Abstract
Study Objectives:
The present study aimed to investigate the effects of treadmill exercise on sleep deprivation (S-D)-induced impairment of hippocampal dependent long-term memory, late phase long-term potentiation (L-LTP) and its signaling cascade in the cornu ammonis 1 (CA1) area.
Experimental Design:
Animals were conditioned to run on treadmills for 4 weeks then deprived of sleep for 24 h using the columns-in-water method. We tested the effect of exercise and/or S-D on behavioral performance using a post-learning paradigm in the radial arm water maze (RAWM) and in vivo extracellular recording in the CA1 area. The levels of L-LTP-related molecules in the CA1 area were then assessed both before and after L-LTP induction.
Measurements and Results:
After 24 h of S-D, spatial long-term memory impairment in the RAWM and L-LTP suppression was prevented by 4 weeks of regular exercise. Regular exercise also restored the S-D-associated decreases in the basal levels of key signaling molecules such as: calcium/calmodulin kinase IV (CaMKIV), mitogen-activated protein kinase (MAPK/ERK), phosphorylated cAMP response element-binding protein (P-CREB) and brain derived neurotrophic factor (BDNF), in the CA1 area. After L-LTP induction, regular exercise also prevented the S-D-induced down regulation of BDNF and P-CREB protein levels.
Conclusions:
The results suggest that our exercise protocol may prevent 24-h S-D-induced impairments in long-term memory and LTP by preventing deleterious changes in the basal and post-stimulation levels of P-CREB and BDNF associated with S-D.
Citation:
Zagaar M; Dao A; Levine A; Alhaider I; Alkadhi K. Regular exercise prevents sleep deprivation associated impairment of long-term memory and synaptic plasticity in the CA1 area of the hippocampus. SLEEP 2013;36(5):751-761.
Keywords: Sleep deprivation, forced exercise, LTP, RAWM, BDNF, CREB, ERK1/2
INTRODUCTION
Sleep contributes significantly to the process of memory and neuronal plasticity.1,2 In fact, adequate sleep is essential for fostering connections among neuronal networks for memory consolidation in certain brain areas. The hippocampus is especially sensitive to sleep,3 with its widespread functional connections to the cortex and its ability to modulate large-scale network activity across different brain regions.4 There are studies showing that sleep can increase hippocampus dependent memory,5 while hippocampal activity during sleep has been reported to increase after a learning task.6 Much of the current evidence supporting a role for sleep in memory consolidation is from sleep deprivation (S-D) studies, which show that suppression of sleep can negatively impact memory formation in rodents7–9 and humans.10 In particular, S-D seems to impair the ability to retain new information and disrupt memory consolidation to a great extent.3,11–13 In support of this, our lab has shown that 24-h S-D has an especially detrimental effect, which manifests as impaired short-term spatial memory and suppressed expression of hippocampal early long-term potentiation (LTP) and key molecules of its signaling cascade.7
An understanding of hippocampal electrophysiology is essential in assessing the functional integrity of neural pathways on the cellular level. Specifically, the detrimental effect of S-D and how it can be prevented is thought to lie in the hippocampus's unique functional arrangement and its role in spatiotemporal coding of information.14 The pyramidal cells in the CA1 area of the hippocampus are especially unique in that they seem to code for spatial arrangement in rats.15 Hippocampal neurons communicate with each other to form networks, which are arranged in functionally related circuits.16 As experimental models are concerned, long-term potentiation is generally considered the closest cellular model for storing new information within these hippocampal neuronal networks.17 In the CA1 area of the hippocampus, there are two major phases of LTP that require NMDA receptor activation by glutamate, early (E-LTP) and late (L-LTP), which correlate with short-term and long-term memory, respectively.18,19 Typically, multiple tetanic stimulation is required to induce L-LTP and consists of 3-4 trains of high frequency stimulation, so as to produce a more massive and focused Ca2+ influx into the post-synaptic membrane. Similar to E-LTP, L-LTP is subserved by its own distinct kinase cascade including calcium/calmodulin kinase IV (CaMKIV), and mitogen-activated protein kinase (MAPK/ERK), which phosphorylate cAMP response element-binding protein (CREB).20–22 Active (phosphorylated) CREB stimulates the expression of target genes including brain derived neurotrophic factor (BDNF), which through tyrosine kinase B (TrkB) receptor signaling is able to cycle back and regulate CREB activity.20–22
Even though research has elucidated much of the underlying physiology of sleep and its role in cognitive function, the impact that environmental intervention such as physical activity has on S-D-induced cognitive impairment remains elusive. Accumulating evidence has shown that physical activity (e.g., aerobic exercise) exerts beneficial effects on neuronal function in humans as well as laboratory animals.23,24 In particular, exercise has been shown to exert positive effects on cognitive function most often in the presence of brain injury or disease-induced impairments including stroke, Alzheimer disease and Parkinson disease.24,26,27 Our lab has recently shown that 4 weeks of tread-mill exercise prevents S-D-induced impairment in spatial learning and short-term memory and maintains E-LTP expression and its associated signaling cascade in the CA1 area of the hippocampus.7 Recent studies have revealed that exercise-induced adaptations in skeletal muscle can initiate various signaling mechanisms that can lead to physiologic changes in both muscle and neuronal networks. Indeed, it is now accepted that the nervous system has evolved feedback mechanisms with peripheral structures such as muscles or joints to ultimately influence brain function and adaptability.28,29 The functional consequences of these adaptations in the hippocampus can be manipulated by the type, amount, and duration of exercise.30,31 For instance, regular aerobic exercise leads to (1) improved performance in spatial memory tasks,32 (2) increased endogenous neurotrophin levels (e.g., brain derived neurotrophic factor [BDNF]),33,34 which in turn, can enhance hippocampal plasticity and its signaling cascade. However, despite the beneficial effects that exercise has on physical and mental health, only 32.5% of the US population actually perform 30 min of exercise daily.
Given the opposing effects of S-D and exercise on cognitive function, it is reasonable to suggest that preconditioning the brain with regular exercise could prevent or attenuate the deleterious effects of S-D on learning and memory. This is especially pertinent, as the long-term effects of exercise on S-D-induced learning and memory impairment have not been fully investigated. The present series of experiments was designed to test whether 4 weeks of treadmill exercise in rats can prevent the cognitive impairment associated with S-D.
MATERIALS AND METHODS
We used 45- to 48-day-old male Wistar rats (Charles River Laboratories, Wilmington, MA), weighing 176-200 g at the beginning of all experiments. Rats were assigned into 4 groups: sedentary, exercise, sedentary/sleep deprived, and exercise/ sleep deprived. Water and food were available ad libitum and animals were kept on a 12h/12h light-dark cycle. Prior to electrophysiological recording, the rats were anesthetized with urethane (1.2 g/kg) and later euthanized in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. All animal manipulations were done in accordance with the National Research Council's Guide for The Care and Use of Laboratory Animals and on approval of The University of Houston Institutional Animal Care and Use Committee.
Exercise Protocol
Rats ran on Eco 3/6 Treadmills (Columbus Instruments, USA) at 0° inclination Monday through Friday during the light cycle between 09:00 and 14:30 for a total of 4 weeks using the protocol outlined as described in Zagaar, et al.7 Rats were familiarized with the treadmills the day before the first day of exercise to reduce the impact of any environmental stress, as described in our previous report. All rats were observed while exercising to ensure they ran throughout the exercise sessions and to monitor for any signs of confounders, such as pain or exhaustion. The rats were offered water at the end of each exercise session.
Sleep Deprivation
The modified multiple platform (columns-in-water) technique was utilized to sleep deprive rats as described.7,35 This model is attractive when testing cognitive outcomes because it is relatively free of isolation stress, immobility stress, and forced activity. Briefly, the model consisted of a large aquarium with 20 platforms 5 cm in diameter that rose 1 cm above the water surface. The rats could move freely from one platform to another and balance on the platform until they began REM sleep with its inherent muscle paralysis, which would cause the rats to contact the water and awaken. In this model, cage mates (4-6 rats) were placed together in the aquarium to maintain social stability. The rats had free access to clean water bottles and food pellets baskets hanging from the top of the aquarium.
Radial Arm Water Maze (RAWM)
The RAWM is a behavioral water maze that is a cross between the radial arm maze and the Morris water maze.7 All behavioral experiments were carried out in a dimly lit room with visual cues placed on the surrounding walls. Briefly, after assigning a rat a specific goal arm, the rat was allowed 1 minute to locate the hidden platform, which was submerged about 1 cm under the water in their corresponding goal arm. Errors were tallied (up to 7) if a rat swam more than halfway into an arm other than the goal arm or if a rat entered more than half of the goal arm but failed to approach the platform. If the rat failed to locate the platform within the time allotted, the rat was gently guided to the platform and scored with 7 errors. Once the rat reached the platform, it was allowed 10-15 sec sitting time on the platform before the next trial began. To test the long-term memory, we used a post-learning sleep deprivation protocol, where all 4 groups first went through the 2 sets of 6 learning trials (trials #1-12). After the end of the 12th trial, we sleep deprived the rats in the sleep deprivation and exercise/sleep deprived groups for 24 h; immediately after that, we subjected the rats to long-term memory test.
Electrophysiology
Standard procedures for in vivo recording from area CA1 of anesthetized rats were performed as previously described.7,9 A new set of all 4 groups were anesthetized with urethane (1.2 g/kg) (Sigma Aldrich) and placed in a stereotaxic frame (nose bar at 0.0), after which 2 holes were drilled for placing of electrodes. The rats were grounded through a subcutaneous wire to prevent noise in the recording.
For recording from area CA1, a concentric bipolar stimulating electrode was placed in the CA3 subregion of the left hippocampus at a 5° angle toward the midline to stimulate the Schaffer collaterals/commissural pathway, which connects the CA3 subregion with the area CA1 of hippocampus. A glass capillary recording electrode filled with 1% fast green in 2 M NaCl, was placed in the area CA1 of the right hippocampus.
The stimulating electrode was connected to a stimulator through an isolation unit whereas the recording electrode was connected to an amplifier (Axoclamp 2A, USA). After proper positioning of both electrodes, a maximum population spike (pspike; postsynaptic response) was obtained by stimulating the left CA3 and recording in the right CA1 and was subsequently allowed to stabilize for 30 min without stimulation. After obtaining a stable baseline by giving a test stimulus every 30 s for 20 min, we administered multiple high frequency stimulation (MHFS) trains to the Schaffer collaterals/commissural pathway to evoke L-LTP. The specific MHFS protocol used in these experiments consisted of 4 trains with 2.5-min intervals between the trains; each train consisted of 8 pulses (400 Hz) given every 10 sec for a period of 30 seconds. Evoked population spikes were recorded from the CA1 of the right hippocampus for 5 h after MHFS.7
Hippocampus Dissection
Immediately after rats were sacrificed under urethane anesthesia, brain dissection was performed on a dry ice-filled Petri dish with the filter paper soaked with 0.2M sucrose for the basal, unstimulated (no LTP induction), and stimulated (LTP induction) experiments. In basal dissection, the right hippo-campus of each rat was removed and separated into the CA1 and DG areas.7 With unstimulated and stimulated dissections we considered the temporal part as an internal control, since most of the stimulation went into the septal portion of the same right hippocampus.36 Therefore, the temporal side was considered as unstimulated and used as an internal control in the same animal. Accordingly, the right CA1 area of the hippocampus was removed; the 2 left and right tips were trimmed off, and the small part in the middle of the hippocampus (the CA3 area) was discarded to minimize overlapping of areas. Then the septal and temporal sides were separated and stored at -80°C for later processing.
Homogenization and Immunoblotting
Preparation of tissue for immunoblot analysis was carried out as reported.7,37 Briefly, tissue samples were homogenized in 200 μL of lysis buffer cocktail containing protease and phosphatase inhibitors by using polytron homogenizer PRO250 (PRO Scientific, Oxford, CT) at a medium speed, then sonicated using an ultrasonicator (Vibra cell, Sonics & Materials Inc., Newtown, CT). Total protein was estimated by Micro BCA assay (Pierce Chemical Rockford, IL), after which homogenates were subjected to SDS-PAGE using the high-throughput E-PAGE 48 Protein Electrophoresis System (Invitrogen Corp). The same quantity of protein (20-25 μg) from each homogenate was added to 2.5 μL E-PAGE loading buffer and 1 μL NuPAGE reducing agent, then heated at 70°C for 10 min. The samples were then loaded onto the pre-cast E-PAGE 48 wells gel. The proteins were transferred to PVDF membranes through the dry iBlot gel transfer system (Invitrogen Corp.). To measure the levels of a protein, we incubated with polyclonal or monoclonal primary antibodies as outlined below, and the immunoreactive bands were detected by a horseradish peroxidase-conjugated secondary antibody. Subsequently, the blots were developed using chemiluminescence reagent, detected in an Alpha Innotech imaging system, and quantified by densitometry.
We used the following antibodies in this study: rabbit polyclonal antibody (anti P-CREB; Millipore, MA; 1:1000 dilution and anti-CREB; Santa Cruz Biotechnology, CA; 1:100 dilution), rabbit polyclonal antibody for BDNF (Santa Cruz Bio-technology, CA; 1:500 dilution), for CAMKIV (Cell Signaling Technology, MA; 1:1000 dilution) and for P/total-MAPK/ ERK Rabbit monoclonal antibody (Cell Signaling Technology, MA; 1:1000. Mouse and rabbit polyclonal antibody for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Millipore, Temacula, CA; 1:2000 dilutions) was used to detect GAPDH (loading control) protein level, and the band intensities of all signaling molecules were expressed as a ratio to the GAPDH intensity.
Statistics
All of the groups were compared using one-way analysis of variance (ANOVA), followed by the Tukey post hoc test. P values < 0.05 were considered significant, as reported previously.7 All values were represented as mean ± standard error of the mean (SEM). Analyses were conducted using GraphPad Prism (5.0).
RESULTS
Behavioral Experiments
Regular exercise prevents spatial long-term memory deficit associated with sleep deprivation.
To test long-term memory, we used a post-learning long-term memory protocol as described,9,38 where all 4 groups went through the 2 blocks of 6 learning trials. Immediately after the end of the 12th trial, the sleep deprived and exercised/sleep-deprived rat groups were placed in the modified multiple platform aquarium for 24 h. Immediately after that, we subjected the rats to the long-term memory test.
Twenty-four hours of S-D after the end of trial number 12, the sleep-deprived group committed significantly (F3,42 = 19.65, P < 0.001) more errors (5 ± 0.37) in finding the hidden platform than the control group (1.4 ± 0.40; Figure 1) indicating marked impairment of long-term memory. In contrast, the exercised/ sleep deprived group showed a significantly reduced number of errors (P < 0.001; 2.1 ± 0.35) compared to the sleep-deprived group but was similar to control (Figure 1). However, in normal rats, exercise produced no significant difference in long-term memory performance in the RAWM compared to control rats.
Figure 1.
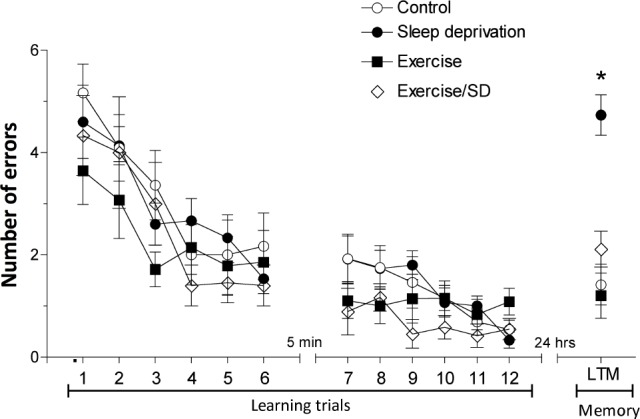
Effect of regular exercise on S-D-impaired long-term memory. Without S-D, all groups performed similarly during learning phase. Following 24-h S-D, the sleep-deprived rats showed more errors in finding the hidden platform than control rats. Regular exercise prevented long-term memory impairment induced by 24-h S-D. Each point is the mean ± SEM of 12-15 rats. *Significant difference (P < 0.001) from all other groups.
Electrophysiological Experiments
Regular exercise prevents sleep deprivation-induced impairment of late phase long-term potentiation (L-LTP) in the CA1 area.
The late phase of LTP is said to be a cellular correlate of long-term memory. To support our behavioral data, in which regular exercise prevented long-term memory deficit induced by 24-h S-D, we determined the impact of 4 weeks of regular exercise on the magnitude of L-LTP decline in sleep-deprived rats.
Multiple high frequency stimulation (MHFS) of the Schaffer-collaterals pathway evoked L-LTP as a marked increase in both fEPSP slope and pspike amplitude that were maintained for ≥ 5 h. However, in the sleep deprived group, after an initial relatively small increase in fEPSP slope and pspike amplitude they gradually declined toward baseline at 5 h (fEPSP = 98.87% ± 9.29% and pspike = 117.2% ± 12% of baseline; Figure 2). In contrast, 5 h after applying MHFS the exercised/sleep deprived group showed a sustained increase (P < 0.001) in the fEPSP slope (133.1% ± 5.93% of baseline) and pspike amplitude (189.6% ± 17.8% of baseline) similar to those of the exercised (fEPSP = 141.2% ± 17.8% and pspike = 220.3% ± 9.9% of baseline) and control groups (fEPSP = 143.37% ± 8.25% and pspike = 192% ± 23.84% of baseline, respectively Figure 2A-B). It is important to note that although the fEPSP slope and pspike amplitude values in the sleep-deprived group were slightly increased after MHFS, they were nevertheless consistently and significantly decreased compared with all other groups. The finding that the sleep-deprived group showed sustained impairment of L-LTP expression seems to correlate with our behavioral results that S-D impaired long-term memory in the RAWM.
Figure 2.
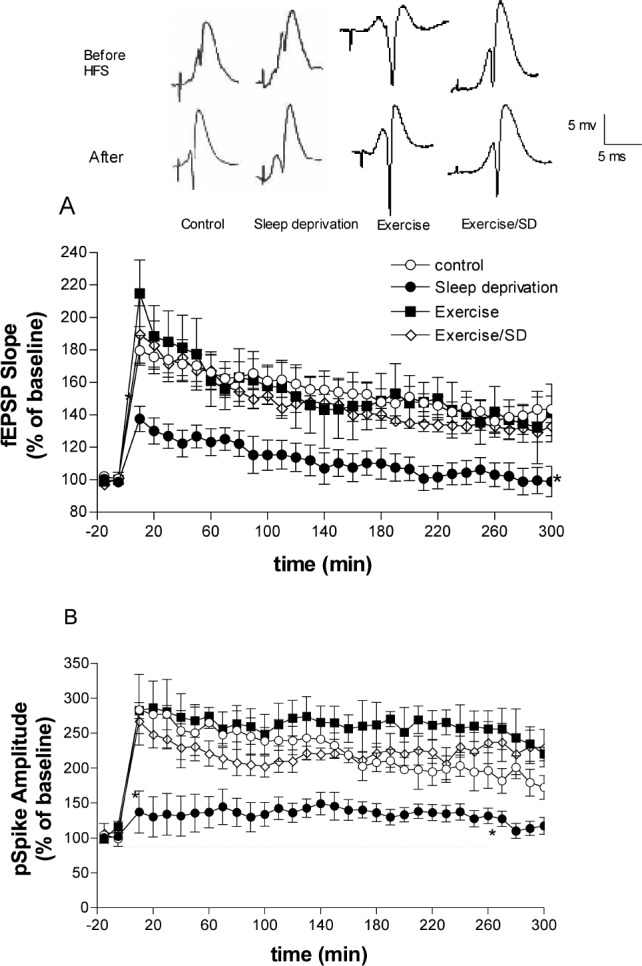
Late-phase long term potentiation (L-LTP) in the CA1 area of the hippocampus evoked by MHFS applied at time zero to the Schaffer collateral/commissural pathway of anesthetized rats. L-LTP was measured as increases in the slope of fEPSP and amplitude of pspike expressed as percentage of the baseline values (before MHFS). (A) fEPSP slope values in the sleep-deprived rats are significantly lower than those of the other groups at all time points after applying MHFS, but regular exercise prevents fEPSP slope suppression. (B) Regular exercise prevents the S-D-induced reduction in pspike amplitude. *All points between are significantly different from all groups (P < 0.05-0.01, n = 4-8 rats/group). Insets are representative experiments; calibrations, 5 mV/5 ms, apply to all traces.
Basal levels of L-LTP-related signaling molecules
It is widely accepted that cAMP response element binding protein (CREB) and CaMKIV are the key molecules implicated in long-term memory and L-LTP. In this section, we examined the effects of S-D and/or regular exercise on the protein levels of CREB and CaMKIV. The immunoblot method was used to measure the levels of CREB and CaMKIV in the total homogenate of CA1 area.
Basal levels of P-CREB and total-CREB in the CA1 area
Manipulation of CREB produces deleterious alterations in LLTP.20,39–41 Sleep loss for as little as 8 h reduces the gene expression of CREB.42 In the present study, we detected a significant reduction in the basal levels of P-CREB (P < 0.05-0.001; 0.32 ± 0.04; Figure 3A) and total CREB (P < 0.01-0.001; 0.4 ± 0.08; Figure 3B) in the CA1 area of the sleep-deprived group compared with all other groups. The S-D-induced decrease in both P-CREB and total-CREB levels was prevented with regular exercise, as these levels were significantly (P < 0.001) elevated in the exercised/sleep-deprived group (1.1 ± 0.21; 1.17 ± 0.01, respectively) compared to sleep-deprived rats (Figure 3 A, B). It is important to note that although the levels of P-CREB and total-CREB were increased in both exercised (1.13 ± 0.14; 1.24 ± 0.11, respectively) and exercised/sleep-deprived groups compared to control, this difference was significant (P < 0.05) only for P-CREB in the exercised group. The ratio of P-CREB: total-CREB (Figure 3C) was not significantly different among the groups.
Figure 3.
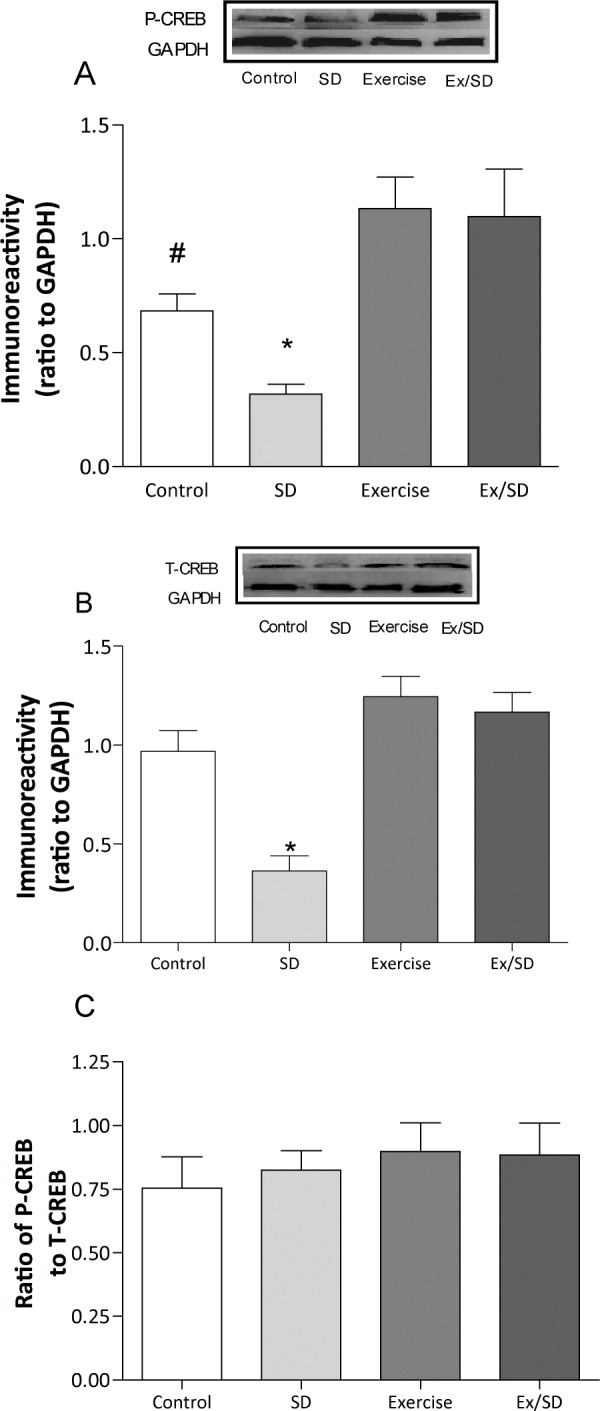
Regular exercise prevented the S-D-induced reduction in the basal levels of P-CREB (A) and total CREB (B). There were no significant changes in the ratio P-CREB: total CREB (C). *Significant difference from all other groups (P < 0.01-0.001). #Significant difference from all groups except exercise/sleep-deprived rats (P < 0.05). Values are mean ± SEM; n = 4-7/group. Insets are representative blots.
Basal levels of calcium/calmodulin-dependent protein kinase IV (CaMKIV)
CaMKIV is involved in the activation of CREB during the expression of L-LTP.43,44 In this study, the sleep-deprived group showed significantly (P < 0.05) reduced basal levels of CaMKIV (0.84 ± 0.1) compared to all other groups. However, the S-D-induced reduction in the basal levels of CaMKIV was not seen in the exercised/sleep-deprived group (1.62 ± 0.23; P < 0.05; Figure 4). Exercise did not seem to significantly elevate CaMKIV levels compared to that of the control group.
Figure 4.
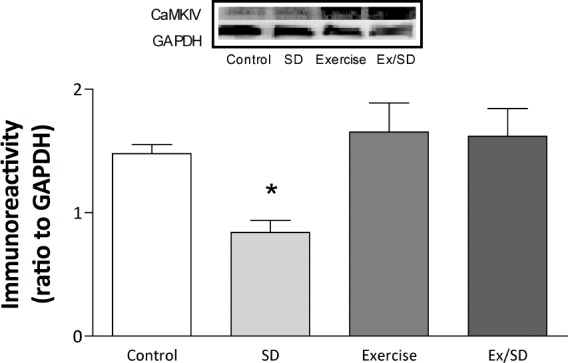
Regular exercise prevented S-D-induced decreases in the basal levels of CaMKIV in hippocampal CA1 area, measured in the total homogenate. Values are mean ± SEM; n = 5-6/group. *Significant difference from all groups (P < 0.05-0.01). Insets are representative blots.
Basal levels of P- and total mitogen activated kinase (MAPK/ERK)
The MAPK/ERK pathway is activated by learning and is instrumental in long-term memory consolidation due to its activation of CREB.45 As a key modulator of synaptic plasticity and its underlying cascade, MAPK is influenced by many active molecules ranging from phosphatases such as calcineurin and neurotrophins such as BDNF.46
The basal protein levels of active MAPK/ERK (P-MAPK/ ERK) in the sleep-deprived group (0.7 ± 0.1) were not significantly different from the control group (1.0 ± 0.2). However, P-MAPK/ERK levels were significantly higher in exercised/ sleep-deprived (1.69 ± 0.12) and exercised (1.9 ± 0.1) animals compared to both the control and sleep-deprived groups (P < 0.05-0.001; Figure 5A). Moreover, the basal protein levels of total-MAPK in the control (1.64 ± 0.18) and sleep-deprived (1.39 ± 0.19) groups were significantly (P < 0.05-0.01) lower than the exercised (2.7 ± 0.11) and exercised/sleep-deprived groups (2.5 ± 0.28; Figure 5B).
Figure 5.
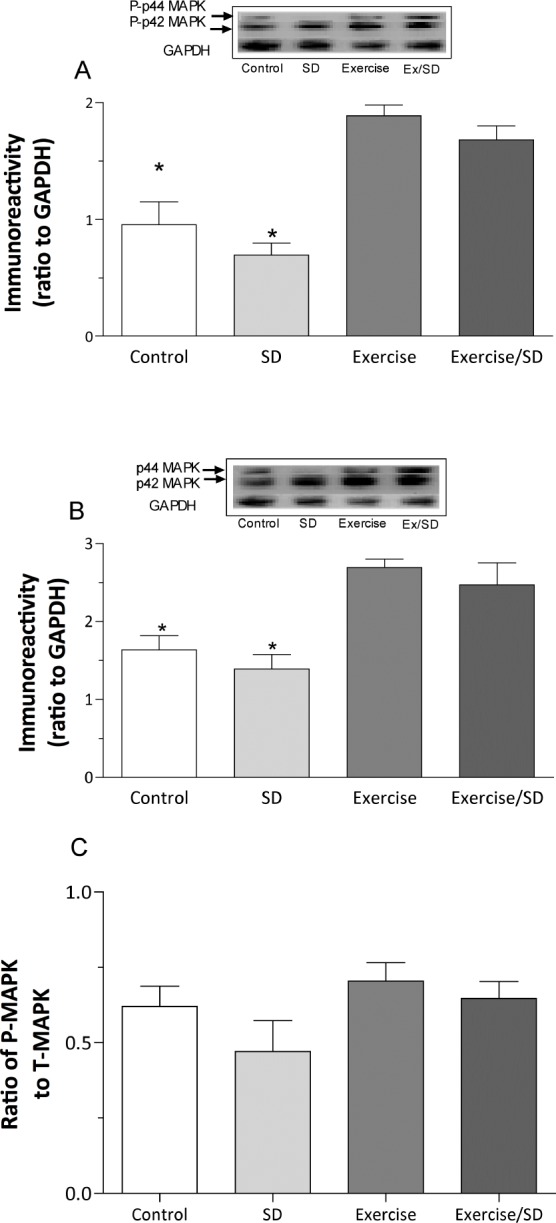
Regular exercised animals had significantly elevated basal levels of P-MAPK/ERK (A) and total MAPK/ERK (B) above that of sleep-deprived and control animals in hippocampal CA1 area, measured in the total homogenate. (C) Although no significant differences were seen in the P-MAPK: T-MAPK ratio, the ratio was slightly reduced in the sleep-deprived group compared to other groups. *Significant difference from exercised and exercised/sleep-deprived groups only (P < 0.05-0.001). Values are mean ± SEM; n = 4-7. Insets are representative blots.
Levels of signaling molecules during the expression of L-LTP in CA1 area
In order to ascertain the changes in L-LTP-related molecules that could be responsible for the protective effect of exercises on L-LTP impairment in sleep deprived rats in the CA1 area, we determined the protein levels of P-CREB, total-CREB, CaMKIV, and BDNF 5 h after induction of L-LTP.
Levels of P-CREB and total-CREB during L-LTP
Phosphorylation and activation of CREB is essential for L-LTP and long-term memory.41 Five hours after the induction of L-LTP in the CA1 area by MHFS, the levels of PCREB were increased in all groups except in the stimulated (S)-sleep-deprived group (0.8 ± 0.14; Figure 6A). However, during the same period after MHFS the exercised/sleep-deprived group showed significantly increased P-CREB levels (1.61 ± 0.18; P < 0.05-0.01) compared to unstimulated control and the S-sleep-deprived groups, in effect preventing the S-D-induced suppression of activated CREB (Figure 6A). Similarly, both S-control (1.5 ± 0.12) and S-exercised (1.68 ± 0.23) groups also showed significantly (P < 0.05) increased P-CREB levels compared to unstimulated (basal) control (0.99 ± 0.05). The protein levels of total-CREB in all stimulated groups were similarly increased compared to the unstimulated control group (P < 0.05-0.01; Figure 6B). The ratio of P-CREB: total CREB (Figure 6C) was significantly (P < 0.01) reduced in the sleep-deprived group compared to all other groups, which along with the reduced P-CREB levels we observed, may suggest that S-D negatively alters the phosphorylation process of CREB. Furthermore, these results suggest that regular exercise prevents the S-D-induced inhibition of CREB phosphorylation.
Figure 6.
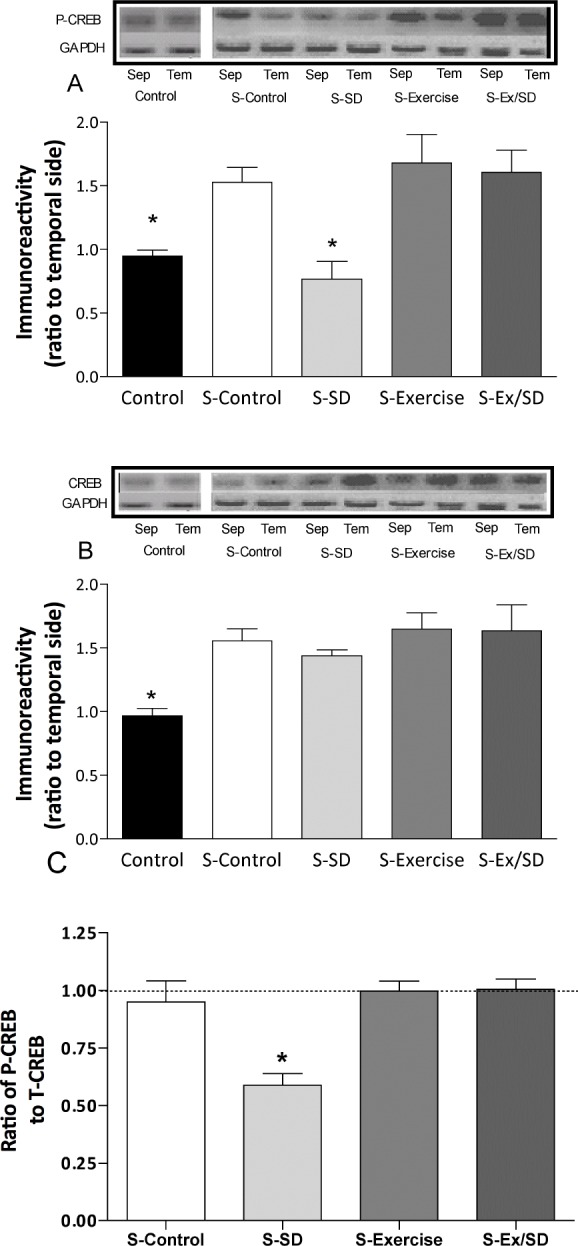
Effect of regular exercise and/or S-D on the levels of P- and total-CREB in the hippocampal CA1 area after L-LTP expressed as ratio of septal side to temporal side. (A) MHFS stimulation of the Schaffer collaterals synapses in the hippocampus caused a significant increase in the levels of P-CREB in the CA1 of all stimulated groups except the S-sleep-deprived animals. (B) The levels of CREB in all stimulated groups were significantly increased compared to the un-stimulated control group. (C) The ratio of P/T-CREB was significantly reduced in sleep-deprived rats. In order to reduce individual variations, the temporal side of the same hippocampus was used as an “internal control.” Protein levels in the septal side were normalized as a percentage of those of the temporal side. *Significant difference from all other stimulated groups (P < 0.05-0.01, n = 6-8 rats/group). Insets are bands from representative blots.
Levels of CaMKIV during L-LTP
Five hours after the induction of L-LTP in the CA1 area by MHFS, all stimulated groups showed a trend toward increased levels of CaMKIV compared to the unstimulated control group, although this was to a lesser extent in the sleep-deprived group (Figure 7). After MHFS both exercised/sleep deprived and exercised (1.33 ± 0.2 and 1.29 ± 0.2, respectively) groups showed slight increases in CaMKIV levels compared to the S-sleep deprived group. These findings may suggest regular exercise and/or S-D do not substantially modulate protein expression of CaMKIV during L-LTP in the CA1 area.
Figure 7.
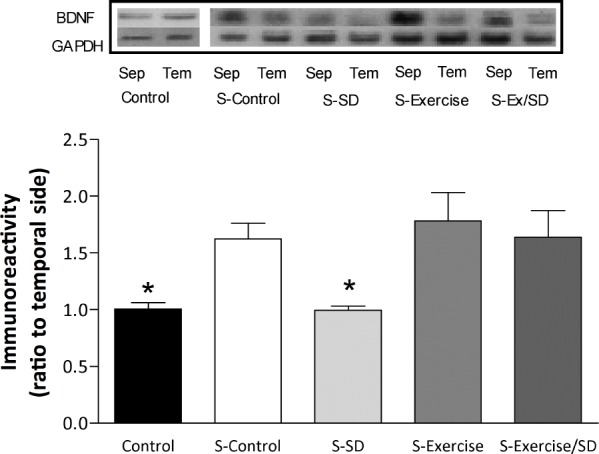
Effect of regular exercise and/or S-D on the levels of CaMKIV in the hippocampal CA1 area after L-LTP expressed as ratio of septal side to temporal side. The levels of CaMKIV in all stimulated groups were slightly (not significant) increased compared to the unstimulated control group. The temporal side serves as an “internal control” for the septal side of the hippocampus (n = 4-7 rats/group). Insets are bands from representative blots.
Levels of BDNF during L-LTP
The BDNF gene, which is a target of CREB, plays an essential role in the persistence of long-term memory.45 In the current study, MHFS failed to produce an increase in BDNF levels in S-sleep-deprived rats (1.07 ± 0.07) compared to unstimulated control (Figure 8). However, in exercised/sleep-deprived rats, levels of BDNF were markedly increased (approximately 64%, 1.64 ± 0.24; P < 0.05) compared to unstimulated control. Similar increases were seen in the S-control (62.4%; 1.6 ± 0.14; P < 0.05) and S-exercised (78%; 1.78 ± 0.25; P < 0.01) groups (Figure 8). In addition, the exercised and exercised/sleep-deprived groups showed significantly (P < 0.01) higher BDNF protein levels than S-sleep-deprived animals, suggesting that regular exercise can normalize the activity-dependent regulation of BDNF expression in the CA1 area.
Figure 8.
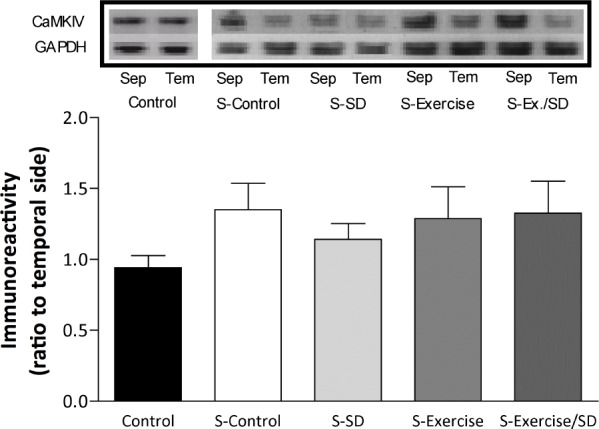
BDNF levels after L-LTP in the CA1 area of the hippocampus expressed as ratio of septal side to temporal side. The levels of BDNF after MHFS are significantly increased in all stimulated groups except the sleep-deprived group compared to the unstimulated control group. *Significant difference compared to all stimulated groups (P < 0.05-0.01; n = 5-7 rats/group). Insets are bands from representative blots.
DISCUSSION
Although numerous studies have shown that physical activity and S-D seemingly produce opposite effects on cognitive function and synaptic plasticity, the combined effect of exercise and S-D on hippocampus-dependent learning and memory has not been fully investigated. In this study, we tested the effect of regular treadmill exercise on S-D-induced long-term memory impairment using three experimental approaches: behavioral, electrophysiological, and molecular. Our behavioral assessment (i.e., RAWM task) revealed that 4 weeks of regular tread-mill exercise could protect against S-D-induced impairments in the long-term memory test. Moreover, regular exercise prevented the S-D-induced deficit of CA1 L-LTP, believed to be a cellular correlate of long-term memory. In correlation with this, analysis of the levels of signaling molecules by western blot indicated that regular exercise prevented the decrease in the basal protein levels of BDNF, CREB, and CAMKIV in the CA1 area of sleep-deprived rats. Additionally, the failure of MHFS to increase the levels of these molecules in the sleep-deprived group is prevented by regular treadmill exercise.
Sleep deprivation in its various forms has been shown to adversely affect the ability to retain new information and disrupt memory consolidation.3,11,12 The harmful effect of sleep loss on cognition has been demonstrated using various models. In the current study, we sleep-deprived rats using the modified multiple platform model, which depends on the loss of muscle tone during REM sleep. This model produces a marked decrease (90% to 95%) in rapid eye movement (REM) sleep but can also affect NREM sleep, as has been confirmed in studies using electroencephalographic recording to monitor sleep-deprived subjects.48–50 In addition to our own, many S-D studies support our current findings showing that suppression of certain sleep stages negatively impacts different types of memory independent of any possible stressors inherent to the aquarium environment such as social anxiety, or humidity.7–9
Nevertheless, a perfect control is not always possible in S-D studies; thus one of the better ways to control for the environment using our technique is to use wide platforms. The rats on these wider platforms (12 cm in diameter) are subjected to the same environment but allowed to sleep relatively undisturbed. Previous experiments have shown that the cognitive ability of rats kept on the wide platforms in the aquarium for 24 h is not significantly different than that of the home cage control rats.37 Our lab has also shown that stress fundamentally affects the CA1 differently than the DG. For instance, psychosocial stress or hypothyroidism impairs hippocampal-dependent LTP in area CA1 but not in the DG area,51–53 whereas S-D affects LTP in both the CA1 and DG areas of the hippocampus.37,54 In agreement, one study looking at plasma levels of corticosterone, a common surrogate marker of stress, showed that rats tested for 72 h on wide platforms did not have elevated corticosterone compared to home cage control rats.55 Also, other studies show that sleep depriving socially stable cage mates in the sleep deprivation tank results in decreased levels of stress hormones (such as plasma corticosterone and ACTH) compared to socially unstable groups.56 Altogether, these findings may indicate that memory impairment seen in rats sleep deprived using the columns-in-water method is actually from prolonged wakefulness as opposed to any nonspecific stress resulting from being in the aquarium environment.
In the current study, we used the RAWM to evaluate spatial long-term memory performance. Although the RAWM is a cross between the radial arm maze (RAM) and the Morris water maze (MWM), the RAWM removes the disadvantages of food deprivation and odors associated with the RAM apparatus and eliminates the issue of thigmotaxis with the MWM by confining the animal to a preestablished route.57,58 Reports have consistently shown that the RAWM task is sensitive to certain treatments and conditions that negatively affect hippo-campal dependent learning and memory such as hypothyroidism,59 epilepsy,60 chronic stress,52 and a combination of stress and β-amyloid peptides.61,62 To this extent, our current findings show that 24-h S-D post training impairs long-term memory in the RAWM task, which is consistent with previous reports.8,9 However, we uniquely report that 4 weeks of regular exercise prevents the 24-h S-D-induced impairment of long-term memory as tested in the RAWM. Similarly, many behavioral studies have shown improved retention in hippocampus dependent tasks after moderate exercise including: object recognition,63 MWM,64 RAM,65 RAWM,7,30 as well as in hippocampus independent anxiety-related behaviors, such as the elevated plus maze66 and open field apparatus.35 However, in normal rats, exercise produced no significant difference in long-term memory performance in the RAWM compared to control rats, which may indicate that regular exercise does not affect normal memory but instead protects against S-D-induced impairment.
Hippocampal LTP is considered a useful model for information processing and storage among neuronal networks as the expression properties of LTP in an animal seem to share similar characteristics with those of learning and memory.17 Accumulating evidence demonstrates that LTP as well as memory are impaired during aging,58 chronic stress,52 Alzheimer disease,61 and with pharmacological blockade of receptors and molecules involved in synaptic plasticity.67 More significantly, there is growing evidence that confirms the detrimental impact of S-D on hippocampal LTP.9,68 Our current electrophysiological investigations show that S-D causes significant impairment of L-LTP in the pyramidal cells of area CA1 in anaesthetized rats. These findings are consistent with previous reports showing S-D induced impairment across different phases of LTP expression (i.e., E-LTP and L-LTP).9,37,69,70 However, in the current study we showed that regular exercise, which has been shown to modulate synaptic plasticity,7,71 prevents 24-h S-D-induced impairment of L-LTP. This finding correlates well with our behavioral results showing the same exercise regimen prevented long-term memory impairment induced by the same period of sleep loss. However, it is important to note that some studies show no benefit with exercise on LTP expression in the CA1 area.64 The possibility remains that exercise induced enhancement of LTP may only manifest when the threshold for LTP induction is already impaired at baseline such as in aged27,72 or S-D animals.7
The negative impact that S-D has on L-LTP and synaptic plasticity is thought to be a product of the underlying deleterious changes in intracellular signaling molecules that regulate expression and function of glutamate receptors including NMDA68 and AMPA receptors.73 For instance, molecular studies have shown that the expression of key signaling proteins and trophic factors (e.g., CREB, BDNF) involved in LTP and memory are impaired in the hippocampus after 8, 24, and 48 hours of S-D.37,42 This is consistent with our present results in the CA1 area, where 24-hour S-D caused a deficit in certain L-LTP signaling molecules (e.g., CaMKIV and P-CREB and T-CREB), which was prevented by regular exercise. There is growing consensus regarding how moderate exercise can exert a positive influence on synaptic plasticity, possibly through modulating its signaling cascade.
A key molecule in this cascade is CREB, a transcription factor involved in the regulation of cAMP response element (CRE) containing genes and also a major enabler of activity-dependent protein synthesis that is synapse-specific. Accumulating evidence has shown that long-term memory and expression of L-LTP requires CREB activation74–76 and that many signaling cascades converge on CREB.77 In the present study, we found a significant trend toward decreased basal protein levels of total-CREB and P-CREB compared to exercise groups in the CA1 of sleep-deprived rats. Additionally, regular exercise not only prevented S-D-induced decreases in the levels of total-CREB and P-CREB in the hippocampus but slightly enhanced their levels in normal rats as well. Coupled with the fact that the PCREB: total CREB ratio was largely unchanged, it is likely that regular exercise induces P-CREB up-regulation by increasing the availability of overall CREB that can be phosphorylated by upstream signaling kinases (e.g., MAPK, PKA, CaMKIV) in the CA1.
Additonally, both CaMKIV and MAPK/ERK are implicated in long-term memory and LTP by virtue of their ability to activate CREB.78–80 Although our results showed reduced basal protein levels of CaMKIV in the CA1 of the sleep deprived group, this was not the case with P- and total-MAPK/ERK, which remained similar to control. This finding may signify that reduced CaMKIV availability in sleep-deprived rats is a key factor in deranged CREB phosphorylation. In contrast, regular exercise normalized the levels of CaMKIV and enhanced the levels of MAPK/ERK in sleep-deprived rats, which supports the notion that exercise could contribute to normal CREB phosphorylation and intact L-LTP in this brain region through increased availability of these molecules at baseline. As for MAPK/ERK, it is possible that 24 hours S-D hinders the MAPK phosphorylation process and decreases the availability of activated MAPK due to increased protein phosphatase activity or decreased levels of other key activators such as BDNF, both of which are negatively altered during S-D.11,81 However, the fact that the levels of P- and total-MAPK/ERK were largely unchanged in control and sleep-deprived rats could further suggest that the MAPK/ ERK pathway may not be particularly influenced by 24-h S-D as much as it is by our exercise regimen.
The role of BDNF is significant because it can influence synaptic plasticity by raising the efficiency of synaptic transmission through activation of different protein kinases (e.g., CaMKII) and CREB.82–84 Much attention has been given to BDNF as a major mechanism behind the benefit of exercise because it has the ability to increase BDNF availability, which through its TrkB receptor can subsequently increase levels of downstream signaling molecules involved in L-LTP including P-CREB, CaMKIV, and P-MAPK/ERK.21,77,84 Previous reports indicate basal protein expression of BDNF is markedly decreased after 24 h of S-D,37,42 whereas our moderate exercise regimen was able to prevent this down regulation of BDNF expression in the CA1.7 Furthermore, the increased availability of these molecules at baseline seems to overlap with the enhanced basal synaptic transmission we had previously reported in the CA1 area with the same exercise regimen.7 Therefore, it is possible that the enhanced basal synaptic transmission in the exercised/ sleep-deprived rats would provide the necessary synaptic machinery and signaling molecules to normalize LTP expression.
During L-LTP expression, these same signaling molecules are integral in translating the initial influx of Ca2+ through the NMDA channel, into persistent changes in synaptic plasticity.85 Our present results show that L-LTP expression in sleep-deprived rats was impaired, possibly through failure to increase the levels of P-CREB and related molecules. The levels of total-CREB were increased in all stimulated groups compared to un-stimulated control, although the magnitude of the increase was slightly less in sedentary normal and sleep-deprived rats. The inability of MHFS to increase the phosphorylation of CREB could be responsible for the impairment of L-LTP and long-term memory observed in sleep-deprived rats. Furthermore, 24 hours of S-D decreased the P-CREB: total CREB ratio in sleep-deprived rats five hours after MHFS compared to all other groups, which along with the reduced P-CREB levels we observed may suggest that S-D negatively alters the phosphorylation process of CREB. Interestingly, the levels of CaMKIV in the CA1 were similarly increased across all groups after MHFS, which is consistent with other reports.9,44 This may imply that exercise does not influence the activity-induced expression of CaMKIV as much as its basal expression in the CA1.
Similarly, five hours after MHFS, the protein levels of BDNF were increased in the CA1 area of all stimulated groups with the exception of the stimulated sleep-deprived group, which indicated that regular exercise prevented the S-D-induced decrease in BDNF availability. We previously reported similar findings during E-LTP, where BDNF expression was up-regulated in both exercised groups compared to the unstimulated control and sleep deprived groups, although to a lesser extent.7 It is well known that BDNF activates CREB-dependent protein synthesis, while the BDNF gene itself is considered to be a target of CREB-induced transcription.83 As a consequence, BDNF synthesis can be stimulated upon CREB activation during L-LTP, which supports our findings that the levels of BDNF and CREB were elevated in parallel in both exercised and exercised/sleep-deprived rats compared to those in both stimulated sleep-deprived and unstimulated control rats in the CA1. Therefore, the failure of MHFS to enhance the BDNF levels in sleep-deprived rats and the significantly higher levels of BDNF in stimulated exercised/sleep-deprived rats may suggest that regular exercise exerts a protective effect on L-LTP expression mechanisms in the presence of S-D in the CA1 area. This finding could support the view that regular exercise develops the synaptic machinery through BDNF modulation of CaMKII and CREB to increase the resilience of synapses86,87 during the fatigue of prolonged wakefulness.
Although the protective mechanism of regular exercise against S-D-induced impairment of spatial memory and LTP is still unclear, our result may indicate that prior regular exercise can prevent the S-D-induced impaired phosphorylation process of CREB by up-regulating BDNF and activating the BDNF-TrkB signaling cascades. This is plausible as pharmacological blockade of BDNF-TrkB signaling abolishes exercise-enhanced memory performance in rodents.81 Exercise, like BDNF, has been found to induce the expression of a wide array of plasticity-related genes,7,25,71,87 which may prime synapses structurally and functionally to facilitate activity dependent plasticity in the presence of sleep deprivation-induced insult.7 In this regard, it is conceivable that exercise may increase the availability of growth factors such as BDNF, which could go on to facilitate synaptic plasticity by increasing levels of subsequent rate-limiting kinases (i.e., CaMKII) as well as regulators (e.g., MAPK/ERK, CaMKIV) to more effectively activate CREB related gene expression. Indeed, the fact that regular exercise can induce sustained increases in P-CREB and MAPK/ ERK that last well beyond the end of exercise is consistent with how BDNF levels can also remain elevated for weeks after exercise.30,71 Also, the fact that the P-CREB:T-CREB ratio in the exercised/sleep-deprived group are similar to those of control further suggests that exercise-induced molecular changes may help to maintain the production of phosphorylated CREB during LTP and positively influence memory and synaptic plasticity. Similar to our previous findings, we did not find that our exercise regimen produced an enhancement in long-term memory or L-LTP induction in normal animals.7 These results may be due to differences in the intensity and duration of exercise, stage of memory tested, or the experimental protocol. Certainly, the possibility remains that the RAWM task in this study is sensitive to S-D-induced impairments in spatial learning and memory rather than enhancements due to exercise. Our findings are also supported by human studies that show moderate aerobic exercise regimens with a duration ranging between 20 and 60 min improves cognitive processes and allows for optimal performance of simple and complex behavioral tasks in young adults.88
In conclusion, we provide behavioral, electrophysiological and molecular evidence that 4 weeks of treadmill exercise prevents 24-h S-D-induced impairment in hippocampal dependent long-term memory L-LTP expression and its relevant signaling molecules. The protective effect of exercise at the cellular level may be attributed to its ability to promote the production of BDNF and prevent the S-D-induced decrease in rate-limiting signaling molecules such as P-CREB before and during expression of LTP. Thus, it is possible that moderate exercise can set the stage for activity dependent plasticity by conditioning and fine-tuning synaptic sites at the molecular level to be more susceptible to learning or LTP.
DISCLOSURE STATEMENT
This was not an industry supported study. This work supported by a grant from the University of Houston. The authors have indicated no financial conflicts of interest.
AUTHOR AFFILIATION CHANGE
Dr. Alhaider's present affillation is College of Clinical Pharmacy, King Faisal University, Al-Hofuf, Kingdom of Saudi Arabia.
REFERENCES
- 1.Blissitt PA. Sleep, memory, and learning. J Neurosci Nurs. 2001;33:208–15. doi: 10.1097/01376517-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 3.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 4.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neo-cortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–9. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai DJ, Shuman T, Gorman MR, Sage JR, Anagnostaras SG. Sleep selectively enhances hippocampus-dependent memory in mice. Behav Neurosci. 2009;123:713–19. doi: 10.1037/a0016415. [DOI] [PubMed] [Google Scholar]
- 6.Gais S, Albouy G, Boly M, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–83. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zagaar M, Alhaider I, Dao A, et al. The beneficial effects of regular exercise on cognition in REM sleep deprivation: Behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;45:1153–62. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Aleisa AM, Helal G, Alhaider IA, et al. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- 9.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci. 2011;46:742–51. doi: 10.1016/j.mcn.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64:934–46. doi: 10.1007/s00018-007-6457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 12.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang RH, Hu SJ, Wang Y, Zhang WB, Luo WJ, Chen JY. Paradoxical sleep deprivation impairs spatial learning and affects membrane excitability and mitochondrial protein in the hippocampus. Brain Res. 2008;1230:224–32. doi: 10.1016/j.brainres.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Huxter JR, Senior TJ, Allen K, Csicsvari J. Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat Neurosci. 2008;11:587–94. doi: 10.1038/nn.2106. [DOI] [PubMed] [Google Scholar]
- 15.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh AR. Towards a network theory of cognition. Neural Netw. 2000;13:861–70. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 17.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 19.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 20.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 21.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 23.Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14:2564–71. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- 24.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3:S30–37. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil. 1999;80:661–7. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2011;33:828. e1–828.e17. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2009;30:493–503. doi: 10.1007/s10571-009-9488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–17. doi: 10.1111/j.1365-2796.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 30.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–65. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 32.Grace L, Hescham S, Kellaway LA, Bugarith K, Russell VA. Effect of exercise on learning and memory in a rat model of developmental stress. Metab Brain Dis. 2009;24:643–57. doi: 10.1007/s11011-009-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khabour OF, Alzoubi KH, Alomari MA, Alzubi MA. Changes in spatial memory and BDNF expression to concurrent dietary restriction and voluntary exercise. Hippocampus. 2009;20:637–45. doi: 10.1002/hipo.20657. [DOI] [PubMed] [Google Scholar]
- 34.Kim SE, Ko IG, Kim BK, et al. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–65. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Vollert C, Zagaar M, Hovatta I, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: Potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Papatheodoropoulos C, Kostopoulos G. Decreased ability of rat temporal hippocampal CA1 region to produce long-term potentiation. Neurosci Lett. 2000;279:177–80. doi: 10.1016/s0304-3940(99)01002-2. [DOI] [PubMed] [Google Scholar]
- 37.Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep. 2010a;33:437–44. doi: 10.1093/sleep/33.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleisa AM, Alzoubi KH, Alkadhi KA. Post-learning REM sleep deprivation impairs long-term memory: Reversal by acute nicotine treatment. Neurosci Lett. 2011;499:28–31. doi: 10.1016/j.neulet.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Alarcon JM, Malleret G, Touzani K, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–59. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Barco A, Patterson S, Alarcon JM, et al. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–37. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 42.Guzman-Marin R, Ying Z, Suntsova N, et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–19. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–14. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 44.Tokuda M, Ahmed BY, Lu YF, et al. Involvement of calmodulin-dependent protein kinases-I and -IV in long-term potentiation. Brain research. 1997;755:162–6. doi: 10.1016/s0006-8993(97)00189-3. [DOI] [PubMed] [Google Scholar]
- 45.Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–33. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–44. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bekinschtein P, Cammarota M, Katche C, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–16. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–27. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Mohammed HS, Aboul Ezz HS, Khadrawy YA, Noor NA. Neurochemical and electrophysiological changes induced by paradoxical sleep deprivation in rats. Behav Brain Res. 2011;225:39–46. doi: 10.1016/j.bbr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Nicotine blocks stress-induced impairment of spatial memory and long-term potentiation of the hippocampal CA1 region. Int J Neuropsychopharmacol. 2006;9:417–26. doi: 10.1017/S1461145705005912. [DOI] [PubMed] [Google Scholar]
- 52.Gerges NZ, Stringer JL, Alkadhi KA. Combination of hypothyroidism and stress abolishes early LTP in the CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res. 2001;922:250–60. doi: 10.1016/s0006-8993(01)03181-x. [DOI] [PubMed] [Google Scholar]
- 53.Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res. 2004;155:77–84. doi: 10.1016/j.bbr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci. 2010;31:1368–76. doi: 10.1111/j.1460-9568.2010.07175.x. [DOI] [PubMed] [Google Scholar]
- 55.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc Natl Acad Sci U S A. 2006;103:19170–5. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68:309–16. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 57.Paul CM, Magda G, Abel S. Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009;203:151–64. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 58.Shukitt-Hale B, McEwen JJ, Szprengiel A, Joseph JA. Effect of age on the radial arm water maze-a test of spatial learning and memory. Neurobiol Aging. 2004;25:223–9. doi: 10.1016/s0197-4580(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 59.Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19:66–78. doi: 10.1002/hipo.20476. [DOI] [PubMed] [Google Scholar]
- 60.Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009;217:378–87. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer's disease. Biol Psychiatry. 2009;65:918–26. doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 62.Tran TT, Srivareerat M, Alkadhi KA. Chronic psychosocial stress accelerates impairment of long-term memory and late-phase long-term potentiation in an at-risk model of Alzheimer's disease. Hippocampus. 2010;21:724–32. doi: 10.1002/hipo.20790. [DOI] [PubMed] [Google Scholar]
- 63.O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE. Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int. 2006;48:9–16. doi: 10.1016/j.neuint.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood BN, Foley TE, Day HE, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDonald RJ, Hong NS, Craig LA, Holahan MR, Louis M, Muller RU. NMDA-receptor blockade by CPP impairs post-training consolidation of a rapidly acquired spatial representation in rat hippocampus. Eur J Neurosci. 2005;22:1201–13. doi: 10.1111/j.1460-9568.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 68.Ravassard P, Pachoud B, Comte JC, et al. Paradoxical REM sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/ MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–40. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–7. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 70.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 71.O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippo-campus. Hippocampus. 2009;19:1019–29. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- 72.Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, Meerlo P. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res. 2010;19:280–8. doi: 10.1111/j.1365-2869.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 73.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–78. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 74.Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–41. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 75.Tully T. Regulation of gene expression and its role in long-term memory and synaptic plasticity. Proc Natl Acad Sci U S A. 1997;94:4239–41. doi: 10.1073/pnas.94.9.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–60. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 77.Ho N, Liauw JA, Blaeser F, et al. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–72. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–83. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 79.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 80.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 81.Boulanger L, Poo MM. Gating of BDNF-induced synaptic potentiation by cAMP. Science. 1999;284:1982–84. doi: 10.1126/science.284.5422.1982. [DOI] [PubMed] [Google Scholar]
- 82.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–47. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 83.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–31. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 85.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–76. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 86.Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–30. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 87.Lin TW, Chen SJ, Huang TY, et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97:140–7. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol (Amst) 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]


