Abstract
Study Objectives:
We hypothesize that extremes of sleep duration are associated with elevated C-reactive protein (CRP), a pro-inflammatory marker for cardiovascular disease risk.
Design:
Cross-sectional.
Setting:
Population-based research.
Participants:
Nationally representative sample of 2007-2008 National Health and Nutrition Examination Survey participants (n = 5,587 adults).
Interventions:
None.
Measurements and Results:
Associations between CRP and self-reported total sleep time (TST) were examined. Explanatory models considered contributions of sex, age, race/ethnicity, body mass index (BMI), and BMI squared (BMI2). Models also explored the role of insomnia symptoms, sleep apnea, active medical illness, and antidiabetic/antihypertensive treatment. Differential patterns among race/ethnicity groups were examined using interactions and stratified analyses. Nonlinear relationships between CRP and TST were assessed using polynomial and multinomial regression models (< 5, 5, 6, 7, 8, 9, and > 9 h). Linear and squared terms were significant in all models in the complete sample, with notable differences by sex and ethnoracial group. Overall, in models adjusted for sociodemographics and BMI, different patterns were observed for non-Hispanic white (elevated CRP for < 5 h and > 9 h), black/African-American (elevated CRP for < 5 h and 8 h), Hispanic/Latino (elevated CRP for > 9 h), and Asian/ Other (higher in 9 and > 9 h and lower in 5 h and 6 h) groups. Ethnoracial groups also demonstrated patterning by sex.
Conclusion:
In a representative sample of American adults, elevated CRP was associated with extreme sleep durations. Sex, race/ethnicity, sleep disorders, and medical comorbidity influenced these associations. Differences in CRP along these dimensions should be considered in future research on sleep related disparities influencing cardiometabolic disease risk.
Citation:
Grandner MA; Buxton OM; Jackson N; Sands M; Pandey A; Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. SLEEP 2013;36(5):769-779.
Keywords: C-reactive protein, cardiovascular disease, epidemiology, inflammation, sleep duration
INTRODUCTION
The recognition of increased population prevalence of insufficient sleep duration1 has led to efforts to address this public health issue.2,3 Evidence of a pro-inflammatory role for sleep loss,4–14 and the consequential cardiovascular consequences,15–17 is being discovered. Recently, clinical observational and experimental sleep deprivation studies have shown an association between sleep loss and pro-inflammatory processes that promote the development of atherosclerotic plaques.4,11
C-reactive protein (CRP), a systemic marker of chronic, low-grade inflammation,18 is associated with sleep duration. Laboratory studies have shown that elevated CRP results from acute sleep deprivation. Epidemiologic studies have found that short10,14,19 and/or long20,21 (extreme) sleep durations are associated with elevated CRP.
Despite these findings, the literature linking CRP to sleep duration is inconsistent. In addition to a number of studies with positive findings (mentioned previously), a number of studies have found either conflicting or negative results.13,22,23 Some important limitations of these studies may explain these inconsistencies.
First, there is a strong floor effect for CRP levels in the general population. Although this can be modeled using data transformations, it still reduces variability and limits statistical power. Previous studies have detected a range of levels in relatively large samples,24 although these samples are often selected for being at higher than normal risk of cardiovascular disease (CVD). Categorizing respondents as having had a recent cardiac event yields tertiles of CRP levels reflecting positive (protected), normal, and elevated risk of cardiometabolic disease that are not representative of a general population with a lower rate of morbidity or predisease states.25 Similarly tertiled values from a sample of the general population may be lower and may not yield such useful categories for subsequent cardiovascular event prediction. Second, CRP levels and relationships between CRP and health outcomes are known to vary by sex and race/ ethnicity.26,27 Despite this, very few studies of sleep duration and CRP have explored interactions with these demographic factors or have had the statistical power to evaluate them. Third, many studies evaluate sleep duration groups that combine a number of possible durations (e.g., short sleepers represented as < 6 h in many cases). They are rarely powered to detect effects at greater extremes of sleep duration. In summary, the inconsistencies present in the literature may be due to a lack of statistical power and/or analysis strategies that do not take into account important confounders such as sex and/or race/ethnicity. Significant race and sex differences exist in the population distribution of CRP,10,26–28 which may contribute to CVD disparities.
Accordingly, the current study examines the independent association between sleep duration and CRP levels in a nationally representative U.S. adult sample, considering the effects of several known confounders. It also examines whether this relationship depends on sex and/or race/ethnicity. The hypotheses for the study were that: (1) CRP is positively associated with short and long sleep duration; (2) the relationship between sleep duration and CRP is different for men and women; and (3) the relationship between sleep duration and CRP depends on race/ethnicity.
METHODS
Data Source
The participants in this study were respondents of the 2007-2008 National Health and Nutrition Examination Survey (NHANES), a national survey conducted by the Centers for Disease Control and Prevention, reporting the health and nutritional characteristics of children and adults.29 Detailed description of the NHANES methods have been previously described, and can be found by accessing the surveys, manuals, and procedures on the NHANES website (http://www.cdc.gov/nchs/nhanes). For example, the CRP laboratory manual is available.30 In brief, participants were administered questionnaires assessing demographic, socioeconomic, nutritional, health, and other domains, during in-person interviews conducted in the home. Additionally, physical examinations were performed in mobile medical facilities to collect medical and physiologic data; additional laboratory tests were also performed from blood and urine samples collected on site. To compensate for underrepresentation, African Americans, Hispanics, and adults older than 60 years were oversampled.29
The NHANES is representative by design. Sampling in this survey was performed to ensure generalizability to the entire population across all ages. Given the complexity of the survey design coupled with variable probabilities of selection, the data used in the following analyses were also weighted to control for representativeness by following the procedures outlined in the current NHANES Analytic and Reporting Guidelines.31 For the current study, analyses included adults ages 18-80+ years (mean = 49.3, standard deviation = 18.6) with complete data on all independent and dependent variables (n = 5,587). All respondents provided informed consent. Consent forms are available online (http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/current_nhanes_07_08.htm).
Measures
C-Reactive Protein
Detailed specimen collection and processing instructions are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual.30 This method quantified CRP (hsCRP) by latex-enhanced nephelometry. Particle-enhanced assays were based on the reaction between a soluble analyte and the corresponding antigen or antibody bound to polystyrene particles. For the quantification of CRP, particles consisting of a polystyrene core and a hydrophilic shell were used to link anti-CRP antibodies covalently. A dilute solution of test sample was mixed with latex particles coated with mouse monoclonal anti-CRP antibodies. CRP present in the test sample forms an antigen antibody complex with the latex particles. An automatic blank subtraction was performed. CRP concentrations were calculated by using a calibration curve. Data reduction of the signals was performed by using a storable logit-log function for the calibration curve-performed data reduction of the signals. These assays were performed on a Behring Nephelometer for quantitative CRP determination (Siemens Heathcare Diagnostics, Inc. New Castle, DE). The lower detectable limit was 0.02. For the purposes of analysis, CRP was assessed as a continuous variable, log-transformed, and as a binary variable (top 5% relative to bottom 95%).
Sleep Duration
Sleep duration was assessed with the survey item, “How much sleep do you usually get at night on weekdays or work-days?” Responses were coded in whole numbers.
Sleep Disorders
Insomnia and sleep apnea were assessed symptomatically and by history of diagnosis. Difficulty falling asleep (hallmark symptom of insomnia) was assessed with the item, “In the past month, how often did you have trouble falling asleep?” Responses were categorized as, “Never,” “Rarely (one time a month),” “Sometimes (two to four times a month),” “Often (five to 15 times a month),” and “Almost Always (16-30 times a month).” Responses were dichotomized as either representing a current problem (“Often” or “Almost Always”) or not. Snorting and/or gasping (hallmark symptom of sleep apnea) was assessed with, “In the past 12 months, how often did you snort, gasp, or stop breathing while you were asleep?” Responses were categorized as “Never,” “Rarely (1-2 nights/ week),” “Occasionally (3-4 nights/week),” or “Frequently (5 or more nights/week).” Responses were diagnosed as either representing a likely clinical problem (“Occasionally” or “Frequently”) or not. Previous diagnosis of insomnia and/or sleep apnea was assessed with the question, “Have you ever been told by a doctor or other health professional that you have a sleep disorder?” If yes, they were asked, “What was the sleep disorder?” Options included “Insomnia” or “Sleep Apnea” (used for the current analyses), as well as “Restless Legs Syndrome” or “Other.”
Covariates
The following variables were chosen as covariates: sex, age, race/ethnicity (non-Hispanic white, Black/African-American, Hispanic/Latino, and Asian/Other), and body mass index (BMI), measured objectively as part of the NHANES physical examination. These covariates were chosen because they have been used previously to study the relationship between sleep duration and CRP.21
Health Status
Because CRP levels are inflated in individuals with certain medical conditions or active illness, the following were also assessed as part of the NHANES interview (yes/no): history of diabetes, current treatment for diabetes, history of hypertension, current treatment for hypertension, hypercholesterolemia, current treatment for hypercholesterolemia, heart failure, coronary artery disease, cancer, chronic kidney disease, rheumatoid arthritis, and gastrointestinal and viral illness. Smoking status was also assessed by self-report (any smoking in the past 30 days), as was alcohol use (number of days consumed alcohol in the past year).
Statistical Analyses
CRP levels were operationalized as either continuous (mg/ dL, log-transformed) or categorical (top 5% versus bottom 95%) in different models. The top 5% represented the subpopulation with clinically relevant elevated CRP (> 1.4 mg/dL). Sleep duration was also assessed both continuously (hours of nightly sleep) and categorically (< 5, 5, 6, 7, 8, 9, > 9 h). This resulted in four sets of analyses: (1) continuous CRP relative to continuous sleep duration; (2) continuous CRP relative to categorical sleep duration; (3) categorical CRP relative to continuous sleep duration; and (4) categorical CRP relative to categorical sleep duration. This allows for analysis of linear and nonlinear relationships.
The nonlinear effects of continuous sleep duration on continuous CRP were modeled using polynomial regression with terms for linear and squared sleep duration. A two-degree polynomial was chosen due to the parabolic nature of the relationship of CRP to sleep duration. Relationships between CRP and categorical sleep duration were modeled using multinomial linear regression to examine how mean log CRP levels change across sleep duration categories relative to normative sleepers (7 h).
High CRP (binary) was defined as having a CRP value greater than the 95th percentile, which was 1.42 mg/dL. The nonlinear effects of continuous sleep duration on binary CRP were modeled using logistic regression with terms for linear and squared sleep duration. Categorical sleep duration was modeled using logistic regression to examine how odds of having a high CRP level change across sleep duration categories, relative to the proportion among normative-duration sleepers (7 h). A reference group of 7 h (instead of 7-8 h or 8 h) was chosen for four reasons: (1) the largest controlled population study of sleep duration associated with mortality risk found that the lowest risk group was 7-h sleepers; (2) the lowest levels of CRP were found among 7-h sleepers; (3) 7 h was the most common sleep duration category; and (4) this allows for an equal number of groups on either side of the reference group for analyses of U-shaped associations.
All analyses were conducted for the overall sample, as well as stratified by sex and race/ethnicity, because of the well-established sex differences in the role of CRP levels in health outcomes. All analyses were conducted hierarchically by including additional covariates in each subsequent model of CRP and sleep duration. Model 1 was unadjusted for covariates. Model 2 included the covariates of age, race, sex, BMI, and BMI squared (BMI2). Model 3 included Model 2 covariates and measures of insomnia and sleep apnea described previously. Model 4 included Model 3 covariates and health status covariates (described previously). Interactions with race/ethnicity were explored in Models 2, 3, and 4, because previous studies have shown differential levels of CRP relative to race/ethnicity.
Because an almost unlimited number of factors could potentially increase CRP, it is unlikely that any model could fully account for these. Further, adding many covariates introduces the problem of compounded measurement error, especially for self-reported measures. In addition, many of those factors that are associated with elevated CRP could be at least partially a consequence of changes in sleep, including these as covariates and then examining the unique variance explained by sleep leads to a situation of overcontrolling. An overcontrolled model underestimates the effects of the outcome of interest (in this case, sleep duration). For the current paper, Model 4 was specifically meant as an over-controlled model. This model is included for the sake of introducing a maximally conservative approach that likely underestimates the effects associated with sleep (not a fully-adjusted model that simply accounts for confounders). Therefore, the results of Model 4 should be interpreted with appropriate caution.
As a validity check, all analyses were re-run excluding the six individuals with CRP levels > 10 mg/dL, a level suggestive of active infection rather than chronic low-grade inflammation. Also, all analyses were re-run to include pregnancy status as a covariate. This did not affect any results (all statistically significant or nonsignificant results remained so, and effect sizes did not change appreciably), thus reported results include these individuals.
RESULTS
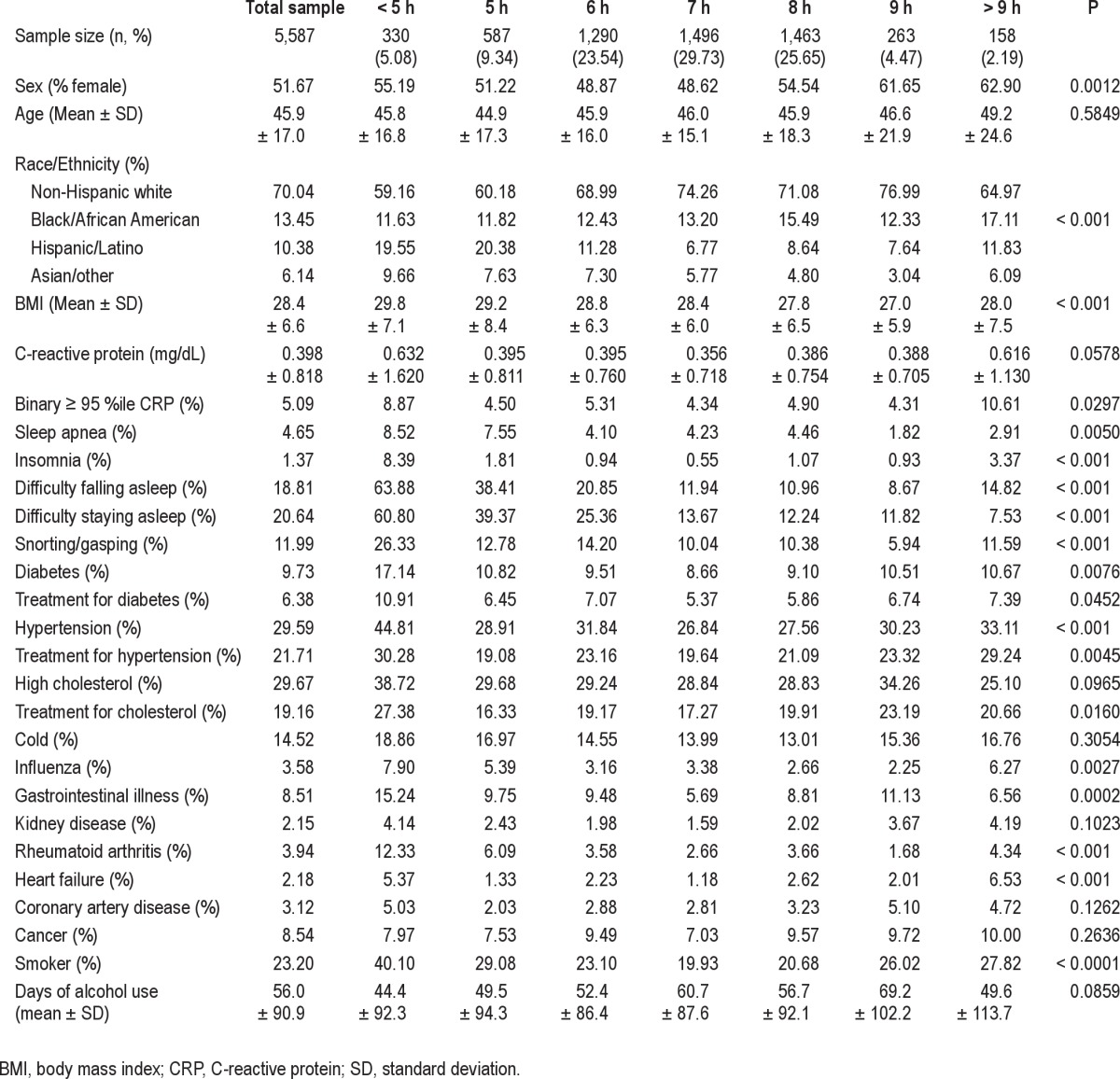
Sample Characteristics
Characteristics of the sample are reported in Table 1. Sex was differentially distributed across sleep duration categories, with the lowest percentage of females among the 7-h sleepers and the highest among > 9 h sleepers. Race/ethnicity was also differentially distributed, with the greatest proportion of non-white respondents among the < 5-h and 5-h sleepers. Characteristics are also reported for the stratified sample, separate for men (Table S1) and women (Table S2).
Table 1.
Characteristics of the sample, stratified by sleep duration category
Sleep Duration Associated With Log CRP
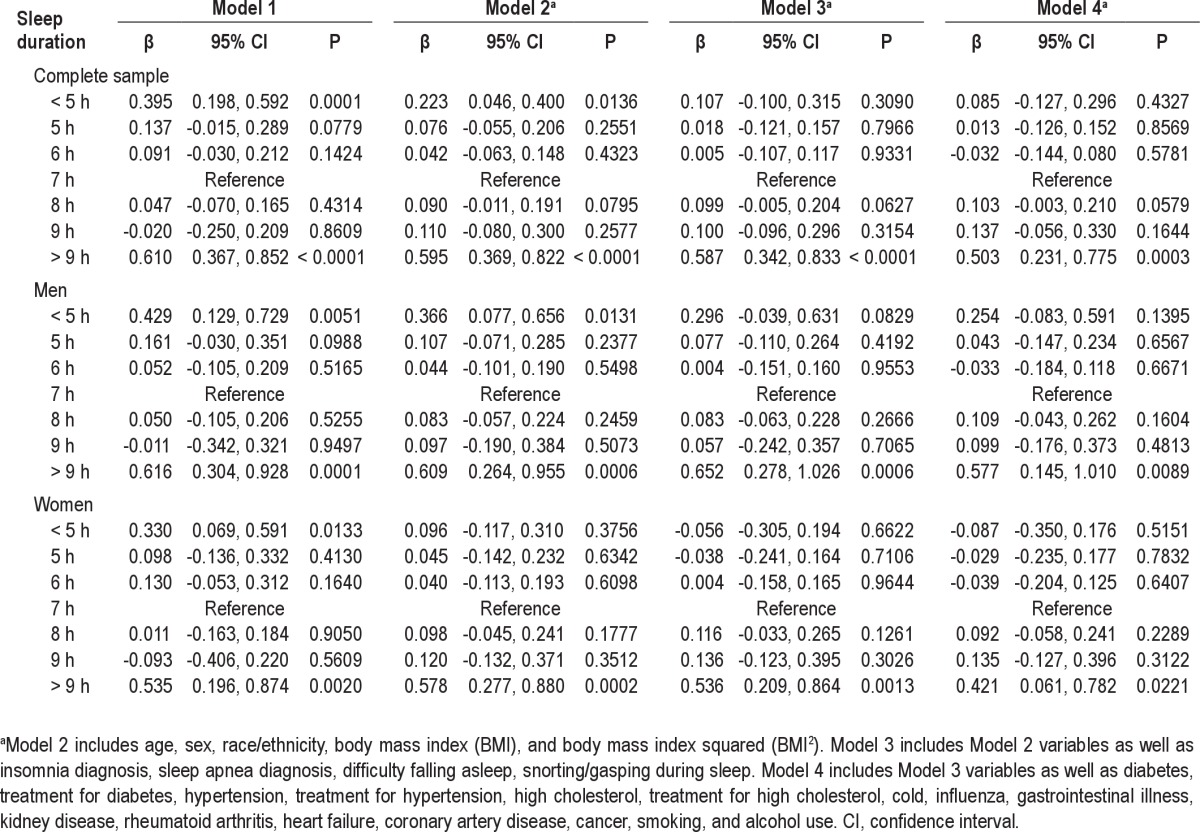
Results from regression analyses for categorical sleep duration are displayed in Table 2. In the unadjusted model (Model 1), the shortest (< 5 h) and longest (> 9 h) sleep durations were associated with higher log CRP, and this pattern was maintained in Model 2, which included adjustment for age, sex, race/ethnicity, BMI, and BMI2. In Model 3, which included diagnosis and symptoms of insomnia and sleep apnea, only the relationship with long sleep remained significant, and this was maintained in Model 4, the overcontrolled model that included health status covariates. When men were considered separately, for < 5-h sleepers, elevated CRP was observed in Models 1 and 2; for > 9-h sleepers, elevated CRP was present in all models. Among women, for < 5-h sleepers, elevated CRP was only significant in Model 1; for > 9-h sleepers, elevated CRP was observed in all models.
Table 2.
Regression coefficients (β) and 95% confidence interval for log C-reactive protein across sleep duration categories
When modeled using polynomial terms, both linear and squared terms were significantly associated with log CRP in all models in the complete sample and in men, when assessed separately. For women, these terms were significant in Models 1 and 2 only. These results are displayed in Table S3.
The relationships between continuous CRP and sleep duration for categorical analyses are displayed graphically in Figure 1. Data displayed reflect the complete sample, men and women for Model 1 versus Model 3.
Figure 1.
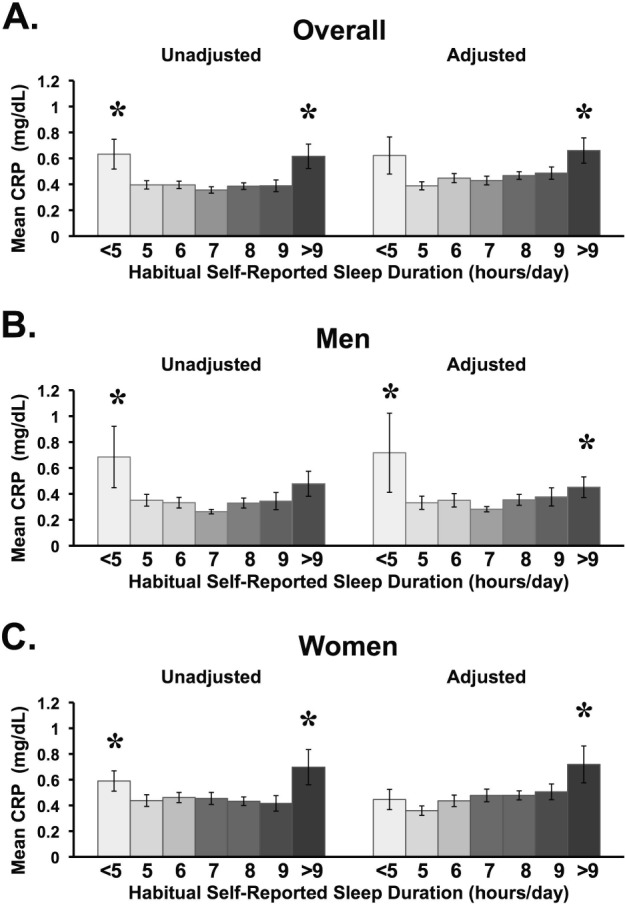
Nonlinear relationship between sleep duration and mg/dL of C-reactive protein (CRP), represented in untransformed values for the complete sample (A), men alone (B), and women alone (C). Unadjusted figures represent Model 1. Adjusted figures represent Model 3, which includes age, sex, race/ethnicity, body mass index (BMI), body mass index squared (BMI2), insomnia diagnosis, sleep apnea diagnosis, difficulty falling asleep, and snorting/gasping during sleep. A significant U shape to the distribution was found for Model 1 and Model 3 for the complete sample and for men, and a U shape was found for Model 1 but not Model 3 in women. Note that the U shape was significant for the complete sample, even if there was no elevation in the shortest sleep duration category. Asterisks (*) represent P < 0.05 difference from 7 h. Error bars = SEM.
Sleep Duration Associated With Binary CRP
Table S4 depicts polynomial regression results for binary log CRP. The linear term was significant in Model 1 only for the complete sample, and Models 1 and 2 for men (not for women). The squared term was significant in Model 1 and for the overall sample, and Models 1, 2, and 3 for men (not for women).
Table S5 depicts relationships between high CRP and categorical sleep duration. In the complete sample, < 5-h sleepers were more likely to have high CRP levels in Model 1 only, whereas the > 9-h sleepers were more likely to have high CRP levels in all models. This pattern was similar in men, where high CRP was more common among < 5-h sleepers in Models 1 and 2, and for > 9-h sleepers in all models. No relationships were significant among women.
The relationships between categorical CRP and sleep duration, for both categorical and polynomial sleep duration analyses, are displayed graphically in Figure S1. Data displayed reflect the complete sample, men and women for Model 1 versus Model 3.
Interactions With Race/Ethnicity
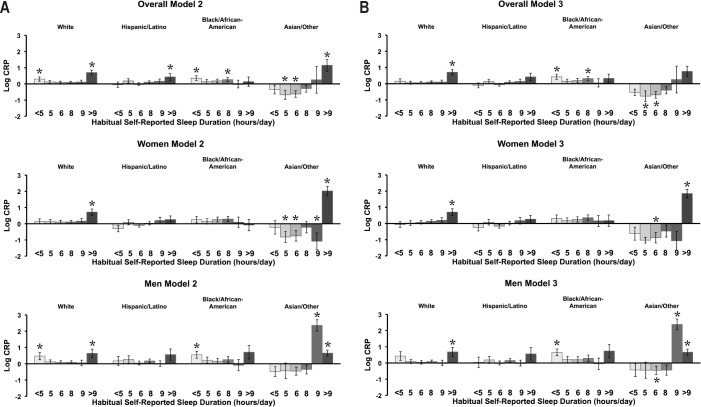
The relationship between continuous CRP and sleep duration depended on race/ethnicity. Significant interactions were demonstrated in the complete sample, men and women for Model 2 (P interaction = 0.003, < 0.001, and < 0.001, respectively), Model 3 (P interaction = 0.003, < 0.001, and < 0.001, respectively), and Model 4 (P interaction = 0.028, < 0.001, and < 0.001, respectively). Figure 2 depicts the nature of this interaction for both Models 2 and 3, for the complete sample, men separately, and women separately.
Figure 2.
Stratified results of sleep duration × race/ethnicity interactions for C-reactive protein (CRP). (A) Results for Model 2 (which includes age, sex, body mass index [BMI] and BMI2) are displayed for the complete sample (interaction P = 0.003), men (interaction P < 0.001), and women (interaction P < 0.001). (B) Results for Model 3 are presented for the complete sample (interaction P = 0.003), men (interaction P < 0.001), women (interaction P < 0.001). *P < 0.05 relative to 7 h. Error bars = SEM.
In Model 2, among non-Hispanic white respondents, elevated CRP was observed in < 5-h and > 9-h sleepers. Among black/African American respondents, elevations were observed among < 5 and 8-h sleepers. Among Hispanic/Latino respondents, elevated CRP was observed in > 9-h sleepers only. Finally, among Asian/Other respondents, elevated CRP was observed in > 9-h sleepers, and lower CRP was observed in 5- and 6-h sleepers, relative to 7-h sleepers. With the addition of insomnia and sleep apnea covariates (Model 3), some of these relationships were changed. Among non-Hispanic white respondents, for example, elevated CRP was observed only in > 9-h sleepers. The black/African American group still showed elevations among < 5-h sleepers and 8-h sleepers. No elevations were observed in Hispanic/Latino respondents. Among Asian/Other respondents, lower CRP was seen in 5-and 6-h sleepers.
Among men, in Model 2, the non-Hispanic white group demonstrated elevated CRP in < 5-h and > 9-h sleepers; in Model 3, only the > 9-h sleepers showed increased CRP. The black/ African American group demonstrated elevated CRP in < 5-h sleepers in both Model 2 and Model 3. The Hispanic/Latino group did not show any elevations in either Model. Asian/Other respondents showed elevated CRP among 9- and > 9 h sleepers in both Model 2 and Model 3, with decreased CRP seen among 6-h sleepers in Model 3.
Among women, the non-Hispanic white group showed elevated CRP at > 9 h in Models 2 and 3. Hispanic/Latino and black/African-American women did not demonstrate elevations in CRP at any sleep duration in either model. Asian/Other respondents demonstrated elevated CRP among > 9-h sleepers in Model 2 and Model 3, with lower CRP found among 5-, 6-, and 9-h sleepers in Model 2 and 6-h sleepers in Model 3.
Because the Asian/Other group demonstrated a pattern that is most different from the other groups, interaction terms were reassessed (post hoc), including data from only the non-Hispanic white, black/African-American, and Hispanic/Latino groups. These analyses demonstrated that the significant interactions that were observed were driven by the Asian/Other group.
DISCUSSION
The current study examined the relationship between CRP and habitual sleep duration in a large, nationally representative, and diverse sample of adult men and women age 18-80+ years. Significant sleep*sex and sleep*race/ethnicity interactions were observed; thus, analyses were performed on the entire sample and stratified by sex and by race/ethnicity. Overall, elevated CRP was consistently found to be elevated among long sleepers (> 9 h) after adjusting for demographics, socioeconomics, BMI, insomnia, sleep apnea, smoking, alcohol use, medications, and comorbidities. CRP was also elevated among very short sleepers (< 5 h), although these were often not significant in models adjusting for sleep and medical comorbidities. Previous literature investigating the association between sleep duration and sleep disturbance and CRP by race/ethnicity group are scarce, and many previous studies are limited with regards to racial and ethnic diversity and overall power to measure these differences.22 14,32
The current study modeled the relationship between CRP levels and sleep duration both continuously and categorically. No previous studies have taken this approach. The benefit of a continuous analysis is that even if individual sleep duration categories do not significantly differ from the reference group, the shape of the distribution can be described using linear and squared terms. In the current study, a U shape was found for both men and women, in unadjusted models, models adjusted for typical covariates, and models that also included sleep disorders. In the case of the overcontrolled model, the U shape was found for the overall sample, although when the sample was stratified based on sex, this was seen only in men; in women, a positive linear relationship was found. Because the long sleep finding is most robust, the relationship between CRP and sleep duration may be best characterized as J-shaped (rather than U-shaped). This characterization is supported by examination of the categorical sleep duration analyses. In this case, the longest sleep duration category was associated with elevations in CRP among the overall sample and both men and women in all models; the shortest sleep duration category, on the other hand, was associated with elevated CRP, but only in models that included typical covariates (and among women only in the unadjusted model).
Differences Among Men and Women
Among women, our findings suggest a significant association between long sleep duration and CRP in linear regression models adjusted for demographics, BMI, and sleep disorders (Table 2, Figure 1, Figure S1). Among men, a nonlinear association between sleep duration and CRP was demonstrated (Table S3), suggesting evidence of a J-shaped association (Figure 1, Figure S1). In the categorical sleep duration analysis among men, a significant association was observed between long sleep and CRP (Table 2).
This study provides evidence of a linear association among women such that longer sleep is significantly associated with increased CRP, which is consistent with previous literature on sleep and inflammation.21,32–35 Within the Nurses' Health Study, association between long sleep duration (≥ 9 h) and CRP concentrations were still significant after adjusting for age and BMI risk factors, family history of diabetes, glycemic control, and medication use.35 Findings from the study by Williams and colleagues35 suggested 44% higher CRP levels among long sleepers compared with those sleeping 7 h. This study was conducted among women with type II diabetes, a disease that is known to be associated with increased CRP levels, and so our findings build on this existing evidence, but extend to populations with generally good overall health.
Sex differences were reported for sleep duration and CRP among 4,600 adults in the Whitehall Study (P for interaction < 0.05).32 Contrary to our findings, Miller and colleagues32 reported a null association between short and long sleep duration and CRP among men in that cohort after adjusting for age, employment status, BMI, smoking, lipid levels, and blood pressure. Among women, long sleep (≥ 9 h) was associated with a 35% increase in CRP levels after adjusting for age, marital status, BMI, smoking, systolic blood pressure, and triglyceride levels.32 Evidence of a significant association between short sleep and CRP among women was also reported in fully adjusted models. Among the women in the current study, short sleep was significantly associated with CRP in unadjusted models; however, this was attenuated and no longer significant after adjusting for BMI and additional sleep disorders (Table 2, Table S3), indicating that obesity and sleep disturbance were key drivers of this attenuation. Miller and colleagues32 did not adjust for insomnia or sleep apnea, and so it is difficult to know how presence of these sleep disorders might affect their results.
It is important to consider measures of sleep quality when assessing the association between sleep duration and CRP. Measures of sleep quality (i.e., characteristics separate from duration), such as sleep continuity disturbances and/or sleep disorders, have been shown to be strong predictors of the metabolic syndrome, a condition strongly correlated with greater CRP levels.36 With regard to sex differences, it has been suggested that symptoms of sleep disturbances are associated with a greater risk of CVD and high blood pressure in females compared with their male counterparts.37,38 Previous findings have also suggested that self-reported sleep disturbance among healthy adults with no history of sleep disorders is associated with increased fibrinogen and inflammatory markers among women but not men.33 Contrary to our findings, Suarez33 did not report a significant association between sleep duration and CRP among men, but the small sample size (n = 210) and younger age group (mean age 28 years) may account for these differences between studies. In the current study, the greatest proportion of sleep disorders was observed among those sleeping ≤ 5 h compared with those sleeping 7 h per night among both men and women, and women had a greater proportion of self-reported sleep disturbances compared with men (Tables S1 and S2). Among short sleepers, sleep apnea was observed among 10.1% of women and 6.6% of men, and insomnia was observed among 13.2% of women compared with 2.5% of men (Table 1). The effect of sleep comorbidities was stronger in women. When adding sleep disorders as a covariate in the model (Table S3), the association between sleep duration and CRP was no longer significant among women, but persisted among men. This suggests that among women, sleep disturbances have a greater effect on the relationship between levels of CRP and short or long sleep duration.
A number of previous studies investigating the CRP and sleep relationship have not reported sex-specific results, and so it is difficult to determine whether similar patterns would have been observed.21,22 Consistent findings with regard to long sleep were observed among both men and women (n = 614) in the Cleveland Family Study.21 Results indicated that for every additional hour in sleep duration, CRP levels increased by 8% (P = 0.004) after adjusting for age, sex, race, BMI, waist circumference, and severity of sleep apnea. Contrary to our findings among men, Patel and colleagues21 reported no evidence of a U-shaped association in a study of 614 individuals; however, this study did not address sex-specific associations. Our results also differ from the Wisconsin Sleep Cohort (n = 907), which reported no association between short or long sleep duration and CRP in adjusted models.22 Investigators examined self-reported as well as polysomnography-assessed sleep duration, and also evaluated quadratic terms in their analysis. This study also did not report sex-stratified results, however, and these findings are based on a sample of predominantly Caucasian participants.
The role of reproductive hormones must be considered when evaluating the differences by sex. It has been demonstrated that CRP levels vary across the menstrual cycle phase and are significantly greater during menses (CRP, > 3 mg/L; 12.3% versus 7.4% in other phases; P < 0.001).39 It is plausible that cycle phase might modify the association between sleep duration and CRP. Because we could not control for this in our statistical analyses, misclassification of the outcome among women may have occurred, resulting in an attenuation of the effect estimate. In addition, it has been demonstrated that CRP levels are higher among women using hormone replacement therapy (compared with nonusers).40 We did not account for hormone use, and this characteristic could have confounded the associations observed among women.
Differences Among Race/Ethnicity Groups
This study provides evidence of significant differences in patterns of the association between sleep duration and CRP by race/ethnicity group. In the current study, elevated CRP was observed among those individuals sleeping > 9 h for non-Hispanic white and Hispanic/Latino participants, which was significant in fully adjusted models. Among Black/African American participants, our results indicate a significant and strong association between short sleepers (< 5 h) and CRP overall and for men in fully adjusted models. Among the Asian/Other participants, a very strong association was observed among long sleep duration (> 9 h) and elevated CRP, and short sleep duration demonstrated a protective effect on CRP level.
There are very few studies that have examined the association between sleep and CRP by race/ethnicity group. Of these, most are limited in terms of diversity and sample size.22,14,32 Most large U.S. cohorts investigating sleep apnea and other sleep disturbances have been relatively homogeneous, consisting of mostly Caucasian participants.41 Because CVD risk factors and the association between obesity and CRP vary by race/ethnicity,26,42,43 it is plausible differences may persist with regard to the association between sleep and CRP.
It has been reported that non-Hispanic African American women and Hispanic/Latino women have elevated CRP levels compared with other race groups.28 One previous study among women suggested differences in patterns between sleep duration and inflammatory and coagulation markers by race.10 These findings from the Study in Women's Health Across the Nation (SWAN) Study indicated that African American women with higher levels of CRP had shorter polysomnography-recorded sleep duration in multivariate models.10 Previous findings from 1999-2000 NHANES suggested higher CRP levels among African American men and women, as well as Hispanic/Latino women compared with Caucasian control men and women.27 Black/African American men and women have greater rates of CVD mortality compared with other race groups, and improvements in prevention strategies are essential.44,45 This study provides evidence of a strong and significant association between short sleep and CRP observed among Black/African Americans compared with other race groups. Improved sleep may be a more important risk factor among this race group, presenting opportunities for future interventions targeted at reducing inflammation and CVD risk.
Previous reports of sleep duration and CVD mortality among Asian populations have indicated that long sleep (> 9 h) with high levels of sleep disturbance predicts the greatest risk of CVD. 46 One study of sleep duration and CRP in Taiwanese adults has suggested that inflammation was significantly associated with long (> 8 h), but not short (< 6 h) sleep duration.47 These associations were significant when using a high CRP threshold (> 10.0), but not a lower CRP threshold (> 3.0) after adjustment for age, sex, waist circumference, reported health decline, diabetes, arthritis, heart disease, and depression. These findings are consistent with our results; however, it is difficult to compare these findings with those of the current study given that the analysis was limited to three sleep duration categories. It has been previously reported that Asians/Pacific Islanders have the lowest rates of CVD mortality compared with any other race/ethnic groups. Our findings indicate that the protective effect of short sleep on inflammation may contribute to the underlying biologic pathway by which this subpopulation obtains lower rates of CVD mortality. Additional investigations on the protective effect of short sleep and inflammation among individuals of Asian race/ethnicity is warranted.
Limitations
We are limited to a cross-sectional analysis on prevalent subclinical disease and cannot make inferences with regard to causal associations. It is also likely that misclassification exists in our primary exposure variable because self-reported sleep duration has been demonstrated to be only moderately associated with objectively measured sleep (r = 0.47).48 Further, the NHANES survey limits self-reported sleep duration to integer values, which further limit validity.
We recognize that although our study had sufficient sample size at the extremes of sleep duration to detect the effects observed, the individuals at these extremes represent a small portion of the population. For example, < 10% of the sample reported sleep durations in the shortest and longest categories combined. This suggests that although these relationships likely exist in the population, they are most relevant for only a small subset of the general population. We should also note that this distribution (< 10% extreme categories) is consistent with other cohorts that have reported on sleep duration. For example, the Nurses' Health Study49 reported 5% short sleepers and 5% long sleepers and the Osteoporotic Fractures in Men (MrOS) Study reported 8% long sleepers.50
Another issue with the current analyses is that BMI is used as a measure of obesity and associated CVD risk. Although many studies have shown BMI to be a useful measure in this regard, BMI does not account for sex differences in body fat, as well as location of fat distributed in the body, or differences in relationships between adiposity and cardiometabolic disease among ethnoracial groups. Several alternatives to BMI have been proposed, including waist-to-hip ratio and the Index of Body Adiposity ([Hip/Height1.5]-18).51,52 These indicators may better approximate body adiposity and associated cardiometabolic disease. For the purposes of these analyses, BMI was chosen because NHANES did not collect hip circumference, precluding the use of either of these alternative approaches.
It should be noted that introducing medical comorbidities as covariates may present the problem of overcontrolling. For example, only variance explained by sleep duration that is not explained by comorbidities would be evaluated statistical significance in Model 4. However, if short and/or long sleepers are more likely to develop comorbidities, some of the variance explained by those comorbidities may actually be variance explained by the short/long sleep duration that contributed to the presence of those conditions (i.e., rather than being confounders, these variables may be on the causal pathway as mediators/ moderators). Because of this, Model 4 results should be interpreted with appropriate caution and may be underrepresenting the effect of sleep duration on CRP levels.
For the women in this study, we did not evaluate phase of menstrual cycle or hormone-replacement therapy, which might have influenced results. Finally, given that this is an observational study, it is likely that residual confounding exists. However, this would likely attenuate the association between sleep duration and CRP toward the null, and so our findings likely represent an underestimation of the true association.
Strengths
The large sample size of this study represents a key strength, as previous smaller studies have not been powered to adequately assess these associations by sex and ethnoracial group. CRP represents a good biomarker of inflammation and has been shown to be a predictor of subsequent CVD beyond traditional risk factors.24 CRP has been linked to increased myocardial infarction, stroke, CVD mortality, and coronary revascularization procedures.53–61
A number of chronic conditions are known to increase biomarkers of inflammation and be associated with sleep disturbances, such as chronic pain, rheumatoid arthritis, cancer, chronic kidney disease, Crohn's disease, and acute infections.62–69 Unlike previous studies, we accounted for this potential confounding by comorbidities by adjusting for a number of conditions, including diabetes, hypertension, hypercholesterolemia, common cold, influenza, gastrointestinal disease, kidney disease, rheumatoid arthritis, heart failure, coronary artery disease, and cancer.
Existing studies linking short and long sleep duration with inflammation and coagulation have been somewhat inconsistent, particularly with regard to sex and race.13,14,22,70,71 Our study provides novel insight regarding these relationships by using a robust dataset, and findings suggest that putative mechanisms linking sleep quality and quantity to inflammation differ by sex and race/ethnicity. These results corroborate previous findings that long sleep is significantly associated with increased inflammation and also suggest that a nonlinear, U-shaped (or J-shaped) pattern for this association persisted among men, which is contrary to previously reported null results.21,22,32,33 Future studies are needed to replicate these findings, and to examine this association using objective measures of sleep duration.
CONCLUSION
In a nationally representative sample of U.S. adults, a U-shaped (or J-shaped) relationship between CRP and sleep duration was demonstrated, such that those with very short or very long sleep duration were more likely to show elevated CRP. In addition, this pattern was only partially explained by sleep disorders and other medical comorbidities. Further, significant sleep*sex and sleep*race/ethnicity interactions demonstrated that this pattern differs among men and women and among race/ethnicity groups. Thus, the role of sleep duration in proinflammatory processes, as marked by CRP levels, is complex, but the current data suggest that extremes of sleep duration increase pro-inflammatory risk.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Buxton has received research support from Sunovion Inc., has received remuneration for serving as a consultant and/or expert witness for Dinsmore LLC and Wake Forest University Medical Center, and serves on the Science Advisory Board of Matsutani America. He has also received travel costs for a speaking engagement for the National Postdoctoral Association. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Grandner was in part supported by the American Heart Association (12SDG9180007), the National Heart, Lung and Blood Institute (K23HL110216), the National Institute for Environmental Health Sciences (R21ES022931), and a fellowship from the Institute for Translational Medicine and Therapeutics, funded by UL1RR024134 (Penn CTSA). Dr. Buxton was in part supported by National Institute on Aging (P01 AG009975), and the National Heart, Lung, and Blood Institute (R01HL107240). Dr. Sands was in part supported by the National Heart, Lung, and Blood Institute (T32HL007713). We thank the Centers for Disease Control and Prevention for collecting these data and making it available and the NHANES participants for providing data. No off-label uses are described. No conflicts of interest are reported.
SUPPLEMENTAL MATERIAL
Characteristics of the men included in the sample, stratified by sleep duration category
Characteristics of the women included in the sample, stratified by sleep duration category
Nonlinear relationship between sleep duration and log C-reactive protein, represented as unstandardized β and 95% confidence interval
Nonlinear relationship between sleep duration and binary C-reactive protein (≥ 95th percentile)
Odds ratio and 95% confidence interval for high C-reactive protein ≥ 95th percentiile across sleep duration categories
Nonlinear relationship between sleep duration and presence of high C-reactive protein (CRP), represented as percent in each sleep duration category of those in the top five percentile for CRP. This is displayed for the complete sample (A), men alone (B), and women alone (C). Unadjusted figures represent Model 1. Adjusted figures represent Model 4, which includes age, sex, race/ethnicity, body mass index (BMI), body mass index squared (BMI2), insomnia diagnosis, sleep apnea diagnosis, difficulty falling asleep, snorting/gasping during sleep, health status, and smoking. A significant U shape to the distribution was found for Model 1 for the complete sample and for men, and no U shape was found for Model 1 for women or Model 4 in any analysis. Asterisks (*) represent P < 0.05 difference from 7 h.
REFERENCES
- 1.Colten HR, Altevogt BM Institute of Medicine Committee on Sleep Medicine and Research. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: Institute of Medicine, National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Ulmer C, Wolman DM, Johns MME Institute of Medicine Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety. Resident duty hours: Enhancing sleep, supervision, and safety. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 3.Office of Disease Prevention and Health Promotion. Healthy People 2020 Objective Topic Areas. Washington, DC: US Department of Health and Human Services; 2011. [Google Scholar]
- 4.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–47. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogen-esis in mice. Brain Behav Immun. 2012;26:672–9. doi: 10.1016/j.bbi.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisor JP, Schmidt MA, Clegern WC. Evidence for neuroinflammatory and microglial changes in the cerebral response to sleep loss. Sleep. 2011;34:261–72. doi: 10.1093/sleep/34.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–7. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews KA, Zheng H, Kravitz HM, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women's Health Across the Nation Sleep Study. Sleep. 2010;33:1649–55. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflamma-tory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7:S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam JC, Ip MS. Sleep and the metabolic syndrome. Indian J Med Res. 2010;131:206–16. [PubMed] [Google Scholar]
- 18.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, et al. Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study. Sleep Med. 2011;12:997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuomilehto H, Peltonen M, Partinen M, et al. Sleep duration, lifestyle intervention and incidence of type 2 diabetes in impaired glucose tolerance. The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–71. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 25.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 26.Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and hispanic US adults. Obesity (Silver Spring) 2008;16:875–80. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford ES, Giles WH, Myers GL, Mannino DM. Population distribution of high-sensitivity C-reactive protein among US men: findings from National Health and Nutrition Examination Survey 1999-2000. Clin Chem. 2003;49:686–90. doi: 10.1373/49.4.686. [DOI] [PubMed] [Google Scholar]
- 28.Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–73. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services. Hyattsville, MD: National Center for Health Statistics; 2008. [Google Scholar]
- 30.Centers for Disease Control and Prevention. NHANES Laboratory Procedure Manual: C-Reactive Protein. Hyattsville, MD: US Department of Health and Human Services; 2007. [Google Scholar]
- 31.Centers for Disease Control and Prevention. Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 32.Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opp MR. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evol Biol. 2009;9:8. doi: 10.1186/1471-2148-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007;30:1233–40. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 36.Troxel WM, Buysse DJ, Matthews KA, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33:1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 39.Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175:423–31. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–6. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 41.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9:419–36. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–52. [PubMed] [Google Scholar]
- 43.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 44.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 45.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–6. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 46.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: Report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel SR, Blackwell T, Ancoli-Israel S, Stone KL. Osteoporotic rractures in men-Mr OSRG. Sleep characteristics of self-reported long sleepers. Sleep. 2012;35:641–8. doi: 10.5665/sleep.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergman RN. A better index of body adiposity. Obesity (Silver Spring) 2012;20:1135. doi: 10.1038/oby.2012.99. [DOI] [PubMed] [Google Scholar]
- 52.Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring) 2011;19:1083–9. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorenz MW, Karbstein P, Markus HS, Sitzer M. High-sensitivity C-reactive protein is not associated with carotid intima-media progression: the carotid atherosclerosis progression study. Stroke. 2007;38:1774–9. doi: 10.1161/STROKEAHA.106.476135. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 55.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–9. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 56.Cao JJ, Thach C, Manolio TA, et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–70. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 57.Curb JD, Abbott RD, Rodriguez BL, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003;107:2016–20. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 58.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–53. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 59.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 60.Tanne D, Benderly M, Goldbourt U, et al. C-reactive protein as a predictor of incident ischemic stroke among patients with preexisting cardiovascular disease. Stroke. 2006;37:1720–4. doi: 10.1161/01.STR.0000227004.08182.bf. [DOI] [PubMed] [Google Scholar]
- 61.Bos MJ, Schipper CM, Koudstaal PJ, Witteman JC, Hofman A, Breteler MM. High serum C-reactive protein level is not an independent predictor for stroke: the Rotterdam Study. Circulation. 2006;114:1591–8. doi: 10.1161/CIRCULATIONAHA.106.619833. [DOI] [PubMed] [Google Scholar]
- 62.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 63.Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–61. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 64.Fox ER, Benjamin EJ, Sarpong DF, et al. The relation of C-reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol. 2010;11:1. doi: 10.1186/1471-2369-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galarraga B, Khan F, Kumar P, Pullar T, Belch JJ. C-reactive protein: the underlying cause of microvascular dysfunction in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1780–4. doi: 10.1093/rheumatology/ken386. [DOI] [PubMed] [Google Scholar]
- 66.Menefee LA, Cohen MJ, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1:156–72. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 67.Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:169–73. [PubMed] [Google Scholar]
- 68.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–27. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Otterness IG. The value of C-reactive protein measurement in rheumatoid arthritis. Semin Arthritis Rheum. 1994;24:91–104. doi: 10.1016/s0049-0172(05)80003-4. [DOI] [PubMed] [Google Scholar]
- 70.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 71.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the men included in the sample, stratified by sleep duration category
Characteristics of the women included in the sample, stratified by sleep duration category
Nonlinear relationship between sleep duration and log C-reactive protein, represented as unstandardized β and 95% confidence interval
Nonlinear relationship between sleep duration and binary C-reactive protein (≥ 95th percentile)
Odds ratio and 95% confidence interval for high C-reactive protein ≥ 95th percentiile across sleep duration categories
Nonlinear relationship between sleep duration and presence of high C-reactive protein (CRP), represented as percent in each sleep duration category of those in the top five percentile for CRP. This is displayed for the complete sample (A), men alone (B), and women alone (C). Unadjusted figures represent Model 1. Adjusted figures represent Model 4, which includes age, sex, race/ethnicity, body mass index (BMI), body mass index squared (BMI2), insomnia diagnosis, sleep apnea diagnosis, difficulty falling asleep, snorting/gasping during sleep, health status, and smoking. A significant U shape to the distribution was found for Model 1 for the complete sample and for men, and no U shape was found for Model 1 for women or Model 4 in any analysis. Asterisks (*) represent P < 0.05 difference from 7 h.