Abstract
Anxiety disorders in humans reduce both the heart rate variability (HRV) and the sensitivity of the cardiac baroreflex (BRS). Both may contribute to sudden death. To elucidate the mechanisms underlying these alterations, male rats were subjected to social defeat sessions on four consecutive days. Five days later, the rats were found to be in an anxiety-like state. At this time point, we analysed HRV and BRS in the defeated rats, with or without treatment with the anxiolytic chlordiazepoxide (CDZ). HRV was reduced after social defeat, due to changes in the autonomic balance favouring the sympathetic over the parasympathetic component. Spontaneous and pharmacological baroreflex gains were also reduced. CDZ abolished anxiety-like symptoms as well as HRV and BRS alterations. Inhibition of the dorsomedial hypothalamus (DMH) with muscimol reversed all cardiovascular alterations, whereas blockade of the nucleus tractus solitarii (NTS) 5-HT3 receptor by the local or systemic administration of granisetron restored only baroreflex gains and the parasympathetic component of HRV. In conclusion, repeated social defeat in the rat lead to an anxiety-like state that was associated with lasting reduction in HRV and baroreflex gains. The DMH and the NTS were responsible for these chronic cardiovascular alterations. These regions may therefore constitute new therapeutic targets for reducing cardiac dysfunction and fibrillation in anxiety disorders.
Key points
Anxiety disorders reduce both the heart rate variability (HRV) and the sensitivity of the cardiac baroreflex (BRS). This may lead to sudden cardiac death.
To elucidate the mechanisms underlying these alterations, male rats were subjected to social defeat sessions that lead to an anxiety-like state.
In this model, HRV and BRS were reduced, reflex of a shift of the autonomic balance towards sympathetic predominance.
Pharmacological blockade of the dorsomedial hypothalamus (DMH) reversed all cardiovascular alterations, whereas blockade of the nucleus tractus solitarii (NTS) 5-HT3 receptor by the local or systemic administration of granisetron restored only baroreflex gains and the parasympathetic component of HRV.
In conclusion, repeated social defeat in the rat leads to an anxiety-like state, in which the DMH and the NTS are chronically activated and are responsible for dysautonomia. These regions may constitute new targets against sudden cardiac death.
Introduction
Mood disorders are associated with the occurrence of ventricular arrhythmia (Francis et al. 2009; Brugada, 2012). There is a solid body of evidence linking the autonomic nervous system to life-threatening arrhythmia and death from cardiovascular causes (Watkins et al. 1998; Friedman, 2007), as reduction of heart rate variability (HRV) and the baroreceptor-heart rate (HR) reflex (baroreflex sensitivity, BRS) have been shown to be predictive of the occurrence of ventricular fibrillation (Billman et al. 1982; Schwartz, 1998; La Rovere et al. 2001). However, the mechanisms underlying these cardiac alterations are still unknown.
A limited number of studies have been conducted in animal models of these diseases, to analyse cardiac and autonomic dysfunctions. Some of these studies used experimentally (Moffitt et al. 2002) or genetically (Padley et al. 2005) modified animals, while others used systems more closely resembling the physiological reactions observed in humans following the application of a chronic stress (Sgoifo et al. 2002; Grippo et al. 2008). However, only a few of these studies have analysed the cardiac BRS. Analyses of BRS during or at the end of a chronic stress inducing depression suggested that this parameter was not affected (Grippo et al. 2008; Porter et al. 2004). Thus, our principal objective was to analyse the long-term effects on HRV and the BRS during an anxiety-like state induced by repeated social defeat (Rivat et al. 2010), one of the most severe known stressors (Koolhaas et al. 1997). In an experimental design based on anticipation, intruder rats were repeatedly subjected to the threat of an aggressive conspecific resident, which was then allowed to attack and defeat them. This procedure has been shown to induce anxiety-like behaviour 5 days after the last session (Andréet al. 2005; Rivat et al. 2010; Blugeot et al. 2011). Thus, the aim of this study was to analyse the changes in autonomic balance and baroreflex gain associated with the establishment of this chronic stress-induced anxiety. We also explored the mechanisms that link anxiety to cardiovascular dysregulation. The dorsomedial hypothalamus (DMH) has been shown to activate sympathetic premotor neurons in the rostral ventro-lateral medulla (Wang et al. 2010), and to induce inhibition of the cardiac baroreflex response (BRR). This inhibition is mediated by the release of serotonin from the raphe magnus region, which acts onto presynaptic 5-HT3 receptors in the nucleus tractus solitarii (NTS; Merahi et al. 1992; Sévoz-Couche et al. 2003; Netzer et al. 2011). Knowing that DMH activation results also in anxiogenic-like effects (Shekhar, 1993; DiMicco et al. 2002), we hypothesized that the DMH, and consequently serotonergic receptors in the NTS, are chronically activated after social challenge sessions, and that this will alter cardiovascular parameters. We tested this hypothesis by pharmacological blockade of both regions in anaesthetized animals that had or had not been subjected to social defeat, 6 days after the last session.
Methods
Animals
Male Sprague–Dawley rats (Centre d’Elevage R. Janvier, Le Genest-St.Isle, France) weighing 250–300 g were used as experimental intruder animals (n= 157). They were housed in individual cages from 7 days before the beginning of the social defeat procedure until the end of the protocol (D−7 to D10; Fig 1A). Wild-type Groningen male rats (Rattus norvegicus, WTG strain), originally reared at the University of Groningen (The Netherlands) under conventionally clean conditions and weighing 400–500 g were used as the resident rats in confrontation encounters. All animals were kept under controlled environmental conditions (22 ± 1°C; 60% relative humidity; 12 h light/dark cycle; food and water ad libitum). Procedures involving animals and their care were all performed in conformity with the institutional guidelines, which are in compliance with national and international laws and policies (Council directive 87–848, 19 October 1987, Ministere de l’Agriculture et de la Foret, Service Veterinaire de la Sante et de la Protection Animale; permissions 75855 to C. Sévoz-Couche and 6180 to J.-J. Benoliel).
Figure 1. Long-term consequences of the social defeat protocol for physiological and behavioural parameters in non-defeated (ND) and defeated (D) rats with no treatment.
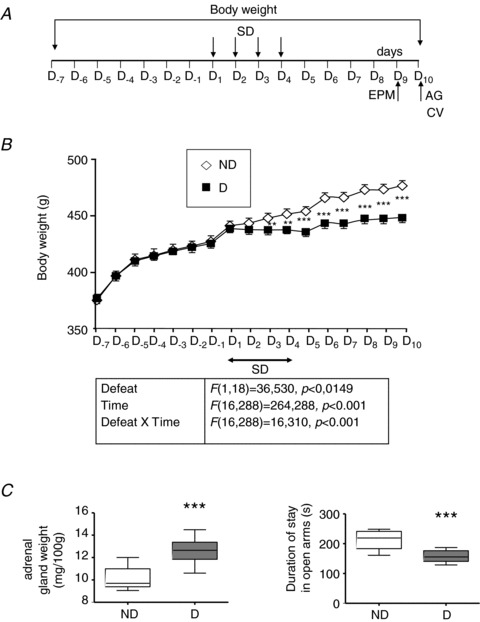
A, the experimental procedure consisted of four daily conditioning sessions (D1–D4) with the same pairs of residents and intruders. The elevated plus-maze test (EPM) was performed 5 days (D9) after the end of the social defeat procedure, and cardiovascular (CV) parameters and adrenal gland (AG) weight were measured on the following day (D10). Body weight was measured daily in ND and D rats, before, during and 6 days after conditioning sessions (D−7 to D10). B, D animals (n= 10) were lighter than ND (n= 10) animals. Differences were first observed the day after the first conditioning session (D2) and were maintained until the end of the protocol (D10). Each point is the mean ± SEM for D or ND rats. **P < 0.005 and ***P < 0.001 vs. ND rats. C, D animals had an increased AG weight and spent less time in the open arms of the EPM. Box–Whisker graphs with minimum and maximum values, lines are the medians. ***P < 0.001 vs. ND rats.
Procedures
Social defeat experimental procedure
Social defeat (Rivat et al. 2010) involved four daily conditioning sessions (Fig. 1A) with the same pairs of residents and intruders (Becker et al. 2001). Briefly, intruders (defeated (D) animals) were placed singly in a protective cage inside the resident home cage, allowing unrestricted visual, auditory and olfactory contact with the resident but precluding close physical contact. The protective cage was then removed with the resident present, allowing physical confrontation with the intruder (D intruders). There were three–four confrontations of 10 s each, during which the intruding animal was always dominated by the resident rat. For non-defeated (ND) intruders, the intruder had access to the entire resident home cage in the absence of the resident. This social defeat procedure has been shown to induce an anxiety-like state 5 days after the last conditioning session (Rivat et al. 2010). Here, we checked that all D animals presented basic signs of anxiety not observed in ND rats.
Body weight
The body weights of D and ND rats were measured daily at 09.00 h, before (7 days, D−7 to D1), during (4 days, D1–D4) and after (6 days, D5–D10) the social defeat procedure.
Elevated plus-maze test
Five days after the end of the fourth conditioning sessions (D9; Fig. 1A), the elevated plus-maze test was used to evaluate anxiety-related behaviour in animals. This test has been described in detail elsewhere (Rivat et al. 2010). The time spent in the various arms, and the numbers of entries into the open and closed arms of the plus-maze were recorded with custom-made software. The total number (open + closed) of arm entries was taken as an indicator of general activity.
Adrenal gland weight
At the end of all experiments (D10; Fig. 1A), adrenal glands were removed, dissected free of adhering fat and weighed. Organ weights are expressed relative to total body weight (in mg (100 g body weight)−1).
General procedures for the measurement of cardiovascular parameters
On the morning after elevated plus-maze tests (D10; Fig. 1A), animals were anaesthetized with pentobarbital sodium (Ceva Santé Animale, Libourne, France; 60 mg kg−1, i.p.; Sévoz-Couche et al. 1998). The depth of anaesthesia was assessed regularly by pinching a hind paw and monitoring the stability of the arterial blood pressure (BP) and HR recordings. In case of withdrawal reflex and/or significant variations of these parameters, a supplementary dose of pentobarbital was given (10 mg kg−1, i.v.). Systemic BP and mean BP (MBP) were monitored via a femoral artery catheter. HR was calculated from the ECG (R-wave pulses) and displayed as mean frequency per minute (bin size = 1 s), and the mean R–R interval duration (RR, ms) was calculated.
HRV analysis
Spectral (frequency domain) analysis
ECG waveform data were imported offline into Spike CED (version 6.0). The criteria for segment (90 s) selection for HRV analysis were stationarity and a lack of ectopic beats. Power spectra were obtained by Fourier transformation (size 256, Hanning window, giving a final frequency resolution of 0.04 Hz). Low- and high-frequency (LF and HF, respectively) powers were calculated within the frequency ranges of 0.2–0.7 Hz and 0.7–2.5 Hz, respectively, and the LF-to-HF ratio (LF/HF), a measure of the autonomic ‘balance’ (Friedman, 2007), was determined. An increase in LF/HF ratio indicates a rise in sympathetic activity (Pagani et al. 1986). HF power is exclusively under parasympathetic (vagal) control, and the peak frequency in the HF domain corresponds to the respiratory sinus arrhythmia (RSA; Stein et al. 1994; Porges et al. 2007).
Temporal (time domain) analysis
The root mean square of successive R–R interval differences (rMSSD, ms), which specifically quantifies parasympathetic activities (Stein et al. 1994), was calculated.
Spontaneous baroreflex activation
We used the sequence method to calculate spontaneous HR BRS (spontaneous BRS; Laude et al. 2004). Spontaneous BRS was calculated as the mean slope of R–R interval sequences for all sequences detected during 90 s segments of data.
Pharmacological baroreflex activation
The administration of nitroprusside (100 μg kg−1, i.v.) followed by phenylephrine (10 μg kg−1, i.v.) made it possible to evaluate the maximal BRR (maximal BRR in mmHg−1= 100 × (ΔHR/HR baseline)/ΔMBP]) and to generate baroreceptor function curves, by fitting a sigmoid logistic function to the data. The maximal (i.e. pharmacological maximal BRS) and rectilinear (pharmacological linear BRS) baroreflex slopes were calculated from the baroreceptor curves (Netzer et al. 2011).
Animals were killed at the end of the procedures for cardiovascular parameter analyses by a lethal dose of anaesthesia (pentobarbital sodium, 120 mg kg−1, i.p.).
Experiments
All the procedures listed above were performed in each of the following experiments. Five series of experiments were performed on five different series of rats.
Experiment 1: no treatment
In this experiment, ND (n= 10) and D (n= 10) rats received no treatment.
Experiment 2: chronic anxiolytic treatment
Social defeat was performed from D1 to D4. Then ALZET osmotic pumps supplying vehicle (VEH) or chlordiazepoxide (CDZ; 10 mg kg−1 day−1) were implanted (Rivat et al. 2010) in ND (n= 7 each) and D (n= 7 and 8, respectively) rats on the morning of the day after completion of the social defeat (D5). Briefly, pumps filled with CDZ (ALZET 2ML1) were implanted subcutaneously on the back of the rats under light isoflurane anaesthesia. A small incision was made in the skin between the scapulae. Using a haemostat, a small pocket was formed by spreading the subcutaneous connective tissues apart. The pump was inserted into the pocket. The skin incision was closed with absorbable sutures. Therefore these treatments did not affect body weight before D5. The infusion of VEH or CDZ continued from D5 to D10, to prevent the development of the anxiety-like state (Rivat et al. 2010). At D10, we compared the effects of VEH and CDZ on physiological, behavioural and cardiovascular parameters.
Experiment 3: acute pharmacological blockade of the DMH
On D10, anaesthetized animals with pentobarbital sodium were placed in a stereotaxic frame, with the head fixed in the flat skull position. Microinjections of saline or muscimol (MUSC; 500 pmol in 0.1 μl) into the DMH were performed at the following coordinates: P 3.0, L 0.5 and V 8 mm from bregma (Netzer et al. 2011), in ND (n= 6 and 10, respectively) and D (n= 7 and 13, respectively) animals. Injections were made bilaterally to maximize the effect and because DMH control of HR has been shown to be assymetric (Xavier et al. 2009). We compared the effects of DMH saline and MUSC on cardiovascular parameters, and we expressed changes as variations from the baseline.
Experiment 4: acute pharmacological blockade of NTS 5-HT3 receptors
As described above, a micropipette filled with either saline or a selective 5-HT3 receptor antagonist (granisetron (GRANI) 250 pmol in 0.1 μl) was inserted into the NTS. Microinjections of saline or GRANI were performed at the level of the calamus scriptorius (L 0.5 and V 0.5 mm; Sévoz-Couche et al. 2003) of ND (n= 8 each) and D (n= 8 each) rats on D10. Injections were made bilaterally to maximize the effect (Sévoz-Couche et al. 2003). We compared the effects of NTS VEH and GRANI on cardiovascular parameters, and we expressed changes as variations from the baseline.
Experiment 5: acute pharmacological blockade of peripheral and central 5-HT3 receptors
GRANI is a 5-HT3 receptor antagonist known to cross the blood–brain barrier (Huang et al. 1998). Saline or GRANI (10 μg kg−1) was administered systemically (0.1 ml) in ND (n= 10 each) and D (n= 10 each) anaesthetized rats on D10. We compared the effects of i.v. VEH and GRANI on cardiovascular parameters, and we expressed changes as variations from the baseline.
The effects of MUSC and GRANI lasted for at least 30 min (Sévoz-Couche et al. 2003; Netzer et al. 2011). HRV and spontaneous BRS parameters were analysed 20 min before and 10 min after treatments, from 90 s segments of recording. The pharmacological BRS was performed 5 min after the HRV and spontaneous BRS analyses, therefore 15 min before and 15 min after treatments.
Drugs
MUSC and sodium nitroprusside (Sigma Chemicals, St Louis, USA), phenylephrine hydrochloride (Merck Sharp and Dohme-Chibret, Paris, France) and GRANI (SmithKline-Beecham, Harlow, UK) were dissolved in saline. The pH of all solutions microinjected into the NTS was adjusted to 7.4.
Histological localization of microinjection sites
In experiments 3 and 4, microinjection sites were identified by the location of the micropipette track in 70 μm-thick sections of brain tissue previously fixed in 10% formalin solution and cryoprotected in 20% sucrose solution for 5 days. Only rats with the injection point correctly positioned in the DMH or NTS were considered for data analysis.
Statistical analysis
In Experiment 1, a one-way (subjects) repeated (time)-measures ANOVA was used to compare body weights throughout the protocol, and a one-way (subjects) ANOVA was used to compare other behavioural, physiological and cardiovascular parameters.
In Experiment 2, a two-way (subjects, treatments) repeated (time)-measures ANOVA was used to compare body weights throughout the protocol, and a two-way (subjects, treatments) ANOVA was used to compare the effects of VEH and treatments on other behavioural, physiological and cardiovascular parameters.
In Experiments 3–5, a one-way (subjects) repeated (time)-measures ANOVA was used to compare body weights throughout the protocol, a one-way (subjects) ANOVA was used to compare time spent in the open arms of the elevated plus maze and adrenal gland weight, a two-way (subjects, treatments) ANOVA was used to compare the effects of VEH and treatments on cardiovascular parameters, and a paired Student's t test was used to compare cardiovascular parameters before and after treatment in the same animals.
All the statistical results from the two-way ANOVA were given in Supplemental Tables 1–5.
Bonferroni correction was applied to all ANOVAs, and results were considered significant if P < 0.05.
Results
Experiment 1: long-lasting physiological, behavioural and cardiovascular parameters in D (n= 10) and ND (n= 10) rats with no treatments
Before the start of the social defeat procedure (Fig. 1A), D and ND animals had similar body weights (Fig. 1B). After social defeat sessions, D rats weighted less (−20%) than ND rats. This divergence began from the day after the first conditioning session (D2) and persisted until the end of the procedure (D10).
Adrenal gland weight was higher (Fig. 1C) and time spent in the open arm was lower (Fig. 1D) in D animals, as previously reported (Rivat et al. 2010). We found no statistically significant difference in the total number of entries between ND and D rats (ND: 30.90 ± 2.99 and D: 27.70 ± 4.39, P= 0.07). Thus, the observed difference in time spent in the open arms between these two groups of rats was not due to a change in general activity.
Cardiovascular parameters
HR and MBP
The mean R–R interval was significantly lower in D rats than in ND rats (Fig. 2Aa), corresponding to an increase in HR (345 ± 7 vs. 321 ± 3 bpm, respectively, P= 0.021). HR was positively correlated with adrenal gland weight in D animals (Fig. 2Ab). D and ND animals had similar basal MBP (D: 102 ± 3 vs. ND: 105 ± 2 mmHg, respectively, P= 0.68).
Figure 2. Long-term consequences of the social defeat protocol for heart rate variability (HRV) in non-defeated (ND) and defeated (D) rats with no treatment.
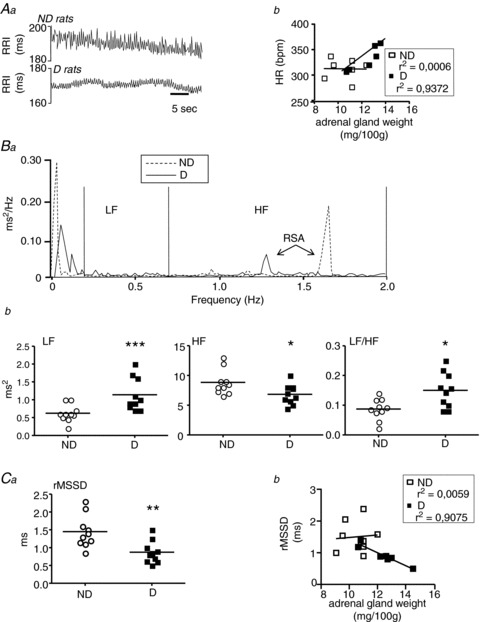
A, representative R–R interval time series (Aa) showed that the mean R–R interval was lower (indicating an increase in heart rate (HR)) in D animals than in ND animals, and that fluctuations around the mean were much smaller than those in controls. The increase in HR seen in D rats was positively correlated with adrenal gland weight (Ab). B, frequency domain analysis of HRV. Analysis of the power spectra of R–R intervals (Ba) showed a lower high-frequency domain (HF) value in D rats, associated with an increase in low-frequency domain (LF) and LF/HF (Bb). Therefore, a decrease in parasympathetic tone was observed, together with an increase in sympathetic activity, in D rats. Data plots of all animals, lines are the means for D or ND animals. *P < 0.05 and ***P < 0.001 vs. ND rats. C, time domain analysis of HRV. The root mean square of successive R–R interval differences (rMSSD) was smaller for D than for ND rats (Ca). Modification in rMSSD was negatively correlated with adrenal gland weight (Cb). Data plots of all animals, lines are the means for D or ND animals. **P < 0.005 vs. ND rats.
HRV
We performed power spectral analysis on the ECG (Fig. 2Ba). Compared with ND animals, D rats had a reduced HF power associated with an increased LF power and LF/HF ratio (Fig. 2Bb). There was thus an increase in sympathetic tone associated with a decrease in parasympathetic tone in animals subjected to social defeat. The altered parasympathetic activity was confirmed by a reduction in rMSSD in D rats (Fig. 2Ca), which was negatively correlated with adrenal gland weight (Fig. 2Cb).
Baroreflex measurements
Spontaneous BRS was significantly lower in D than in ND animals (Fig. 3Aa), and was negatively correlated with adrenal gland weight (Fig. 3Ab). During the pharmacological induction of the baroreflex with nitroprusside followed by phenylephrine, the maximal BRR was lower in D (0.37 ± 0.04 mmHg−1) than in ND (0.62 ± 0.05 mmHg−1, P < 0.001) rats. Accordingly, the pharmacological maximal BRS slope calculated from the sigmoid baroreflex curves was lower in D rats (Fig. 3B, left), as was the pharmacological linear BRS (Fig. 3B, right). Values and statistics are given in Fig 3B (lower panel).
Figure 3. Long-term consequences of the social defeat protocol for baroreflex parameters in non-defeated (ND) and defeated (D) rats with no treatment.
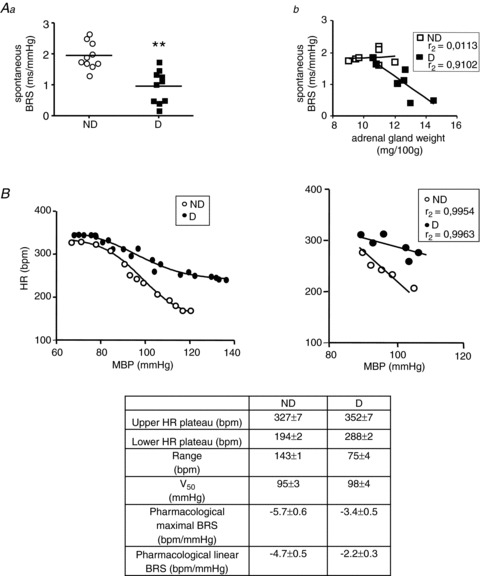
A, spontaneous baroreflex sensitivity (BRS), measured by the sequence method, was lower in D than in ND animals (Aa), and this decrease was inversely correlated with the development of an anxiety-like state (Ab). Data plots of all animals, lines are the means for D or ND rats. *P < 0.005 vs. ND intruders. B, the administration of nitroprusside followed by phenylephrine made it possible to generate baroreceptor function curves by fitting a sigmoid logistic function to the data (left). The maximal BRS calculated from the slope of these curves was lower in D than in ND rats. In the same manner, the linear BRS calculated from the linear part of the sigmoid curves (right) was lower in D rats. The values shown in the table are the means ± SEM for D and ND rats.
To prevent the development of the anxiety-like state (Rivat et al. 2010), and to observe the effects of this prevention on cardiovascular parameters, an anxiolytic treatment was given after completion of the social defeat (D5).
Experiment 2: effects of VEH or chronic anxiolytic treatment in D (n= 7 and 8, respectively) and ND (n= 7 each) rats
In the group of D rats, CDZ treatment maintained normal body weight gain (Fig. 4A), adrenal gland weight and behaviour in the plus maze (Fig. 4B), while no change was observed in ND animals.
Figure 4. Effects of vehicle (VEH) and chlordiazepoxide (CDZ) treatment on physiological, behavioural and cardiovascular parameters in non-defeated (ND) and defeated (D) rats.
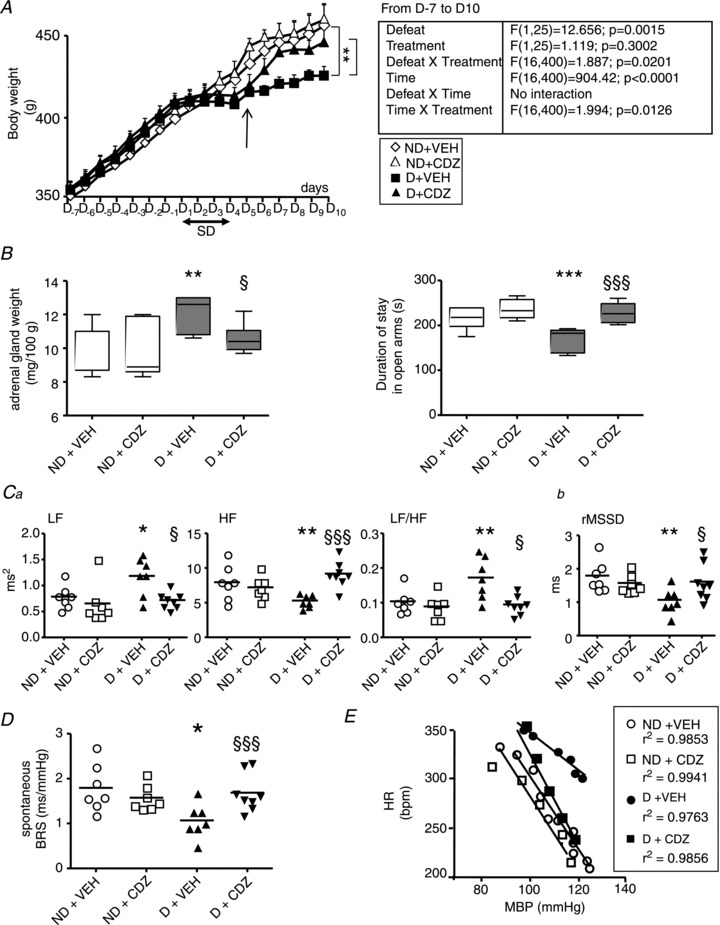
Compared with VEH, the anxiolytic treatment (10 mg kg−1 day−1) given at D5 (A, arrow) prevented the decrease in body weight gain (A), the increase in adrenal gland weight and the decrease in the time spent in open arms (B), the changes in frequency (Ca) and time (Cb) domain parameters for HRV, and the decrease in spontaneous (D) and pharmacological linear (E) BRS in D rats, but not in ND rats. B, box–Whisker graphs with minimum and maximum values, lines are the medians. **P < 0.005 and ***P < 0.001 vs. ND + VEH; §P < 0.05 and §§§P < 0.001 vs. D + VEH. C and D, data plots of all animals, lines are the means for D and ND rats. *P < 0.05 and **P < 0.005 vs. ND + VEH; §P < 0.05 and §§§P < 0.001 vs. D + VEH.
Cardiovascular parameters
HR
HR was significantly higher in D than in ND treated with VEH (D + VEH: 354 ± 7 and ND + VEH: 320 ± 7 bpm, P= 0.021). Anxiolytic treatment reduced HR in D rats (D + CDZ: 321 ± 8 bpm, P= 0.006), whereas HR remained unaffected in ND animals (ND + CDZ: 320 ± 7 bpm, P= 0.91).
HRV and baroreflex measurements
LF and LF/HF were higher, and HF and rMSSD were lower in D than in ND rats with VEH (Fig. 4Ca and Cb). These alterations were suppressed by CDZ (Fig. 4Ca and Cb). The treatment had no effect in ND rats.
The lower spontaneous BRS seen in D animals with VEH treatment was also suppressed with CDZ (Fig. 4D). CDZ did not affect spontaneous BRS in ND rats (Fig 4D). Similar results were obtained with pharmacological linear BRS (ND + VEH: −4.0 ± 0.2 bpm mmHg−1 vs. ND + CDZ: −3.5 ± 0.1 bpm mmHg−1, P= 0.67; D + VEH: −2.5 ± 0.3 bpm mmHg−1 vs. D + CDZ: −4.4 ± 0.4 bpm mmHg−1, P= 0.001; Fig. 4E).
The DMH has been shown to be responsible for similar alterations in acute stress (Sévoz-Couche et al. 2003; Netzer et al. 2011). We therefore analysed the possible involvement of the DMH in this model of chronic stress.
Experiment 3: effects of VEH and MUSC injections into the DMH on cardiovascular parameters in D (n= 7 and 13, respectively) and ND (n= 6 and 10, respectively) rats
HR and MBP
Unlike saline, the changes in HR (ΔHR/baseline HR) and MBP (ΔMBP/baseline MBP) after MUSC microinjection were greater in D rats than in ND animals (Fig. 5Aa and Ab). HR between-group differences in animals with saline (ND + DMH VEH: 315 ± 5 and D + DMH VEH: 349 ± 8 bpm, P= 0.004) were eliminated after MUSC (ND + DMH MUSC: 310 ± 7 and D + DMH MUSC: 290 ± 11 bpm, P= 0.75). Parameters returned to baseline after 10 min in D rats, but only after 2 min in ND rats.
Figure 5. Effects of microinjection into the dorsomedial hypothalamus (DMH) of vehicle (VEH) or muscimol (MUSC) on cardiovascular parameters in non-defeated (ND) and defeated (D) rats.
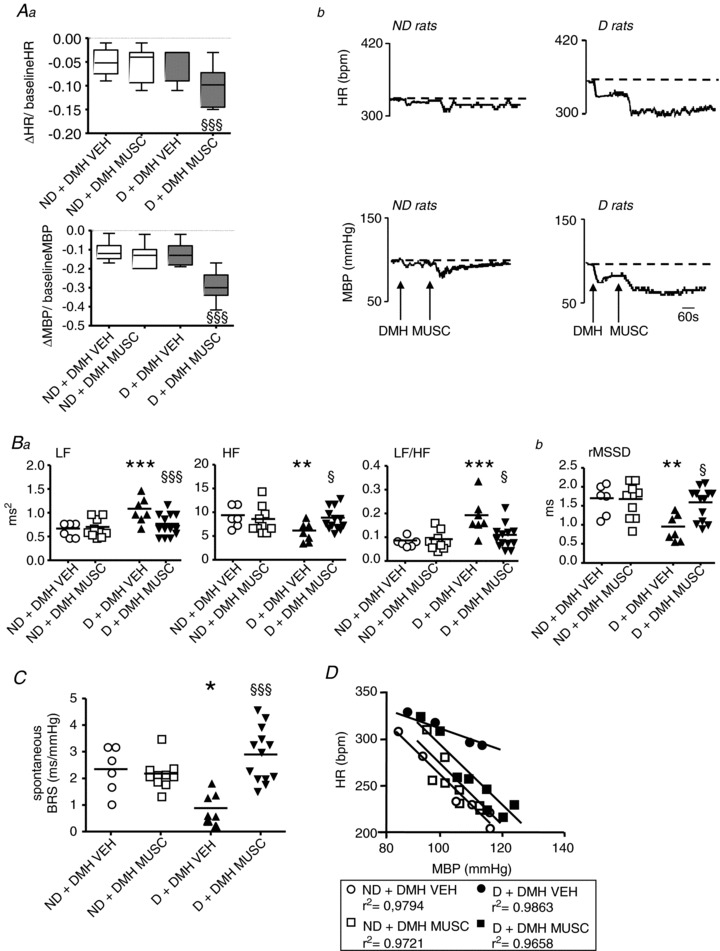
A, MUSC induced larger reductions in heart rate (HR) and mean blood pressure (MBP) from baselines than saline in D but not in ND rats (Aa). Representative tracings of the effects of MUSC are shown in Ab. Box–Whisker graphs with minimum and maximum values, lines are the medians. §§§P < 0.001 vs. D + VEH. B–D, compared with VEH, DMH MUSC prevented the changes in frequency (Ba) and time (Bb) domain parameters for heart rate variability (HRV), spontaneous (C) and pharmacological linear (D) baroreflex sensitivity (BRS) observed in D rats, but had no effect on ND rats. Data plots of all animals, lines are the means for D and ND rats. *P < 0.05, **P < 0.005 and ***P < 0.001 vs. ND + VEH; §P < 0.05 and §§§P < 0.001 vs. D + VEH.
HRV
Compared with saline, while no change was observed in ND rats, MUSC prevented the changes in HRV in D rats (Fig. 5Ba and Bb). The same was observed when the comparisons were made in D rats before and after the MUSC injection (LF: 1.55 ± 0.07 vs. 0.77 ± 0.06 ms2, respectively, P < 0.001; HF: 5.46 ± 0.56 vs. 8.84 ± 0.64 ms2, respectively, P < 0.001; LF/HF: 0.17 ± 0.01 vs. 0.11 ± 0.01, respectively, P= 0.008; rMSSD: 1.02 ± 0.10 vs. 1.60 ± 0.12 ms, respectively, P < 0.001).
Baroreflex measurements
While no change in the spontaneous BRS was observed in ND rats, MUSC compared with saline restored the BRS in D rats (Fig. 5C). The same was observed when the comparison was made in D rats before and after the MUSC injection (0.87 ± 0.13 vs. 2.80 ± 0.28 bpm s−1, before and after MUSC, respectively, P < 0.001).
While no change in linear BRS was observed in ND (ND + DMH VEH: −4.3 ± 0.2 bpm mmHg−1 vs. ND + DMH MUSC: −3.9 ± 0.1 bpm mmHg−1, P= 0.3) rats, MUSC compared with saline prevented the low linear BRS in D rats (D + DMH VEH: −2.0 ± 0.1 bpm mmHg−1 vs. D + DMH MUSC: −4.5 ± 0.2 bpm mmHg−1, P= 0.005; Fig. 5D). The same was observed when the comparison was made in D rats before and after the MUSC injection (−2.7 ± 0.2 vs.−4.5 ± 0.2 bpm mmHg−1, respectively, P= 0.005).
Downstream from the DMH, presynaptic 5-HT3 receptors in the NTS are responsible for decreasing the baroreflex in conditions of acute stress (Sévoz-Couche et al. 2003; Netzer et al. 2011). We therefore analysed the possible role of these receptors in our model of chronic stress.
Experiment 4: effects of VEH and GRANI injections into the NTS on cardiovascular parameters in D (n= 8 each) and ND (n= 8 each) rats
HR and MBP
The microinjection of GRANI into the NTS, unlike that of saline, induced a greater reduction in MBP in D than in ND rats, but had no effect on HR in either group of rats (Fig. 6Aa and Ab). Parameters returned to baseline after 10 min in D rats, but only after 2 min in ND rats.
Figure 6. Effects of microinjection into the nucleus tractus solitarii (NTS) of vehicle (VEH) or granisetron (GRANI) on cardiovascular parameters in defeated (D) and non-defeated (ND) rats.
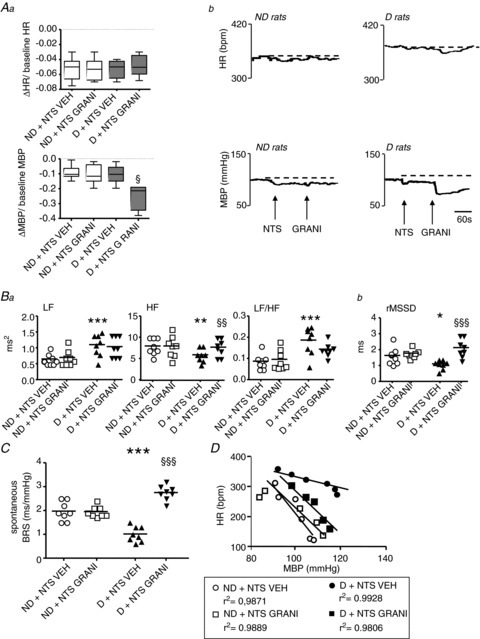
A, GRANI injection into the NTS induced a larger decrease in mean blood pressure (MBP) but not in heart rate (HR) than saline in D rats, but had no effect on either of these parameters in ND (Aa). Representative tracings of the effects of GRANI injection into the NTS are given in Ab. Box–Whisker graphs with minimum and maximum values, lines are the medians. §P < 0.05 vs. D + VEH. B, compared with VEH, the injection of GRANI into the NTS abolished the reduction in high-frequency domain (HF) but not the increase in low-frequency domain (LF)/HF in D rats (Ba), and had no effect in ND rats (Ba), suggesting that GRANI reversed the decrease in parasympathetic activity but not the increase in sympathetic activity. This treatment also eliminated the decrease in time domain HRV analysis (Bb) normally induced in D rats, and had no effect on ND rats. Data plots of all animals, lines are the means for D and ND rats. *P < 0.05, **P < 0.005 and ***P < 0.001 vs. ND + VEH; §§P < 0.005 and §§§P < 0.001 vs. D + VEH. C and D, the low spontaneous baroreflex sensitivity (BRS) (C) and pharmacological linear (D) BRS in D rats with VEH was prevented in D rats with i.v. GRANI, and was unchanged in ND rats. ***P < 0.001 vs. ND + VEH; §§§P < 0.001 vs. D + VEH.
HRV
While HRV parameters were unchanged in ND rats, GRANI compared with saline restored HF and rMSSD in D animals, but not LF and LF/HF (Fig. 6Ba and Bb). The same was observed when the comparisons were made in D rats before and after the GRANI injection (HF: 5.40 ± 0.49 vs. 7.66 ± 0.70 ms2, respectively, P= 0.036; LF: 1.12 ± 0.09 vs. 1.03 ± 0.11 ms2, respectively, P= 0.40; LF/HF: 0.18 ± 0.01 vs. 0.14 ± 0.01, respectively, P= 0.09; rMSSD: 1.10 ± 0.11 vs. 2.10 ± 0.22 ms, respectively, P= 0.004).
Baroreflex measurements
While no change in the spontaneous BRS was observed in ND animals, GRANI compared with saline restored the spontaneous BRS in D rats (Fig. 6C). The same was observed when the comparison was made in D rats before and after the GRANI injection (1.01 ± 0.13 vs. 2.75 ± 0.10 ms mmHg−1, before and after NTS GRANI, respectively, P < 0.001).
Compared with saline, GRANI into the NTS had no effect in ND rats (ND + VEH: −4.1 ± 0.2 vs. ND + GRANI: −3.9 ± 0.4 bpm mmHg−1, P= 0.52), but prevented the low pharmacological linear BRS in D rats (D + VEH: −2.9 ± 0.1 bpm mmHg−1 vs. D + GRANI: −4.5 ± 0.2 bpm mmHg−1, P= 0.01; Fig. 6D). The same was observed when the comparison was made in D rats before and after the GRANI injection (−3.1 ± 0.3 vs.−4.5 ± 0.2 bpm mmHg−1, respectively, P= 0.009).
We have shown that the chronic activation of medullary 5-HT3 receptors by social defeat procedure alters baroreflex alterations. As GRANI can cross the blood–brain barrier (Huang et al. 1998), we investigated the possibility that the systemic administration of this substance could also prevent baroreflex alteration in D rats.
Experiment 5: effects of systemically administered VEH or GRANI on cardiovascular parameters in D (n= 10 each) and ND (n= 10 each) rats
HR and MBP
Compared with saline, the systemic administration of GRANI induced a greater reduction in MBP in D than in ND rats, but had no effect on HR in either group of rats (Fig. 7Aa and Ab). Parameters returned to baseline after 3 min in D rats, but only after 1 min in ND rats.
Figure 7. Effects of the systemic administration of vehicle (VEH) or granisetron (GRANI) on cardiovascular parameters in defeated (D) and non-defeated (ND) rats.
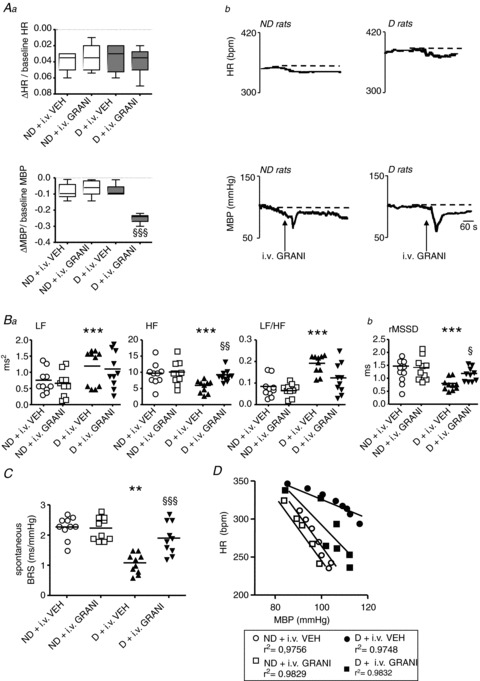
A, systemic administration of GRANI induced a larger decrease in mean blood pressure (MBP) but not in heart rate (HR) than saline in D rats, but had no effect on either of these parameters in ND rats (Aa). Representative tracings of the effects of i.v. GRANI are given in Ab. Box–Whisker graphs with minimum and maximum values, lines are the medians. §§§P < 0.001 vs. D + VEH. B, compared with VEH, i.v. GRANI abolished high-frequency domain (HF) decrease but not the increase in low-frequency domain (LF)/HF in D rats, and had no effect on ND rats (Ba). This treatment also eliminated the decrease in time domain heart rate variability (HRV) analysis (Bb) normally induced in D rats, and had no effect on ND rats. Data plots of all animals, lines are the means for D and ND rats. ***P < 0.001 vs. ND + VEH; §P < 0.05 and §§P < 0.005. C and D, the low spontaneous (C) and pharmacological linear (D) baroreflex sensitivity (BRS) in D rats with VEH was increased in D rats with i.v. GRANI, and was unchanged in ND rats. **P < 0.005 vs. ND + VEH; §§§P < 0.001 vs. D + VEH.
HRV
While no changes in HRV parameters were observed in ND rats, systemic GRANI treatment compared with saline restored HF and rMSSD in D rats, but not LF and LF/HF (Fig. 7Ba and Bb). The same was observed when the comparisons were made in D rats before and after the administration of GRANI (HF: 5.59 ± 0.54 vs. 9.24 ± 0.58 ms2, respectively, P= 0.006; LF: 1.16 ± 0.14 vs. 1.10 ± 0.17 ms2, respectively, P= 0.59; LF/HF: 0.15 ± 0.01 vs. 0.12 ± 0.01, respectively, P= 0.19; rMSSD: 0.80 ± 0.07 vs. 1.8 ± 0.08 ms, respectively, P= 0.001).
Baroreflex measurements
While no changes in spontaneous BRS were observed in ND rats, compared with saline, i.v. GRANI restored the spontaneous BRS in D rats (Fig. 7C). The same was observed when the comparison was made in D rats before and after the GRANI administration (1.09 ± 0.12 vs. 1.9 ± 0.16 ms mmHg−1, before and after i.v. GRANI, respectively, P < 0.001).
While no change in pharmacological linear BRS was observed in ND rats (ND + VEH: −4.1 ± 0.2 bpm mmHg−1 vs. ND +i.v. GRANI: −3.9 ± 0.1 bpm mmHg−1, P= 0.72), GRANI administration compared with saline prevented the low linear BRS in D rats (D + VEH: −2.1 ± 0.1 bpm mmHg−1 vs. D +i.v. GRANI: −4.0 ± 0.2 bpm mmHg−1, P= 0.005; Fig. 7D). The same was observed when the comparison was made in D rats before and after the administration of GRANI (−2.5 ± 0.2 vs.−4.0 ± 0.2 bpm mmHg−1, respectively, P= 0.009).
Experiments 3–5: body weight, elevated plus-maze test results and adrenal gland weight in D (n= 56) and ND (n= 52) animals in experiments with central microinjection or systemic administration
We found that the body weight of D rats measured from D−7 to D10 was less than that of ND rats from the day after the first conditioning session (D2) to the end of the procedure (Supplemental Fig. 1A). We also assessed that adrenal gland weight was higher and time spent in the open arms of the maze was less in D than ND rats (Supplemental Fig. 1B and C, respectively).
Discussion
Our findings demonstrate that chronic social defeat based on anticipation leads, at distance of stressor application, to overall cardiovascular modifications that resemble those seen in anxiety: changes to autonomic balance associated with a decrease in BRS. This dysregulation, which is prevented by anxiolytic treatment, is due to chronic activation of the DMH and NTS 5-HT3 receptors.
Experiments 1 and 2: physiological and cardiovascular modifications after social defeat
Only a few studies have evaluated the cardiovascular modifications induced by social defeat (Sgoifo et al. 2002; Beig et al. 2009). Most have described HRV, without identifying the mechanisms involved. Moreover, because these studies were performed during, or just after, application of the stressor (Porter et al. 2004; Grippo et al. 2008), they only evaluated the stress effect, not the effect of an anxiety state. In the present study, experiments were performed 6 days after the end of four consecutive days of social defeat. An absence of body weight gain, an increase in adrenal gland weight and less time spent in the open arms at that time point indicated an anxiety-like state (Andréet al. 2005; Rivet et al. 2010), making it possible to evaluate the consequences of chronic stress-induced anxiety on cardiovascular function.
As described in patients with high or low anxiety scores (Watkins et al. 1998), BP was not affected by the social defeat procedure. However, D animals had a higher HR and a lower HRV than controls. We first analysed rMSSD and HF power, two measures that are vagally mediated (Schwartz, 1998; Porges et al. 2007). We found that both parameters were reduced. The LF/HF ratio, which provides an indirect estimate of sympathovagal balance, increased, as did LF. These results indicate a shift in the autonomic balance towards sympathetic predominance. We also observed a shift of the RSA peak to lower frequencies (Fig. 2Ba). Modifications of the RSA peak frequency, unlike HF and rMSSD (Denver et al. 2007; Sin et al. 2010; Overbeek et al. 2012), may reflect changes in the depth and/or rate of respiration (Denver et al. 2007). Further studies are needed to determine whether rats with an anxiety-like profile present modifications in basal respiratory period and tidal volume, although no such changes were found in patients with anxiety (Watkins et al. 1998) or depression (Berger et al. 2012).
Spontaneous BRS was much lower in D than in ND animals. In addition, the pharmacological range and maximal BRS calculated from the sigmoid curve were smaller in animals presenting an anxiety-like profile. All these data suggest that the reduction in the parasympathetic activity due to chronic stress-induced anxiety results from a diminished BRS. CDZ treatment, which prevented the development of an anxiety-like profile, also prevented the reduction in HRV and BRS. Consistent with these findings, we found that reduced HRV and BRS were positively correlated with an increase in adrenal gland weight. Thus, the baroreflex changes may result from the anxiety state induced by the chronic stress.
Experiment 3: role of the DMH in the cardiovascular modifications induced by social defeat
DMH activation induces a sympathetically mediated increase in HR and MBP (Netzer et al. 2011). We found that microinjections of MUSC into the DMH resulted in significantly larger decreases in HR and MBP in D rats, suggesting that both HR and MBP were higher in animals subjected to chronic stress. D rats presented an increase in HR but not in MBP, consistent with findings in patients with high anxiety scores (Watkins et al. 1998). There may perhaps be compensatory mechanisms at work on MBP. The injection of MUSC into the DMH prevented both the increase in sympathetic activity and the decrease in vagally mediated HRV in rats subjected to social defeat. In addition, this treatment restored spontaneous and pharmacological baroreflex parameters. These effects lasted at least 30 min, indicating that compensatory mechanisms on HR occurred after MUSC (as HR returned at basal levels after only 10 min). The DMH is clearly overactivated and involved in all the cardiovascular modifications seen in animals subjected to social challenge, and is therefore a key component of the central pathway activated during the anxiety induced by chronic stress.
During acute stress, the DMH acts on the rostral cuneiform nucleus that, in turn, activates the dorsolateral periaqueductal grey (Netzer et al. 2011). Downstream to this structure, serotonin is released from the B3 region to the NTS to activate 5-HT3 receptors and produce baroreflex inhibition (Sévoz-Couche et al. 2003). We investigated whether 5-HT3 receptor blockade could prevent the sympathetic overactivity and baroreflex reduction induced by chronic stress.
Experiments 4 and 5: role of the NTS in the cardiovascular modifications induced by social defeat
We targeted the NTS receptors by administering GRANI in two ways: by local microinjection into the NTS, the central region with the highest density of 5-HT3 receptors (Laporte et al. 1992); or systemically. In both experiments, GRANI induced a larger decrease in BP (but not in HR) in D rats than in controls, as expected given that the activation of NTS 5-HT3 receptors induces an increase in MBP only (Sévoz-Couche et al. 1998). NTS 5-HT3 receptors are therefore chronically activated after chronic stress. The effects of these receptors on MBP suggested that NTS 5-HT3 receptor activation may have been at the origin of the stress-induced increase in sympathetic activity. However, this seems unlikely, as GRANI tended to reduce both LF and the LF/HF ratio, albeit non-significantly, suggesting that sympathetic overactivity persists after NTS 5-HT3 receptor blockade. We also showed that intravenous and intra-NTS treatments with GRANI prevented the reduction in spontaneous and pharmacological baroreflex parameters in D animals. Vagally mediated rMSSD and HF, which are normally low in animals subjected to chronic stress, were similar to those in controls after both treatments. Taken together, these results suggest that the reduction of the BRR may be responsible for the lower level of vagal activity observed in animals with social stress-induced anxiety. 5-HT3 receptors are localized presynaptically on non-cardiovascular (probably from gastrointestinal origin; Leslie et al. 1990) NTS vagal afferents. When activated, these receptors trigger glutamatergic activation of local GABAergic interneurons, thereby inhibiting by GABAA receptors second-order baroreflex neurons (Sévoz-Couche et al. 2003) that project to the nucleus ambiguous on pre-ganglionic vagal neurons responsible for the production of the cardiac response (Loewy, 1990). It is likely that chronic activation of NTS 5-HT3 receptors due to overactivity of serotonin neurons in the B3 region (containing the raphe magnus and the lateral paragigantocellularis nuclei) and/or hypersensitivity of 5-HT3 and GABAA receptors is at the origin of the chronic baroreflex inhibition.
In conclusion, the long-term changes in HRV associated with social defeat-induced anxiety result from an increase in sympathetic tone associated with a decrease in parasympathetic tone. The latter is possibly due to a decrease in BRS, which may lead to sudden cardiac death (Khaykin et al. 1998). The central pathway involved in these changes involves DMH activation, leading to a decrease in baroreflex gain via NTS 5-HT3 receptor excitation and an increase in sympathetic tone independently of the NTS. Specific treatment for mood disorders restores vagal cardiac function only partially (Carnevali et al. 2011). Thus, in patients with high anxiety scores and in patients with induced dysautonomia, as observed after ischaemic stroke for example (Soros & Hachinsli, 2012), systemic treatment with the 5-HT3 receptor antagonist GRANI – a potent anti-emetic (Audhuy et al. 1996) with a highly safe profile (Aapro, 2004) – could be used to restore parasympathetic activity and, thus, reduce the likelihood of adverse cardiac events.
Acknowledgments
We would like to thank Dr Paul Pilowsky (Macquarie University, Sydney, Australia) for his help concerning HRV analysis, and Dr Pascal Carrive (UNSW, Sydney, Australia) for his judicious suggestions on language and style, and interesting comments on the results. This work was supported by grants from INSERM and UPMC.
Glossary
- BP
blood pressure
- BRR
cardiac baroreflex response
- BRS
baroreflex sensitivity
- CDZ
chlordiazepoxide
- D
defeated rats
- DMH
dorsomedial nucleus of the hypothalamus
- GRANI
granisetron
- HF
high-frequency domain
- HR
heart rate
- HRV
heart rate variability
- LF
low-frequency domain
- MBP
mean blood pressure
- MUSC
muscimol
- ND
non-defeated rats
- NTS
nucleus tractus solitarii
- rMSSD
root mean square of successive R–R interval differences
- RSA
respiratory sinus arrhythmia
- VEH
vehicle
Author contributions
C.S-C. conception and design, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content & final approval of the version to be published. C.Br., F.C., D.L., S.D.B., analysis and interpretation of data, drafting the article & final approval of the version to be published. C.Be., analysis and interpretation of data, revising the article critically for important intellectual content & final approval of the version to be published. J-J.B., conception and design, revising the article critically for important intellectual content & final approval of the version to be published.
Supplementary material
Supplemental Tables 1--5
Supplemental Fig. 1A
References
- Aapro M. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist. 2004;9:673–686. doi: 10.1634/theoncologist.9-6-673. [DOI] [PubMed] [Google Scholar]
- Andre J, Zeau B, Pohl M, Cesselin F, Benoliel JJ, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats: behavioral and biochemical studies. J Neurosci. 2005;25:7896–7904. doi: 10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhuy B, Cappelaere P, Martin M, Cervantes A, Fabbro M, Rivière A, Khayat D, Bleiberg H, Faraldi M, Claverie N, Aranda E, Auclerc G, Audhuy B, Benhammouda A, Bleiberg H, Cals L, Cappelaere P, Cattan A, Cervantes A, Chevallier B, Conroy T, Cupissol D, De Grève J, Diaz-Rubio E, Seitz JF, et al. A double-blind, randomised comparison of the anti-emetic efficacy of two intravenous doses of dolasetron mesilate and granisetron in patients receiving high dose cisplatin chemotherapy. Eur J Cancer. 1996;32:807–813. [PubMed] [Google Scholar]
- Becker C, Thiébot MH, Touitou Y, Hamon M, Cesselin F, Benoliel JJ. Enhanced cortical extracellular levels of cholecystokinin-like material in a model of anticipation of social defeat in the rat. J Neurosci. 2001;21:262–269. doi: 10.1523/JNEUROSCI.21-01-00262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beig MI, Baumert M, Walker FR, Day TA, Nalivaiko E. Blockade of 5-HT2A receptors suppresses hyperthermic but not cardiovascular responses to psychosocial stress in rats. Neuroscience. 2009;159:1185–1191. doi: 10.1016/j.neuroscience.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Berger S, Kliem A, Yeragani V, Bär KJ. Cardio-respiratory coupling in untreated patients with major depression. J Affect Disord. 2012;139:166–171. doi: 10.1016/j.jad.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel JJ, Becker C. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada J. Psychosis, depression, and high risk for sudden cardiac death: time for co-operation between psychiatrists and cardiologists. Eur Heart J. 2012;33:687–688. doi: 10.1093/eurheartj/ehr405. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Bondarenko E, Sgoifo A, Walker FR, Head GA, Lukoshkova EV, Day TA, Nalivaiko E. Metyrapone and fluoxetine suppress enduring behavioral but not cardiac effects of subchronic stress in rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1123–R1131. doi: 10.1152/ajpregu.00273.2011. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;74:286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Francis JL, Weinstein AA, Krantz DS, Haigney MC, Stein PK, Stone PH, Gottdiener JS, Kop WJ. Association between symptoms of depression and anxiety with heart rate variability in patients with implantable cardioverter defibrillators. Psychosom Med. 2009;71:821–827. doi: 10.1097/PSY.0b013e3181b39aa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Evaluation of baroreceptor reflex function in the chronic mild stress rodent model of depression. Psychosom Med. 2008;70:435–443. doi: 10.1097/PSY.0b013e31816ff7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Chen KC, Chen CF, Tsai TH. Simultaneous measurement of blood and brain microdialysates of granisetron in rat by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1998;716:251–255. doi: 10.1016/s0378-4347(98)00274-6. [DOI] [PubMed] [Google Scholar]
- Khaykin Y, Dorian P, Baker B, Shapiro C, Sandor P, Mironov D, Irvine J, Newman D. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Can J Psychiatry. 1998;43:183–186. doi: 10.1177/070674379804300209. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Laporte AM, Koscielniak T, Ponchant M, Vergé D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodozacopride and [3H]zacopride as radioligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- La RovereMT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Sxhwartz PJ, ATRAMI investigators. Autonomic Tone and Reflexes After Myocardial Infarction Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- Laude D, Elghozi JL, Girard A, Bellard E, Bouhaddi M, Castiglioni P, Cerutti C, Cividjian A, Di Rienzo M, Fortrat JO, Janssen B, Karemaker JM, Lefthériotis G, Parati G, Persson PB, Porta A, Quintin L, Regnard J, Rüdiger H, Stauss HM. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study) Am J Physiol Regul Integr Comp Physiol. 2004;286:R226–R231. doi: 10.1152/ajpregu.00709.2002. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Reynolds DJ, Andrews PL, Grahame-Smith DG, Davis CJ, Harvey JM. Evidence for presynaptic 5-hydroxytryptamine3 recognition sites on vagal afferent terminals in the brainstem of the ferret. Neuroscience. 1990;38:667–673. doi: 10.1016/0306-4522(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Anatomy of the autonomic nervous system: an overview. In: Loewy AD, Spyer KM, editors. Central Regulation of the Autonomic Function. Oxford University Press, New-York; 1990. pp. 1–16. [Google Scholar]
- Merahi N, Orer HS, Laporte AM, Gozlan H, Hamon M, Laguzzi R. Baroreceptor reflex inhibition induced by the stimulation of serotonin3 receptors in the nucleus tractus solitarius of the rat. Neuroscience. 1992;46:91–100. doi: 10.1016/0306-4522(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Moffitt JA, Grippo AJ, Holmes PV, Johnson AK. Olfactory bulbectomy attenuates cardiovascular sympathoexcitatory reflexes in rats. Am J Physiol Heart Circ Physiol. 2002;283:H2575–H2583. doi: 10.1152/ajpheart.00164.2002. [DOI] [PubMed] [Google Scholar]
- Netzer F, Bernard JF, Verberne AJ, Hamon M, Camus F, Benoliel JJ, Sévoz-Couche C. Brain circuits mediating baroreflex bradycardia inhibition in rats: an anatomical and functional link between the cuneiform nucleus and the periaqueductal grey. J Physiol. 2011;589:2079–2091. doi: 10.1113/jphysiol.2010.203737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek TJ, van Boxtel A, Westerink JH. Respiratory sinus arrhythmia responses to induced emotional states: effects of RSA indices, emotion induction method, age, and sex. Biol Psychol. 2012;91:128–141. doi: 10.1016/j.biopsycho.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Padley JR, Overstreet DH, Pilowsky PM, Goodchild AK. Impaired cardiac and sympathetic autonomic control in rats differing in acetylcholine receptor sensitivity. Am J Physiol Heart Circ Physiol. 2005;289:H1985–H1992. doi: 10.1152/ajpheart.00430.2005. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Porges SW, Heilman KJ, Bazhenova OV, Bal E, Doussard- Roosevelt JA, Koledin M. Does motor activity during psychophysiological paradigms confound the quantification and interpretation of heart rate and heart rate variability measures in young children. Dev Psychobiol. 2007;49:485–494. doi: 10.1002/dev.20228. [DOI] [PubMed] [Google Scholar]
- Porter JP, Phillips A, Rich J, Wright D. Effect of chronic stress on the cardiac baroreflex in the post-weanling rat. Life Sci. 2004;75:1595–1607. doi: 10.1016/j.lfs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J. 1998;19:F72–F80. [PubMed] [Google Scholar]
- Sévoz-Couche C, Comet MA, Hamon M, Laguzzi R. Role of nucleus tractus solitarius 5-HT3 receptors in the defense reaction-induced inhibition of the aortic baroreflex in rats. J Neurophysiol. 2003;90:2521–2530. doi: 10.1152/jn.00275.2003. [DOI] [PubMed] [Google Scholar]
- Sévoz-Couche C, Nosjean A, Franc B, Hamon M, Laguzzi R. Dorsal medullary 5-HT3 receptors and sympathetic premotor neurones in the rat. J Physiol. 1998;508:747–762. doi: 10.1111/j.1469-7793.1998.747bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Meerlo P, Costoli T, Manghi M, Stilli D, Olivetti G, Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ECG, body temperature and physical activity. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Res. 1993;627:9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- Sin PY, Galletly DC, Tzeng YC. Influence of breathing frequency on the pattern of respiratory sinus arrhythmia and blood pressure: old questions revisited. Am J Physiol Heart Circ Physiol. 2010;298:H1588–H1599. doi: 10.1152/ajpheart.00036.2010. [DOI] [PubMed] [Google Scholar]
- Sörös P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11:179–188. doi: 10.1016/S1474-4422(11)70291-5. [DOI] [PubMed] [Google Scholar]
- Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Wang R, Koganezawa T, Terui N. Differential responses of sympathetic premotor neurons in the rostral ventrolateral medulla to stimulation of the dorsomedial hypothalamus in rabbits. Brain Res. 2010;1356:44–53. doi: 10.1016/j.brainres.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P, Krishnan R, Sherwood A. Anxiety and vagal control of heart rate. Psychosom Med. 1998;60:498–502. doi: 10.1097/00006842-199807000-00018. [DOI] [PubMed] [Google Scholar]
- Xavier CH, Nalivaiko E, Beig MI, Menezes GB, Cara DC, Campagnole-Santos MJ, Fontes MA. Functional asymmetry in the descending cardiovascular pathways from dorsomedial hypothalamic nucleus. Neuroscience. 2009;164:1360–1368. doi: 10.1016/j.neuroscience.2009.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


