Abstract
By regulating inhibition at dendrodendritic synapses between mitral and granule cells (GCs), noradrenergic neurons extending from the brainstem provide an input essential for odour processing in the olfactory bulb (OB). In the accessory OB (AOB), we have recently shown that noradrenaline (NA) increases GABA inhibitory input on to mitral cells (MCs) by exciting GCs. Here, we show that GCs in the main OB (MOB) exhibit a similar response to NA, indicating a common mechanism for noradrenergic regulation of GC→MC inhibition throughout the OB. In GCs of the MOB, NA (10 μm) produced a robust excitatory effect that included a slow afterdepolarization that followed a train of action potentials evoked by a current stimulus. The depolarization and slow afterdepolarization in GCs were blocked by the α1A-adrenergic receptor (AR) selective antagonist WB 4101 (30 nm) and mimicked by the α1A-AR selective agonist A 61603 (1 μm). In recordings from MCs, A 61603 (30 nm–1 μm) produced a sizeable increase in the frequency of spontaneous and miniature IPSCs, an effect completely abolished by the GABAA receptor antagonist gabazine (5 μm). Likewise, activation of β-ARs increased the frequency of spontaneous IPSCs; however, this effect was smaller and confined to the first postnatal weeks. NA enhanced inhibition in MCs across a broad concentration range (0.1–30 μm) and its effects were completely abolished by a mixture of α1- and β-AR antagonists (1 μm prazosin and 10 μm propranolol). Furthermore, the general α2-AR agonist clonidine (10 μm) failed to affect sIPSC frequency. Thus, the NA-mediated increase in GC→MC inhibition in the OB results mostly from activation of the α1A-AR subtype.
Key points
Here, in mouse brain slices, we examined the cellular effects of noradrenaline to better understand its influence on olfactory bulb processing.
β- and α1-adrenergic receptor activation increases GABA currents in mitral cells in an age-dependent manner; the β-adrenergic effect is prominent only during early postnatal weeks, while the α1 effect is present at all ages.
This study focused on the α1-mediated increase in GABA inhibitory currents in mitral cells and found noradrenaline acts on the α1A-adrenergic receptor subtype to produce long-lasting excitation of granule cells.
The enhancement of inhibition by noradrenaline was consistent across a broad concentration range; at all concentrations, noradrenaline increased inhibitory currents in mitral cells.
Our studies highlight the important role of α1A-adrenergic receptor subtypes in increasing inhibition at dendrodendritic synapses, suggesting a synaptic mechanism for noradrenergic modulation of olfactory driven behaviours.
Introduction
The olfactory bulb (OB) receives significant noradrenergic input from the locus coeruleus (LC) that is instrumental for a broad range of olfactory behaviours, particularly those that depend on a strong association with social odours (Shipley et al. 1985; Brennan & Keverne, 1997). In the accessory OB (AOB), a region involved in the processing of chemosignals that trigger aggressive and reproductive behaviours, noradrenaline (NA) is thought to promote synaptic changes that underlie a female mouse's ability to learn a stud's odour during mating, also known as the Bruce effect (Brennan & Keverne, 2004). In the main OB (MOB), NA plays a similarly important role in several aspects of odour processing and odour memory formation, including discrimination of perceptually similar odours and long-term suppression of odour responses (Sullivan et al. 1989, 1992; Doucette et al. 2007; Shea et al. 2008).
Despite the importance of noradrenergic regulation of OB function, the underlying mechanisms facilitating short- and long-term plasticity by NA are not fully understood. In the OB, the most abundant neuronal type, the granule cells (GCs), form ubiquitous dendrodendritic synapses with projection neurons, mitral and tufted cells (hereafter referred to as MCs) (Shepherd et al. 2007). Lateral and recurrent inhibition at dendrodendritic synapses is an important physiological mechanism contributing to olfactory processing within the OB (Schoppa & Urban, 2003). The presence of noradrenergic fibres throughout the GC and MC layers suggests dendrodendritic synapses as a plausible target for the neuromodulatory action of NA (McLean et al. 1989). However, different studies have suggested contrasting mechanisms of action.
Adrenergic receptors (ARs) are divided into three major classes (α1, α2 and β), and their activation has been shown to produce either excitatory or inhibitory effects in MCs of the MOB (Salmoiraghi et al. 1964; McLennan, 1971; Jahr & Nicoll, 1982; Perez et al. 1987; Trombley & Shepherd, 1992; Mouly et al. 1995; Okutani et al. 1998; Ciombor et al. 1999; Hayar et al. 2001). Furthermore, recent studies in the MOB of rats have indicated that the effect of NA on OB neurons is concentration-dependent whereby low concentrations of NA decrease the frequency of GABA currents recorded in MCs and higher doses increase their occurrence (Nai et al. 2009). In contrast, in the AOB, we have shown that the overall effect of NA is to inhibit MCs by producing a long-lasting excitation in GCs (Araneda & Firestein, 2006; Smith et al. 2009). Lastly, the plasticity mechanisms underlying the facilitation of memory formation by NA may be different between the MOB and AOB. While NA increases gamma frequency oscillations in MCs of the MOB (Gire & Schoppa, 2008; Pandipati et al. 2010), an action associated with reduced inhibition, the proposed plasticity mechanisms in the AOB can be explained solely by enhancement of inhibitory input on to MCs (Leszkowicz et al. 2012).
Here, we report that GCs in the MOB exhibit an excitatory response to NA similar to that observed in the AOB, including a stimulus-elicited slow afterdepolarization (sADP). Pharmacological characterization indicated that the α1A-AR subtype mediates this effect. NA increased inhibitory GABA currents in MCs at all concentrations tested by activating α1A- and β-ARs. However, the β-AR effect was not observed in older animals, and at all ages tested, the α1A-AR response was significantly robust. Our results indicate a common mechanism for regulation of GC-MC synapses throughout the OB: NA activates α1A-ARs, enhancing inhibition of MCs, providing new evidence for the cellular events underlying the important olfactory behaviours facilitated by the noradrenergic system.
Methods
Slice preparation
All animal procedures were done in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Maryland. Experiments were performed in sagittal slices containing the OB obtained from postnatal day 4 (P4) to P99 C57BL/6 mice as previously described (Smith et al. 2009). Mice were anaesthetized with isoflurane and decapitated, and the brain was quickly removed and placed in oxygenated (95% O2 and 5% CO2) ice-cold ACSF containing low Ca2+ (1 mm) and high Mg2+ (3 mm). Sections (250–300 μm thick) of the OB were sliced using a Leica vibrating microslicer (Redding, CA, USA). The slices were then transferred to an incubation chamber containing normal ACSF (see below) and left to recuperate first at 37°C for 45 min and then at room temperature for another hour. In all experiments, unless otherwise indicated, the extracellular solution was ACSF of the following composition (in mm): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2 CaCl2 and 1 MgCl2, 3 myo-inositol, 0.3 ascorbic acid, 2 sodium pyruvate and 15 glucose, continuously oxygenated to pH ∼7.4 and with an osmolarity of ∼305 mosmol l−1.
Data acquisition and analysis
After incubation, the slices were transferred to a recording chamber mounted on the stage of an Olympus BX51 microscope. MCs and GCs were identified based on their size and position in the slices using a 40× water immersion objective. In the AOB, the lateral olfactory tract (LOT) separates the MC from GC layers. In the MOB, MCs are confined to a single layer, while GCs, which have smaller somata are found in a separate layer. Experiments were performed at room temperature using standard patch pipettes (3–8 MΩ resistance). Membrane potential and currents were recorded in current and voltage clamp mode using a dual EPC10 amplifier (HEKA, Bellmore, NY, USA). We performed data analysis using macros written for Igor Pro (Wavemetrics, Wosego, OR, USA) and Mini60 software (Synaptosoft Inc., Decatur, GA, USA).
In current-clamp experiments, the sADP in GCs was elicited using a depolarizing current stimulus (500 ms every 30 s) of variable intensity, adjusted to produce 3–12 action potentials. We measured the sADP as the most positive membrane potential value after the end of the pulse and it generally occurred within a few seconds (<3 s) after the stimulus. The pre-stimulus membrane potential was subtracted from each of these values; therefore, the reported values of afterhyperpolarization and sADP correspond to the ΔV (in mV). Similarly, changes in membrane potential (ΔVm) in the presence of drugs correspond to the most depolarized value, which in many cases was the cell's threshold, minus the baseline membrane potential. The duration of the sADP was quantified by fitting a single exponential from the peak of the response, and is expressed as the τ of decay.
GABA IPSCs were recorded at 0 mV in MCs and the kinetic analysis was performed using Mini60 software. The frequency and amplitude of IPSCs were determined in 2 min segments right before (control) and 1.5–2 min segments right after drug application. Fold increase in IPSC frequency is expressed as the ratio of the change in IPSC frequency produced by the drug to the basal frequency (IPSCdrug– IPSCbasal)/(IPSCbasal). Statistical significance for frequency changes and fold increases in the presence of drugs was assessed using the Student's paired t test and shifts in the cumulative probability distributions were determined with the Kolmogorov–Smirnov test. For the dose–response data, the reported fold increase in sIPSC frequency was normalized to the value at the highest concentration tested and fit using a Hill equation routine in Igor Pro. The values reported correspond to results from at least six different trials and error bars indicate the standard error of the mean.
Solutions and pharmacological agents
We determined the effect of NA on GC excitability using current-clamp recordings with pipettes containing the following internal solution (in mm): 120 potassium gluconate, 10 sodium gluconate, 4 NaCl, 10 Hepes-K, 10 sodium phosphocreatine, 2 Na-ATP, 4 Mg-ATP and 0.3 GTP adjusted to pH 7.3 with KOH. To determine the effect of NA on GABA IPSC frequency in MCs, we used voltage clamp recordings, with pipettes containing (in mm): 125 caesium gluconate or caesium methylsulphate, 4 NaCl, 2 MgCl2, 2 CaCl2, 10 EGTA, 10 Hepes, 2 Na-ATP, 4 Mg-ATP and 0.3 GTP adjusted to pH 7.3 with CsOH. This Cs based internal solution allows for stable recording of outward GABA currents with minimal influence from ionotropic glutamate currents, which reverse close to the holding potential of 0 mV. The osmolarity of the internal solutions ranged 290–305 mosmol l-1. The following drugs were bath applied during experiments: l-(–)-NA (+)-bitartrate salt monohydrate (NA), (R)-(–)-1-(3-hydroxyphenyl)-2-methylaminoethanol hydrochloride (phenylephrine, PE), 4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]-1,2-benzenediol hydrochloride (isoproterenol, ISO), N-[5-(4,5-dihydro-1H-imidazol-2-yl)-2-hydroxy-5, 6, 7, 8-tetrahydronaphthalen-1-yl]methanesulphonamide hydrobromide (A 61603), 2-[(2,6-dichlorophenyl)amino-2-imidazoline hydrochloride (clonidine, CLO), 2-(2,6-dimethoxyphenoxyethyl) aminomethyl-1, 4-benzodioxane hydrochloride (WB 4101), 1-(4-Amino-6, 7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine hydrochloride (prazosin), (RS)-1-[(1-methylethyl)amino]-3-(1-naphthalenyloxy)-2-propanol hydrochloride (propranolol), dl-2-amino-5-phosphonopentanoic acid, 6-cyano-7-nitroquinoxaline-2,3-dione disod-ium, 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (gabazine, GBZ), octahydro-12-(hydroxymethyl)-2-imino-5,9:7,10a-dimethano-10aH-[1,3]dioxocino[6,5-d]pyrimidine-4,7,10,11,12-pentol citrate (tetrodotoxin, TTX). The chamber volume and speed of perfusion were adjusted to allow for full exchange of the solution in less than 1 min. Antagonists were applied for at least 10 min before agonists. All drugs were purchased from Tocris Cookson (Minneapolis, MN, USA) with the exception of NA, which we bought from Sigma-Aldrich (St Louis, MO, USA).
Results
Noradrenaline increases GABA release in the main olfactory bulb by exciting granule cells
We have previously shown that NA produces a robust depolarization of GCs in the AOB (Smith et al. 2009). GCs consist of a heterogeneous group of interneurons and we wondered whether GCs in the MOB exhibited a similar response to NA. Therefore, we conducted whole-cell recordings in sagittal slices of the OB, where both subregions are preserved (MOB and AOB, Fig. 1A), using the current-clamp mode in GCs and voltage clamp for MCs. As shown in Fig. 1B, a submaximal concentration of NA widely used in studies with similar slice preparations (10 μm, see also below), produced a robust excitatory effect in MOB GCs (ΔVm: 12.8 ± 0.9 mV, n= 33). This excitatory response had a slow onset (time to peak: 184 ± 17 s, n= 5) and lasted several minutes (>10 min). In agreement with a common cellular mechanism of action on GCs throughout the OB, NA also induced the appearance of an sADP, following a stimulus-elicited train of action potentials, in GCs of the MOB (Fig. 1C; ΔVm: 6.5 ± 0.6 mV, n= 27). Importantly, the kinetics of this sADP were similar to those of the sADP in GCs of the AOB ((Smith et al. 2009); time to peak: 2.1 ± 0.5 s; τ of decay: 10.1 ± 4.2 s; n= 7).
Figure 1. NA increases GABA release in the MOB by exciting GCs.
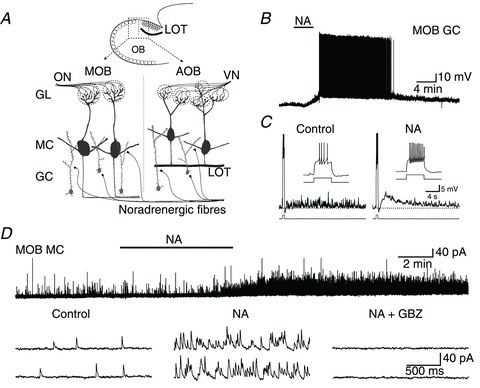
A, diagram of a sagittal slice of the OB showing the relative positions of the MOB and AOB and neurons we recorded from. LOT (dark line) forms a boundary that defines the AOB. Sensory neurons relay peripheral information via the ON and VN to GL in the MOB and AOB, where they synapse with MCs (dark grey). GCs (light grey) form numerous dendrodendritic synapses with MCs, influencing their output through GABAergic inhibition. Noradrenergic fibres innervate both the MOB and AOB where they are thought to regulate dendrodendritic synaptic activity. In the AOB, the LOT separates GCs from MCs. B, recording from a GC in the MOB where bath application of NA (10 μm) depolarized the cell to threshold and resulted in several minutes of spontaneous firing (−60 mV resting membrane potential). C, in another MOB GC, NA (10 μm, 100 s application) produced a slow afterdepolarization following a stimulus-induced train of action potentials (65 pA, 500 ms). Shown are the responses to current pulses before and after application of NA with expansions of the stimulus-elicited spike trains displayed in the insets. Similar to NA-induced excitatory responses in GCs of the AOB (see text and (Smith et al. 2009), the peak of this slow afterdepolarization (ΔVm: 9 mV) occurred ∼3 s after the end of the stimulus pulse and had a slow decay. The dotted lines indicate the pre-stimulus membrane potential (control: −78 mV, NA: −68 mV; calibration bar: 3 s, 10 mV). D, top trace: recording from an MC in the MOB where NA (10 μm) dramatically increased the frequency of sIPSCs with a time course similar to that of the depolarization of GCs (control: 0.9 Hz, NA: 19.5 Hz). Bottom traces: representative sIPSCs from the above recording pre- and postapplication of NA (left and centre traces, respectively). This cell's sIPSCs were abolished by the GABAA receptor antagonist GBZ (right traces; 5 μm). The holding potential was 0 mV in this and all following voltage clamp experiments. AOB, accessory olfactory bulb; GBZ, gabazine; GC, granule cells; GL, glomeruli, LOT, lateral olfactory tract; MC, mitral/tufted cells; MOB, main olfactory bulb; NA, noradrenaline; ON, olfactory nerve; sIPSC, spontaneous IPSCs; VN, vomeronasal nerve.
In accordance with this excitatory effect on GCs, voltage clamp recordings indicated that NA (10 μm) produced a 7.9 ± 1.7-fold increase in the frequency of spontaneous IPSCs (sIPSCs) in MOB MCs (Fig. 1D; control: 1.3 ± 0.3 Hz, NA: 9.0 ± 1.8 Hz, P < 0.0005, n= 12). This effect had a slow onset (153 ± 18 s, n= 5) and was long-lasting, closely mimicking the time course of the depolarization recorded in GCs. The basal and NA-stimulated sIPSCs were completely abolished in the presence of the GABAA receptor antagonist GBZ (5 μm) indicating that they correspond to GABA activated currents (Fig. 1C; NA + GBZ: 0.0 ± 0.0 Hz, n= 5). In addition, NA also increased the frequency of miniature IPSCs (mIPSCs) measured in the presence of TTX (0.5 μm) and blockers of fast excitatory transmission (100 μm dl-2-Amino-5-phosphonopentanoic acid, an NMDA receptor antagonist, and 10 μm 6-Cyano-7-nitroquinoxaline-2,3-dione disodium, an AMPA receptor antagonist; data not shown). Under these conditions, NA produced an 8.3 ± 3.5-fold increase in frequency (control: 0.9 ± 0.3 Hz, NA: 5.9 ± 1.8 Hz, P < 0.02, n= 8), further suggesting a direct effect of NA on MOB GCs.
Multiple adrenergic receptor subtypes increase GABA release in the olfactory bulb in an age-dependent manner
To further characterize the excitatory effect of NA on sIPSC frequency in the MOB, we examined the actions of PE (10 μm) and ISO (10 μm), two general agonists of α1- and β-ARs, respectively. In recordings from 2- to 4-week-old slices, we found that both ISO and PE, tested in the same MCs (Fig. 2A, left traces), produced significant increases in sIPSC frequency, with the effect of PE being greater than that of ISO (Fig. 2A, right graph; control: 1.7 ± 0.2 Hz; ISO: 3.0 ± 0.4 Hz, P < 0.0002; PE: 5.3 ± 0.7 Hz, P < 0.000004; n= 21). In the AOB, we observed similar effects in animals of the same age range, including a larger effect for PE (data not shown; control: 1.5 ± 0.2 Hz; ISO: 2.4 ± 0.4 Hz, P < 0.00003; PE: 6.5 ± 0.9 Hz, P < 0.000003; n= 19).
Figure 2. NA increases sIPSC frequency in MCs by activating α1- and β-ARs in an age-dependent manner.
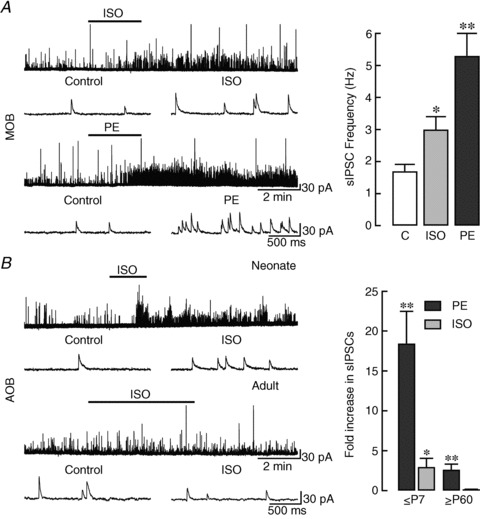
A, left panel: recording of sIPSCs in an MC in the MOB of a P14 slice. Top traces: ISO (10 μm), a β-AR agonist, produced a ∼1-fold increase in sIPSC frequency (control: 0.5 Hz, ISO: 1.2 Hz). Bottom traces: the effect of PE (10 μm), an α1-AR agonist, was much greater in the same cell, increasing sIPSC frequency by ∼15-fold (control: 0.6 Hz, PE: 10.2 Hz). Representative traces in an expanded time scale, before and after drug application, are shown below. Right panel: summary of the magnitude of the α1- and β-AR-mediated effects on sIPSC frequency in MCs of the MOB. Though both ISO and PE increased the frequency of sIPSCs, the effect of PE was larger (*P < 0.00002; **P < 0.000001). The reported averages are from cells in P14–28 slices where both PE and ISO were applied (n= 21). B, left panel: recordings of sIPSCs from MCs in the AOB of slices from P4 (neonate, top traces) and P70 (adult, bottom traces) animals. While β-AR activation produced large increases in sIPSC frequency in young mice, ISO had no effect in older animals. Representative traces in an expanded time scale, before and after ISO application, are shown below. Right panel: summary of the effects of PE (black bars) and ISO (grey bars) on sIPSC frequency in the AOB of young and old mice. In both young (≤P7; n= 14) and old (≥P60; n= 10) mice, PE produced a significant increase in sIPSC frequency (**P < 0.0002). In contrast, in the same cells ISO significantly increased the frequency only in the younger group (*P < 0.05). AR, adrenergic receptor; AOB, accessory olfactory bulb; ISO, isoproterenol; MC, mitral/tufted cells; MOB, main olfactory bulb; PE, phenylephrine; sIPSC, spontaneous IPSCs.
Interestingly, comparison of recordings obtained at different ages revealed that the responses to PE and ISO were largest in slices from younger mice. Thus, as illustrated by recordings from the AOB (Fig. 2B, left traces), when we divided MCs into two age cohorts (≤P7 and ≥P60), we found that the profile of responses was significantly different between young and old mice, particularly with respect to β-AR activation (Fig. 2B, right graph). Table 1 summarizes our findings in both the AOB and MOB, where we found that the sIPSC frequency increases produced by ISO were limited to the neonatal age group (≤P7), while PE significantly increased sIPSC frequency in both young and old mice. These results suggest that activation of both α1- and β-ARs by NA contribute to increased inhibitory input on to MCs in the OB of neonatal mice. Moreover, they highlight the potential importance of the α1-AR subtype in regulating inhibition during olfactory mediated behaviours relevant to early events (e.g. mother–infant interaction), and adulthood (e.g. mating). Therefore, we pursued a more detailed characterization of the α1-AR-mediated response.
Table 1.
Noradrenerqic requlation of sIPSC frequency durinq development
| Control | ISO | Control | PE | ISO Fold Δ | PE Fold Δ | |
|---|---|---|---|---|---|---|
| AOB | ||||||
| ≤P7 | 0.7 ± 0.3 | 1.2 ± 0.4* | 0.5 ± 0.2 | 5.2 ± 1.0** | 3.0 ± 1.1* | 18.5 ± 4.0** |
| ≥P60 | 5.7 ± 1.1 | 5.9 ± 1.1 n.s. | 4.6 ± 1.1 | 11.5 ± 1.3** | 0.1 ± 0.1 n.s. | 2.6 ± 0.7** |
| MOB | ||||||
| ≤P7 | 0.6 ± 0.2 | 1.1 ± 0.3* | 0.9 ± 0.3 | 4.3 ± 0.7** | 2.3 ± 0.7* | 6.2 ± 1.4** |
| ≥P60 | 3.6 ± 0.8 | 3.6 ± 0.8 n.s. | 2.9 ± 0.7 | 5.6 ± 1.6* | 0.04 ± 0.08 n.s. | 0.9 ± 0.2** |
AOB, accessory olfactory bulb; AR, adrenergic receptor; ISO, isoproterenol; MOB, main olfactory bulb; PE phenylephrine; sIPSCs, spontaneous IPSCs. Summary of the age-dependent effects of the α1- and β-AR selective agonists PE and ISO on sIPSC frequency in MCs of the AOB and MOB. Fold increase in sIPSC frequency is expressed as the ratio of the change in sIPSC frequency produced by the drug to the basal frequency (see Methods). In both regions, PE produced significant frequency increases in the young (≤P7) and old (≥P60) age groups, while frequency increases by ISO were restricted to younger mice (*P < 0.02, **P < 0.002, n.s. P > 0.3). Control and agonist sIPSC frequencies (Hz) and fold Δ values are expressed as the average ±s.e.m.
Noradrenaline excites granule cells in the olfactory bulb by activating α1A-adrenergic receptors
Pharmacological characterization of the response to NA indicated that the excitation in GCs results from activation of the α1A-AR subtype (Fig. 3). Both effects of NA in MOB GCs, the long-lasting depolarization (ΔVm: 12.6 ± 2.0 mV, n= 12) and stimulus-induced sADP (Fig. 3A, right panel 2; sADP: 5.4 ± 0.8 mV, n= 9) were greatly reduced by a sub-micromolar concentration of the α1A-AR selective antagonist WB 4101 (30 nm; ΔVm: 1.0 ± 0.6 mV, P < 0.0004; sADP: 0.3 ± 0.2 mV, P < 0.0002). Likewise, a low concentration of the α1A-AR selective agonist A 61603 (1 μm) mimicked the depolarization and sADP of NA, producing responses of comparable magnitude (Fig. 3B; ΔVm: 10.6 ± 1.0 mV; sADP: 5.3 ± 0.6 mV; n= 9). In addition, the time course of the A 61603 induced depolarization and the kinetics of its sADP were similar to that of NA (data not shown; ΔVm time to peak: 152 ± 26 s, n= 5; sADP time to peak: 2.6 ± 0.6 s; τ of decay: 19.0 ± 5.3 s; n= 6).
Figure 3. NA excites GCs in the MOB and AOB by activating α1A-ARs.
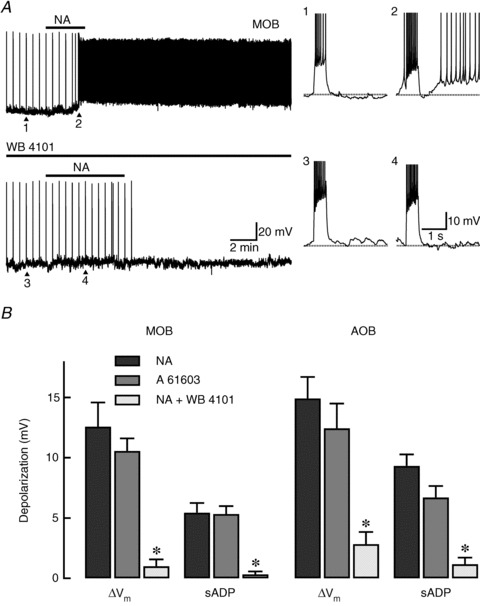
A, top trace: recording from a GC in the MOB where bath application of NA (10 μm) depolarized the cell and produced an sADP that resulted in a series of spontaneous action potentials. In this cell, spike trains, which appear compressed as vertical lines in the time axis, were elicited every 30 s by a depolarizing current stimulus (30 pA, 500 ms). The right traces correspond to those indicated by the arrowheads and are shown in an expanded time scale. In these traces, the dotted lines indicate the pre-stimulus membrane potential. 1: under control conditions, the current stimulus produced seven spikes that were followed by a small afterhyperpolarization (ΔVm: 1 mV). 2: post-application of NA, the same stimulus elicited 10 spikes and an sADP (7 mV) that greatly enhanced the cell's excitability. Bottom trace: in the same cell, application of a low concentration of the α1A-AR selective antagonist WB 4101 (30 nm) completely abolished the depolarization and sADP produced by NA. 3 and 4: expanded responses to the current pulse at the points indicated with arrowheads (control and NA in the presence of WB 4101, the resting membrane potential is −62 mV). B, summary of the α1A-AR-mediated changes in excitability of GCs in the MOB and AOB. The α1A-AR selective antagonist WB 4101 (30 nm, white bars) greatly reduced the NA-induced depolarization (ΔVm, black bars) in the MOB and AOB (n= 12 respectively). Likewise, application of WB 4101 significantly decreased the sADP in both regions (*P < 0.0006; MOB: n= 9; AOB: n= 6). Similarly, the NA-induced depolarization and sADP were mimicked by the α1A-AR selective agonist A 61603 (1 μm, grey bars) in the MOB and AOB (n= 9, respectively). AOB, accessory olfactory bulb; AR, adrenergic receptor; GC, granule cells; MOB, main olfactory bulb; NA, noradrenaline; sADP, slow afterdepolarization.
Consistent with a uniform adrenergic action throughout the OB, the pharmacology of the response to NA in AOB GCs indicated involvement of the same receptor subtype (Fig. 3B). In the AOB, A 61603 (1 μm) depolarized GCs by 12.4 ± 2.0 mV and produced an sADP following current stimulus (sADP: 6.7 ± 0.9 mV, n= 9). The time course of the response to A 61603 was similar to that in the MOB, with a slow onset (time to peak: 132 ± 29 s, n= 5) and comparable kinetics for the sADP (time to peak: 1.8 ± 0.2 s; τ of decay: 12.4 ± 6 s; n= 6). Similarly, the depolarization and sADP induced by NA in the AOB were greatly reduced in the presence of WB 4101 (30 nm; ΔVm: 14.9 ± 1.8 vs. 2.8 ± 1.0 mV, P < 0.00008, n= 12; sADP: 9.3 ± 1.0 vs. 1.2 ± 0.5 mV, P < 0.0006, n= 6).
Activation of α1A-adrenergic receptors increases GABA inhibitory currents in mitral cells
In addition to an excitatory effect on GCs, A 61603 produced a dose-dependent increase in inhibition of MCs in the AOB and MOB. As illustrated in Fig. 4A by an AOB MC, application of A 61603 (30 nm) produced a 2.2 ± 0.7-fold increase in sIPSC frequency (control: 1.5 ± 0.4 Hz, A 61603: 4.3 ± 1.5 Hz, P < 0.03, n= 7). At 100 nm, A 61603 produced a 6.1 ± 2.2-fold increase in sIPSCs (control: 2.6 ± 0.6 Hz, A 61603: 13.4 ± 2.8 Hz, P < 0.006, n= 5), and an 11.6 ± 4.6-fold increase when applied at 1 μm (control: 3.0 ± 1.1 Hz, A 61603: 17.3 ± 3.1 Hz, P < 0.0004, n= 7). The effect we observed in response to 1 μm A 61603 was comparable to that produced by 10 μm NA, and was also completely abolished by GBZ (5 μm; 0.0 ± 0.0 Hz, n= 3; data not shown). Similarly, the increase in sIPSCs had a slow onset (140 ± 24 s, n= 5) and was long-lasting (>10 min). As shown in Fig. 4B (left), A 61603 (1 μm) significantly shifted the cumulative probability function for inter-event intervals in the AOB (P < 0.0001). Similar to NA, the large frequency increases produced by A 61603 occurred without affecting average sIPSC amplitude (Fig. 4B, left inset; control: 22.8 ± 1.9 pA, A 61603: 24.8 ± 2.2 pA, P > 0.1). Likewise, the average amplitude of the A 61603 elicited events was not different from those produced by NA (22.7 ± 3.4 pA, P > 0.6, n= 8).
Figure 4. α1A-AR activation increases GABAergic inhibition on to MCs in the AOB and MOB.
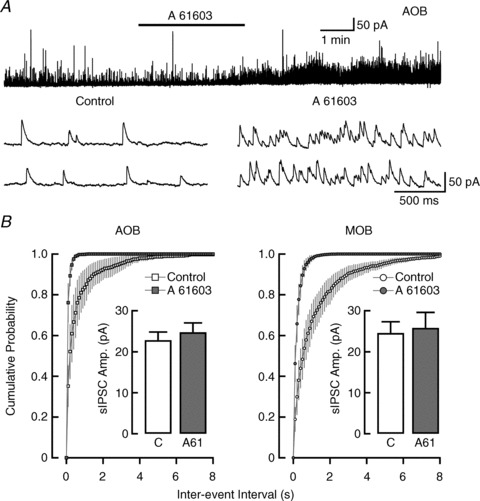
A, top trace: recording from an MC in the AOB, where sIPSC frequency was greatly increased by a low concentration of the α1A-AR selective agonist A 61603 (30 nm; control: 2.1 Hz, A 61603: 8.0 Hz). Bottom traces: representative sIPSCs pre-application (left) and post-application (right) of A 61603 from the above trace. B, average cumulative inter-event interval distributions of sIPSCs in MCs of the AOB (left) and MOB (right). Application of 1 μm A 61603 (filled squares and circles) produced a leftward shift from the control event distribution (open squares and circles) consistent with an increase in sIPSC frequency. Insets: the average sIPSC amplitude in A 61603 (grey bars) was not different from control conditions (white bars) in the AOB (n= 7) or MOB (n= 8). AOB, accessory olfactory bulb; MCs, mitral cells; MOB, main olfactory bulb; sIPSCs, spontaneous IPSCs.
Similar results were obtained in the MOB. At 1 μm, A 61603 produced a 5.1 ± 1.6-fold increase in sIPSC frequency (control: 1.8 ± 0.7 Hz, A 61603: 7.4 ± 1.8 Hz, P < 0.003, n= 8), also shown as a leftward shift in the cumulative probability plot in Fig. 4B (right; P < 0.0001). As in the AOB, the average amplitude of the sIPSCs recorded post-application of A 61603 was not different from control (Fig. 4B, right inset; control: 24.6 ± 2.7 pA, A 61603: 25.8 ± 3.8 pA, P > 0.3) or from the average amplitude of sIPSCs produced by NA (22.6 ± 2.0 pA, P > 0.4, n= 12). The effect of this agonist extended to 100 nm, where A 61603 produced a 5.3 ± 1.7-fold increase in sIPSC frequency in MCs (control: 2.0 ± 0.4 Hz, A 61603: 12.0 ± 4.2 Hz, P < 0.04, n= 5). Lastly, like NA, application of A 61603 (1 μm) also increased the frequency of mIPSCs recorded in the MOB and AOB (data not shown; MOB: 1.9 ± 1.0 vs. 6.3 ± 2.9 Hz, P < 0.05, n= 4; AOB: 1.7 ± 0.8 vs. 9.7 ± 3.4 Hz, P < 0.04, n= 5).
As described above, NA acting on α1-ARs produced a significant increase in inhibitory currents in MCs across all ages in the MOB and AOB. Likewise, A 61603 (100 nm) significantly increased sIPSC frequency in young animals in the MOB (P7; control: 3.0 ± 0.7 Hz, A 61603: 11.0 ± 2.7 Hz, P < 0.03, n= 4). In the same age group, A 61603 produced a 4.3 ± 1.3-fold increase in frequency in the AOB (control: 3.2 ± 1.0 Hz, A 61603: 13.2 ± 1.1 Hz, P < 0.00005, n= 4). Similarly, in older mice (≥P60), A 61603 (100 nm) significantly increased sIPSC frequency in both regions (MOB, control: 2.3 ± 0.7 Hz, A 61603: 6.6 ± 2.5 Hz, P < 0.04, n= 7; AOB, control: 3.3 ± 0.5 Hz, A 61603: 12.3 ± 1.6 Hz, P < 0.0007, n= 5).
Noradrenaline regulates inhibition of mitral cells in a concentration-invariant manner
Previous work has shown that NA produces a concentration-dependent effect on IPSC frequency in the MOB of rats (Nai et al. 2009, 2010). At low concentrations, NA decreased the frequency of IPSCs through activation of α2-ARs, while at higher concentrations it increased inhibition. To examine the possibility that NA may regulate inhibition in the mouse OB in a similar manner, we recorded sIPSCs in response to submicromolar concentrations of NA in both MOB and AOB MCs. Unexpectedly, we found that even at a low concentration (0.3 μm), NA significantly increased sIPSC frequency in both regions of the OB (Fig. 5A traces). In the MOB (top traces), this low dose of NA increased sIPSC frequency by ∼1-fold (control: 3.1 ± 0.6 Hz, NA: 5.7 ± 1.1 Hz, P < 0.005, n= 11), and we observed a similar change in the AOB (bottom traces, control: 2.3 ± 0.8 Hz, NA: 3.8 ± 1.1 Hz, P < 0.03, n= 10). Furthermore, a small, but significant, increase in frequency was observed in the MOB following application of an even lower NA concentration (0.1 μm, 0.3 ± 0.03-fold increase in frequency; control: 2.2 ± 0.9 Hz, NA: 2.9 ± 1.1 Hz, P < 0.04, n= 5, data not shown). We found comparable frequency increases in mIPSC recordings; at 0.1 and 0.3 μm in the MOB, NA produced significant increases in mIPSC frequency (0.3 ± 0.05 Hz vs. 0.4 ± 0.06 Hz, P < 0.02, n= 8 and 0.7 ± 0.2 Hz vs. 0.9 ± 0.3 Hz, P < 0.02, n= 11, respectively). In the AOB, 0.3 μm NA produced a 1-fold increase in mIPSCs (Fig. 5B; control: 0.6 ± 0.1 Hz, NA: 1.2 ± 0.3 Hz, P < 0.04, n= 6).
Figure 5. NA produces a concentration-invariant increase in sIPSC frequency in MCs.
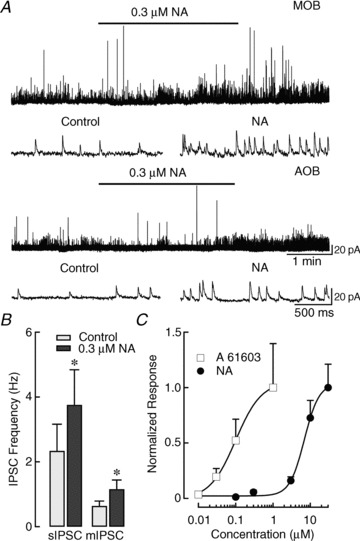
A, top traces: recording from an MC in the MOB where a low concentration of NA increased sIPSC frequency by ∼1-fold (control: 2.5 Hz, NA: 4.5 Hz). Lower traces: recording from an MC in the AOB where a low concentration of NA also increased sIPSC frequency (control: 2.5 Hz, NA: 4.5 Hz). Representative expanded traces pre- and post-application of NA are shown below. B, a low concentration of NA increased both sIPSCs (n= 10) and mIPSCs (n= 6) in the AOB. mIPSCs were recorded in the presence of dl-2-Amino-5-phosphonopentanoic acid, 6-Cyano-7-nitroquinoxaline-2,3-dione disodium and tetrodotoxin (**P < 0.05). C, dose–response curve for the effects of NA (black circles) and A 61603 (white squares) on sIPSC frequency in the AOB. Data were obtained from different cells and normalized to the fold increase in sIPSCs at the highest concentration of agonist. The fitted line was obtained using the Hill equation; A 61603 was more potent than NA (NA, EC50: 6.9 μm; A 61603, EC50: 0.1 μm). At 10 nm, A 61603 produced a small, though not significant, fold increase (control: 2.5 ± 1.0 Hz, A 61603: 4.3 ± 2.6 Hz, P > 0.3, n= 6). Similarly, 0.1 μm NA produced a small, but not significant, fold increase in frequency (control: 1.5 ± 0.5 Hz, NA: 1.9 ± 0.6 Hz, P > 0.1, n= 7). At concentrations greater than these, the frequency increases were statistically significant for both NA and A 61603 (see Results). mIPSCs, miniature IPSCs; MOB, main olfactory bulb; NA, noradrenaline; sIPSCs, spontaneous IPSCs.
This concentration-invariant effect is illustrated in the dose–response curve in Fig. 5C for sIPSC recordings from MCs in the AOB. Across the broad range of concentrations tested, NA produced only increases in inhibition (apparent EC50= 6.9 μm). For comparison, we have included data for A 61603, which, at all concentrations, was more potent than NA in eliciting augmentation of sIPSC frequency (apparent EC50= 0.1 μm). In agreement with these results, in the presence of prazosin (1 μm) and propranolol (10 μm), general α1- and β-AR antagonists, NA failed to affect sIPSC frequency in the MOB (data not shown, control: 1.48 ± 0.26 Hz, NA + blockers: 1.45 ± 0.25 Hz, P > 0.7, n= 9) or AOB (control: 1.42 ± 0.43 Hz, NA + blockers: 1.51 ± 0.51 Hz, P > 0.3, n= 7). Likewise, the α2-AR agonist CLO (10 μm) did not alter sIPSC frequency in the MOB (data not shown, control: 1.93 ± 0.30 Hz, CLO: 1.91 ± 0.27 Hz, P > 0.6, n= 22) or AOB (control: 2.19 ± 0.34 Hz, CLO: 1.97 ± 0.27 Hz, P > 0.1, n= 24). Taken together, these results support an overall enhancement of inhibition in MCs by NA, acting primarily on α1A-ARs, throughout the OB.
Discussion
Here, we show that NA modulates GABAergic inhibition by a common mechanism throughout the OB. NA had a robust excitatory action on GCs in the MOB, including induction of an sADP, a response similar to what we have previously described in the AOB. The long-lasting depolarization produced by NA greatly enhanced the release of GABA from GCs, dramatically increasing inhibitory currents in MCs. The pharmacological profile of these effects was consistent with the activation of α1A-ARs. Two mechanisms contributed to the increase in IPSC frequency in MCs although the degree of enhancement varied with age. During early postnatal days, activation of both β- and α1A-ARs significantly increased sIPSC frequency, but only the α1A-AR-mediated effect remained in older animals. Moreover, in both AOB and MOB MCs, NA increased inhibitory currents over a wide concentration range that included sub-micromolar doses, bringing us to the conclusion that in mice, NA acts to enhance GABAergic inhibition of MCs.
The OB receives a significant noradrenergic projection from the LC (Shipley et al. 1985) and GCs are an important target of neuromodulation by this system (Jahr & Nicoll, 1982; Araneda & Firestein, 2006; Smith et al. 2009; Nai et al. 2010). Although GCs comprise a heterogeneous group of inhibitory neurons (Shepherd et al. 2007), our results indicate that the effect of NA on GC excitability is rather consistent between the AOB and MOB, suggesting that the noradrenergic system regulates activity in the OB network by increasing inhibition. Our results are in agreement with previous findings in the MOB, which also indicate an inhibitory effect of NA on MCs (Salmoiraghi et al. 1964; McLennan, 1971; Mouly et al. 1995). Additionally, stimulation of the LC has been shown to produce several types of responses in the MOB, including inhibition. For example, LC stimulation produced a biphasic effect that promoted depression and subsequent potentiation of dendrodendritic inhibition, increased inhibition of MCs and suppression of MC responses to odours (Perez et al. 1987; Okutani et al. 1998; Shea et al. 2008). Nevertheless, other studies have indicated excitatory noradrenergic effects in the MOB (Jahr & Nicoll, 1982; Trombley & Shepherd, 1992; Ciombor et al. 1999; Hayar et al. 2001; Gire & Schoppa, 2008; Pandipati et al. 2010). In our experiments, blockers of fast synaptic transmission and TTX did not affect the magnitude of the inhibition produced by NA, suggesting a direct excitatory action on GCs. In addition, NA increased the frequency of GABA IPSCs without significantly affecting the amplitude of these currents, further arguing for a presynaptic mechanism. The increase in IPSC frequency is consistent with an enhancement of activity at dendrodendritic synapses between GC and MCs (GC→MC) and inhibition of MCs. However, our experiments do not rule out the possibility of long-term downregulation of dendrodendritic synaptic activity by NA, which could promote a disinhibition of MCs such as that reported in the MOB (Gire & Schoppa, 2008; Pandipati et al. 2010). Interestingly, a recent study in the AOB showed that local infusions of NA failed to disinhibit MCs, despite inducing long-term plasticity similar to that observed during memory formation in female mice (Leszkowicz et al. 2012). These results agree with our observations and further support an inhibitory role of NA in the OB network. We note, however, that in the absence of information regarding AR distribution in OB GCs, we cannot rule out functional differences at the circuit level or differences in the physiological role of this inhibition between the AOB and MOB. For example, MCs in the AOB have shorter secondary dendrites and as a result, most of their dendrodendritic synapses are formed via primary dendrites, unlike in the MOB where dendrodendritic synapses are made mostly with secondary dendrites (Takami & Graziadei, 1991).
Three classes of receptors, α1, α2 and β, each of which is further divided into at least three subtypes, mediate adrenergic responses in the brain (Hein & Kobilka, 1995; Small et al. 2003). Here, we show that nanomolar concentrations of α1A-AR selective agonist mimicked the effect of NA, while a selective antagonist of this receptor significantly reduced the response. These results agree with the reported high expression of the α1A-AR subtype in the OB, especially in the GC layer (Day et al. 1997; Domyancic & Morilak, 1997; Papay et al. 2006). While α1A-ARs have been extensively characterized in the autonomic nervous system, little is known about their physiological function in the brain (Tanoue et al. 2003; Docherty, 2010). In interneurons of the hippocampus and amygdala, activation of α1A-ARs produces increased GABAergic transmission, similar to what we show here in the OB (Braga et al. 2004; Hillman et al. 2007, 2009). In addition, activation of α1A-ARs by NA induced the appearance of an sADP that often resulted in long-lasting spontaneous spiking of GCs. Accumulating evidence supports the notion that this sADP results from activation of transient receptor potential channels, which induce a non-selective cationic current (ICAN) in neurons (Constanti et al. 1993; Haj-Dahmane & Andrade, 1999; Pressler et al. 2007; Smith et al. 2009; Yan et al. 2009). Interestingly, we have previously shown in the AOB that activation of M1 muscarinic acetylcholine receptors (M1 mAChRs) and metabotropic glutamate receptor type 1 (mGluR1) elicits an sADP in GCs with characteristics similar to that produced by the α1A-AR activation described here (Smith et al. 2009). Additionally, M1 mAChR activation produced a similar effect in GCs of the MOB (Pressler et al. 2007). All these receptors subtypes couple through Gq/11 and activate phospholipase C to increase intracellular Ca2+ (Conn & Pin, 1997; Zhong & Minneman, 1999), an essential step in the activation of the sADP in GCs (Pressler et al. 2007; Smith et al. 2009). Together these results suggest that in GCs, similar second messenger pathways activated by different neuromodulatory systems can converge on to a common final target, the activation of ICAN and the subsequent depolarization of GCs.
Similarly, another transient receptor potential current, dependent on NMDA activation, has also been described in MOB GCs (Hall & Delaney, 2002; Stroh et al. 2012). This implies that GCs possess multiple independent mechanisms, including ionotropic and metabotropic signalling, to activate this excitatory current. Interestingly, the kinetics of the depolarizing response produced by NA in GCs had a slow onset. This suggests that activation of ICAN by NA could allow GCs to integrate excitatory inputs over a long period, for example, when mice engage in active sniffing during olfactory behaviours. The sADP activated by NA exhibits similar characteristics to the ADP described in cortical circuits (Egorov et al. 2002). There, the persistent activity accompanying the sADP and the associated increase in intracellular Ca2+ was suggested as an underlying mechanism for learning. Therefore, we propose that the α1A-AR-mediated sADP could serve the same purpose in the OB.
Activation of β-ARs also increased sIPSC frequency; however, this effect was present in young (P4–P28), but not in adult mice (≥P60). This suggests that in early postnatal weeks, NA can increase GABAergic input on to MCs through two different mechanisms. These results are in agreement with behavioural studies showing that odour preference learning in neonatal rats is dependent on β-AR activation (Sullivan et al. 1989, 1992), but see (Yuan et al. 2004). Contrasting the robust depolarization of GCs mediated by α1A-ARs, we have failed to see a significant change in membrane potential induced by β-AR activation (data not shown). However, we cannot rule out the possibility of a direct effect of β-ARs on the release machinery at dendrodendritic synapses without affecting GC somatic membrane potential. In addition, it is possible that during early postnatal weeks the site of action of these receptors could switch within the network. For example, recent work in the MOB showed a change in the expression of M1 mAChRs from MCs to GCs in rats as they aged (Ghatpande & Gelperin, 2009). Nevertheless, in agreement with the NA-induced excitation of GCs, the α1A-adrenergic effect on IPSC frequency was greater than the β-adrenergic effect at all ages, including early postnatal days. Furthermore, we also noticed that the enhanced frequency of inhibitory events in MCs, promoted by the selective activation of these receptors, was significantly greater in younger mice. This age-dependent increase in inhibitory currents on to MCs together with the activation of parallel mechanisms to increase sIPSC frequency (α1A- and β-ARs) suggests that NA produces a stronger overall inhibitory effect in the OB of younger mice. A possible mechanism underlying this observation is that the basal frequency was significantly smaller in younger animals and increased with age. Further experiments are necessary to more clearly define the precise neuronal location of these receptors during early postnatal days using immunostaining methods.
Intriguingly, submicromolar concentrations of NA increased both spontaneous and mIPSC frequency in MCs, an effect observed at all concentrations tested. These results contrast recent reports indicating a dose-dependent effect of NA on GABA IPSC frequency in the MOB (Nai et al. 2009, 2010). These studies showed that at low concentrations, NA reduced the frequency of IPSCs by acting on α2-ARs. At higher concentrations, NA increased IPSC frequency primarily through α1-AR activation. Although we cannot definitively rule out the possibility of a small inhibitory effect on frequency by NA, the frequency of GABA events was not affected by the α2-AR selective agonist CLO in our experiments. Furthermore, in the presence of a selective blockade of α1- and β-ARs, sIPSC frequency was unaltered by NA. The reason for these differences is not clear, but we hypothesize that there could be unexplored variations between the experimental models used in these experiments (mouse vs. rat). One possibility is that these species exhibit different levels of expression and/or distribution of these receptor subtypes within the OB network, as shown in other brain regions (Jean-Charles & Gerard, 2002). Most studies addressing receptor distribution in the OB have been performed in rats and show expression of α1A- and α2- ARs (McCune et al. 1993; Day et al. 1997; Domyancic & Morilak, 1997; Winzer-Serhan et al. 1997a,b; Papay et al. 2006). Similar studies in mice could yield further insight into the reasons underlying the observed physiological differences in the action of NA. These species are common models used to study olfactory function, including the role of neuromodulation in behaviour. Therefore, further studies are necessary to explore the possibility that their olfactory network is regulated in a different manner by the noradrenergic system.
Acknowledgments
We thank Matthew Grant and Spencer Bonar for their early contribution to this work and the members of the Araneda Lab for their helpful comments on this manuscript. This work was funded by a National Institute of Deafness and Other Communicational Disorders Grant DC RO1-DC-009817 to R.C. Araneda. N.C.Z. and T.T. were supported by Howard Hughes Medical Institute undergraduate research fellowships and R.S.S. by an NSF graduate research fellowship.
Glossary
- GC
granule cells
- ICAN
calcium activated non-selective cationic current
- LC
locus coeruleus
- mIPSCs
miniature IPSCs
- MCs
mitral cells
- MOB
main olfactory bulb
- OB
olfactory bulb
- AOB
accessory olfactory bulb
- sADP
slow afterdepolarization
- sIPSCs
spontaneous IPSCs
- NA
noradrenaline
- AR
adrenergic receptor
Author contributions
Conception and design of the experiments: R.C.A., N.C.Z. and R.S.S. Collection analysis and interpretation of data: N.C.Z., T.T. and R.S.S. Drafting the article or revising it critically for important intellectual content: N.C.Z., R.C.A. and R.S.S.
References
- Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci. 2006;26:3292–3298. doi: 10.1523/JNEUROSCI.4768-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neuroscience. 1999;90:595–606. doi: 10.1016/s0306-4522(98)00437-0. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Constanti A, Bagetta G, Libri V. Persistent muscarinic excitation in guinea-pig olfactory cortex neurons: involvement of a slow post-stimulus afterdepolarizing current. Neuroscience. 1993;56:887–904. doi: 10.1016/0306-4522(93)90135-3. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14:539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Ghatpande AS, Gelperin A. Presynaptic muscarinic receptors enhance glutamate release at the mitral/tufted to granule cell dendrodendritic synapse in the rat main olfactory bulb. J Neurophysiol. 2009;101:2052–2061. doi: 10.1152/jn.90734.2008. [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Long-term enhancement of synchronized oscillations by adrenergic receptor activation in the olfactory bulb. J Neurophysiol. 2008;99:2021–2025. doi: 10.1152/jn.01324.2007. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Andrade R. Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. Eur J Neurosci. 1999;11:1973–1980. doi: 10.1046/j.1460-9568.1999.00612.x. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Delaney KR. Contribution of a calcium-activated non-specific conductance to NMDA receptor-mediated synaptic potentials in granule cells of the frog olfactory bulb. J Physiol. 2002;543:819–834. doi: 10.1113/jphysiol.2002.024638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol. 2001;86:2173–2182. doi: 10.1152/jn.2001.86.5.2173. [DOI] [PubMed] [Google Scholar]
- Hein L, Kobilka BK. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34:357–366. doi: 10.1016/0028-3908(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Doze VA, Porter JE. Alpha1A-adrenergic receptors are functionally expressed by a subpopulation of cornu ammonis 1 interneurons in rat hippocampus. J Pharmacol Exp Ther. 2007;321:1062–1068. doi: 10.1124/jpet.106.119297. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Lei S, Doze VA, Porter JE. Alpha-1A adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res. 2009;84:97–109. doi: 10.1016/j.eplepsyres.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Jean-Charles V, Gerard H. Noradrenergic receptors and in vitro respiratory rhythm: possible interspecies differences between mouse and rat neonates. Neurosci Lett. 2002;324:149–153. doi: 10.1016/s0304-3940(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Leszkowicz E, Khan S, Ng S, Ved N, Swallow DL, Brennan PA. Noradrenaline-induced enhancement of oscillatory local field potentials in the mouse accessory olfactory bulb does not depend on disinhibition of mitral cells. Eur J Neurosci. 2012;35:1433–1445. doi: 10.1111/j.1460-9568.2012.08070.x. [DOI] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol. 1989;285:339–349. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- McLennan H. The pharmacology of inhibition of mitral cells in the olfactory bulb. Brain Res. 1971;29:177–184. doi: 10.1016/0006-8993(71)90026-6. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Elaagouby A, Ravel N. A study of the effects of noradrenaline in the rat olfactory bulb using evoked field potential response. Brain Res. 1995;681:47–57. doi: 10.1016/0006-8993(95)00280-4. [DOI] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Hayar A, Linster C, Ennis M. Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol. 2009;101:2472–2484. doi: 10.1152/jn.91187.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Linster C, Ennis M. Activation of alpha1 and alpha2 noradrenergic receptors exert opposing effects on excitability of main olfactory bulb granule cells. Neuroscience. 2010;169:882–892. doi: 10.1016/j.neuroscience.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okutani F, Kaba H, Takahashi S, Seto K. The biphasic effects of locus coeruleus noradrenergic activation on dendrodendritic inhibition in the rat olfactory bulb. Brain Res. 1998;783:272–279. doi: 10.1016/s0006-8993(97)01371-1. [DOI] [PubMed] [Google Scholar]
- Pandipati S, Gire DH, Schoppa NE. Adrenergic receptor-mediated disinhibition of mitral cells triggers long-term enhancement of synchronized oscillations in the olfactory bulb. J Neurophysiol. 2010;104:665–674. doi: 10.1152/jn.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM. Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: alpha1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol. 2006;497:209–222. doi: 10.1002/cne.20992. [DOI] [PubMed] [Google Scholar]
- Perez H, Hernandez A, Almli CR. Locus coeruleus stimulation modulates olfactory bulb evoked potentials. Brain Res Bull. 1987;18:767–770. doi: 10.1016/0361-9230(87)90213-9. [DOI] [PubMed] [Google Scholar]
- Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci. 2007;27:10969–10981. doi: 10.1523/JNEUROSCI.2961-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoiraghi GC, Bloom FE, Costa E. Adrenergic mechanisms in rabbit olfactory bulb. Am J Physiol. 1964;207:1417–1424. doi: 10.1152/ajplegacy.1964.207.6.1417. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci. 2008;28:10711–10719. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329:294–299. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- Smith RS, Weitz CJ, Araneda RC. Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb. J Neurophysiol. 2009;102:1103–1114. doi: 10.1152/jn.91093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh O, Freichel M, Kretz O, Birnbaumer L, Hartmann J, Egger V. NMDA receptor-dependent synaptic activation of TRPC channels in olfactory bulb granule cells. J Neurosci. 2012;32:5737–5746. doi: 10.1523/JNEUROSCI.3753-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Takami S, Graziadei PP. Light microscopic Golgi study of mitral/tufted cells in the accessory olfactory bulb of the adult rat. J Comp Neurol. 1991;311:65–83. doi: 10.1002/cne.903110106. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Koshimizu TA, Shibata K, Nasa Y, Takeo S, Tsujimoto G. Insights into alpha1 adrenoceptor function in health and disease from transgenic animal studies. Trends Endocrinol Metab. 2003;14:107–113. doi: 10.1016/s1043-2760(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci. 1992;12:3985–3991. doi: 10.1523/JNEUROSCI.12-10-03985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development – I. Alpha 2A messenger RNA expression. Neuroscience. 1997a;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development – II. Alpha 2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1997b;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Yan HD, Villalobos C, Andrade R. TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J Neurosci. 2009;29:10038–10046. doi: 10.1523/JNEUROSCI.1042-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Mutoh H, Debarbieux F, Knopfel T. Calcium signaling in mitral cell dendrites of olfactory bulbs of neonatal rats and mice during olfactory nerve stimulation and beta-adrenoceptor activation. Learn Mem. 2004;11:406–411. doi: 10.1101/lm.75204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999;375:261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]


