Abstract
Study Objectives:
To compare the efficacy of problem-solving therapy (PST) combined with behavioral sleep strategies to standard cognitive therapy (CT) combined with behavioral sleep strategies in the treatment of insomnia.
Design:
A six-week randomized controlled trial with one month follow-up.
Setting:
The Australian National University Psychology Clinic, Canberra, Australia.
Participants:
Forty-seven adults aged 18-60 years recruited from the community meeting the Research Diagnostic Criteria for insomnia.
Interventions:
Participants received 6 weeks of treatment including one group session (sleep education and hygiene, stimulus control instructions and progressive muscle relaxation) followed by 5 weeks of individual treatment of PST or CT.
Measurements and Results:
Primary outcomes included sleep efficiency (SE) from sleep diaries, the Insomnia Severity Index (ISI), and the Pittsburgh Sleep Quality Index (PSQI). Secondary measures assessed dysfunctional sleep beliefs, problem-solving skills and orientations, and worry. Both treatments produced significant post therapy improvements in sleep which were maintained at 1 month follow-up (on SE Cohen d = 1.42, 95% CI 1.02-1.87 for PST; d = 1.26, 95% CI 0.81-1.65 for CT; on ISI d = 1.46, 95% CI 1.03-1.88 for PST; d = 1.95, 95% CI 0.52-2.38 for CT; for PSQI d = 0.97, 95% CI 0.55-1.40 for PST and d = 1.34, 95% CI 0.90-1.79 for the CT). There were no differences in PST and CT in the size or rate of improvement in sleep although CT produced a significant faster rate of decline in negative beliefs about sleep than PST and there was a trend (P = 0.08) for PST to produce a faster rate of improvement in negative problem orientation than CT.
Conclusions:
The results provide preliminary support for problem solving treatment as an equally efficacious alternative component to cognitive therapy in psychological interventions for insomnia.
Citation:
Pech M; O'Kearney R. A randomized controlled trial of problem-solving therapy compared to cognitive therapy for the treatment of insomnia in adults. SLEEP 2013;36(5):739-749.
Keywords: Insomnia, problem-solving therapy, cognitive behavior therapy, cognitive therapy, non-pharmacological treatment
INTRODUCTION
Insomnia is an insidious disorder of sleep which disrupts daytime functioning and has serious long-term health consequences. Current practice parameters1 recommend treatment with psychological therapy in preference to sleep medications, which have been shown to be effective only in the short-term. A variety of non-pharmacological interventions have been tested for their efficacy in treating insomnia. Of these, cognitive behavior therapy for insomnia (CBT-I), a therapy package involving various combinations of behavioral and cognitive strategies, is the most widely tested, highly recommended and commonly utilized.2–4
CBT-I has been found to be safe and effective for a large proportion of people with insomnia, both primary and secondary subtypes, with longer lasting effects than hypnotic medications.5 A recent meta-analysis6 of CBT-I reported pooled effects sizes relative to waitlist, treatment as usual, or pill placebo controls on diary measures of sleep efficiency of 0.86 (95% CI 0.66-0.95) immediately post treatment, 0.81 (95% CI 0.35-1.15) at 3 months post-treatment, and 0.54 (95% CI 0.23-0.20) at 12 months. This review estimated pooled prior to post effect sizes for CBT-I on sleep efficiency at 0.82 (95% CI 0.62-0.93). Despite these generally favorable outcomes, some evidence shows that 20% to 35% of patients do not respond to CBT-I7 and of those who do respond; the average improvement is about 50% to 60%.8 These considerations indicate the need to develop and test other psychological interventions which may optimize outcomes and which offer clinicians effective options for treating patients with insomnia.
CBT-I usually consists of 4-6 weekly individual or group sessions which combine behavioral strategies such as sleep hygiene, stimulus control, sleep restriction, or relaxation therapy with a cognitive therapy (CT) component.9 While there is independent evidence for the effectiveness of the behavioral interventions such as stimulus control, cognitive therapy, although included as a component of CBT-I in the controlled trials1 has not been shown to be an active component of the treatment. In the trials the CT component involves standard cognitive restructuring of unrealistic beliefs and irrational fears specifically about sleep or the loss of sleep. There is some evidence to support targeting sleep specific beliefs.10 The exclusive focus on these beliefs in the current CT component of evaluated CBT-I program may, however, neglect other critical cognitive factors implicated in the maintenance of insomnia. Worry, defined as general negative and uncontrollable thoughts about future outcomes, has been show to increase vulnerability to developing insomnia,11–15 disrupt sleep onset and maintenance,15,16 and increase over time in insomnia, influencing the chronicity of the condition.17 In addition experimental studies testing the involvement of worry in insomnia support these connections.18–24 These data suggest that worry may be an important target for intervention in insomnia.
While worry is conceptualized is varying ways, one understanding of the nature of worry which has implications for treatment is that people with excessive levels of worry have dysfunctional problem-solving abilities, including negative beliefs regarding problems and their own ability to actively solve them.25,26 This negative problem orientation has been found to predict worry scores independent of a person's mood state.25 Furthermore, beliefs relating to helplessness and hopelessness, closely resembling a negative problem orientation in insomnia sufferers, have been found to remain elevated following treatment for insomnia using CT strategies.26,27 Taken together these findings suggest that while CT addresses problematic beliefs specific to sleep, it does not impact on general problem orientation and, consequentially, on the risks to continuing sleep problems due to excessive worry. Strategies focusing on reducing worry by improving problem solving abilities may reduce the role played by these factors in sleep difficulties and expand options for effective psychological treatment for insomnia. Some worry-focused strategies have been shown to confer benefits in treating insomnia.28–31 A “constructive worry” strategy involving setting aside a period of time to list current problems and the next step to their resolution prior to going to bed was found to reduce pre-sleep arousal in the early evening.31,32 Other recent research found that a constructive worry component added to the treatment effect of a behavioral intervention for insomnia by further reducing insomnia severity and worry levels.33 This emerging evidence suggests that there is merit in exploring insomnia interventions which focus on the reduction of worry and improvement of problem-solving.
Problem-solving therapy (PST) has been found to be an effective therapy for a range of psychological conditions including major depressive disorder and generalized anxiety disorder.34 PST is a behaviorally orientated approach to teaching problem-solving skills that are generalizable to a range of stressful problem situations. It aims to both enhance rational problem solving skills and improve problem-solving self-efficacy, by promoting a more positive problem orientation.35 It is a skills-based therapy, easily taught in a brief course of treatment. Despite its potential benefits, to our knowledge no study to date has assessed PST for its efficacy as a therapy or component of treatment for insomnia.
The present study compares the efficacy of a psychological intervention for insomnia which involves behavioral components combined with problem solving therapy (PST) to the usual CBT-I intervention with combines behavioral components with standard cognitive therapy (CT). We predicted that the intervention with PST would be at least as effective as the intervention with CT and that both treatments would result in significant and clinically important improvements in sleep and daytime functioning. The study also examines dysfunctional beliefs about sleep and problem-solving skills and problem orientation as secondary outcomes specific to CT and PST, respectively.
MATERIALS AND METHOD
Participants
Participants were recruited by way of pamphlets mailed to local general practitioners in Canberra and surrounds, in addition to newspaper advertisements and postings on both online and physical community noticeboards. Recruitment was staggered between November 2010 and August 2011, and treatment was completed by October 2011. The project was registered with the Australian New Zealand Clinical Trial Registry http://actr.org.au, (Registry No. ACTRN12610000123044) and ethical approval was granted by The Australian National University's Human Research Ethics Committee. All participants gave informed written consent and participated in the treatment on a voluntary basis, however, they were offered $10 - $15 reimbursement for completing research forms for a related study exploring worry content in insomnia, which they completed pre-intervention.
Following brief initial screening via online or telephone survey, potential participants were invited to attend The Australian National University for comprehensive clinical assessment interviews (approximately 90 min in duration). Participants were asked about their sleep, medical and mental health, and substance and medication use, to establish whether they met inclusion criteria for participation. The Duke Structured Interview for Sleep Disorders (DSISD)36 and the Mini International Neuropsychiatric Interview 6.0 (MINI)37 were utilized to screen for sleep status and mental health status respectively.
To be included in the trial, participants met the following criteria: (1) aged between 18 and 60 years old, and (2) assessed as having current insomnia as defined by the Research Diagnostic Criteria for Insomnia38 for ≥ 3 months. The Research Diagnostic Criteria as developed by Edinger and colleagues (2004) for the American Academy of Sleep Medicine suggests a standardized criteria for insomnia as follows: (1) the individual reports one or more sleep related complaints (e.g. difficulty initiating or maintaining sleep, early waking or non-restorative sleep), (2) the difficulty occurs despite adequate opportunity for sleep, (3) the individual reports at least one daytime impairment (e.g. fatigue; poor attention, concentration or memory; poor social, vocational or school performance; mood disturbance or irritability; proneness for errors or accidents at work or while driving; tension headaches or gastrointestinal symptoms in response to sleep loss; and excessive concerns or worries about sleep).38 Participants were excluded if (1) they met criteria for a sleep disorder or disturbance other than insomnia on the DSISD (e.g., sleep apnea, restless legs syndrome, period limb movement disorder, narcolepsy, jet lag, nightmare disorder, shift-work or other circadian rhythm disturbances, parasomnias or hypersomnia), (2) they had a serious psychological or psychiatric condition as determined by their responses on the MINI (e.g. high risk of suicide, bipolar affective disorder, psychotic disorder, obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder), (3) or if they showed evidence of an alcohol or other substance abuse or dependence disorder. Any participants excluded on this basis were given a sleep hygiene information sheet and referred to an appropriate local service. To be more inclusive of the general insomnia population, participants were included in the trial if they reported comorbid symptoms of a major depressive disorder, anxiety disorders not listed in the exclusion criteria, or reported comorbid medical conditions which were stabilized (with or without treatment) at the time of assessment. Participants were also accepted if they were currently taking sleeping medication, the use of which was monitored via logging in their weekly sleep diaries. Participants were asked not to commence other new treatments, medical or psychological, during their participation in the trial.
Figure 1 shows participant flow from enrolment in the study to follow-up and inclusion in statistical analyses. In total, of the 140 participants initially screened, 53 were found eligible to participate, and of these, 47 elected to take part in the trial. Six participants withdrew prior to treatment commencement and randomization, citing work commitments or time constraints as the primary reasons for declining participation. In total, 23 (6 males) participants were assigned to CT, and 24 (12 males) participants were allocated to PST. Over half of participants (57.45%) presented with comorbid medical or psychological conditions, ranging from one to three co-occurring conditions each. Of the total number of comorbidities, 34.28% had chronic pain disorders, 14.29% met criteria for a mood disorder (4 participants with major depressive disorder and one with generalized anxiety disorder), 14.29% had pulmonary conditions (primarily mild asthma), 11.43% gastrointestinal conditions, 8.57% heart conditions (including arrhythmias), 5.75% each with endocrine and autoimmune disorders, and one participant (2.86%) with a sinus condition. There was no between group difference in the proportion of males (χ2 = 2.84, P = 0.092) or on mean age (Mean age PST = 44.50; Mean age CT = 33.91; t = 1.32, P = 0.19). The groups did not differ on the number of comorbidities (Mean No. PST = 0.63, Mean No. CT = 0.91; t = 1.26; P = 0.21) or duration of insomnia in years (Mean duration PST = 12.42, Mean duration CT = 15.13; t = 0.83; P = 0.41).
Figure 1.
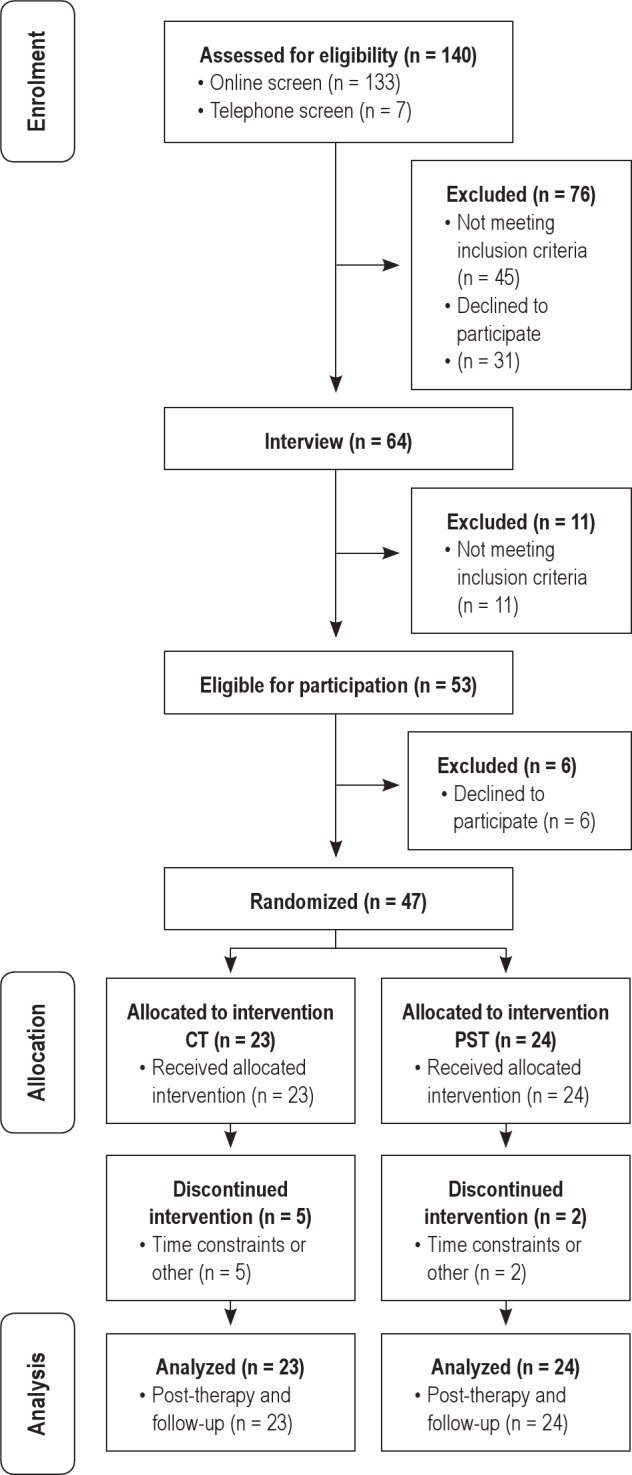
Flow of participants from enrolment to analysis.
Apparatus and Materials
Sleep Diary
Participants completed a daily sleep diary for each week of study including for one week prior to start of treatment and for one week prior to follow-up assessment. The form was completed within 30 min of waking and assessed sleep onset latency ([SOL] minutes taken to fall asleep), time in bed (TIB), number of nighttime awakenings (WAKE), duration of nighttime awakenings after sleep onset (WASO), total sleep time (TST), and sleep efficiency ([SE] percentage of time in bed spent asleep). Participants were also asked to log the frequency of their relaxation practice, and whether they used medication or alcohol to aid sleep for each night. Mean values were obtained across one week to assess a representative sample of nights.
Insomnia Severity Index
The ISI39 is a 7-item self-report measure designed as a brief screening tool for subjective insomnia severity and validated as a clinical research outcome measure. Items cover the severity of insomnia symptoms, satisfaction with sleep pattern, level of interference with functioning, appearance of impairment noticeable by others, and level of worry or distress over the past 2 weeks. Scores are rated on a 5-point Likert scale ranging from not at all to extremely. Total scores range from 0-28 with cutoff scores suggested by the authors of 0-7 for nonclinical insomnia, 8-14 for sub-threshold insomnia, 15-21 for clinical insomnia with moderate severity, and 22-28 for severe clinical insomnia.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI40 is a 19-item retrospective self-report measure assessing sleep quality and disturbances over a 1-month period. It consists of a combination of free entry and 4-point Likert scale items. It has been widely used in clinical practice and research, with validity in distinguishing individuals with and without sleep disturbance.41 Domains of sleep quality assessed include subjective sleep quality, latency, duration, efficiency, frequency and severity of sleep disturbances, use of sleep medication, and perceived impact on daytime function. Each of these component scores has a range from 0-3 and summed together yield a global sleep quality rating ranging from 0-21. Higher global scores indicate poorer quality sleep.
Social Problem-Solving Inventory–Revised: Short-Form (SPSI-R:S)
The SPSI-R:S42 is a 25-item short version of the original SPSI 52-item scale43 designed to assess problem-solving skills and orientations. The SPSI-R assesses problem-solving skills and beliefs using a 5-point Likert scale ranging from not at all true of me to extremely true of me (0-4) for general statements concerning difficult life problems. The measure has 5 subscales including rational problem-solving (RPS), impulsivity/carelessness style (ICS), avoidance style (AS), positive problem orientation (PPO), and negative problem orientation (NPO). Participants receive a standardized total score (M = 100), along with standardized scores for each subscale, utilizing age-based norms. Total SPSI score and positive (PPO) and negative problem orientation (NPO) subscale scores are used here.
Dysfunctional Beliefs and Attitudes about Sleep-16 (DBAS-16)
The DBAS-1644 is a 16-item short version of the original DBAS 30-item scale,45 which was designed to assess sleep-specific cognition. The DBAS-16 consists of 16 sleep-related thoughts across a range of domains which are rated on an 11-point Likert scale ranging from strongly disagree to strongly agree (0- 10). The domains include: (1) expectations about sleep requirements; (2) beliefs about the causes and consequences of insomnia; (3) issues of worry and helplessness regarding insomnia; and (4) biological attribution of insomnia (including beliefs about the usefulness of sleep medication). Total score on the DBAS is used as an index of severity of negative beliefs about sleep.
Penn State Worry Questionnaire (PSWQ)
The PSWQ46 is a 16-item self-report form assessing general tendency to worry. Respondents indicate on a 5-point scale to what degree each item is typical of them, with responses ranging from not at all typical of me to very typical of me. Scores are added (5 items are reverse scored) and total scores range from 16-80, with higher scores being indicative of a greater degree of worry.
Procedure
Pre-Intervention
Participants were screened for eligibility to enter the trial, first via brief survey, then through clinical interview as described above. Included participants completed baseline measures during their pre-treatment interview. Participants took a one-week sleep diary home and were instructed to complete this during the week following the initial interview. Treatment commenced as soon as feasible after the initial interview. This was on average within 14 days of assessment.
Interventions
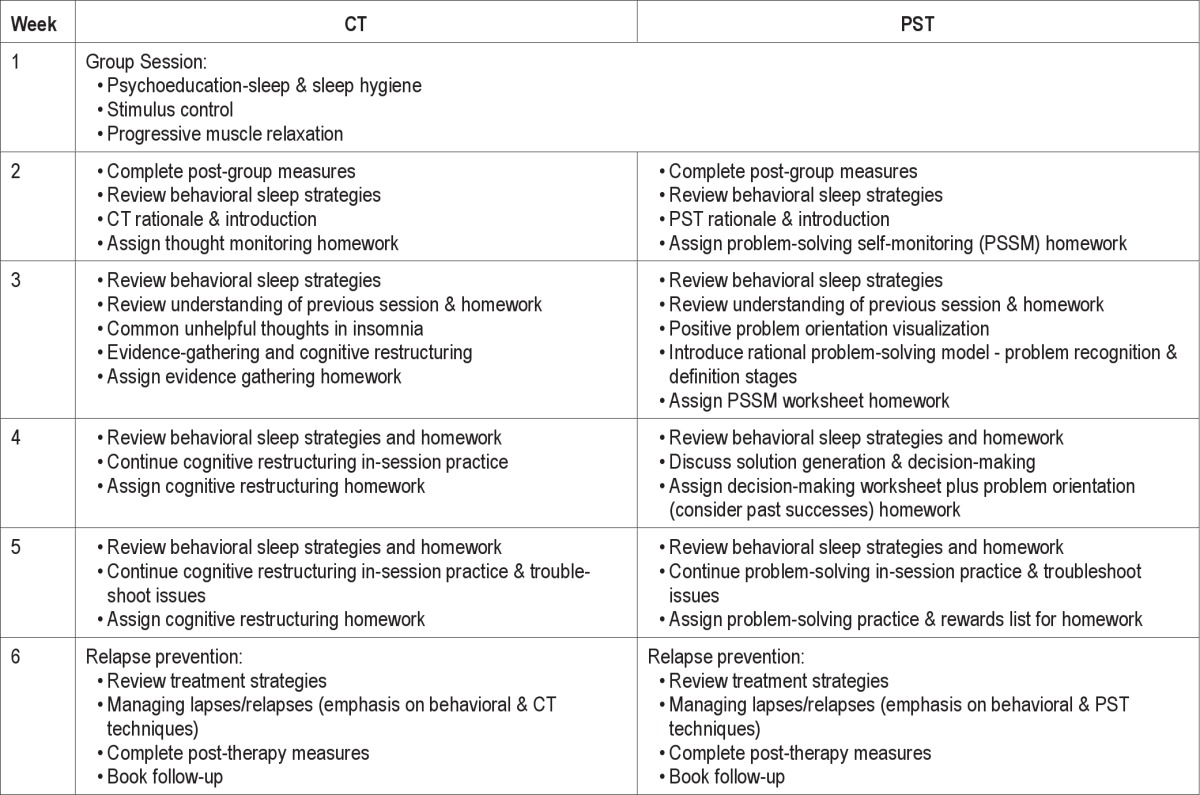
All treatment sessions took place at The Australian National University Psychology Clinic during business hours. Table 1 describes the session components for the two treatments. In each treatment the first session was a 90-min group session with 3-6 participants of educational and behavioral components including psychoeducation on sleep, and instructions in sleep hygiene, stimulus control, and progressive muscle relaxation. A sleep restriction component was not included in the present study because of the wider inclusion of participant comorbidi-ties for which this strategy is often contraindicated. Participants were provided with a workbook including notes from the session and a compact disc with a recording of the relaxation (approximately 14 min in duration), which they were instructed to use for daily practice. Group delivery of behavioral sleep strategies is empirically supported.47
Table 1.
Session-by-session treatment description
Following the group session, each participant was randomly allocated to PST or CT for 5 weeks of individual therapy. Allocation to the two treatments was randomized by a colleague independent to the trial, using a computerized random numbers generator. Assignment was concealed from the researchers using sealed envelopes, which were opened only when allocation took place (i.e., after each participant had attended their group treatment session).
Individual Sessions
Participants received 5 individual sessions. In each of these sessions the behavioral strategies were reviewed, patient concerns implementing the strategies were addressed, and specific instructions in either PST or CT provided.
Problem-Solving Therapy (PST)
PST included education about the importance of effective problem-solving in stress management and health, information about problem-solving styles and orientations, instruction in rational problem-solving techniques (e.g., problem definition, generation and selection of solutions, action planning and evaluation of solution attempts), and enhancement of problem orientation using a variety of strategies (e.g., guided visualization, practice in positive problem statements, reflecting on previous successful problem-solving attempts, encouraging and rewarding persistence). Participants were provided with problem-solving worksheets for between session tasks, along with handouts of the information covered. Participants were offered the choice to focus on their specific sleep problem, or on any other current problem of their choosing which was causing general distress or disruption to their sleep. Week 5 was largely allocated to guided practice and review, and Week 6 consisted of relapse prevention strategies combining behavioral sleep strategies from the group session with PST.
Cognitive Therapy (CT)
CT consisted of psychoeducation about the role of dysfunctional beliefs about sleep in maintaining insomnia, identification of client-specific dysfunctional beliefs about sleep, teaching of cognitive restructuring techniques, and guided practice in balancing unhelpful beliefs about sleep using specific client examples. Thought monitoring records were utilized to facilitate guided and individualized practice, and client handouts were provided including summaries of the main information covered. Week 5 was largely allocated to guided practice and review, and Week 6 consisted of relapse prevention strategies combining behavioral sleep strategies from the group session with CT.
Follow-up
Participants were invited to return for a 30-min follow-up session one month after treatment completion, during which they completed the follow-up measures and were offered an opportunity to discuss their progress and address any issues.
Treatment Consistency
To ensure treatment consistency, manuals were adapted from existing manuals for both CT9,32,48 and PST35 conditions. The study manuals are available upon request from the primary author. All sessions were conducted by the first author, a clinically trained and provisionally registered psychologist, who was provided with weekly clinical supervision by the second author, a fully registered psychologist with endorsement in clinical psychology. Individual treatment sessions were digitally audio-recorded by the therapist and used in supervision to ensure fidelity to the treatment protocol and to allow for independent review for treatment consistency. In addition, after each session participants completed a session evaluation form by endorsing whether specific sleep, problem solving, and cognitive therapy strategies were discussed in the session.
The recordings of 25 randomly selected sessions (approximately 10% of the total 211 of sessions available) were also reviewed and rated by an independent rater, blind to the nature of the study hypotheses, the treatments, and the participant's allocation to a treatment. Sessions were blocked such that each treatment had a similar number of sessions reviewed (12 CT sessions and 13 PST sessions) and those selected were spread across the 5 sessions of individual treatment. The rater was provided with the same list of strategies provided to participants after each session and asked to tick a strategy if it was utilized within each session (i.e., if the strategy was discussed at some length, rather than just being mentioned once, or if the therapist asked about or provided instruction in any particular strategy). Brief training was provided in the task to ensure the rater's understanding of the task.
Outcomes
There were 4 assessments; initial clinical interview (Pre), one week following participation in the group session (Post Group), one week after the completion of the sixth therapy session (Post), and one month following the completion of therapy (Follow-up). During each assessment, participants completed one week of sleep diary, in addition to self-report measures. Post group results were concealed from the therapist to reduce potential bias by having participants place their completed forms in a sealed envelope, which was not opened and scored until they had completed the 6 weeks of the trial. The main primary outcomes were sleep efficiency (SE) calculated from the sleep diary, ISI total, and PSQI total. In addition, specific sleep outcomes from the sleep diary, SOL (minutes taken to fall asleep), WAKE (number of nighttime awakenings), WASO (duration of nighttime awakenings after sleep onset in minutes), TST (total sleep time in minutes), and MED/AL (number of nights used medication or alcohol to help sleep) were calculated. Secondary outcomes were SPSI-R total, positive problem orientation (PPO), and negative problem orientation (NPO) from the SPSIR, DBAS total score, and PSWQ total score.
Analysis Plan
The relative effectiveness of PST and CT on the primary and secondary sleep outcomes was assessed with mixed effects regression. This method allows estimates of each individual's trajectory of change across time (pre, post group, post, and follow-up assessments) and also considers between-treatment differences in the trajectories of change across time (time × group interaction). It utilizes all existing data at the individual and treatment level rather than requiring imputation for missing values. In addition, because regression considers time as a continuous variable, it better accommodates varying intervals between measurement points, as is the case in our study.
Secondary outcomes were also analyzed with time by treatment type regression. Because we predicted therapy specific effects on these outcomes with PST improving the problem solving outcomes (SSPI, PPO, NPO) and CT improving sleep belief outcomes (DBAS), these analyses used 3 time points (post group, post, follow-up).
Categorical outcomes were also calculated. Criteria were selected based on previous suggestions.5,49,50 Participants were considered recovered if they had (1) SE ≥ 85% or (2) an ISI score < 8. Pre to post and pre to follow-up raw and standardized (Cohen's d) effect sizes for each therapy were calculated on primary sleep outcomes. Analyses for the primary sleep outcomes (SE, ISI, PSQI), using mixed effects regression was also done for participants who completed all sessions (per-protocol analysis).
RESULTS
Overview
All analyses were conducted using PASW Statistics 18-20 (formerly SPSS).
Preliminary Analyses
Of the 47 participants randomized to PST or CT, 40 (85.1%) participated in all 6 weeks of treatment. Five participants withdrew from CT, and 2 withdrew from PST. Reported reasons for withdrawal included work or other commitments, lack of motivation, or lack of time. No further participants were lost to follow-up.
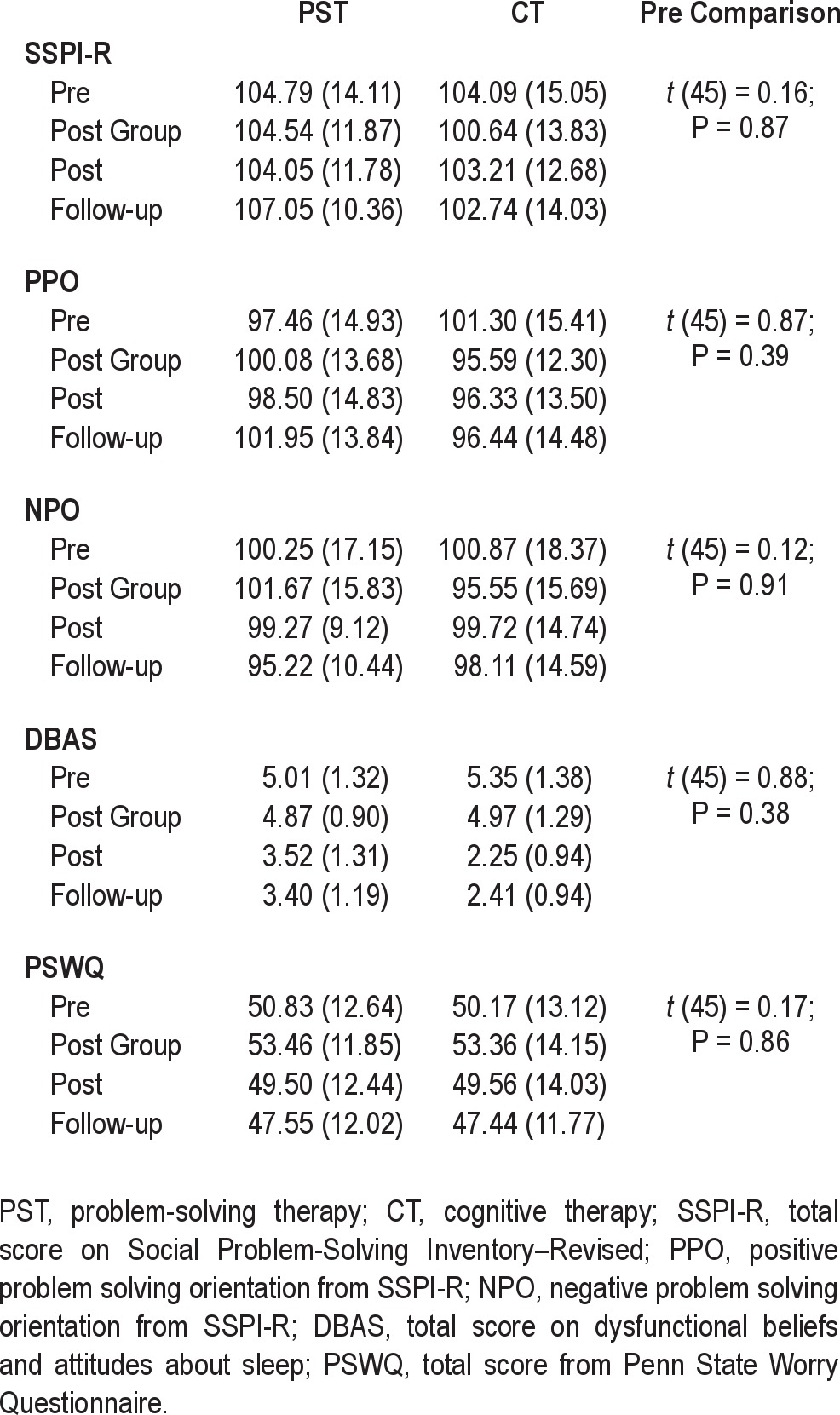
Descriptive data for each sleep outcome at each assessment for each therapy are presented in Table 2. Participants in the 2 treatments did not differ significantly on pre-treatment primary sleep outcomes (SE, ISI, PSQI) or on sleep diary scores (SOL, WAKE, WASO, TSTS, MDE/AL). Table 3 presents descriptive information for each secondary outcome (SPSI-R, PPO, NPO, PSWQ, DBAStot) at each assessment for the 2 treatments. There were no significant between treatment differences on any of the secondary outcomes before treatment.
Table 2.
Means (SD) for each sleep outcome measure by group for each assessment and significance of difference between groups prior to intervention
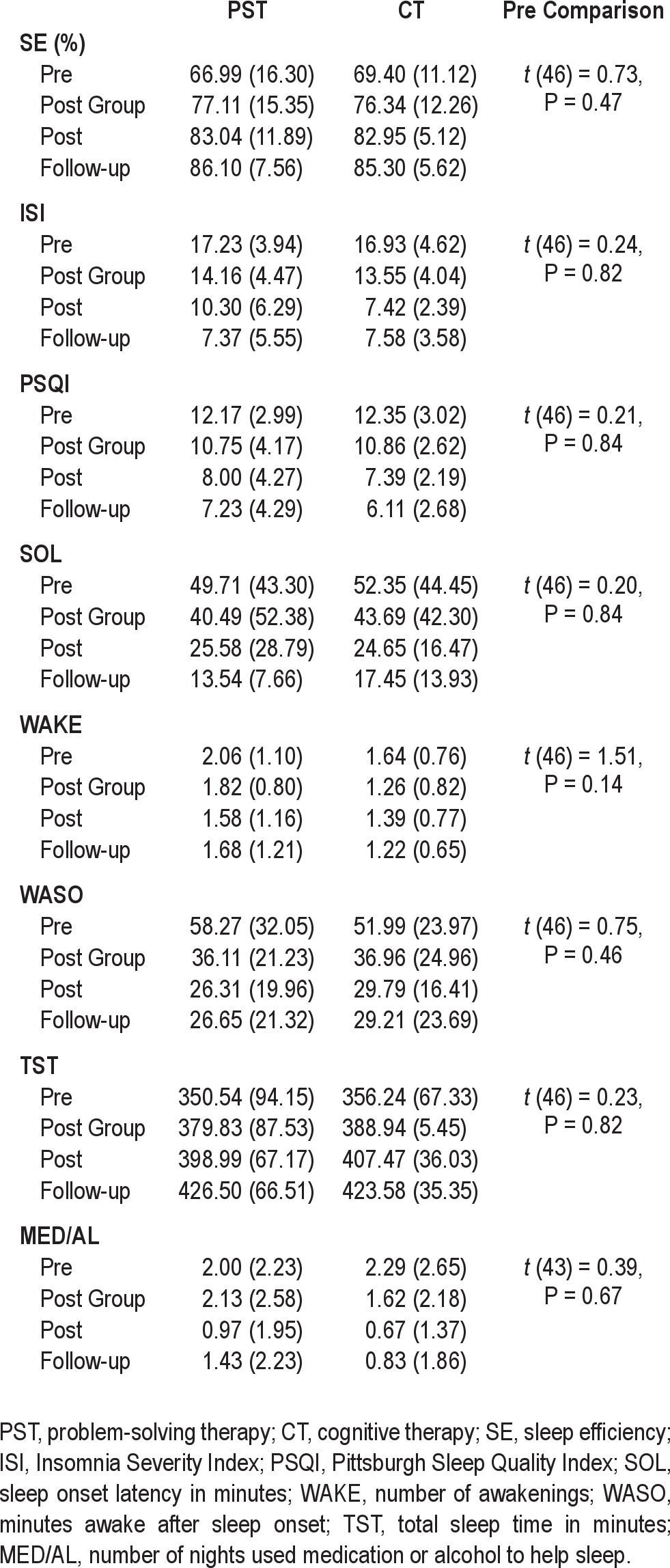
Table 3.
Means (SD) for each secondary outcome measure by group for each assessment and significance of difference between groups prior to intervention
Treatment Consistency Check
Figure 2 presents the mean number of sleep, problem solving and cognitive strategies identified by participants in each treatment for each individual session. There were no differences between the treatments in the number of behavioral strategies received by participants overall or for any of the sessions. Participants in the problem-solving treatment reported receiving significantly more problem-solving strategies than participants in the cognitive therapy treatment, both overall and for each session. Correspondingly, participants in the cognitive therapy treatment reported receiving significantly more cognitive therapy strategies than participants in the problem-solving treatment both overall and for each session.
Figure 2.
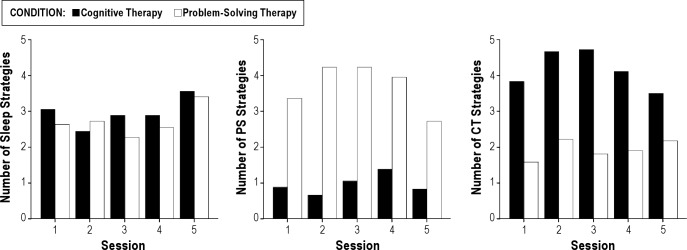
Mean number of behavioral sleep, problem solving (PS), and cognitive therapy (CT) strategies endorsed by participants in each group for each individual therapy session.
For the sessions reviewed by the judge, χ2 analysis was performed on the distribution of strategies used across treatment sessions to test for treatment consistency. For both treatments, a similar number of general sleep strategies were used, and the treatments did not differ significantly in the total number of strategies utilized. A 2 × 2 χ2 analysis on the treatment specific strategies across treatments showed that each treatment used strategies consistent with PST or CT (χ2 = 45.20, P < 0.001), signifying minimal contamination across groups and appropriate adherence to the treatments.
Main Outcomes
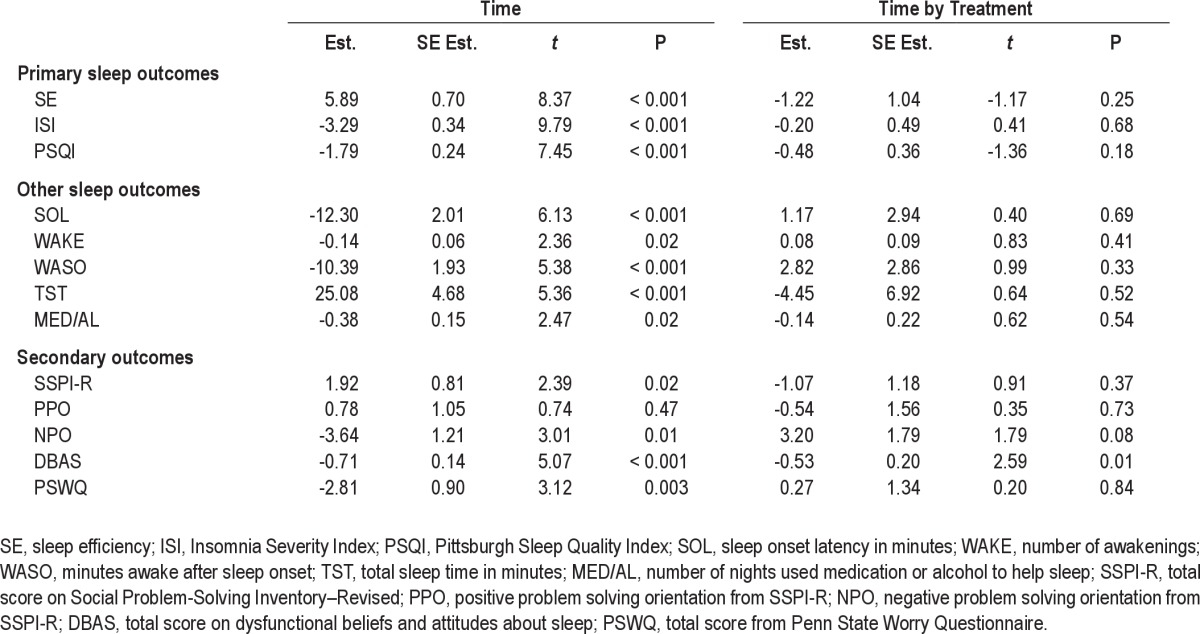
Table 4 presents the estimates from the regression model for time and time × treatment interactions for all the outcomes. For sleep outcomes, time includes 4 assessments (pre, post group, post, follow-up), while for secondary outcomes, 3 points are used (post group, post, follow-up).
Table 4.
Mixed effects regression estimates (Est.), standard error of estimate (SE Est.), t, and P value for time and time by treatment interaction for all sleep and secondary outcomes
Sleep Outcomes
There was significant effect for time for the 3 primary sleep outcomes (SE, ISI, PSQI), indicating that participants reported significant improvements in sleep efficiency (SE) and sleep quality (PSQI) and significant reduction in insomnia severity (ISI). The regression estimates indicate that from pre-treatment to follow-up an average participant had a 17.7% improvement in sleep efficiency, almost a 10-point reduction in insomnia severity on the ISIS, and 5.4-point improvement in self-rated sleep quality on the PSQI. These effects did not vary between the PST and CT, with non-significant time × treatment interactions effects for the 3 primary outcomes.
A similar pattern of result was found for the individual sleep outcomes from the sleep diary. There were significant effects of time for SOL, WAKE, WASO, TST, and MED/AL, indicating overall positive treatment effects on all sleep measures, but no differences between PST and CT on the rate of improvement in these outcomes.
The analysis using only participants who completed the 6 sessions showed an identical pattern of results with significant improvements over time (for SE, Est. = 5.90, P < 0.001; ISI, Est. = 3.26, P < 0.001; PSQI, Est. = 1.79, P < 0.001), no between treatment difference (SE, Est. = 4.83, P = -0.25; ISI, Est. = -0.02, P = 0.99; PSQI, Est. = -0.93, P = 0.47), and no differential effect for time for each treatment (SE, Est. = -1.46, P = 0.18; ISI, Est. = -0.15, P = 0.75; PSQI, Est. = -0.49, P = 0.18).
Categorical Outcomes
Based on the SE criterion of ≥ 85%, 21 CT participants and 22 PST participants had clinical insomnia on entry to the trial. In the CT condition, 33% of participants at post and 56% at follow-up no longer met this criterion. In the PST condition, 59% no longer met this criterion post-therapy and 54% at follow-up. Chi-Square analysis revealed no signifi-cant differences between the treatments in relation to the proportion of responders on the SE criterion at post (χ2 (1) = 2.63, P = 0.11) or follow-up (χ2 (1) = 0.01, P = 0.95). Based on ISI criteria, 16 CT participants had pre-intervention scores classed as clinical insomnia (≥ 15), whereas 21 PST participants exceeded this threshold. In the CT treatment, 100% of participants were classed as recovered at post and 95% at follow-up. In the PST treatment, ISI scores reflecting recovery were found for 82% of participants post-therapy and 87% at follow-up. Chi-square analyses comparing the number of responders across treatments revealed no significant differences at post (χ2 (2) = 5.87, P = 0.12) or one-month follow-up (χ2 (2) = 2.26, P = 0.32).
We calculated pre to follow-up effect sizes using mean difference and standardized mean difference (Cohen d) for each treatment separately in order to estimate the relative size of the treatment benefits and compare the results with benchmark effect sizes of CBT-I from previous studies. For SE, the pre to follow-up effect sizes for PST was a mean improvement of 19.11% (Cohen d = 1.42; 95% CI 1.02-1.87), and for CT 15.24% (Cohen d = 1.26; 95% CI 0.81-1.65). For ISI, the respective effect sizes were a mean improvement of 9.87 points (Cohen d = 1.46; 95% CI 1.03-1.88) for PST and 9.36 points (Cohen d = 1.95; 95% CI 0.52-2.38) for CT, while for PSQI the effect sizes were a mean improvement of 4.94 points (Cohen d = 0.97; 95% CI 0.55-1.40) for PST and 6.34 points (Cohen d = 1.34; 95% CI 0.90-1.79) for the CT group.
Secondary Outcomes
Table 4 also provides the estimates from the regression analysis of the time and time by treatment interaction for the secondary outcomes. There were overall time effects for PSWQ and for the SSPI-R, NPO, and DBAStot, but not for PPO, indicating significant reductions in worry, negative problem orientation, and in negative beliefs about sleep and improvements in overall problem solving skills across post-group, post-treatment, and follow-up assessments. For SSPI-R and PSWQ these effects were not related to treatment. For DBAStot, however, there was a significant time × treatment effect indicating that the rate of reduction in negative beliefs about sleep was significantly faster for CT than the PST group. There was also a notable trend for a time by treatment interaction for NPO (P = 0.08) suggesting a faster rate of decline in negative problem orientation in PST compared to CT across post group, post, and follow-up assessments.
DISCUSSION
This study compared a psychological intervention for insomnia which combined usual behavioral sleep strategies and problem solving training to a CBT intervention combining the same behavioral strategies with cognitive therapy. We found that both interventions produced clinically important benefits enhancing sleep efficiency, reducing insomnia severity and improving sleep quality. These gains were maintained for both treatments, with most participants being classed as good sleepers on the basis of sleep diary reports and greater than 80% of participants self-rating subjective recovery from insomnia at one month follow-up. The size of these treatment effects from pre-treatment to post treatment and pre-treatment to follow-up for both interventions were in keeping with effect sizes reported in previous efficacy studies of CBT-I.5,6,51–53
The results of the study also provide preliminary support for the usefulness of problem solving training as a feasible alternative component to cognitive therapy in psychological treatments for insomnia. When combined with behavioral strategies, problem solving training had equivalent dropout rates, proportion of treatment responders, degree of improvement to sleep and daytime function, and pattern of improvement over time to the CBT-I intervention combining behavioral strategies with cognitive therapy. While there were similar improvements in levels of worry for both interventions, there were changes specific to each treatment consistent with the understandings of how problem solving treatment and cognitive therapy facilitate change. The cognitive therapy intervention produced a significantly more rapid reduction in negative beliefs about sleep than the problem solving training intervention. Similarly, there was trend evidence that problem solving treatment reduced general negative beliefs about problem solving effectiveness more than the cognitive therapy treatment. Considering that both treatments produced improvements in sleep outcomes comparable to those found in previous trials of CBT-I, the differential impact on these associated outcomes for problem solving training and cognitive therapy treatment suggest that change in specific negative beliefs about sleep or about general problems solving abilities are not necessary to reduce insomnia at least at one month follow-up. Whether specific cognitive change is required to maintain improvements in the longer term is unclear.
This relatively short follow-up period is an acknowledged limitation to conclusions from the current study. In addition, because we did not predict a superiority of one treatment over the other, the size of the sample may have not have been large enough to detect a real difference between the treatments in their impact on the sleep outcomes. We attempted to strengthen inferences about outcome equivalence by including a number of measures to ensure the two interventions provided to participants were distinct so that any real differences in effect on sleep should have been realized in the study. In particular, both interventions were fully manualized from existing versions of problem solving treatment and cognitive therapy, the therapist was supervised in the delivery of each intervention by an experienced clinician including supervisory observations of sessions, the results from patient endorsement and blind independent judge's assessment demonstrated fidelity of the individual sessions to the specific intervention components, as well as distinctiveness of the interventions delivered in the individual sessions. In addition, both the intention to treat outcomes and the per-protocol analysis indicated no differences between the interventions in the extent of the benefits to the participants. The decision to only compare PST combined with behavioral strategies to a version of the recommended psychological interventions for insomnia (CBT-I) makes it difficult to rule out the alternative conclusion that the improvements in both groups were solely due to the behavioral sleep strategies. While we accept this criticism of the study, the tested and recommended CBT-I protocols include a cognitive therapy component. In addition, our results include specific impacts for each treatment on negative beliefs about sleep (CT) or about problem solving self-efficacy (PST) consistent with the putative mechanism by which the two interventions effect change, suggesting both are active treatment components. Whether the PST or CT components enhance the efficacy of the behavioral components, however, remains an untested question.
In view of these limitations, the current results should be viewed as preliminary and conclusions from them as tentative at this point. If its efficacy is equivalent to CT, it is arguable that PST confers several advantages over CT as a component of psychological treatment for insomnia. As PST is a psychological skills-based intervention aimed at treating a range of stress-related conditions, it may be particularly useful for the treatment of insomnia presenting with common comorbidities such as generalized anxiety disorder, depression, chronic pain, and other medical illness, which may account for 40% to 75% of insomnia presentations.54–57 PST may also aid the application of behavioral interventions, with its focus on building a positive problem orientation, motivation to approach problems constructively, and self-efficacy for solving problems successfully. Addressing negative problem orientation seems particularly important in populations of chronic insomnia sufferers, as it has been clinically noted that this population commonly become resigned to beliefs regarding the permanence of their problems.58 PST may also be administered in a general practice context, as recent evidence shows the effectiveness of PST in treating emotional disorders, particularly depression, when administered by general or nurse practitioners,59,60 and its use for this purpose is currently advocated in Australia.61 This might address key issues surrounding the current lack of accessible treatments for insomnia, which is particularly pertinent to the dissemination of CBT-I. Given that PST is focused on building broader rational and positive coping skills, the potential benefit for relapse prevention and maintenance of functional coping when faced with stress in the longer-term is worth noting.
CONCLUSION
Insomnia is a stress-related disruption to sleep, perpetuated by cognitive and physiological hyperarousal and associated learning. Interventions targeting problem solving have been shown to give an additive benefit to existing behavioral treatments. The present study adds preliminary evidence demonstrating the efficacy of behavioral strategies and problem-solving treatment for insomnia when directly compared to behavioral strategies and cognitive therapy. It would be productive for future research to further examine problem-solving interventions and their application to insomnia, in particular, to explore the role and improvement of negative problem orientation.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded by an Australian Postgraduate Award and the Research School of Psychology, The Australian National University. Australian New Zealand Clinical Trials Registry No. ACTRN12610000123044. The authors have indicated no financial conflicts of interest. The first author undertook this research in partial fulfillment of a Doctorate in Psychology at The Australian National University.
ACKNOWLEDGMENTS
The authors thank all participants who took part in the trial and The Australian National University for supporting the research.
ABBREVIATIONS
- CBT
cognitive behavior therapy
- CT
cognitive therapy
- DBAS
Dysfunctional Beliefs and Attitudes about Sleep Scale
- DSISD
Duke Structured Interview for Sleep Disorders
- ISI
Insomnia Severity Index
- MINI
Mini International Neuropsychiatric Interview
- PSQI
Pittsburgh Sleep Quality Index
- PSWQ
Penn State Worry Questionnaire
- PST
problem-solving therapy
- SE
sleep efficiency
- SOL
sleep onset latency
- SPSI- R:S
Social Problem-Solving Inventory–Revised: Short-form
- TST
total sleep time
- WAKE
number of awakenings
- WASO
wake after sleep onset
REFERENCES
- 1.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine Report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 2.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 3.Unbehaun T, et al. Management of insomnia: Update and new approaches. Nat Sci Sleep. 2010;2:127–38. doi: 10.2147/nss.s6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dautovich ND, McNamara J, Williams JM. Tackling sleeplessness: Psychological treatment options for insomnia. Nat Sci Sleep. 2010;2:23–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia: An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–56. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 6.Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9:24–34. [Google Scholar]
- 7.Harvey AG. A cognitive theory and therapy for chronic insomnia. J Cogn Psychother. 2005;19:41–59. [Google Scholar]
- 8.Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65(Suppl 16):33–40. [PubMed] [Google Scholar]
- 9.Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. New York: Springer; 2003. [Google Scholar]
- 10.Jansson-Fröjmark M, Linton SJ. The role of sleep-related beliefs to improvement in early cognitive behavioural therapy for insomnia. Cogn Behav Ther. 2008;37:5–13. doi: 10.1080/16506070801907013. [DOI] [PubMed] [Google Scholar]
- 11.Spielman AJ, Glovinsky PB. In: The varied nature of insomnia: in Case Studies In Insomnia. Hauri P.J., editor. New York: Plenum Publishing Corporation; 1991. pp. 1–15. [Google Scholar]
- 12.Spielman AJ. Assessment of insomnia. Clin Psychol Rev. 1986;6:11–25. [Google Scholar]
- 13.Harvey AG, Tang NK, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25:593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002 Aug;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AG, Greenall E. Catastrophic worry in primary insomnia. J Behav Ther Exp Psychiatry. 2003;34:11–23. doi: 10.1016/s0005-7916(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 17.Jansson M, Linton SJ. The development of insomnia within the first year: A focus on worry. Br J Health Psychol. 2006;11(Pt 3):501–11. doi: 10.1348/135910705X57412. [DOI] [PubMed] [Google Scholar]
- 18.Levey AB, et al. Articulatory suppression and the treatment of insomnia. Behav Res Ther. 1991;29:85–9. doi: 10.1016/s0005-7967(09)80010-7. [DOI] [PubMed] [Google Scholar]
- 19.Haynes SN, Adams A, Franzen M. The effects of presleep stress on sleep-onset insomnia. J Abnorm Psychol. 1981;90:601–6. doi: 10.1037//0021-843x.90.6.601. [DOI] [PubMed] [Google Scholar]
- 20.Harvey AG, Payne S. The management of unwanted pre-sleep thoughts in insomnia: distraction with imagery versus general distraction. Behav Res Ther. 2002;40:267–77. doi: 10.1016/s0005-7967(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 21.Harvey AG. Trouble in Bed: The Role of Pre-Sleep Worry and Intrusions in the Maintenance of Insomnia. J Cogn Psychother. 2002;16:161–77. [Google Scholar]
- 22.Gross RT, Borkovec TD. Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther. 1982;13:112–6. [Google Scholar]
- 23.Tang NKY, Harvey AG. Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behav Res Ther. 2004;42:27–39. doi: 10.1016/s0005-7967(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 24.Ansfield ME, Wegner DM, Bowser R. Ironic effects of sleep urgency. Behav Res Ther. 1996;34:523–31. doi: 10.1016/0005-7967(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 25.Borkovec TD, et al. Preliminary exploration of worry: Some characteristics and processes. Behav Res Ther. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- 26.Dugas MJ, et al. Worry and problem solving: Evidence of a specific relationship. Cognit Ther Res. 1995;19:109–20. [Google Scholar]
- 27.Carney CE, Edinger JD. Identifying critical beliefs about sleep in primary insomnia. Sleep. 2006;29:444–53. [PubMed] [Google Scholar]
- 28.Harvey AG, Farrell C. The efficacy of a Pennebaker-like writing intervention for poor sleepers. Behav Sleep Med. 2003;1:115–24. doi: 10.1207/S15402010BSM0102_4. [DOI] [PubMed] [Google Scholar]
- 29.Espie CA, Lindsay WR. Cognitive strategies for the management of severe sleep-maintenance insomnia: A preliminary investigation. Behavioural Psychotherapy. 1987;15:388–95. [Google Scholar]
- 30.Mooney P, Espie CA, Broomfield NM. An experimental assessment of a Pennebaker writing intervention in primary insomnia. Behav Sleep Med. 2009;7:99–105. doi: 10.1080/15402000902762386. [DOI] [PubMed] [Google Scholar]
- 31.Carney CE, Waters WF. Effects of a structured problem-solving procedure on pre-sleep cognitive arousal in college students with insomnia. Behav Sleep Med. 2006;4:13–28. doi: 10.1207/s15402010bsm0401_2. [DOI] [PubMed] [Google Scholar]
- 32.Edinger JD, Carney CE. Overcoming Insomnia: A Cognitive-Behavioral Therapy Approach. New York: Oxford University Press; 2008. [Google Scholar]
- 33.Jansson-Fröjmark M, Lind M, Sunnhed R. Don't worry, be constructive: A randomized controlled feasibility study comparing behaviour therapy singly and combined with constructive worry for insomnia. Br J Clin Psychol. 2012;51:142–57. doi: 10.1111/j.2044-8260.2011.02018.x. [DOI] [PubMed] [Google Scholar]
- 34.Malouff JM, Thorsteinsson EB, Schutte NS. The efficacy of problem solving therapy in reducing mental and physical health problems: A meta-analysis. Clin Psychol Rev. 2007;27:46–57. doi: 10.1016/j.cpr.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 35.D'Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Positive Approach to Clinical Intervention. 3rd ed. New York: Springer Publishing Company; 2007. [Google Scholar]
- 36.Edinger JD, Wyatt JK, Olsen MK, et al. Reliability and validity of the Duke structured interview for sleep disorders for insomnia screening. Sleep. 2009;32:A265. Abstract Supplement. [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 38.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 39.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as a clinical outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 42.D'Zurilla TJ, Nezu AM, Maydeu-Olivares A. The social problem-solving inventory-revised (SPSI-R): Technical manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 2002. [Google Scholar]
- 43.D'Zurilla TJ, Nezu AM. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990 Jun;Volume 2(issue 2):156–63. [Google Scholar]
- 44.Morin CM, Valliéres A, Ivers H. Dysfunctional Beliefs and Attitudes about Sleep (DBAS): Validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morin CM. Dysfunctional beliefs and attitudes about sleep: Preliminary scale development and description. Behav Ther. 1994;17:163–4. [Google Scholar]
- 46.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 47.Bastien CH, Morin CM, Ouellet MC, et al. Cognitive behavioural therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72:653–9. doi: 10.1037/0022-006X.72.4.653. [DOI] [PubMed] [Google Scholar]
- 48.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York, NY: Springer; 2008. [Google Scholar]
- 49.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;7:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 51.Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364:1959–73. doi: 10.1016/S0140-6736(04)17480-1. [DOI] [PubMed] [Google Scholar]
- 52.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 53.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 54.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 55.Harvey AG. Insomnia: Symptom or diagnosis? Clin Psychol Rev. 2001;21:1037–59. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 56.Mai E, Buysse DJ. Insomnia: Prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3:167–74. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth T. Insomnia: Definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(Supplement):S7. [PMC free article] [PubMed] [Google Scholar]
- 58.Collier E, Skitt G, Cutts H. A study on the experience of insomnia in a psychiatric inpatient population. J Psychiatr Ment Health Nurs. 2003;10:697–704. doi: 10.1046/j.1365-2850.2003.00654.x. [DOI] [PubMed] [Google Scholar]
- 59.Hassink-Franke LJ, van Weel-Baumgarten EM, Wierda E, et al. Effectiveness of problem-solving treatment by general practice registrars for patients with emotional symptoms. J Prim Health Care. 2011;3:181–9. [PubMed] [Google Scholar]
- 60.Huibers MJ, Beurskens AJ, Bleijenberg G, van Schayck CP. The effectiveness of psychosocial interventions delivered by general practitioners. Cochrane Database Syst Rev. 2003;(2):CD003494. doi: 10.1002/14651858.CD003494. [DOI] [PubMed] [Google Scholar]
- 61.Pierce D, Gunn J. Using problem-solving therapy in general practice. Aust Fam Physician. 2007;36:230–3. [PubMed] [Google Scholar]


