 |
David MacLean obtained his BSc in Neuroscience from the University of Toronto, where he first began studying AMPA receptors. He completed his PhD at McGill University where he focused on the allosteric regulation of kainate receptors by external ions. Currently, he is a postdoctoral fellow at the Center for Membrane Biology at the University of Texas in Houston, working under Dr Vasanthi Jayaraman. The primary focus of his research is the mechanism of action of auxiliary proteins on AMPA receptors.
The structural mechanisms of gating for ionotropic glutamate receptors are, arguably, better understood than other ligand-gated ion channels. Based on detailed electrophysiological, computational and crystallographic study, there is a plausible framework for the structural basis of gating in these channels, especially AMPA receptors (Traynelis et al. 2010). The accepted model is that they assemble as a dimer of dimers, with each subunit possessing a clamshell-like ligand-binding domain (LBD) braced against its neighbour. Agonist binding in this clamshell leads the cleft to clamp down around the ligand (state AR in Fig. 1). This agonist-bound cleft-closed dimer-intact state may represent an unstable pre-open conformation (state AR* in Fig. 1) from which the AMPA receptor (AMPAR) has three routes of escape. The upper lobe can swing down, breaking the dimer interface apart and leaving the agonist bound but the channel non-responsive (i.e. desensitized, AD in Fig. 1); the lower lobe can swing up, pulling open the pore and leading to channel activation (state AO); or the cleft can simply re-open. With appropriate rate constants, this scheme can broadly reproduce AMPAR macroscopic responses at saturating concentrations. Of course the real situation is more complex with four subunits and multiple open states (Tomita et al. 2005). Moreover, recent fluorescence resonance energy transfer (FRET) experiments on isolated soluble LBDs reveal that the LBD occupies multiple closed cleft conformations with distinct degrees of cleft closure (i.e. multiple AR* states; Landes et al. 2011). Despite these and other complexities, this scheme represents a straightforward, though coarse grained, way to think about AMPAR gating.
Figure 1. Kinetic scheme of AMPA receptor gating.
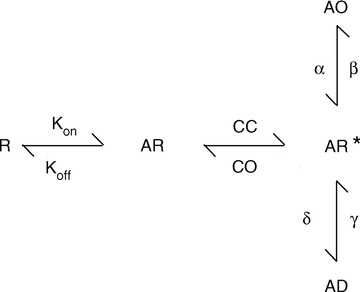
Agonists bind to state ‘R’ leading to the formation of an agonist-bound cleft open state ‘AR’. Subsequent cleft closure leads to the formation of an unstable pre-open state ‘AR*’ with the agonist bound, cleft closed and channel closed. From this state, transitions to the channel open ‘AO’ or channel desensitized ‘AD’ can occur.
How can the effect of the prototypical auxiliary proteins, the transmembrane AMPAR auxiliary proteins (TARPs), be modelled in this scheme? TARPs produce a number of ‘gain of function’ changes in the gating of AMPARs, for example slowing deactivation and desensitization decays whilst speeding recovery from desensitization (Tomita et al. 2005; Priel et al. 2005). In the case of slower deactivation rates, increases in β or decreases in cleft opening (CO) (i.e. a more stable AR* state) both slow deactivation decays. A larger value for β does this by increasing the chances of the channel opening multiple times before the agonist can dissociate while a smaller CO would simply keep the agonist bound longer, also increasing the chances of multiple openings, thus prolonging the decay time in response to short agonist applications. The slowing of desensitization decays in response to long agonist applications can also be reproduced by increasing β, as each time the channel shuts it has a greater chance of re-opening than falling into desensitization. However, reducing CO alone will not slow desensitization. To reproduce the requisite slowing of desensitization, both CO and δ must be reduced. In modelling the effects of TARPs, it may be simpler to alter as few rates as possible, but there is no reason receptor behaviour follows this rule. Indeed, a notable feature of TARPs is their speeding of recovery from desensitization (Priel et al. 2005), and neither increasing β nor reducing CO can account for this effect. Rather, γ, the rate of recovery from desensitization, must become larger. Therefore, it is not at all unexpected that the reverse reaction, δ, becomes smaller.
It is difficult on the basis of kinetic data alone to distinguish an increase in β from a reduction in CO. Of course, such reasoning is strongly dependent on the model used, and the real value of the model in Fig. 1 is in its reflection of the structural changes underlying AMPAR gating. So in terms of the physical mechanism, how might TARPs modulate AMPAR gating? One attractive possibility is that TARPs promote pre-open states that are more stable and have a larger degree of LBD closure. Isolated, soluble LBDs have multiple resolvable agonist-bound conformations with differing degrees of domain closure at the single molecule level (Landes et al. 2011). A mutation, T686S in the GluA2 AMPA subunit which attenuates electrostatic interactions between the LBD lobes produces soluble LBDs which are more flexible and dynamic. These mutant LBDs populate closed-cleft conformations similar to the wild-type but with a broader distribution of occupancies (Landes et al. 2011). When this mutation (or the more dramatic T686A) is introduced into full-length receptors the resulting channels have reduced agonist potency, lower probability of opening or occupying larger conductances, and faster deactivation (although no change to or even slower desensitization; Zhang et al. 2008). In other words, expanding the range and destabilizing pre-gating closed-cleft conformations produces, in large part, the opposite effect of TARPs. Therefore, a preliminary structural hypothesis of TARP modulation is that they favour more closed conformations of the LBD and stabilize these more productive states.
There is no direct evidence either for this cleft closure hypothesis or for one in which TARPs enhance channel opening rates. And, if TARPs work by either mechanism, the issue of how they speed recovery from desensitization remains unaddressed since neither mechanism alone can account for this observation. TARPs are just the first in the recent wave of newly identified auxiliary proteins, each having a slightly different effect on AMPAR gating. So while our understanding of glutamate receptors is arguably more advanced than that of other ligand-gated channels, the growing list of auxiliary proteins highlights how much we have yet to learn about native AMPAR/auxiliary protein complexes.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/7/1585
Acknowledgments
This work was supported by an American Heart Association grant, number 11GRNT7890004, to Dr Vasanthi Jayaraman. I wish to thank Dr Catherine Lichten for a critical reading of the manuscript.
References
- Landes CF, Rambhadran A, Taylor JN, Salatan F, Jayaraman V. Structural landscape of isolated agonist-binding domains from single AMPA receptors. Nat Chem Biol. 2011;7:168–173. doi: 10.1038/nchembio.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Adensik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cho Y, Lolis E, Howe JR. Structural and single channel results indicate that the rates of ligand binding domain closing and opening directly impact AMPA receptor gating. J Neurosci. 2008;28:932–943. doi: 10.1523/JNEUROSCI.3309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


