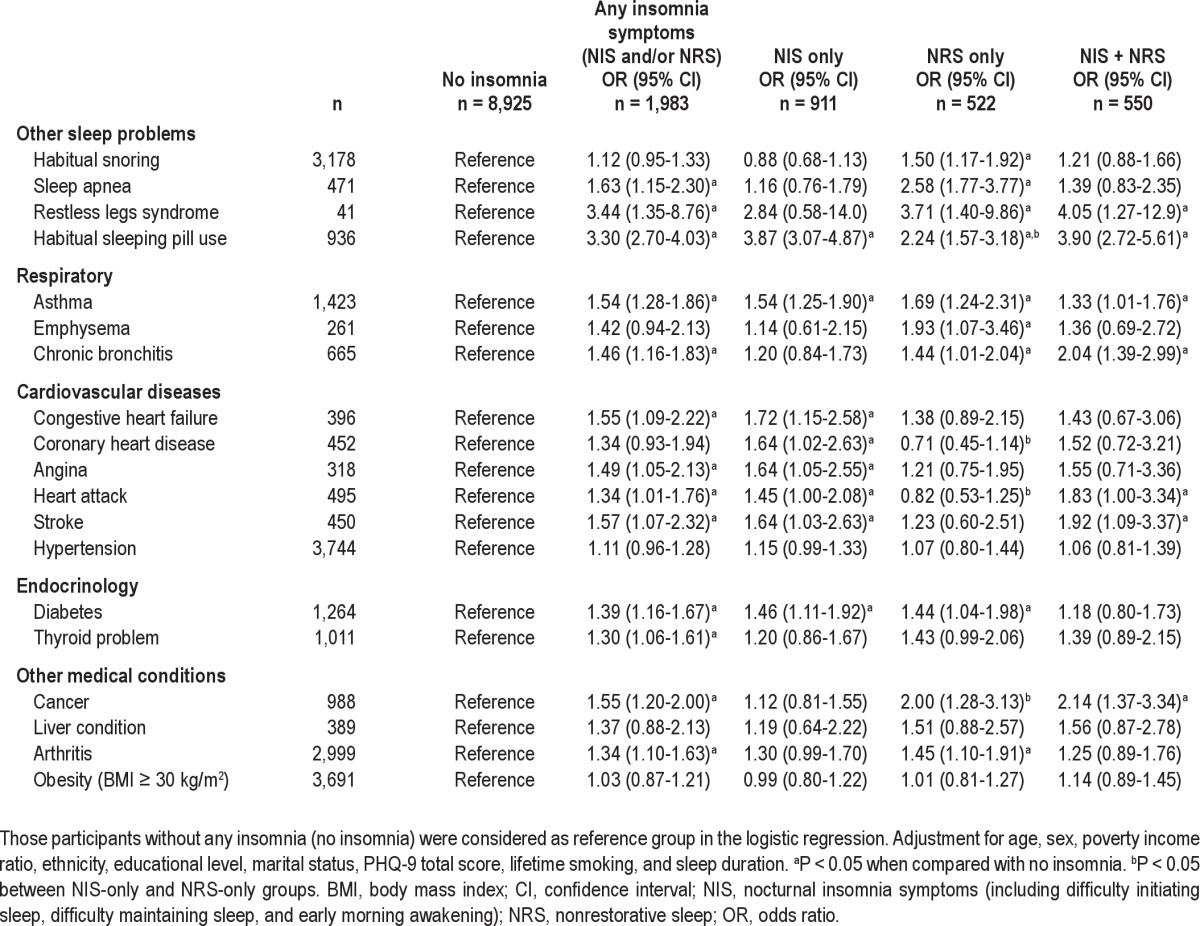
Table 1.
Associations between insomnia subgroups and other sleep problems and medical disorders (odds ratio, 95% confidence interval)
RESULTS
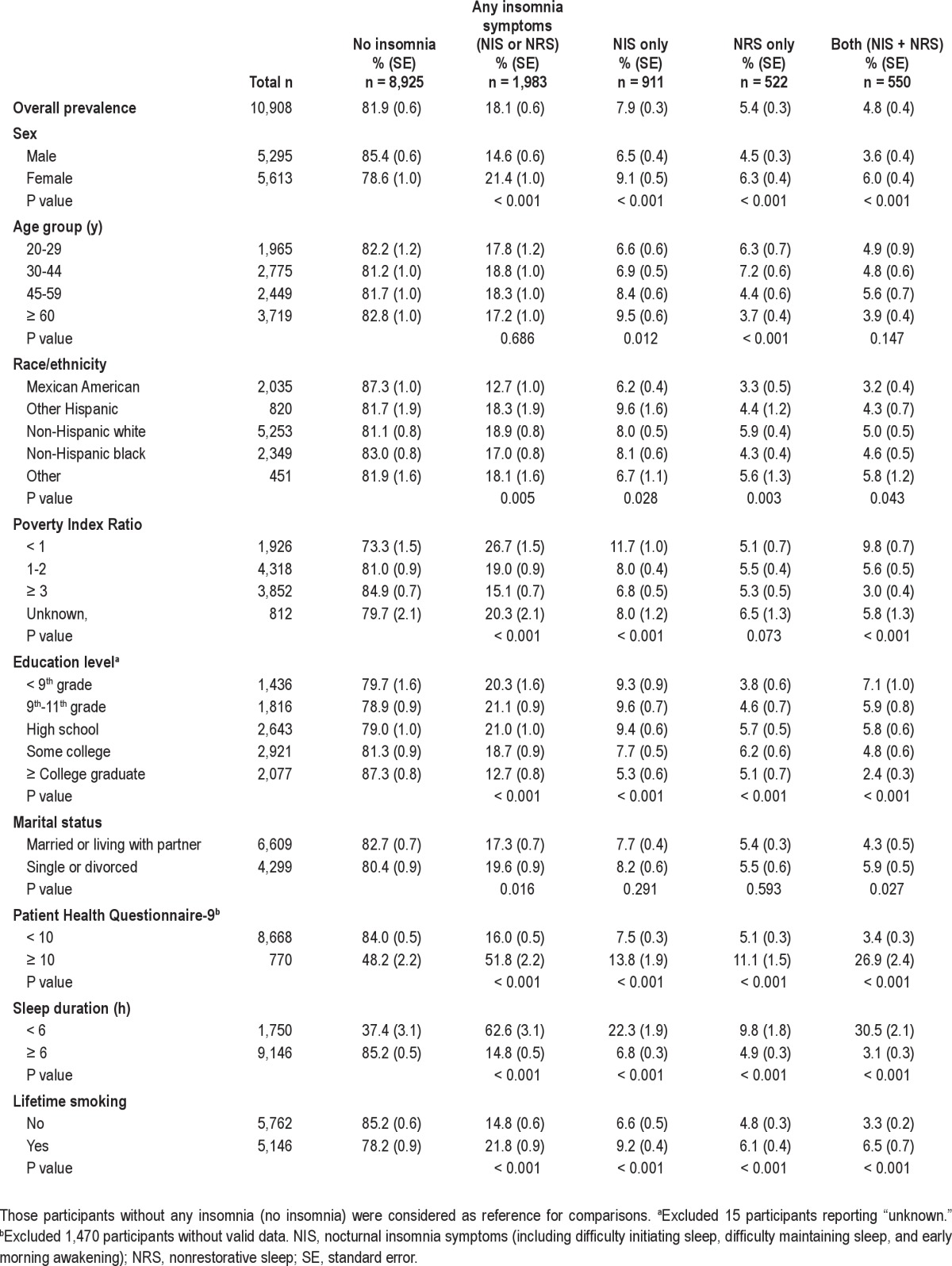
The 1-month prevalence estimates were 18.1% for any insomnia symptoms, 7.9% for NIS-only, 5.4% for NRS-only, and 4.8% for NIS + NRS groups (Table 2). Approximately 26% of those with any insomnia symptoms (persons with NIS or NRS) had both NIS and NRS. The associations between sociodemographic variables, depression, short sleep duration, and the sleep disorders groups are shown in Table 2. Females, non-Hispanic whites, poorer people, and those with lower educational levels were more likely to report insomnia symptoms than controls. Higher prevalence rates of both NIS only and NRS only were found in females and among those with depressive symptoms (PHQ-9 ≥ 10), a lifetime history of smoking, and short sleep duration when compared with those participants without any insomnia symptoms (P < 0.001). However, there were different patterns of demographic correlates of the NIS-only and NRS-only groups when compared with controls without insomnia. For example, the prevalence of NIS only increased with age (from 6.6% in group age 20-29 y to 9.5% in group age ≥ 60 y) whereas the prevalence of NRS decreased with age (from 6.3% in group age 20-29 y to 3.7% in group age ≥ 60 y). Lower PIR was associated with a higher prevalence of NIS only (from 11.7% in PIR < 1 to 6.8% in PIR ≥ 3, P < 0.001) but not NRS only (P > 0.05). Regarding ethnic differences, the prevalence of NIS only was highest in Hispanics other than Mexican Americans, but the prevalence of NRS only was highest in non-Hispanic whites. Lower education level was associated with a higher prevalence of NIS only but lower prevalence of NRS only. Participants with NIS only were older and had a lower PIR and shorter sleep duration than those with NRS only directly (P < 0.05) (data not shown).
Table 2.
One-month prevalence of insomnia subgroups by demographic and clinical characteristics (n = 10,908)
The associations between insomnia symptoms and a series of common medical conditions and sleep problems are shown in Table 1. Those with any insomnia symptoms were significantly more likely to have a series of medical conditions. However, there were differences between the NIS-only and NRS-only groups in the patterns of associations; the NIS-only group was significantly associated with all cardiovascular diseases, except for hypertension (adjusted ORs ranging from 1.45-1.72; P < 0.05), whereas the NRS-only group was not associated with any of these cardiovascular diseases (P > 0.05). In contrast, the NRS-only group was significantly associated with chronic obstructive pulmonary disease (emphysema or chronic bronchitis) and sleep problems (including habitual snoring, sleep apnea, and restless legs syndrome), whereas the NIS-only group was not. In summary, of the 15 medical and four sleep problems measured, only four of them were associated with both NIS only and NRS only, one was associated with neither NIS only nor NRS only, but 13 were associated with either NIS only or NRS only in the final models (Table 3). The NIS-only group was strongly associated with coronary heart disease, heart attack, and habitual sleeping pill use, and weakly associated with habitual snoring, sleep apnea, and cancer when compared with the NRS-only group directly. The NIS + NRS group was not more strongly associated with sleep problems or medical conditions in terms of the number of problems when compared with the NIS-only and NRS-only groups. This finding indicates that it is unlikely that there is an additive association between NIS + NRS on comorbidity in medical conditions and sleep problems.
Table 3.
Association between insomnia subgroups and increased C-reactive protein level (odds ratio, 95%CI)
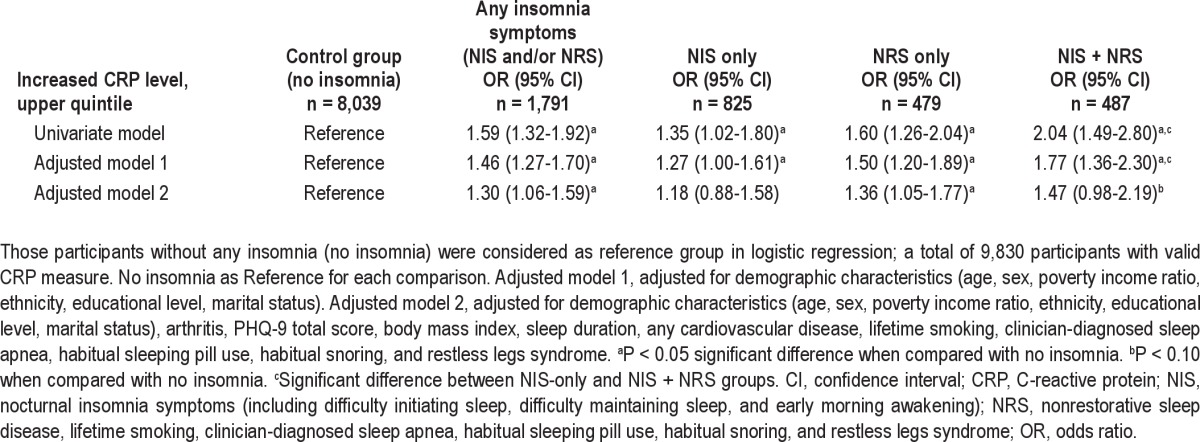
Insomnia symptoms were associated with the high CRP risk quintile with an OR of 1.30 (95% CI: 1.06-1.59) in the fully adjusted model (Table 3). Furthermore, Table 3 also shows that different insomnia symptoms were more often in the CRP risk quintile in the univariate model (ORs ranging from 1.35-2.04, P < 0.05) when compared with normal sleep. However, after adjustment for potential confounding factors, the association persisted in the NRS-only group, but not in the NIS-only group. This indicates that the significant association between any insomnia symptoms and CRP risk quintile was likely to be attributable to NRS. Similar results were found when comparing the mean differences in CRP level across groups (Figure 1). The NIS + NRS group had the highest CRP level, followed by the NRS-only group, NIS-only group, and normal sleep group in all models. In the univariate analyses, NIS-only, NRS-only, and NIS + NRS groups had higher CRP levels than the normal sleep group. However, the differences in mean CRP level between the NIS-only and normal sleep groups did not maintain statistical significance in adjusted models. There was also a trend (P = 0.06) for the NRS-only group to have a higher CRP level than the normal sleep group in the fully adjusted model (adjusted model two).
The association between the insomnia symptom groups and the FOSQ–general productivity subscale score was examined using a GLM procedure. In the univariate analysis (data not shown), there were significant differences across groups in the FOSQ-general productivity subscale score. The No Insomnia group had the highest mean FOSQ-general productivity sub-scale score (3.88), followed by NIS-only (3.80), NRS-only (3.65), and NIS + NRS (3.45) groups (P < 0.001 in post hoc analyses). However, there was no difference in the FOSQ-general productivity subscale score between the No Insomnia and NIS-only groups after adjustment for sociodemographics, PHQ-9 total score, and sleep duration (Figure 2). NRS-only and NIR + NRS groups had lower scores than both No Insomnia and NIS-only groups in the final model.
Figure 2.
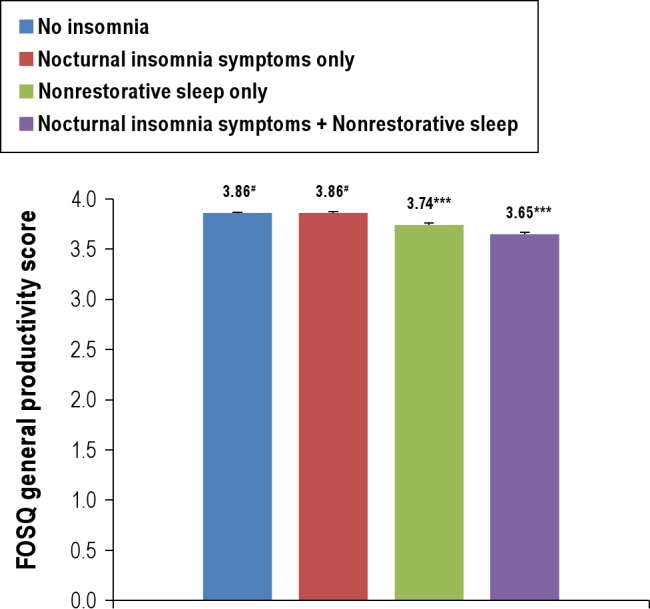
Comparison in mean Functional Outcomes of Sleep Questionnaire general productivity scores across groups. Nocturnal insomnia symptoms include difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening. ***P < 0.001 when compared with any other group. #No statistically significant difference between normal sleep and nocturnal insomnia symptoms only (P = 0.670). Multivariable analysis was adjusted for age, sex, poverty income ratio, ethnicity, educational level, marital status, habitual sleep duration, habitual snoring, PHQ-9, lifetime smoking, clinician-diagnosed sleep apnea, restless legs syndrome, habitual sleeping pill use, and number of medical conditions. Error bars = SEM.
DISCUSSION
The current study demonstrates that less than half of participants reporting NRS symptoms also had NIS symptoms. Furthermore, there are more differences than similarities in the sociodemographic features, medical comorbidity, other sleep problems, CRP level, and functional impairment of NRS-only and NIS-only groups. In view of the limited understanding of the nature of NRS,23 our findings suggest that NRS should be further investigated to determine whether this symptom may index a different subgroup of people with insomnia in the general population. Another interesting finding is that although those with both NIS and NRS symptoms do not have more sleep problems or medical comorbidity, they have substantially more functional impairment than those with either insomnia symptom alone or normal controls.
Differences in Demographic Correlates between the NIS-Only and NRS-Only Groups
The sociodemographic correlates of insomnia symptoms have been examined in previous studies of aggregate NIS and NRS. The current study confirms the associations of any insomnia symptoms with female sex,8 lower family income, lower education level, and single or divorced marital status.24 However, there were substantial differences in the sociodemographic characteristics (age, PIR, education level, and ethnicity) of those with NIS only compared with those with NRS only; in fact, some associations with demographic correlates were in the opposite direction. It is generally assumed that the prevalence of insomnia increases with age due to somatic symptoms and comorbid medical and mental problems.24 However, in agreement with several population-based studies,25–27 this finding could be an artifact of less impairment associated with insomnia with increasing age as reported by Roth et al. in the American Insomnia Study.4 This study showed that the prevalence of insomnia symptoms increases with age4; however, the low rate of insomnia disorder in the elderly patients despite the high prevalence of insomnia symptoms suggests that insomnia symptoms are better tolerated in elderly patients.4
Differences in Associations with Sleep Problems between the NIS-Only and NRS-Only Groups
Although insomnia symptoms have been correlated with obstructive sleep apnea (OSA),28 our results revealed that NRS, but not NIS, was associated with self-reported sleep apnea and the core symptom of sleep apnea (habitual snoring). This suggests that the associations between insomnia symptoms and OSA found in previous studies may be attributable to NRS. NRS was also associated with habitual sleeping pill usage, even though sleeping pills have not been shown to be effective in treating NRS. However, we could not investigate the order of the association between NRS and sleeping pill use. Given the significant associations of NRS with all sleep problems assessed in this study, we speculate that NRS might be an integrated indicator of insufficient sleep quantity or sleep quality as induced by other sleep problems. Indeed, the International Classification of Sleep Disorders, Second Edition (ICSD-2) suggests that in addition to being a core symptom of insomnia, NRS is also a nonspecific consequence of various sleep disturbances, including OSA, sleep deprivation, and restless legs syndrome.5 In this regard, NRS is probably a heterogeneous complaint.
Differences in Comorbidity of Medical Conditions between the NIS-Only and NRS-Only Groups
The current study confirms the findings of two previous studies that insomnia symptoms are pervasively associated with several medical conditions.29,30 Insomnia symptoms are commonly associated with chronic medical conditions and may be consequences of some of these conditions.29,31–33 The different patterns for the associations of most of the medical conditions between the NIS-only and NRS-only groups indicate, however, that there are likely to be different underlying mechanisms for the associations between NIS and NRS and other chronic medical conditions. For example, persons with NIS are more likely to have cardiovascular diseases (CVD), whereas persons with NRS more often have chronic obstructive pulmonary diseases (emphysema or chronic bronchitis), cancer, and thyroid disease. Although it is interesting that there are clear differences between the NIS-only and NRS-only groups in their associations with CVD, it is consistent with the demographic association between younger age and NRS alone. Recent evidence suggests that similar to short sleep duration, NIS is a risk factor for the onset and mortality associated with CVD.34,35 Although the longitudinal associations between NRS and CVD have not been tested in previous prospective studies, the current study indicates a lower prevalence of CVD in persons with NRS than in persons with NIS. With regard to cancer, three prospective studies have shown that nocturnal insomnia symptoms are a prevalent and persistent problem in patients with cancer.36–38 However, the findings in the current study suggest that “pure NIS” is not related to self-reported cancer.
Differences in CRP Level between the NIS-Only and NRS-Only Groups
According to ICSD-2, NRS has been considered as a consequence of other sleep disorders, including restless legs syndrome and OSA.1 However, the current study demonstrates that the relationship between NRS alone and increased CRP level persisted after controlling for these sleep disorders. This indicates that increased CRP level in NRS might be directly linked to the pathogenesis of NRS. Indeed, NRS is a core symptom of chronic fatigue syndrome (CFS) and some forms of major depression,39 both of which have been shown to be associated with increased peripheral inflammatory markers.40 However, CFS was not measured in the current study. Future studies should examine the role of CFS in the higher CRP level in NRS. In addition, further prospective studies with comprehensive measures of these domains are warranted to examine the causal relationship between NRS and increased CRP level.
Differences in Functional Impairment between the NIS-Only and NRS-Only Groups
Previous studies have demonstrated that insomnia symptoms exert significant effect on functional impairment.1,4,41,42 NRS is the only insomnia subtype that was significantly associated with all five measures of role functioning after excluding or controlling for 12-months Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) axis I disorders in the National Comorbidity Survey Replication study.1 Hence, it has been argued that the effect of insomnia symptoms on functional impairment may be confounded by medical and mental comorbidity.43 In line with these findings, the current study demonstrates that medical comorbidity may explain impairment associated with NIS but not with NRS. In addition, the lowest FOSQ general productivity score found in NIS + NRS group in the current study suggests that NIS may exacerbate the functional impairment associated with NRS, although there is no association between NIS and FOSQ general productivity score in the final model. Nonetheless, the effect sizes in the differences between groups were rather small. In this regard, the clinical significance of these differences might be modest.
Clinical Implications and Future Directions
The current study suggests that insomnia symptoms are heterogeneous,5–7 even though different insomnia symptoms may be highly correlated with each other.1,14 However, different insomnia symptoms have not been assessed or reported separately in most previous studies.2,3 In particular, few epidemio-logic studies have assessed NRS separately. For example, little is known about NRS in terms of operational measures,44 natural history,23 and effective management.16 A recent study has shown that 31.9% of those with NRS have a persistent course,45 which would therefore also suggest that NRS would be associated with substantial impairment and disability. However, we are unaware of previous epidemiologic studies that stratified NIS and NRS to explore the differences in their sociodemographic correlates, mental and physical comorbidity, biomarkers, and functional impairments.
The close associations of NRS with medical conditions and depression found in the current study support the stratification of NRS into NRS secondary to medical conditions, NRS secondary to psychiatric conditions, and NRS alone.46,47 In addition, as suggested by previous research,43 the strong associations of NRS with other sleep problems and sleeping pill use also suggest the importance of consideration of other sleep problems and treatments when investigating pathophysiologic mechanisms of NRS alone. In addition, as noted by Vernon et al.,44 a standardized, operational diagnostic tool for NRS should be developed to meet the need to address the variability in definitions used in previous studies. The currently available treatments for insomnia, including pharmacologic managements and psychological therapy, are designed for NIS rather than NRS, even though NRS is associated with a greater level of functional impairment.1,30 Our findings of the high comorbidity of medical conditions and sleep problems in the NRS-only group indicate that the management of other sleep problems and medical conditions might also benefit those with NRS symptoms, and may consequently improve daytime functioning.
Limitations
First, the self-reported information on insomnia symptoms, other sleep problems, and most of the medical conditions without further objective measures or clinical assessment may have led to reporting bias. Similar to previous studies of NRS,44 the validity and reliability of the measure of NRS in the current study has not been established. In addition, the NRS item in the current study that referred to “feeling unrested” during the day rather than specifically targeting this symptom upon awakening may also have tapped people who suffer from chronic fatigue, which has been associated with elevated CRP. Second, the measure of general productivity was derived from FOSQ, which was designed to assess the functional outcomes related to sleep disorders. Therefore, the effect of NRS and NIS on other functional impairment, such as physical functioning, cannot be addressed in the current study. Third, insomnia symptoms in the current study were rated for the past month. In view of the waxing and waning course of insomnia,48 other studies over longer time frames, particularly with prospective designs, are necessary to replicate our findings. Finally, due to the relatively small numbers, we were not able to stratify NIS into DIS, DMS, and EMA. However, these three symptoms of NIS have low stability across time.49 In addition, all of the associations that were tested in our analyses were not prespecified and hence might have raised false positive results.
CONCLUSIONS
Both NIS and NRS are common sleep problems in the US adult population. There are more differences than similarities between NIS and NRS in sociodemographic characteristics, sleep disorders and medical comorbidity, increased inflamma-tory response, and functional impairment. These findings suggest that sources of heterogeneity in the correlates of NRS and NIS should be investigated in future studies.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by an intramural grant (1Z011Z01MH002870) from the National Institute of Mental Health. Dr. Hickie is supported by a National Health and Medical Research Council Australia Fellowship (No. 511921). He was a director of Headspace: The National Youth Mental Health Foundation until January 2012. He is a member of the new Australian National Mental Health commission and was previously the CEO of Beyondblue: The National Depression Initiative. He has led depression and other mental health research projects that have been supported by a variety of pharmaceutical partners. Current investigator-initiated studies are supported by Servier and Pfizer. Dr. Hickie has served on the board, advisory council, or consulted for Bupa Australia, Drinkwise Australia, Western Australia (Labor) Government, DOHA Australian Government, Sydney Magazine, and Sydney City Council. He has also received travel compensation from RANZCP 7, Wyeth, Eli Lilly, Servier, Astra Zeneca, Price Waterhouse Cooper, American Psychiatric Association, RSL National congress, Chinese Society of Psychiatry and Neurology, Australian General Practice Network, and Focus-Sunshine Coast Research. He has received research support or received other support from Servier, Pfizer, Servier, and Astra Zeneca. Dr. Lamers is supported by a Rubicon Fellowship from the Netherlands Organisation for Scientific Research (NWO). The other authors have indicated no financial conflicts of interest. The views and opinions expressed in the report are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or US Government.
Footnotes
A commentary on this article appears in this issue on page 633.
REFERENCES
- 1.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60:1364–71. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166:1775–82. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 3.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–32. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 4.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 6.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. 2003 [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 8.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Li RH, Wing YK, Ho SC, Fong SY. Gender differences in insomnia-a study in the Hong Kong Chinese population. J Psychosom Res. 2002;53:601–9. doi: 10.1016/s0022-3999(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 10.Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–55. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 11.Roth T, Zammit G, Lankford A, et al. Nonrestorative sleep as a distinct component of insomnia. Sleep. 2010;33:449–58. doi: 10.1093/sleep/33.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohayon MM, Bader G. Prevalence and correlates of insomnia in the Swedish population aged 19-75 years. Sleep Med. 2010;11:980–6. doi: 10.1016/j.sleep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165:35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Sagales T. Prevalence of insomnia and sleep characteristics in the general population of Spain. Sleep Med. 2010;11:1010–8. doi: 10.1016/j.sleep.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Riemann D. Nonrestorative sleep: have we finally found it? Sleep. 2010;33:417–8. doi: 10.1093/sleep/33.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone KC, Taylor DJ, McCrae CS, Kalsekar A, Lichstein KL. Nonrestorative sleep. Sleep Med Rev. 2008;12:275–88. doi: 10.1016/j.smrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. National Center for Health Statistics (NCHS) Accessed at http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm and http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08. htm. [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005-2008. National Center for Health Statistics (NCHS) Accessed at http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/sp_slq_d. pdf and http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/SLQ_E.htm. [Google Scholar]
- 20.Seicean S, Neuhauser D, Strohl K, Redline S. An exploration of differences in sleep characteristics between Mexico-born US immigrants and other Americans to address the Hispanic Paradox. Sleep. 2011;34:1021–31. doi: 10.5665/SLEEP.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson KB, Richardson AK, He J, Lateef TM, Khoromi S, Merikangas KR. Headache and biomarkers predictive of vascular disease in a representative sample of US children. Arch Pediatr Adolesc Med. 2010;164:358–62. doi: 10.1001/archpediatrics.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 23.Roth T. What is the nature of nonrestorative sleep? Sleep Med. 2010;11:963–4. doi: 10.1016/j.sleep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 25.Ohayon MM, Caulet M, Guilleminault C. How a general population perceives its sleep and how this relates to the complaint of insomnia. Sleep. 1997;20:715–23. doi: 10.1093/sleep/20.9.715. [DOI] [PubMed] [Google Scholar]
- 26.Ohayon MM, Caulet M, Priest RG, Guilleminault C. DSM-IV and ICSD-90 insomnia symptoms and sleep dissatisfaction. Br J Psychiatry. 1997;171:382–8. doi: 10.1192/bjp.171.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier H, Los F, Boichut D, et al. Evaluation of severe insomnia in the general population: results of a European multinational survey. J Psycho-pharmacol. 1999;13:S21–4. doi: 10.1177/026988119901304S04. [DOI] [PubMed] [Google Scholar]
- 28.Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137:1449–63. doi: 10.1378/chest.09-1485. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS) Sleep. 2011;34:997–1011. doi: 10.5665/SLEEP.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S. The impact and prevalence of chronic insomnia and other sleep disturbances associated with chronic illness. Am J Manag Care. 2006;12:S221–9. [PubMed] [Google Scholar]
- 33.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 34.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580–6. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 37.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–9. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 38.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–31. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- 40.Raison CL, Lin JM, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327–37. doi: 10.1016/j.bbi.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Stein MB, Belik SL, Jacobi F, Sareen J. Impairment associated with sleep problems in the community: relationship to physical and mental health comorbidity. Psychosom Med. 2008;70:913–9. doi: 10.1097/PSY.0b013e3181871405. [DOI] [PubMed] [Google Scholar]
- 42.Ramsawh HJ, Stein MB, Belik SL, Jacobi F, Sareen J. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. J Psychiatr Res. 2009;43:926–33. doi: 10.1016/j.jpsychires.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14:69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Vernon MK, Dugar A, Revicki D, Treglia M, Buysse D. Measurement of non-restorative sleep in insomnia: a review of the literature. Sleep Med Rev. 2010;14:205–12. doi: 10.1016/j.smrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Lam SP, Li SX, Li AM, Wing YK. The longitudinal course and impact of non-restorative sleep: a five-year community-based follow-up study. Sleep Med. 2012;13:570–6. doi: 10.1016/j.sleep.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson K, Shapiro C. Nonrestorative sleep: Symptom or unique diagnostic entity? Sleep Med. 2012;13:561–9. doi: 10.1016/j.sleep.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Roth T. Investigating nonrestorative sleep. Sleep Med. 2012;13:557–8. doi: 10.1016/j.sleep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 49.Hohagen F, Kappler C, Schramm E, Riemann D, Weyerer S, Berger M. Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening--temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep. 1994;17:551–4. [PubMed] [Google Scholar]


