Abstract
Study Objectives:
We assessed the relationship between sleep duration, snoring and colorectal cancer risk.
Design:
Prospective cohort studies.
Setting:
United States.
Participants:
A total of 30,121 men aged 41 to 79 years in the Health Professionals Follow-up Study and 76,368 women aged 40 to 73 years in the Nurses' Health Study.
Interventions:
None.
Measurements and Results:
We queried information on sleep duration and snoring in 1986/87. Cox proportional hazards regression models were used to estimate multivariable hazard ratios (HRs, 95% CIs). We documented 1,973 incident colorectal cancer cases (709 men and 1,264 women) over a 22-year follow-up period. Compared to sleep an average 7 h, ≥ 9 h of sleep was significantly associated with a higher risk of colorectal cancer among men (HR = 1.35, 95% CI: 1.00, 1.82), and to a lesser degree, among women (HR = 1.11, 95% CI: 0.85, 1.44). The risk associated with longer sleep was restricted to individuals who regularly snored (men: HR = 1.80, 95% CI: 1.14, 2.84; women: HR = 2.32, 95% CI: 1.24, 4.36) and to overweight individuals (i.e., BMI ≥ 25 kg/m2) (men: HR = 1.52, 95% CI: 1.04, 2.21; women: HR = 1.37, 95% CI: 0.97, 1.94). Short sleep duration (≤ 5 h) was not associated with an increased risk of colorectal cancer in the entire sample or in subgroups stratified by snoring or BMI.
Conclusions:
Longer sleep duration was associated with an increased risk of developing colorectal cancer among individuals who were overweight or snored regularly. This observation raises the possibility that sleep apnea and its attendant intermittent hypoxemia may contribute to cancer risk.
Citation:
Zhang X; Giovannucci EL; Wu K; Gao X; Hu F; Ogino S; Schernhammer ES; Fuchs CS; Redline S; Willett WC; Ma J. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. SLEEP 2013;36(5):681-688.
Keywords: Sleep duration, snoring, colorectal cancer, incidence, prospective study
INTRODUCTION
Despite progress in prevention,1 colorectal cancer remains the third most commonly diagnosed cancer and the third leading cause of death due to cancer in both women and men in the United States.2 Efforts to continue identifying modifiable risk factors are warranted.
Both short and long sleep duration has been associated with an increased risk of cardiovascular disease,3,4 breast cancer,5–7 and overall mortality.8,9 One recent case-control study10 reported a significant association between short sleep duration and an increased risk of colorectal adenomas, precursors of colorectal cancer. This finding suggested a potential role of sleep duration in colorectal carcinogenesis, although the association with long sleep duration was not tested due to small number of cases in that study. Short or long sleep duration might affect colorectal cancer risk through associations with weight gain,11,12 obesity,11 diabetes,13 and insulin resistance,14 which are all risk factors for colorectal cancer.15,16 However, while a meta-analysis and a recent study described associations between long8 and short sleep duration and overall cancer risk,17 to the best of our knowledge, no published data have examined the relation of sleep duration to risk of colorectal cancer specifically and whether the associations differ by overweight/obesity status (a factor associated with insulin resistance). In addition, although intermittent hypoxemia, which occurs with sleep disordered breathing, has been associated with tumorigenesis,18,19 studies of cancer incidence have not considered whether the association of sleep duration and cancer may be modified by sleep disordered breathing.
We therefore examined whether sleep duration was associated with risk of colorectal cancer in large prospective cohorts of men (the Health Professionals Follow-up Study)20 and women (the Nurses' Health Study).21 We hypothesized that individuals with either shorter or longer sleep duration had increased risk of colorectal cancer. Furthermore, we specifically evaluated whether this relationship was modified by regular snoring (a marker of sleep apnea or disordered breathing) or overweight.
METHODS
Study Population and Assessment of Sleep Duration
The Health Professionals Follow-up Study (HPFS)20 is a prospective cohort study of 51,529 U.S. male professionals who were aged 40 to 75 years at baseline in 1986. The Nurses' Health Study (NHS)21 is a prospective cohort study of 121,700 registered female nurses who were aged 30 to 55 years at baseline in 1976 in the U.S. A biennial questionnaire has been sent to participants in each cohort since 1986 and 1976, respectively, to collect information on demographics, lifestyle factors, and disease endpoints. The follow-up rate has been > 90% for NHS and HPFS. Both studies have been approved by the institutional review board at the Harvard School of Public Health and Brigham and Women's Hospital, Boston, Massachusetts. Return of the questionnaires was considered to imply informed consent, and we also obtained written consent from each participant to obtain and review medical records.
In 1986, NHS participants were asked to “indicate total hours of actual sleep in a 24-hour period” and “do you snore?” The same question was asked in the HPFS 1987 questionnaire and was subsequently included in both cohorts in 2000. Response categories for sleep duration were identical between the two cohorts: “5 hours or less, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, or 11 hours or more.” The validity of self-reported sleep duration in NHS has been described in detail elsewhere.9 Briefly, self-reported average time spent sleeping was highly reproducible and correlated well with sleep duration as assessed by sleep diaries (Spearman r = 0.79; P < 0.0001).9 Response categories for snoring were “Regularly, occasionally, never” in NHS and “Every night, most nights, a few nights a week, occasionally, never” in HPFS. We defined regular snorers as those who reported snoring regularly in NHS and those who snored every night, most nights, or a few nights a week in HPFS. We defined non-snorers as those who only occasionally or almost never snored.
A total of 83,027 women answered the sleep duration question in 1986. In HPFS, 33,579 men reported sleep duration. Participants in both cohorts who did not answer the sleep question did not differ substantially from respondents according to age, BMI, physical activity, endoscopy screening, family history of colorectal cancer, alcohol consumption, or major dietary factors. We further excluded participants with ulcerative colitis, and those who died or reported colorectal cancer or any other cancer (except non-melanoma skin cancer) before 1986 for NHS and 1988 for HPFS. A total of 76,368 women and 30,121men remained to form the baseline population for this analysis.
Identification of Incident Colorectal Cancer Cases
In both cohorts, participants reported cancer and other disease outcomes on biennial questionnaires. Researchers received participants' permission to obtain their medical records and pathological reports and, while blinded to exposure information, abstracted the information on anatomic location, stage, and histological type of the cancer. Colorectal cancer and sub-sites were defined according to the International Classification of Diseases, Ninth Revision (ICD-9).22
Assessment of Other Variables
We obtained information on potential colorectal cancer risk factors such as height, body weight, physical activity (MET-h/w), cigarette smoking, aspirin use, family history of colorectal cancer, snoring, antidepressant drug use, and menopausal status and postmenopausal hormone use (women only) on the biennial questionnaires. In addition, almost every 4 years, we collected information on dietary factors using baseline and subsequent validated food frequency questionnaires in the NHS23 and in the HPFS.24 These factors included consumption of red meat, processed meat, alcohol, folate, calcium, and vitamin D. In 1988, women participating in NHS were asked how many years in total (never, 1-2, 3-5, 6-9, 10-14, 15-19, 20-29, and ≥ 30 years) they had worked rotating night shifts.25
Statistical Analyses
We calculated person-time for each participant from the date of baseline questionnaire return to the date of death, loss to follow-up, colorectal cancer diagnosis, or the end of follow-up (May 31, 2008, for the NHS; January 31, 2008, for the HPFS), whichever came first. We used a Cox proportional hazards regression model26 to calculate hazard ratios (HRs, 95% CIs) and adjusted simultaneously for age (in months) and year of questionnaire return. We observed no violation of the proportional hazard assumption based on the likelihood ratio test that compared the model with and without the interaction terms between sleep duration and age or follow-up time. We conducted all analyses using the SAS software (Version 9.2; SAS Institute Inc). All statistical analyses were two-sided, with a P-value < 0.05 indicating significance.
In addition to age, in multivariate models, we adjusted for established or potential risk factors for colorectal cancer including BMI and diabetes (see Table 2 for variable categorizations). In women, we used the information provided in the 1986 questionnaire for these factors. In men, for non-dietary factors, we used information provided in the 1988 questionnaire; for dietary factors, we used the information provided in the 1986 questionnaire because no food frequency questionnaire was administered in 1988. In addition, we conducted sensitivity analysis adjusting for marital status, education level, sedentary activity (TV watching as a surrogate), waist to hip ratio, history of hypertension, depression (antidepressant use as a surrogate), and cardiovascular disease. Since results were essentially unchanged with adjustment for these latter factors, they were not included in the final model. Because sleep duration may be influenced by undiagnosed cancer, we conducted lag-time analyses, excluding cases that occurred within the first 4 years after assessment of sleep duration.
Table 2.
Hazard ratios (95% CIs) of colorectal cancer according to hours of sleep per day in the Health Professionals Follow-up Study (1988-2008)
To test the priori hypotheses, we specifically evaluated whether the sleep duration and colorectal cancer association was stronger among regular snorers or overweight individuals. As exploratory analyses, we also evaluated whether the association with sleep duration differed by alcohol consumption (< 10 g/d, ≥ 10 g/d in women; < 15 g/d, ≥ 15 g/d in men), family history of colorectal cancer (yes, no), physical activity (< median, ≥ median, in each cohort), menopausal status (premenopausal, postmenopausal), and years of shift work (0-14, ≥ 15 years, women only). In these stratified analyses, given the relatively small number of cases for short sleepers (i.e., ≤ 5 h) in HPFS, we used ≤ 6 h as the lowest category. Because initial analyses did not reveal significant associations with short sleep, further post hoc analyses only examined associations for the long sleepers (≥ 9 h) compared to the reference group (7 h). We constructed cross-product terms between the 2 sleep duration groups (i.e., ≥ 9 vs. 7 h) and each of the variables considered for effect modification and tested whether beta-coefficients of the cross product terms were statistically significant using a Wald test. We further conducted stratified analysis by history of endoscopy/sigmoidoscopy screening (yes, no) to assess potential detection bias. Lastly, using information on sleep duration collected from the baseline and year 2000, we calculated HRs for colorectal cancer diagnosed after 2000 according to 4 cross-classified categories of sleep duration (i.e., optimal: defined as 7 h vs. suboptimal: defined otherwise). We also conducted interaction analyses to evaluate whether the associations with “changes” in sleep duration in these 2 time periods differ by BMI at baseline or weight change in these 2 time periods.
RESULTS
We documented a total of 1,973 incident colorectal cancer cases: 1,264 cases in NHS (1986-2008) and 709 cases in HPFS (1988-2008). At baseline, the median age was 53 for women and 56 for men. Selected lifestyle and potential confounding factors were compared across hours of sleep duration (Table 1). Individuals with ≥ 9 h of sleep per day tended to smoke more, consume more alcohol, and were less likely to be physically active. In contrast, the short sleepers (≤ 5 h), especially for the male health professionals, tended to smoke less, consume less alcohol, and to be more physically active. In addition, among women, those with ≥ 15 years of rotating night shift work were most likely to report short sleep duration (≤ 5 h).
Table 1.
Age-standardized characteristics by sleep duration in the Health Professionals Follow-up Study (in 1988) and in the Nurses' Health Study (in 1986)
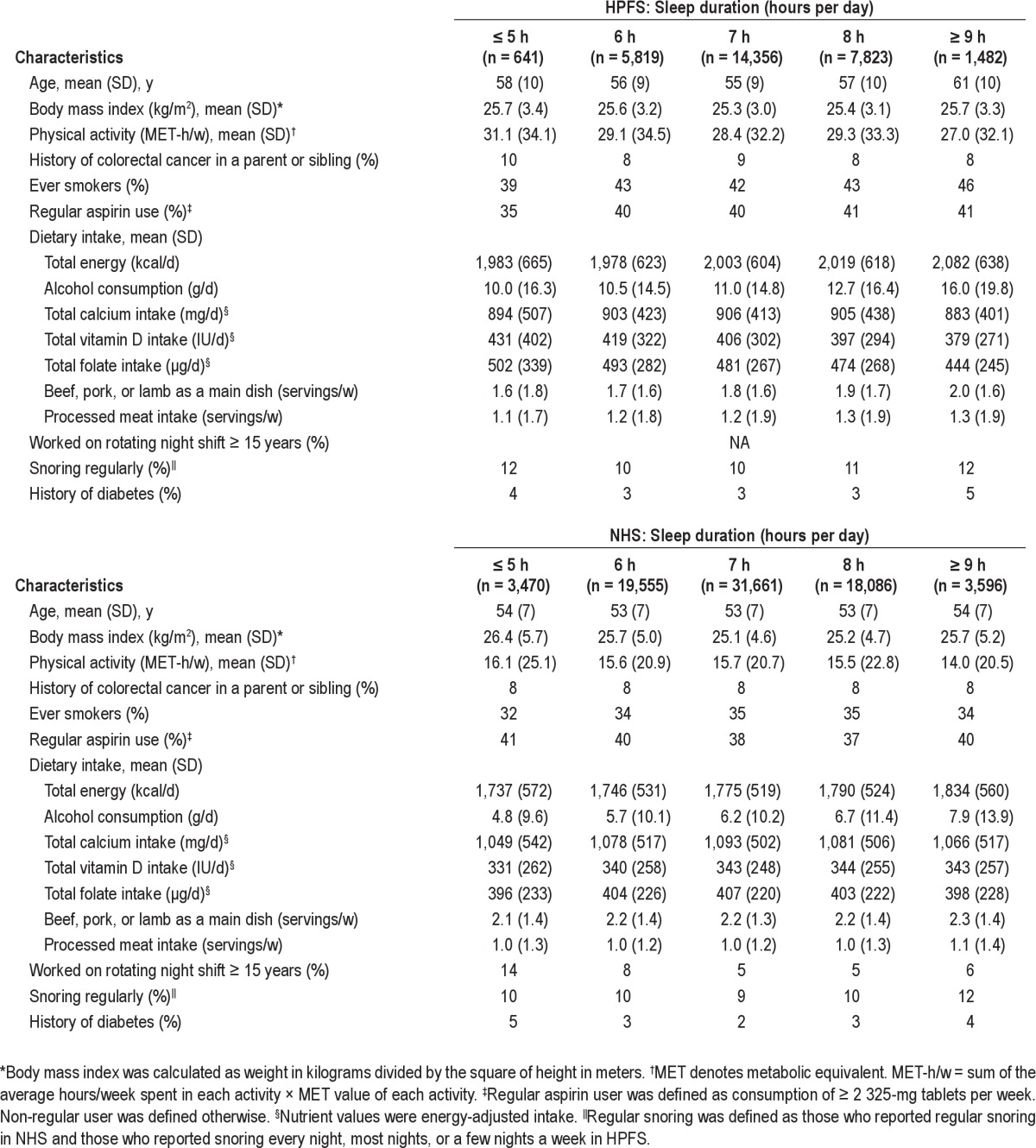
Habitual regular snoring was slightly more frequent in those with long or short sleep hours compared with individuals with median of 7 h of sleep (Table 1). Regular snorers had a higher mean BMI than non-snorers (28.3 vs. 25.1 kg/m2 in men; 26.0 vs. 25.3 kg/m2 in women) and tend to be overweight (65% vs. 41% in men; 55% vs. 37% in women).
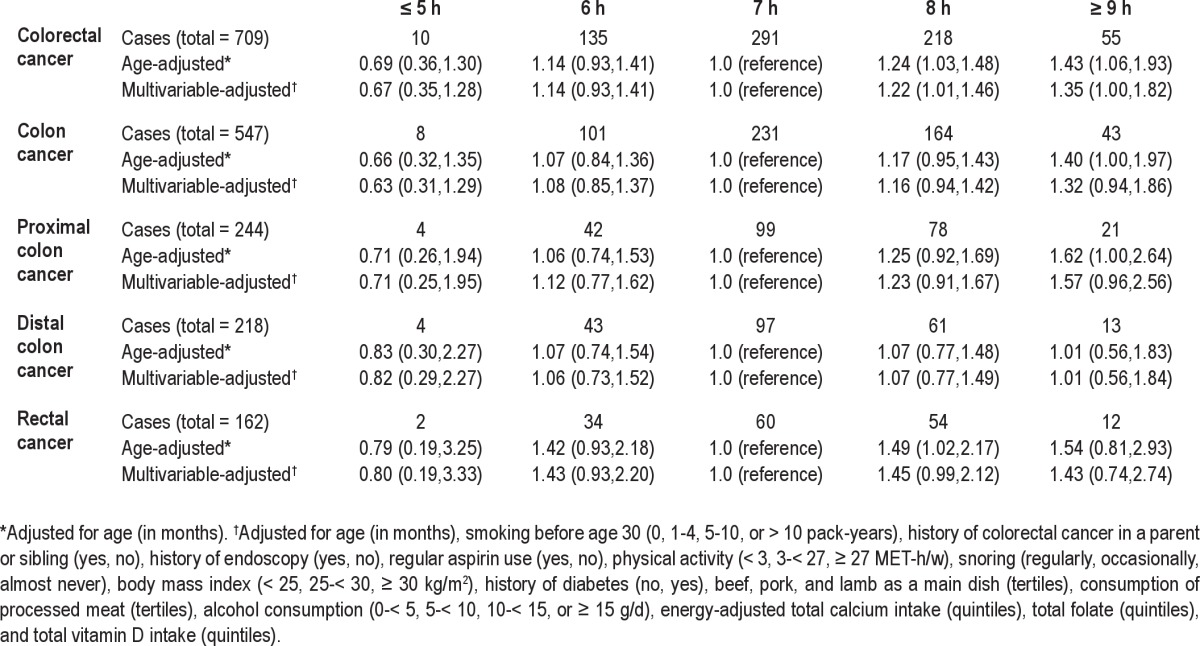
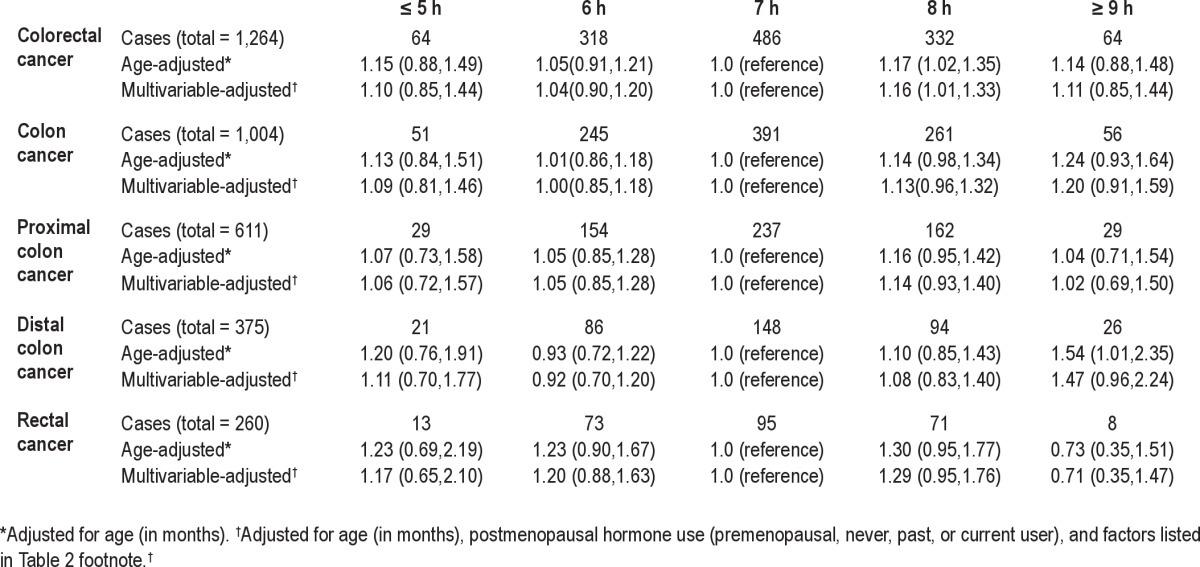
The hazard ratios of colorectal cancer in relation to sleep duration are presented in Table 2 for men and Table 3 for women. In both men and women, the age-adjusted results were quite similar to the multivariable-adjusted results. In men, compared to those with 7 h of sleep, men with ≥ 9 h of sleep per day had a suggestively increased risk of colorectal cancer (HR = 1.35; 95% CI: 1.00, 1.82) (Table 2). Results were similar when stratified by endoscopy/sigmoidoscopy screening or excluding cases within the first 4 years after assessment of sleep duration (data not shown). In women, the hazard ratios for both ≥ 9 h and ≤ 5 h of sleep were generally above 1.0 although not statistically significant (Table 3). No apparent or consistent pattern was seen by cancer sub-sites. In addition, no clear patterns were seen when sleep duration were examined in the 2 time periods using cross-classified sleep variables from baseline and 2000; results did not differ by BMI at baseline or weight change in these 2 time periods (data not shown).
Table 3.
Hazard ratios (95% CIs) of colorectal cancer according to hours of sleep per day in the Nurses' Health Study (1986-2008)
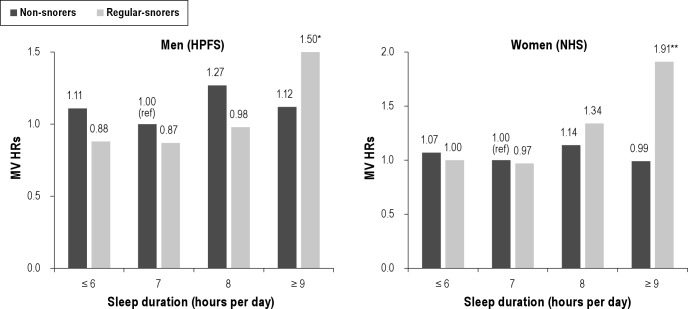
We found no association between snoring and colorectal cancer risk in men or women; the multivariate-adjusted HRs were 0.79 (95% CI: 0.59, 1.06) for men who snored every night and 1.07 (95% CI: 0.87, 1.32) for women who regularly snored. However, when the association with sleep duration stratified by snoring, we found that the positive association with ≥ 9 h of sleep appeared to be restricted to individuals who regularly snored in both men (HR = 1.80; 95% CI: 1.14, 2.84) and women (HR = 2.32; 95% CI: 1.24, 4.36; Table 4). The interaction between sleep duration and snoring was borderline significant for women (P = 0.05; Figure 1).
Table 4.
Multivariable hazard ratios* for colorectal cancer by sleep duration and BMI and snoring for men in the Health Professionals Follow-up Study (1988-2008) and for women in the Nurses' Health Study (1986-2008)
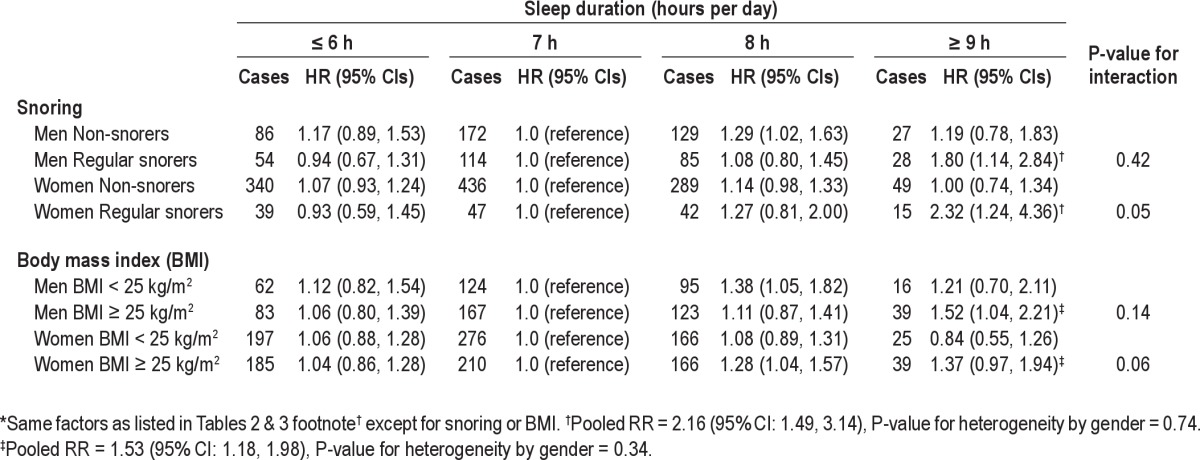
Figure 1.
Multivariable hazard ratios for the joint classification of sleep duration and snoring in the Health Professionals Follow-up Study (1988-2008) and the Nurses' Health Study (1986-2008). Same covariates as Tables 2 & 3 except snoring. Left: Men (HPFS). *MV HR = 1.50 (95% CI: 0.99, 2.27; n = 28 cases). Pinteraction = 0.42. Right: Women (NHS). **MV HR = 1.91 (95% CI: 1.13, 3.21; n = 15 cases). Pinteraction = 0.05.
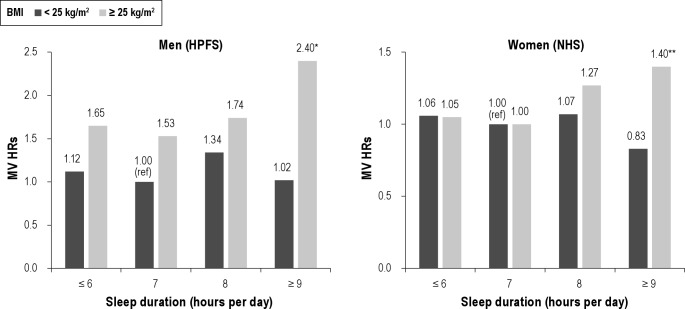
We further found that the positive association with ≥ 9 h of sleep was restricted to overweight individuals (i.e., BMI ≥ 25 kg/m2) (HR = 1.52; 95% CI: 1.04, 2.21 for men; HR = 1.37; 95% CI: 0.97, 1.94 for women). Figure 2 shows the joint associations of BMI and sleep duration.
Figure 2.
Multivariable hazard ratios for the joint classification of sleep duration and BMI in the Health Professionals Follow-up Study (1988-2008) and the Nurses' Health Study (1986-2008). Same covariates as Tables 2 & 3 except BMI. Left: Men (HPFS) *MV HR = 2.40 (95% CI: 1.65, 3.49; n = 39 cases). Pinteraction = 0.14. Right: Women (NHS) **MV HR = 1.40 (95% CI: 1.00, 1.97; n = 39 cases). Pinteraction = 0.06.
Finally, an suggestive increased colorectal cancer risk associated with sleep ≤ 5 h was only observed among women who had ≥ 15 years of shift work (HR = 1.50; 95% CI: 0.94, 2.39; n = 19 cases). No apparent pattern was seen by alcohol consumption, family history of colorectal cancer, physical activity, or menopausal status (women only).
DISCUSSION
In these two large cohorts of middle-aged to elderly men and women, we found that men with long sleep duration (≥ 9 h per day) had a significantly increased risk of developing colorectal cancer compared with those with 7 h of sleep. In subgroup analyses, men or women who were overweight or who were regular snorers and who reported sleeping ≥ 9 h per day were at an approximately 1.4 to 2-fold increased risk of developing colorectal cancer compared to overweight or regular snorers with 7 h of sleep per day. These associations were independent of known colorectal cancer risk factors.
To the best of our knowledge, our study is the first to report a significant positive association of colorectal cancer with long sleep duration, especially among those who are overweight or regular snorers. There are several potential mechanisms for these positive associations. Individuals with ≥ 9 h of sleep may spend less time in physical activity and more time in sedentary behaviors. However, adjustment for physical activity and sedentary behaviors (TV watching as a surrogate) did not alter our findings. Alternatively, individuals reporting long sleep duration may spend more time in bed but actually get less sleep and experience poorer sleep quality due to frequent awakenings. Low sleep efficiency and reduced slow wave sleep may result in increased cortisol secretion, inflammation and insulin resistance27–29 and may contribute to the development of obesity.30 Longer sleep also may reflect exposures to high levels of somnogenic cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor (TNF-α), which may have direct pro-inflammatory effects on tumorigenesis.18,19 Thus, an association between long sleep and colorectal cancer may reflect the effects of increased inflammatory mediators among individuals who sleep longer.
A novel finding of this study was evidence for a stronger association with long sleep hours among regular snorers. Snoring is a cardinal symptom of sleep apnea, and has been used as a surrogate for sleep disordered breathing, a common disorder associated with recurrent episodes of sleep disruption and intermittent hypoxemia. Sleep disruption may reduce sleep quality, increase sleepiness, and result in longer reported sleep durations. Intermittent hypoxemia has been shown in animal models to promote tumor growth, possibly through release of proangiogenic mediators resulting in cell proliferation.31–33 There has been little prior work that has addressed the association of snoring or sleep disordered breathing with cancer. Among the two studies we identified, one case-control study has reported an increased, albeit nonsignificant increase in colonic polyps in those reporting a doctor diagnosis of sleep apnea.10 In the other Wisconsin Sleep Cohort, sleep disordered breathing was associated with increased total cancer mortality.34
Prior studies have reported “U” shaped associations between sleep duration and mortality,8,9,35 cardiovascular disease,3,4 obesity,11 and diabetes.13 One recent case-control study in a sample of patients undergoing routine colonoscopy screening reported that when compared with individuals sleeping ≥ 7 h per night, individuals reporting < 6 h per night had a 47% increase in risk of developing adenomas (OR = 1.47, 95% CI: 1.05, 2.06).10 In our study, the hazard ratios for the short sleepers (i.e., ≤ 5 h of sleep) were all above 1.0 in women, but not statistically significant; in men, the hazard ratios were less than one. In contrast to many other studies, several health habits were more favorable in the short sleep sleepers compared to those sleeping longer in the male health professionals. In particular, men sleeping < 5 h per night reported the highest levels of physical activity and both men and women who were short sleepers reported lower energy consumption than the long sleepers. Since both cohorts were health professionals, it is possible that the correlates of sleep duration differ from those in more general population samples and these health professionals with shorter sleep choose to sleep less in order to exercise or work longer.
Several potential limitations merit discussion. First, objective measurements of sleep duration, snoring, sleep quality, and sleep disordered breathing were unavailable. Self-reported sleep duration and snoring data obtained from questionnaires may result in misclassification, which could bias the results to either direction. In addition, long sleep duration may represent multiple comorbidities36 and may have been influenced by undiagnosed subclinical diseases, raising concerns of reverse causality. Moreover, although changes in sleep pattern might occur during up to 22 years of follow-up, given that colorectal cancer takes decades to develop, the sleep duration queried in this study might capture the relevant long-term sleep habits. Second, we cannot rule out unmeasured residual confounding36,37 as a possible explanation of the observed association. Third, our study had a large sample size overall, but we still had limited power examining the potential effect of short sleep hours on colorectal cancer risk in men. Lastly, our study populations were mainly of European origin, and results may not be generalizable to other ethnic groups.
Strengths of this study include its prospective design with long follow-up time, which minimized the potential selection or recall bias. Paralleled analyses among men and women showed consistent results of longer sleep duration and risk of colorectal cancer among regular snorers or overweight individuals, suggesting that these findings were unlikely entirely due to chance. Finally, the measurement of multiple risk factors for colorectal cancer allowed us to evaluate the potential independent effect of sleep duration.
In summary, longer sleep duration was associated with an increased risk of developing colorectal cancer among individuals who were overweight or snored regularly. Given the sparse data, more research is warranted to evaluate whether sleep duration or sleep quality is a novel risk factor for colorectal cancer and to understand the mechanisms behind this association. The novel observation of increased risk among regular snorers who sleep long raises the possibility that sleep apnea and its attendant intermittent hypoxemia may contribute to cancer risk. In addition, large prospective studies examining potential effect of sleep duration on colorectal adenomas will also be informative.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK,OR, PA, RI, SC, TN, TX, VA, WA, WY. This study was funded by the NIH grants CA87969, 1U54CA155626-01, and CA55075.
REFERENCES
- 1.Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029–43. e10. doi: 10.1053/j.gastro.2010.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 5.Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595–600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841–7. doi: 10.1002/cncr.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 14.Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep. 2009;32:615–22. doi: 10.1093/sleep/32.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–79. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 17.von Ruesten A, Weikert C, Fietze I, Boeing H. Association of Sleep Duration with Chronic Diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. PLoS One. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 19.Krueger JM, Fang J, Taishi P, Chen Z, Kushikata T, Gardi J. Sleep. A physiologic role for IL-1 beta and TNF-alpha. Ann N Y Acad Sci. 1998;856:148–59. doi: 10.1111/j.1749-6632.1998.tb08323.x. [DOI] [PubMed] [Google Scholar]
- 20.Willett W. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 21.Colditz GA, Coakley E. Weight, weight gain, activity, and major illnesses: the Nurses' Health Study. Int J Sports Med. 1997;18(Suppl 3):S162–70. doi: 10.1055/s-2007-972709. [DOI] [PubMed] [Google Scholar]
- 22.Puckett CD. The Educational Annotation of ICD-9-CM; Diseases and Procedures Tabular Lists. Reno. 1986;Volume 1 [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semi-quantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 25.Schernhammer ES, Laden F, Speizer FE, et al. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 27.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 28.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–84. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 30.Vicennati V, Pasqui F, Cavazza C, Pagotto U, Pasquali R. Stress-related development of obesity and cortisol in women. Obesity (Silver Spring) 2009;17:1678–83. doi: 10.1038/oby.2009.76. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Song X, Wang X, et al. Effect of chronic intermittent hypoxia on biological behavior and hypoxia-associated gene expression in lung cancer cells. J Cell Biochem. 2010;111:554–63. doi: 10.1002/jcb.22739. [DOI] [PubMed] [Google Scholar]
- 32.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215–7. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 33.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275:2991–3002. doi: 10.1111/j.1742-4658.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- 34.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redline S, Foody J. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124:2049–51. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 36.Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev. 2007;11:337–9. doi: 10.1016/j.smrv.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]