Abstract
Study Objectives:
Drug treatment for obstructive sleep apnea (OSA) is desirable because at least 30% of patients do not tolerate continuous positive airway pressure (CPAP) treatment. The negative pressure reflex (NPR) involving superficially located mechanoreceptors in the upper airway (UA) is an important mechanism for UA patency inhibitable by topical UA anesthesia (lidocaine). The NPR may serve as a target for pharmacological intervention for a topical treatment of OSA. The objective was to determine the effect of pharmacological augmentation of the NPR on UA collapsibility.
Design:
We developed a model of UA collapsibility in which application of negative pressures caused UA collapses in spontaneously breathing α-chloralose-urethane anesthetized pigs as indicated by characteristic tracheal pressure and air flow changes.
Setting:
N/A.
Patients or Participants:
N/A.
Interventions:
N/A.
Measurements and Results:
The potassium channel blocker AVE0118 administered topically to the UA in doses of 1, 3, and 10 mg per nostril sensitized the NPR, shifting the mechanoreceptor response threshold for the genioglossus muscle to more positive pressures (P < 0.001; n = 6 per group) and dose-dependently inhibited UA collapsibility. Ten mg of AVE0118 prevented UA collapses against negative pressures of -150 mbar (P < 0.01) for > 4 h in all pigs, while in control pigs the UA collapsed at -50 mbar or less negative pressures. The effect of AVE0118 was abolished by UA lidocaine anesthesia. Acute intravenous administration of naloxone or acetazolamide was ineffective; paroxetine and mirtazepine were weakly effective and fluoxetine was moderately effective in line with reported clinical efficacy.
Conclusion:
Topical administration of AVE0118 to the UA is a promising pharmacologic approach for the treatment of OSA.
Citation:
Wirth KJ; Steinmeyer K; Ruetten H. Sensitization of upper airway mechanoreceptors as a new pharmacologic principle to treat obstructive sleep apnea: investigations with AVE0118 in anesthetized pigs. SLEEP 2013;36(5):699-708.
Keywords: Animal model, AVE0118, mechanoreceptors, negative pressure reflex, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder characterized by repetitive collapses of the pharyngeal airway during sleep. The prevalence of moderate-severe OSA (AHI > 15) in middle-aged adults is approximately 7% (9% in men, 4% in women), whereas the prevalence of mild OSA is substantially higher.1 OSA is highly prevalent in patients with cardiovascular and metabolic disorders.2,3
The current gold standard treatment is continuous positive airway pressure (CPAP) via a mask that splints open the airway against all collapse-inducing forces. The discomfort associated with CPAP reduces compliance so that only 50-70% of the patients with OSA use it in the long run.4,5 Therefore, a pharmacological treatment would be highly desirable for those who do not tolerate CPAP or who refuse it because they do not suffer from excessive daytime sleepiness but who may carry a higher cardiovascular risk.
OSA results from an anatomically narrow upper airway (UA) in conjunction with an insufficient neuromuscular activation of the UA dilating muscles during sleep, in absolute terms or only relative to the need for a higher tone that would be required in a narrow airway to compensate for the unfavourable narrow anatomy. Simple passive, anatomic abnormality cannot fully explain the genesis of OSA.6 Several studies have shown that the sever ity of OSA is weakly correlated with the severity of the pharyngeal mechanical abnormality.7,8 This strongly suggests that shortcomings in active neuromuscular con trol mechanisms, which may often only be transient, play an important role. Those active neuromuscular mechanisms should be amenable to pharmacological manipulation. The observation that patients with OSA have apnea-free intervals in which genioglossus (GG) muscle activity was only 25-40% higher compared with sleep phases with frequent obstructive apneas9 clearly adds an additional argument that pharmacological stimulation of the UA dilating muscles by an appropriate drug has a chance of protection from obstructive apneas. Moreover, electrical stimulation of the GG muscle has been shown to reduce airway collapsibility.10
Whether effective pharmacological activation of UA dilating muscle activity is possible in patients with OSA remains to be demonstrated because attempts to do so were not convincingly successful despite a number of clinical studies with different pharmacological principles. Only mild to moderate efficacy was achieved.5,11,12 Serotonin uptake inhibition and acetylcholine-esterase inhibition were the only pharmacological interventions that were most consistently effective. However, the efficacy was at best moderate and without any clinical relevance. It is important in this context to mention that none of those or other pharmacological principles ever tested in patients with OSA had been specially designed and developed for OSA. In this article we report that indeed five such drugs tested in patients with OSA have no or only a moderate effect in our new pig OSA model.
Major pharmacological progress in the search for drugs for OSA has been hampered or even precluded by both the lack of appropriate pharmacological models and the lack of innovative pharmacological concepts for anti-OSA drugs. To some extent both flaws were causally related in that it is difficult to establish a new pharmacological model without a positive control (a drug appropriate at least for experimental purposes). Developing new pharmacological concepts often requires testing of numerous diverse pharmacological principles in an appropriate animal model to deduce ideas for more appropriate and more efficacious pharmacological principles. We succeeded in identifying a potent new pharmacological principle that inhibited UA collapsibility in pigs as a functional parameter.
Superficially located mechanoreceptors are present in the UA mucosa to sense UA (negative) pressure during the respiratory cycle. This mechanoreceptor feedback is responsible for most of the UA dilating muscle response and referred to as the negative pressure reflex.13,14 Our new pharmacological concept is derived from the observation that careful local UA anesthesia with sodium channel blockers such as lidocaine (administered topically) was reported to inhibit GG electromyogram (EMG) activity in animals and man and to induce OSA in snorers or to worsen OSA in patients.13,15 This reflex is deemed one of the most potent mechanisms in keeping the UA patent. The basic idea was that because this reflex can be pharmacologically inhibited by topical administration of local anesthetic agents to the UA, there should be a complementary pharmacological possibility of activating it (by topical administration of an appropriate pharmacological principle). Sodium channel blockers inhibit neuronal activity, whereas potassium channel blockers augment neuronal activity by effects on the resting membrane potential or on repolarization.16 Therefore, an appropriate potassium channel blocker could increase UA reflex activity after topical administration. We were able to identify such a compound, AVE0118, which has been characterized as a blocker of potassium channels.17 In this study we investigated whether AVE0118, administered nasally in slow-release formulation, augmented the activity of the negative pressure reflex (NPR) by determining the pressure threshold at which the NPR was activated, and we tried to find out whether an augmented NPR was able to inhibit UA collapsibility induced by strong negative pressure challenges in an new animal model of α-chloraloseurethane anesthetized pigs.
METHODS
Experimental Animals, Anesthesia, and Surgical Procedures
All studies in animals were conducted in accordance with German laws for protection of animals. Furthermore, the investigation conforms to the guidance for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male castrated German Landrace pigs (weight range 20-35 kg, n = 45) were used. To avoid stress responses during ear vein cannulation for the induction of general anesthesia, pigs were sedated in the animal house by intramuscular injection of a mixture of low doses of Rompun® 2% (xylazine HCl, 23.3 mg/mL; Bayer), 0.5 mL and Zoletil® 100 (Virbac). The contents of a vial of Zoletil® 100, 250 mg of tiletamine, and 250 mg of zolazepam as dry powder was dissolved in 10 mL of the solvent. Next, 1.5 mL of this solution was injected intramuscularly per pig.
Experiments were performed on pigs in a supine position under general anesthesia induced and maintained by a mixture of α-chloralose and urethane. Anesthesia was induced by injecting 10 mL of α-chloralose solution (4.2 g/100 mL) intravenously into an ear vein corresponding to a dose of 16.8 mg/kg for a pig of 25 kg body weight followed by 35 mL of urethane solution (20 g/100 mL; 280 mg/kg). Urethane was dissolved in saline (0.9%). Chloralose was dissolved in saline that contained borax (2%) and filtered. Anesthesia was maintained by continuous infusion of 25 mL/h of urethane- and α-chloralose solutions mixed in a 50-mL syringe that was filled with 36 mL of the urethane solution and 14 mL of α-chloralose solution.
Before skin incisions were made bupivacaine 0.5% JENAPHARM®, having a long lasting anesthetic effect, was injected for additional infiltration anesthesia. The incisions were closed and sutured to prevent drying.
A femoral artery and vein were cannulated for blood pressure monitoring, blood gas analysis, and for the administration of the test compounds. Anesthesia was monitored via heart rate, blood pressure, ventilation, electrocardiogram (ECG), determination of blood gases, pulse oximetry (ear), and regular reflex testing for pain. Reflex testing was performed 15 min before each collapsibility test by pinching the pig's tail with a pair of tweezers close to its origin at the level of an intercoccygeal articulation. In case of insufficient anesthesia the pig would move its tail and a bolus dose of 5 mL of the anesthesia maintenance solution would then be applied and, if needed, repeated until this reflex disappeared. Body temperature was monitored and maintained using an infrared lamp. Oxygen was applied if necessary to keep oxygen saturation close to 100% via a tube placed into the outlet of the flowmeter attached to the facial mask (Figure 1). In this open system a flow rate of 2 L/min of oxygen was sufficient to keep saturation close to 100%. The animals spent most of the time with nasal breathing. Occasionally, mucus tended to obstruct the tracheal tubes and had to be removed. In such cases tracheal breathing was periodically allowed for blood gases improvement until physiological values were recovered.
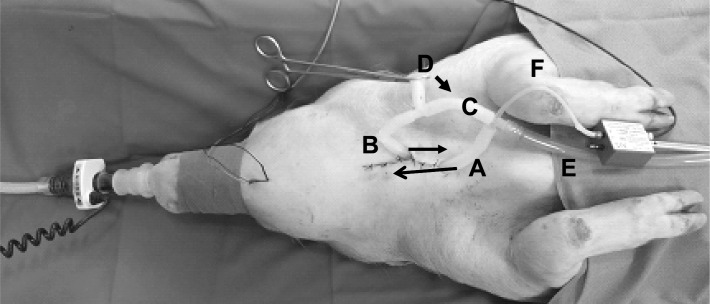
Figure 1.
Breathing circuits in the anesthetized pig. A, rostral tracheal tube; B, caudal tracheal tube; C, tube connecting rostral and caudal trachea; D, tube to atmosphere for tracheal breathing in the open state; E, tube to negative pressure device; F, thin tube for the registration of sublaryngeal pressure that was advanced into the rostral tracheal tube. Arrows on tubes A and B show the direction of the tubes in the trachea. In the setting illustrated in the figure the pig is in a situation of nasal breathing, with the clamp closing the tube to atmosphere. Removing the clamp from position D and putting it onto the connecting tube between rostral and caudal trachael tubes (C, arrow) leads to tracheal breathing, a situation in which actuation of the negative pressure device for the collapsibility test directs the negative pressure to the upper airway in an inspiratory direction via tube E.
A tracheotomy was performed 1-2 cm below the larynx (Figure 1). Care was taken to avoid injury of the laryngeal nerves. Two cannulas (1 cm outer diameter, 1 mm wall thickness) were inserted (approximately 2 cm) into the trachea, one into the rostral part and the other into the caudal part of the trachea so that they could be fixed by a thread around the trachea to seal the connection. As the trachea was dissected, both ends of the thread that fixed the proximal tube were tied around the distal tube to restore the longitudinal tension. Using a T-shaped connection piece, the rostral cannula was connected to a tube to the negative pressure device and to the distal tracheal cannula. The distal tracheal cannula was additionally connected to a tube with an open end to atmosphere via a T-shaped connection piece that served for free tracheal breathing, circumventing the UA. By appropriate opening and clamping of those tubes breathing could be switched from nasal breathing to breathing through the caudal tracheal cannula, circumventing the UA, and the (isolated) UA could be connected to the negative pressure device, causing airflow in the inspiratory direction (Figure 1). A thin tube was advanced into the rostral trachea and connected to a pressure transducer element for the measurement of tracheal (sublaryngeal) pressure. The snout was sealed with adhesive tapes, sparing the nostrils, and covered with a plastic bag onto which a flowmeter (Ohmeda) was fixed at the free end for the measurement of nasal airflow. The part of the bag on the pig's snout was sealed airtight by an elastic ribbon.
Measurement Methods
GG EMG Measurement
For the bipolar registration of the GG EMG, steel needles were placed 4-6 mm deep through the mylohyoid muscle into the GG muscle via a small skin incision midway between the chin and the hyoid bone. The raw EMG signals were amplified, filtered (bandwidth 50 Hz to 10 kHz), rectified, and integrated (moving average with a time constant of 1 sec) using a Hugo Sachs EMG-amplifier.
Collapsibility Tests by Application of Negative Pressure
The negative pressure device consisted of a negative pressure container (50 L) with a manometer that was evacuated by a vacuum pump and activated via a solenoid valve. The device enabled generation of any negative pressure as low as -150 mbar. The pressure level was set and the desired level reached in the device before the pressure challenge. To induce a UA collapse (collapsibility test), breathing was switched from nasal to tracheal breathing and one nostril was closed during application of negative pressure by lateral pressure with two fingers to increase nasal resistance (Figure 1). Next, actuation of the negative pressure device applied the preselected device pressure onto the UA airway via the tube connecting the device to the cranial tracheal cannula. Negative pressure was applied for at least three breaths, which caused a collapse of the UA under control conditions as indicated by the measurements of airflow (to the device) and of sublaryngeal pressure. In pigs such negative pressure challenges were performed with negative pressures of -50, -100, and -150 mbar. The highest pressure of -50 mbar was applied first and maintained for at least three breaths. The next pressure challenge applying the more negative pressures were performed usually after pauses of at least five breaths. A complete collapsibility test at the three pressure levels indicated was performed before administration of the test compound and at regular intervals after administration (up to 4 h after administration of the test compound).
Determination of the Mechanoreceptor Response Threshold
The aim of this investigation was to determine the highest pressure (which is the least negative pressure) at which GG EMG activity appeared, starting from total inactivity during tracheal breathing. After the switch from nasal to tracheal breathing, GG EMG disappeared completely in these anesthetized pigs under control conditions because of the absence of the activating influences of UA negative pressure. In this situation, application of negative pressure from the negative pressure device could elicit GG EMG activity. Both nostrils were closed so that the device pressure prevailed in the UA. Usually the pressure test started with a negative pressure of -20 mbar. If GG EMG activity was present, the pressure was increased to less negative pressure by steps of 5 mbar. If no activity appeared at -20 mbar, the pressure was decreased by steps of 10 mbar. The negative pressure threshold is the negative pressure at which clear GG EMG appeared from total inactivity (during tracheal breathing). The measurements were made before and 1, 2, and 3 h after administration of AVE0118. The measurements were always performed before the collapsibility tests. The values given in Figure 3 are the means of six pigs in each of the four groups (vehicle, 1, 3, and 10 mg).
Figure 3.
Effect of nasal administration of AVE0118 given at time point 0 min on upper airway collapsibility at different levels of negative pressure and on the mechanoreceptor activation pressure threshold (right lower panel) in anesthetized pigs. Percentages of pigs (n = 6 per group) with collapse or mechanosensor activation thresholds (mbar) are given for vehicle (control), 1, 3, and 10 mg per nostril of AVE0118. Results for mechanoreceptor threshold after AVE0118 are significantly different versus vehicle for all doses and timepoints (P < 0.001). Results for collapsibility are significant versus vehicle group for time points > 30-60 min (P < 0.01) for all doses and pressures. At 1-mg duration of inhibition of collapsibility versus control group is 180, 120, and 75 min (medians; P < 0.01) at -50, -100, and -150 mbar, respectively.
The biological signals were recorded by a Hugo Sachs Plug-sys-amplifier system and continuously stored on a computer hard disk by an online data acquisition and analysis system (Hem 4.2 Notocord Systems, Croissy-sur-Seine, France).
Experimental Protocols
Twenty-four pigs were studied to define the effect of a slow-release preparation of AVE0118 (Sanofi-Aventis, Frankfurt, Germany) on upper airway collapsibility: six after nasal administration of a biologically neutral vehicle, and six each after nasal administration of three different doses of AVE0118 (1, 3, and 10 mg per nostril). The slow-release formulation showed almost constant release over 4 h (data not shown). The ribbon and the bag around the snout were temporarily removed for the nasal administration of the study drug. Next, 0.4 mL of vehicle or test compound was slowly instilled into each nostril using a pipette during the inspiratory phase, with the head of the pig in an elevated position. The solution was carefully distributed over the entire circumference of the nostril to enable homogenous nasal distribution. Thereafter, the snout was again covered by the bag and sealed with the elastic ribbon.
Influence of Local Anesthesia on AVE0118 Effects
In a separate group of three pigs it was investigated whether UA local anesthesia with lidocaine abolished the effect of AVE0118 administered to the UA in the same way as described previously. After nasal administration of 10 mg of AVE0118 per nostril and the first demonstration that collapsibility had been inhibited, UA anesthesia with lidocaine was performed to investigate whether the effect of AVE0118 was abolished. Lidocaine 0.4 mL of 100 mg/mL solution (Xylocain-Pumpspray, Astra-Zeneca) was instilled into each nostril with the head of the pig in an elevated position and the negative pressure device was actuated for 5 sec to distribute the solution into more distal parts of the UA. As soon as GG EMG activity had disappeared after administration of lidocaine, collapsibility tests were performed as described previously.
Pharmacologic Validation of the Model
To determine the reliability of the model in discriminating pharmacological effects, we used it to test a number of other drugs for which clinical results from OSA patients have been published.5,11 The drugs examined were: fluoxetine (two doses: 0.5 and 1 mg/kg given as an intravenous bolus); mirtazepine and paroxetine (1 mg/kg intravenous bolus); acetazolamide (3 mg/kg intravenous bolus followed by 6 mg/kg infused over 2 h), and naloxone (40 μg/kg intravenous bolus). Three pigs were used for each drug and each dose (18 pigs in all). The intravenous route was used to ensure reliable drug delivery. The dose of each drug for the pigs (dose/body weight) was determined from that used orally in the relevant OSA patient study divided by an assumed body weight of 75 kg.
Naloxone (Ratiopharm) was used as a ready-to-use drug. The other agents mentioned, urethane and α-chloralose, and borax were purchased from Sigma-Aldrich (89555 Steinheim, Germany). UA collapsibility was determined before administration of these drugs and at regular intervals up to 3 h after administration as with the methods used to study AVE0118.
Euthanasia
At the end of the experiment pigs were euthanized by an overdose of pentobarbital followed by a lethal dose of potassium chloride.
Data Analysis
Mechanoreceptor Threshold Determination
Data are presented as means ± standard error of the mean. The normality (Shapiro-Wilks test) was tested for the negative pressure threshold and global normality was assessed. This was followed by the Levene test for two factors to check homogeneity of variances. Both the test on global normality and on homogeneity of variances was significant. Thus, a rank-transformation was performed with subsequent two-way analysis of variance for the factor treatment and the factor time with repeated measures. The Dunnett test was chosen as posthoc test for comparisons with the vehicle group as control.
Collapsibility Test
Results are presented as percentage of pigs in which the UA collapsed during pressure challenges with different pressure levels of -50, -100, and -150 mbar over the time course of the experiment. For statistical calculation of the treatment effect, the individual time- dependent binary collapsibility data (collapse versus no collapse) were transformed to two continuous time parameters: ‘time till inhibition of collapse’ and ‘duration of inhibition of collapse’. The subsequent statistical analysis on both derived continuous parameters used a standard log-rank test for the factor dose followed by the log-rank multiple comparisons test with Bonferroni-Holm correction versus vehicle group to account for the censoring of the data.
The analyses were performed using SAS statistical software V8.2. All tests were performed at the 0.05 significance level.
RESULTS
Characteristics of the Pharmacologic Pig Model of UA Collapsibility Used
In urethane-chloralose-anesthetized spontaneously breathing pigs, continuous application of negative pressure to the UA for a few breaths caused UA occlusion (referred to as collapse). UA collapse was indicated by an interruption of airflow to the negative pressure device and by a sublaryngeal pressure change from atmospheric pressure to a pressure that approximated the device pressure because the collapsed UA was almost airtight toward its oral end (Figure 2A). GG EMG increased during the negative pressure challenge but was ineffective in opening the UA under control conditions. Pharmacologically augmented inspiratory phasic activation of UA dilating muscle was able to open the closed airway in case of effective stimulation during the inspiratory phase whereas in most cases the UA collapsed again during the expiratory phase because of an expiratory decline in UA dilating muscle activity. The closed airway then opened again with the rise in UA dilating muscle activity with the next inspiratory phase. Figure 2B shows a negative pressure challenge after AVE0118 was administered to the UA. Under these conditions GG EMG increased. A cyclic pattern of airflow to the negative pressure device occurred that was synchronous with the respiratory cycle and resembled tracheal flow during normal breathing. Pressure in the upper tracheal segment during the collapse in the expiratory phase approximated the pressure generated by the negative pressure device whereas it rose to almost atmospheric pressure during an effective inspiratory opening of the UA. Thus, the UA remained collapsed over the entire respiratory cycle in the control situation or after administration of ineffective drugs. After effective pharmacological stimulation of UA-dilating muscle activity, the UA opened with the rising phasic activity during the inspiratory phase, while during the expiratory phase it collapsed again and then opened again with the next inspiratory phase. Tonic GG EMG activity was mostly absent at normal breathing but appeared during the negative pressure challenges (Figure 2). The effect of AVE0118 seemed to mainly depend on phasic inspiratory UA dilating muscle activity.
Figure 2.
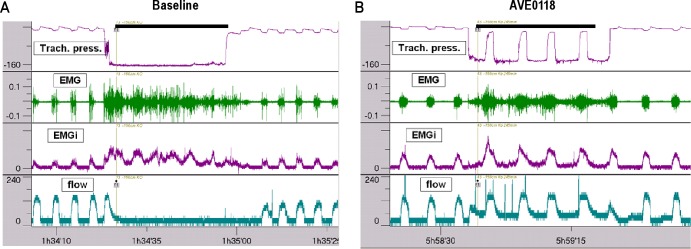
Tracings illustrating a collapsibility test in an anesthetized pig before (A) and after nasal administration of AVE0118, 10 mg per nostril (B). Upper airway (UA) collapse (A) is indicated by an interruption of flow (lowest tracing) and a sublaryngeal pressure close to the negative device pressure (upper tracing) during both the inspiratory and expiratory phase. Second and third tracing, genioglossus (GG) raw electromyogram (EMG) and integrated EMG, respectively. After AVE0118 the UA is open (B) during the inspiratory phase as indicated by flow to the negative pressure device and sublaryngeal pressure approaching atmospheric pressure. Time of application of negative pressure is labeled by a black line. Airflow during this period is directed to the negative pressure device. EMG activity is given in arbitrary units, tracheal pressure in mbar, and airflow in mL/sec.
Effect of AVE0118 on UA Collapsibility and Mechanoreceptor Activation Threshold
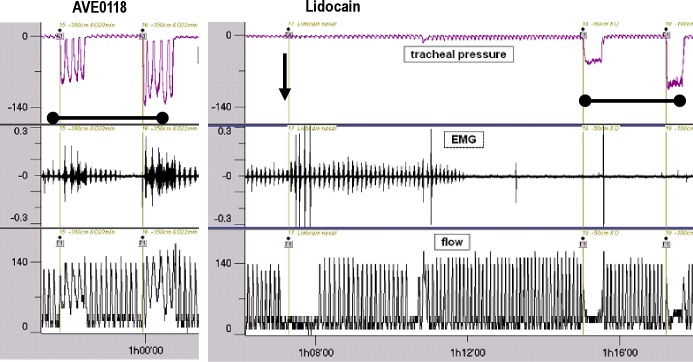
The effects of AVE0118 on UA collapsibility and mechanoreceptor activation threshold are shown in Figure 3. Before administration of AVE0118 or of vehicle, negative pressure application to the UA caused UA collapses at all pressure levels in all six pigs in each of the four groups except for a single pig in the 10-mg group at -50 mbar. Vehicle did not inhibit UA collapsibility during the ensuing experimental period of 4 h (Figure 3). AVE0118 showed a dose-dependent inhibition of collapsibility with regard to the duration of action and the level of negative pressure applied. Collapsibility was fully inhibited after the highest dose of 10 mg for 4 h even at -150 mbar, whereas the effect of the lowest dose of 1 mg was incomplete and its duration of action was shorter. The medians of the ‘time till inhibition of UA collapse’ for the different dose groups of AVE0118 and for the different applied negative pressures were within the time interval of 30-60 min (onset of action), being largest for the strongest negative pressures and smallest for the largest doses (P < 0.01 versus control group). The onset of action of the slow-release formulation used in this experiment was delayed compared with a solution (high free concentration) where efficacy starts very quickly, often within 1 min but at the expense of a shorter duration of action (data not shown). Figure 4 shows the development of raw GG EMG activity in a pig after administration of 10 mg of AVE0118 to each nostril. No inhibition of collapse was observed within the testing period of 240 min for the vehicle group for any level of negative pressure. A similar dose-dependency as for the ‘time till inhibition of UA collapse’ was observed for the medians of the 'duration of inhibition of collapse' (duration of action) for the different dose groups of AVE0118 being statistically significantly different from the vehicle group (P < 0.01).
Figure 4.
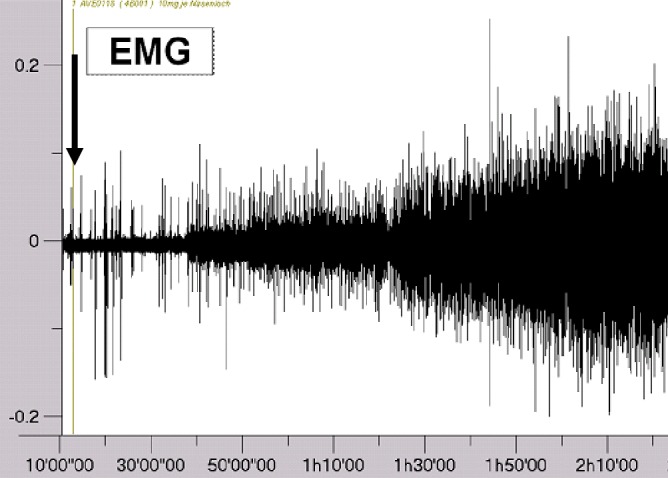
Tracing illustrating the development of raw genioglossus (GG) electromyogram (EMG) activity in a pig after administration of 10 mg of AVE0118 to each nostril (vertical arrow). The onset of action of the nasal formulation is delayed by approximately 30 min in line with the onset of inhibition of collapsibility (see Figure 3). EMG activity is given in arbitrary units.
The mechanoreceptor activation threshold required to elicit visible GG EMG activity from total inactivity during tracheal breathing was significantly shifted to more positive values after AVE0118 at all three doses used (P < 0.001 versus control group) (Figure 3), indicating a sensitization of the reflex, whereas it showed a slight decrease toward more negative values in the control group over the time course of the experiment. At 3 mg, 2 h after administration of AVE0118 as an example, the mechanoreceptor threshold had risen from a value of -22 ± 3.4 mbar at baseline to -4 ± 1.9 mbar (mean ± standard error of the mean; n = 6) and remained more or less at this level for the entire time period from 60-180 min. The curves for the 3-mg and the 10-mg dose curves were almost superimposable. The 1-mg dose only showed a slightly weaker effect compared with the 3-mg and 10-mg dose.
Influence of Local Anesthesia on AVE0118 Effects
Topical administration of lidocaine to the UA, which was performed after the first demonstration of full inhibition of collapsibility by AVE0118, abolished any GG EMG activity, even during the negative pressure challenges where EMG activity is otherwise very high (n = 3). Consequently, collapsibility inhibited by AVE0118 returned after lidocaine, whereas in the experiment described previously, the effect of AVE0118 alone persisted for more than 4 h. Figure 5 shows an original tracing of the effect of lidocaine in a pig.
Figure 5.
Tracings illustrating the effect of topical upper airway (UA) anesthesia with lidocaine in a pig that had received nasal AVE0118, 10 mg per nostril. Before lidocaine genioglossus (GG) electromyogram (EMG) activity was present and collapsibility was inhibited by AVE0118 during application of -100 and -150 mbar negative pressure (left tracings, labeled by a black line). Approximately 5 min after lidocaine administration (vertical arrow) GG EMG activity disappeared and collapsibility returned at negative pressure challenges of -50 and -100 mbar labeled by a black line (right tracings). Note that inspiratory tracheal pressure became more negative after lidocaine administration after GG EMG activity had disappeared (from -5 mbar to -9 mbar) and that even during application of negative pressure GG EMG activity does not appear any more. Airflow measurement is interrupted during the nasal application of lidocaine. EMG activity is given in arbitrary units, tracheal pressure in mbar, and airflow in mL/sec.
Pharmacologic Validation of the Model
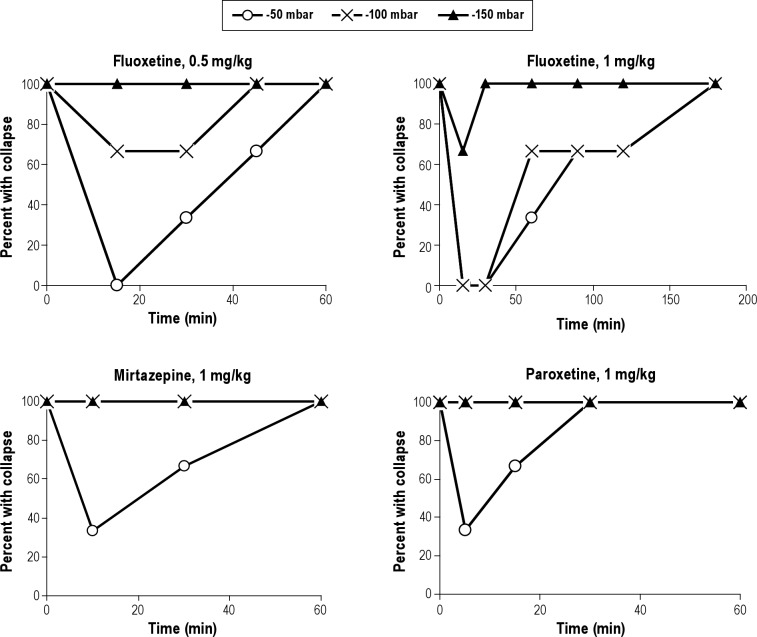
Naloxone (40 μg/kg intravenously; n = 3) and acetazolamide (3 mg/kg intravenous bolus plus infusion of 6 mg/kg in 2 h, n = 3) did not inhibit collapsibility, although in some cases transient and moderate increases in GG EMG activity were observed. Fluoxetine (0.5 and 1 mg/kg intravenously; n = 3 per dose) showed a dose-dependent incomplete inhibition of collapsibility. Collapsibility at 1 mg/kg at -150 mbar was inhibited in one of three pigs at a single timepoint only, whereas at -50 and -100 mbar collapsibility was inhibited for approximately 30 min only in all pigs. After 3 h collapsibility had fully returned. The effect of 0.5 mg/kg fluoxetine was weaker. The effects of paroxetine (1 mg/kg intravenously; n = 3) and mirtazepine (1 mg/kg intravenously; n = 3) were quite weak in that they inhibited only the -50 mbar negative pressure challenge and even this effect was short-lasting. The effects of fluoxetine, mirtazepine, and paroxetine are shown in Figure 6.
Figure 6.
Effect of fluoxetine at 0.5 and 1 mg/kg, and mirtazepine and paroxetine at 1 mg/kg on upper airway collapsibility at different levels of negative pressure in anesthetized pigs. Percentage of pigs with collapse is shown (n = 3 for each drug and dose).
DISCUSSION
In this article we demonstrate a new potential drug for the treatment of OSA, which we characterized in a newly developed pharmacological pig model for OSA. The potassium channel blocker AVE0118 given via nasal administration sensitized and amplified the NPR as indicated by a shift of the threshold to much higher pressures. Given as a slow-release formulation it showed a complete inhibition of UA collapsibility at 10 mg per nostril for more than 4 h, showing its potential for the treatment of OSA. Topical administration of lidocaine to the UA abolished the effect of AVE0118 in accordance with its peripheral mode of action. Collapsibility returned a few min after lidocaine administration when GG EMG had disappeared. To determine the reliability of the new model in discriminating pharmacological effects we used it to test five other drugs for which clinical results from patients with OSA have been published. This comparison is important for the appraisal of the efficacy of AVE0118 in our model with respect to its potential predictive value for clinical efficacy. There was good concordance between clinical data and our experimental results. Fluoxetine, the most potent of those drugs tested, showed moderate efficacy in this model in keeping with previous clinical study data.11 The other drugs were ineffective or weakly effective in our model.
The target of this new pharmacological principle is the NPR, an important mechanism for UA patency.13–15,18 Sensitization of the NPR by AVE0118 in our experiments was indeed indicated by a shift of the mechanoreceptor threshold to less negative pressures that were needed to elicit GG EMG activity from a total inactivity level in tracheal breathing. The fundamental idea behind this new pharmacological concept was that the NPR could be topically activated by an appropriate pharmacological principle (e.g., by inhibition of certain potassium channels) because it can be topically inhibited by local anesthetic agents (i.e., by sodium channel blockers) applied to the UA. Negative pressure-sensitive mechanoreceptors located in the mucosa of the UA are highly sensitive to even small changes in negative pressure. The importance of the NPR for UA patency can also be judged from our experiments where administration of lidocaine abolished any GG EMG activity, even during the negative pressure challenges where it is otherwise very high. Sodium channel blockers inhibit neuronal activity, whereas potassium channel blockers increase neuronal activity by effects on the resting membrane potential (depolarization toward the firing threshold) or on repolarization.16 The expectation was that topical application of an appropriate potassium channel blocker such as AVE011817 would sensitize and amplify the negative pressure reflex to increase UA dilating activity so as to prevent UA collapsibility.
This UA collapsibility model in anesthetized, spontaneously breathing pigs is based on the fact that application of strong negative pressure causes UA occlusion and that pharmacological stimulation of UA dilating muscle activity can keep the UA patent during this negative pressure challenge, at least during the inspiratory phase. It was a major challenge to find an anesthetic procedure that maintains UA muscle activity and the NPR activity in general anesthesia, which was a necessity in our investigations. This is because most anesthetic agents cause deep UA muscle relaxation with a total disappearance of the NPR, which means that no GG EMG activity appears even in response to strong negative pressures. The anesthetic procedure in which UA reflexes were almost fully intact in our pigs used a mixture of α-chloralose and urethane. In the literature UA collapsibility has been mainly investigated under passive conditions in the absence of muscle tone. In other articles it is unclear whether the NPR was still intact in anesthesia. In one of those publications the authors used pentobarbital in spontaneously breathing dogs and measured the influence of hypercapnia and hypoxia on UA collapsibility, which was judged from pressure-flow relationships at negative pressures above -10 mbar.19 In pigs, in our hands, pentobarbital abolished any GG EMG activity, including the increases caused by strong negative pressures. Heavily anesthetized dogs were used to assess UA airway mechanics after selective electrical hypoglossal nerve stimulation,20 which means that the negative pressure was abolished but the UA muscles were stimulated. We used the pig because it is closer to the human situation with regard to UA size and physiology than any other laboratory animal species including rats, rabbits, and dogs. Nevertheless, those articles and others21 were quite valuable for the development of our new method in the pig.21
Although stimulation of GG EMG activity after administration of nasal solutions of AVE0118 could be seen, quantification of these effects turned out to be difficult because baseline EMG activity was strongly variable, probably due to a threshold effect, that seemed to depend on the anesthetic depth and could not be controlled to an extent that would be necessary for reliably measuring baseline GG EMG activity. Moreover, measurement of GG EMG activity does not answer the question as to whether a certain extent of activation is sufficient to keep the UA patent in a critical situation or following a challenge. By contrast, the collapsibility model showed highly reproducible results independent on the baseline GG EMG activity before AVE0118 administration. To cause UA collapses we used strong negative pressures of -50 mbar to -150 mbar. These negative pressures were continuously applied during the inspiratory phase and the expiratory phase, in which, physiologically, UA pressure becomes positive, so that the UA always collapsed in the expiratory phase while in the inspiratory phase it was open or closed depending on UA dilating muscle activity. Practically, we assessed whether the UA would open in the inspiratory phase after expiratory closure. Opening of the collapsed UA against such negative pressures is probably more difficult than keeping the UA open against physiological negative inspiratory pressure of, e.g., -8 mbar. However, large intrathoracic negative pressures of approximately -100 mbar have been reported during obstructive apneas.22 The strong negative pressure used by us caused a strong collapsing force but at the same time a vigorous activation of the NPR.
We also used negative pressures of -20 mbar, which caused an UA collapse in most pigs, but the judgement of whether the UA was open or collapsed was more difficult and not always reliable. The application of three different negative pressure levels of -50, -100, and -150 mbar allowed recognition of graduated responses so as to even identify drugs with weak to moderate experimental (and clinical) efficacy.5,11 Fluoxetine, the most potent drug other than AVE0118, hardly reduced collapsibility at the lowest negative pressure of -150 mbar but at -50 and -100 mbar although for a short time only. Paroxetine and mirtazepine only reduced collapsibility for the -50 mbar negative pressure challenge for a short time interval. Acetazol-amide (acute experiment with 2-h duration) and naloxone did not reduce collapsibility with even the weakest negative pressure challenge, although with naloxone occasionally and transiently signs of increased GG EMG activity could be seen. Our acute experiment with acetazolamide does not exclude a possible effect of chronic administration on UA dilating muscle activity by development of a metabolic acidosis.
AVE0118 sensitized and amplified the NPR as indicated by a shift of the threshold to much higher pressures. The thresholds taken from this negative pressure challenge test are obviously much lower–more negative–than those physiologically present during nasal breathing. After switching from nasal to tracheal breathing GG EMG activity disappeared completely and it reappeared with resumption of normal nasal breathing with minimal inspiratory pressures being between -6 and -9 mbar. This indicates that the true threshold must have been above -9 mbar. The threshold measured with the negative pressure challenges were much lower (group means between -25 and -19 mbar) under control conditions. This could be due to the continuous application of negative pressure through the entire respiratory cycle, including the expiratory phase where pressure during nasal breathing is otherwise positive. Thus, negative pressure mechanoreceptors may be depolarized in the expiratory phase, too, with negative consequences for recovery and restoration of excitability. This could have reduced the number of functional mechanosensors in the subsequent inspiratory phase so as to lower the activation threshold. Nevertheless, the fact that AVE0118 raised the activation threshold from approximately -25 mbar to approximately -5 mbar indicates a sensitization of mechanoreceptors. Unlike the collapsibility test this test for the mechanoreceptor response threshold is not a quantitative or a functional test for the concerted UA dilating muscle activity but a detection of a threshold for the activation of the GG muscle.
Inspiratory GG muscle activation may occur as a reflex to negative airway pressure and by a central drive.21 In humans during sleep, GG activity was found in the absence of negative UA pressure, suggesting the existence of efficient central GG activation.21 By contrast, in our pig model, GG activity disappeared in the absence of UA negative pressure during tracheal breathing. It is likely that in the absence of negative UA pressure in our pigs, anesthesia (compared with sleep) had reduced central GG activation to an extent that it was not able to generate GG activity alone, implying that the NPR was the main mechanism of GG activation. However, this does not exclude the existence of a central GG drive even in our pig model, as rhythmic inspiratory GG activity was present during the negative pressure challenges over a few breaths, although negative pressure was applied during the entire respiratory cycle including the expiratory phase. We presume that in the absence of negative UA pressure, anesthesia reduced central GG activation to an extent that it was not able to generate GG activity alone, implying the existence of a threshold for hypoglossal motoneuron activation.
Our new model has some limitations. General anesthesia and healthy animals are used, tonic expiratory UA dilating muscle activity is not considered, and challenges to induce collapsibility are nonphysiological. A further limitation is that it does not consider the chronic situation of a patient with OSA with tissue changes and possible changes in innervation by chronic snoring and distensions. A new OSA model in sleeping cats has been published recently but results with drugs have not been reported.23 Although this model has the advantage of being more clinically related, training of the animals to the procedures and surgery is quite time consuming, thus probably limiting the number of possible investigations. Our acute pig model was designed for pharmacological purposes and thus offers an attractive throughput. We believe that it is a significant contribution to the search for effective anti-OSA drugs, showing a good concordance between clinical data and our experimental results, with most drugs being ineffective or only moderately effective (fluoxetine). It also has the capacity to identify drugs with moderate efficacy such as fluoxetine. From the compounds tested in this article AVE0118 administered nasally was the most potent drug.
In summary, we present a new pharmacological model for the investigation of the potential of drugs against OSA in spontaneously breathing urethane-α-chloralose anesthetized pigs that is based on UA collapsibility induced by application of strong negative pressures. In this model we investigated a new effective pharmacological principle, which is based on a pharmacological sensitization of UA mechanoreceptors for negative pressure. By topical administration to the UA, potassium channel blocker AVE0118 demonstrated its potential to treat OSA by sensitizing the mechanoreceptor reflex and abolishing UA collapsibility for more than 4 h.
DISCLOSURE STATEMENT
Klaus J. Wirth, Klaus Steinmeyer, and Hartmut Ruetten are employees of Sanofi.
ACKNOWLEDGMENTS
The authors thank Björn Rosenstein for his excellent technical assistance and Joachim Brendel for the first synthesis of AVE0118.
ABBREVIATIONS
- AHI
apnea hypopnea index
- AU
arbitrary units
- CPAP
continuous positive airway pressure treatment
- ECG
electrocardiogram
- EMG
electromyogram
- GG
genioglossus
- h
hour(s)
- mL
milliliter(s)
- min
minute(s)
- NPR
negative pressure reflex
- OSA
obstructive sleep apnea
- sec
second(s)
- UA
upper airway
Footnotes
A commentary on this article appears in this issue on page 635.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26:702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 3.Baguet JP, Barone-Rochette G, Pepin JL. Hypertension and obstructive sleep apnoea syndrome: current perspectives. J Hum Hypertens. 2009;23:431–43. doi: 10.1038/jhh.2008.147. [DOI] [PubMed] [Google Scholar]
- 4.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 5.Hedner J, Grote L, Zou D. Pharmacological treatment of sleep apnea: current situation and future strategies. Sleep Med Rev. 2008;12:33–47. doi: 10.1016/j.smrv.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159:149–57. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 7.Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ, Younes M. Response of genioglossus muscle to increasing chemical drive in sleeping obstructive apnea patients. Sleep. 2011;34:1061–73. doi: 10.5665/SLEEP.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan AS, White DP, Fogel RB. Recent advances in understanding the pathogenesis of obstructive sleep apnea. Curr Opin Pulm Med. 2003;9:459–64. doi: 10.1097/00063198-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dotan Y, Golibroda T, Oliven R, et al. Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J. 2011;38:338–47. doi: 10.1183/09031936.00125810. [DOI] [PubMed] [Google Scholar]
- 11.Kohler M, Bloch KE, Stradling JR. Pharmacological approaches to the treatment of obstructive sleep apnoea. Expert Opin Investig Drugs. 2009;18:647–56. doi: 10.1517/13543780902877674. [DOI] [PubMed] [Google Scholar]
- 12.Kohler M, Stradling JR. Pitfalls of clinical trials on pharmacological treatment for obstructive sleep apnoea: future directions. Expert Opin Investig Drugs. 2011;20:1033–7. doi: 10.1517/13543784.2011.590473. [DOI] [PubMed] [Google Scholar]
- 13.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dematteis M, Levy P, Pepin JL. A simple procedure for measuring pharyngeal sensitivity: a contribution to the diagnosis of sleep apnoea. Thorax. 2005;60:418–26. doi: 10.1136/thx.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deegan PC, Mulloy E, McNicholas WT. Topical oropharyngeal anesthesia in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:1108–12. doi: 10.1164/ajrccm/151.4.1108. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–84. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 17.Gogelein H, Brendel J, Steinmeyer K, et al. Effects of the atrial antiar-rhythmic drug AVE0118 on cardiac ion channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:183–92. doi: 10.1007/s00210-004-0957-y. [DOI] [PubMed] [Google Scholar]
- 18.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol. 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliven A, Odeh M, Gavriely N. Effect of hypercapnia on upper airway resistance and collapsibility in anesthetized dogs. Respir Physiol. 1989;75:29–38. doi: 10.1016/0034-5687(89)90084-4. [DOI] [PubMed] [Google Scholar]
- 20.Yoo PB, Durand DM. Effects of selective hypoglossal nerve stimulation on canine upper airway mechanics. J Appl Physiol. 2005;99:937–43. doi: 10.1152/japplphysiol.00652.2004. [DOI] [PubMed] [Google Scholar]
- 21.Innes JA, Morell JA, Kobayashi I, Hamilton RD, Guz A. Central and reflex neural control of genioglossus in subjects who underwent laryngectomy. Appl Physiol. 1995;78:2180–6. doi: 10.1152/jappl.1995.78.6.2180. [DOI] [PubMed] [Google Scholar]
- 22.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 23.Neuzeret PC, Gormand F, Reix P, et al. A new animal model of obstructive sleep apnea responding to continuous positive airway pressure. Sleep. 2011;34:541–8. doi: 10.1093/sleep/34.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]