Abstract
Previous studies of the cortical control of human facial muscles documented the distribution of corticobulbar projections and the presence of intracortical inhibitory and facilitatory mechanisms. Yet surprisingly, given the importance and precision in control of facial expression, there have been no studies of the afferent modulation of corticobulbar excitability or of the plasticity of synaptic connections in the facial primary motor cortex (face M1). In 25 healthy volunteers, we used standard single- and paired-pulse transcranial magnetic stimulation (TMS) methods to probe motor-evoked potentials (MEPs), short-intracortical inhibition, intracortical facilitation, short-afferent and long-afferent inhibition and paired associative stimulation in relaxed and active depressor anguli oris muscles. Single-pulse TMS evoked bilateral MEPs at rest and during activity that were larger in contralateral muscles, confirming that corticobulbar projection to lower facial muscles is bilateral and asymmetric, with contralateral predominance. Both short-intracortical inhibition and intracortical facilitation were present bilaterally in resting and active conditions. Electrical stimulation of the facial nerve paired with a TMS pulse 5–200 ms later showed no short-afferent inhibition, but long-afferent inhibition was present. Paired associative stimulation tested with an electrical stimulation–TMS interval of 20 ms significantly facilitated MEPs for up to 30 min. The long-term potentiation, evoked for the first time in face M1, demonstrates that excitability of the facial motor cortex is prone to plastic changes after paired associative stimulation. Evaluation of intracortical circuits in both relaxed and active lower facial muscles as well as of plasticity in the facial motor cortex may provide further physiological insight into pathologies affecting the facial motor system.
Key points
Previous studies documented features of corticobulbar projections and of intracortical circuits in the motor cortex innervating lower facial muscles (face M1). However, there have been no studies of the afferent modulation of corticobulbar excitability or of the plasticity of synaptic connections in face M1.
Intracortical circuits, sensorimotor integration and plasticity in face M1 were investigated in healthy volunteers using standard protocols of the transcranial magnetic stimulation technique.
This study showed, for the first time, that face M1 is prone to plastic changes following paired associative stimulation and that its excitability is modulated by afferent stimulation at long latency (200 ms) but not at short latency (20 ms). Furthermore, contralateral predominance of cortical projection to lower facial muscles was confirmed, and the presence of bilateral intracortical inhibitory and facilitatory mechanisms at rest and during voluntary muscle activation was clarified.
These data provide further physiological insight into pathologies affecting the facial motor system.
Introduction
Facial expressions are an important channel of non-verbal communication and require highly co-ordinated control of a large number of small yet complex muscles. In this paper, we present further information about the physiological organization of these muscles using transcranial magnetic stimulation (TMS) of the facial area of the human primary motor cortex (face M1). Previous works have already characterized the optimal position and orientation of the coil over the scalp (Benecke et al. 1988; Meyer et al. 1994; Rödel et al. 2000; Dubach et al. 2004; Yildiz et al. 2007). They have also described the latency and amplitude of facial motor-evoked potentials (MEPs), as well as the cortical silent period in actively contracting muscles (Werhahn et al. 1995; Cruccu et al. 1997). Nevertheless, a number of outstanding questions remains.
The first conoversy concerns the bilaterality and symmetry of cortical command to lower facial muscles. Some TMS studies (Benecke et al. 1988; Meyer et al. 1989, 1994; Werhahn et al. 1995; Urban et al. 1997; Liscić & Zidar, 1998; Yildiz et al. 2004, 2007; Triggs et al. 2005) confirmed findings in monkeys (Jenny & Saper, 1987) which showed that regions of the facial nucleus supplying muscles of the lower face receive a bilateral corticobulbar projection with a contralateral predominance. However, several authors found no ipsilateral responses to cortical TMS in lower facial muscles (Kobayashi et al. 2001), whilst others suggested that any small-amplitude ipsilateral responses were due either to mid-line crossing of peripheral nerves or to volume conduction of EMG activity in contralateral muscles (Cruccu et al. 1990; Paradiso et al. 2005).
A second controversy revolves around the question of whether, in lower facial muscles, the excitability of intracortical inhibitory and excitatory circuits is suppressed during voluntary contraction. Paired-pulse TMS protocols (Kujirai et al. 1993) have been used to measure short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) in the cortical representation of the orbicularis oculi (Kobayashi et al. 2001; Paradiso et al. 2005), depressor anguli oris (DAO; Paradiso et al. 2005), mentalis (Kobayashi et al. 2001), tongue (Muellbacher et al. 2001a; Baad-Hansen et al. 2009), masseter (Ortu et al. 2008a) and digastric muscles (Jaberzadeh et al. 2007). All have found SICI to be present in resting muscles on the contralateral side, but there is debate about the presence of ipsilateral effects and whether SICI/ICF persists during volitional contraction (Kobayashi et al. 2001; Muellbacher et al. 2001a; Paradiso et al. 2005).
Finally, two aspects of neurophysiological organization have yet to be characterized in facial muscles, namely the modulation of corticobulbar excitability by afferent input and the synaptic plasticity in the facial motor cortex. It is now recognized from studies on control of limb movement that M1 plays a critical role in sensorimotor integration (Classen et al. 2000; Tokimura et al. 2000) and in learning of new motor skills (Muellbacher et al. 2001b). The same is likely to be true for the area of facial representation. Indeed, studies in animals have shown that face M1 is involved in the control of learned orofacial movements (Sessle et al. 2007), and human TMS studies have shown that after only 1 h of training in a tongue-protrusion task, subjects show a significant increase of M1 tongue representation and of MEP amplitude as well as a significant decrease in motor threshold (Svensson et al. 2003, 2006). Recent work, using stretch manipulation of the facial skin during speech, has highlighted the importance of peripheral somatosensory afferent inputs in facial muscle motor learning (Ito & Ostry, 2010). However, although hand motor cortex excitability is increased by repetitive nerve stimulation (Ridding et al. 2000; Kaelin-Lang et al. 2002), the only single TMS study that examined effects on corticobulbar output to perioral facial muscles showed no change after repetitive electrical stimulation of the lower facial skin (Yildiz et al. 2004). In the present study, we examined sensorimotor integration with the short-afferent inhibition (SAI) and the long-afferent inhibition (LAI) techniques (Chen et al. 1999; Tokimura et al. 2000; Sailer et al. 2002) and cortical plasticity with the paired associative stimulation (PAS) method (Stefan et al. 2000). In hand muscles, the former methods cause a brief suppression of cortical excitability, whereas the latter is able to induce long-term potentiation-like (LTP-like) increases in excitability (Chen et al. 1999; Classen et al., 2000; Sailer et al. 2002; Stefan et al. 2000, 2002, 2006; Tokimura et al. 2000; Wolters et al. 2003; Ziemann et al. 2004). Similar protocols have been developed recently to study sensorimotor integration (Roy & Gorassini, 2008; Bikmullina et al. 2009) and PAS effects (Stinear & Hornby 2005; Prior & Stinear, 2006; Mrachacz-Kersting et al. 2007; Roy et al. 2007) in the lower limb area, but so far they have never been explored in the facial area.
Aims of the present study were therefore as follows: (i) to reassess the controversy about the symmetry of cortical output to lower facial muscles; (ii) to clarify whether both SICI and ICF mechanisms are involved in modulation of cortical command to ipsi- and contralateral facial motor nucleus, in relation to the muscle state (relaxed versus activated); and (iii) to study sensorimotor integration and plasticity of the primary facial motor cortex by testing SAI, LAI and PAS effects in relaxed lower facial muscles.
Methods
Subjects
Twenty-five healthy volunteers (14 females and 11 males; mean age 32.6 ± 8.6 years; range 20–56 years) participated in the study. All the subjects were right handed except one. Prior to the study, subjects gave their informed written consent, and the procedure, approved by the local ethical committee (NHNN/Institute of Neurology Joint Research Ethics Committee 03/N018 and Bioethics Committee of ASL. no. 1 – Sassari, prot. 987/2) was in accordance with the Declaration of Helsinki. Most of the subjects took part in more than one experiment. None of them had a history of neurological diseases. No side-effects were noted during and after the experiment in any of the individuals tested. Subjects sat in a comfortable chair and were asked to keep their eyes open during the experiments and to stay relaxed but alert.
Electromyographic recordings
In the main experiments (1–7), MEPs evoked by TMS of the left face M1 were recorded from the right and left DAO muscles using 9-mm-diameter Ag–AgCl surface cup electrodes. Recording electrodes were placed at the mid-point between the angle of the mouth and the lower border of the mandible, reference electrodes were placed over the mandible border, 1–2 cm below recording electrodes (Lapatki et al. 2003) and a earth electrode was placed over the chin. This electrode montage assures minimization of EMG contamination, via cross-talk with active nearby muscles, confirmed by control experiments. When required, subjects performed a tonic activation of the DAO at 10% of maximal voluntary contraction (MVC), to be steadily maintained during recordings, with the aid of visual feedback of the rectified and filtered EMG activity.
In control experiment 8, the first (R1) and the second (R2) components of the blink reflex (BR) were recorded from the right orbicularis oculi muscle through 9-mm-diameter surface Ag–AgCl electrodes, with the recording electrode placed over the lower lid and the reference electrode 2 cm from the lateral cantus.
Unrectified and rectified EMG signals were recorded (D360 amplifier; Digitimer Ltd, Welwyn Garden City, UK), amplified (×1000), filtered (bandpass 3–3000 Hz for TMS recordings and 50–5000 Hz for BR recordings) and sampled (5 kHz per channel; window frame length, 250 ms for TMS recordings and 4000 ms for BR recordings) using a CED1401 power analog-to-digital converter (Cambridge Electronic Design, Cambridge, UK) and Signal 4 software on a computer.
Transcranial magnetic stimulation
Transcranial magnetic stimulation of the left face M1 was performed using a figure-of-eight-shaped coil with external loop diameter of 9 cm connected to two Magstim 200 stimulators in a Bistim module (Magstim Co., Whitland, UK). The optimal stimulation site was chosen based on the best motor responses elicited in the contralateral DAO (cDAO), and then marked on a cotton cap fitted over the scalp to ensure that the coil remained in the same place throughout the experiment. The handle of the coil pointed posteriorly and laterally, at approximately 30–45 deg to the interhemispheric line, this being the optimal orientation to evoke cortical responses and to avoid short- and long-latency responses (Dubach et al. 2004). In all experiments, TMS frequency was 0.25 Hz, and motor threshold was expressed as a percentage of the maximal stimulator output (MSO). The resting motor threshold (RMT) was taken as the lowest TMS intensity that elicited, in the relaxed DAO, MEPs of ∼0.05 mV in at least five of 10 consecutive trials (Rothwell et al. 1999). The active motor threshold (AMT) was defined as the lowest intensity able to induce, in at least five of 10 trials, MEPs of >0.1 mV amplitude in the DAO voluntarily activated, by performing a depression of the mouth angles.
Electrical stimulation
Electrical stimulation (ES) was applied to the mandibular branch of the right facial nerve, through a pair of cup electrodes (cathode distal) connected to a constant-current stimulator (model DS7; Digitimer Ltd). Single square-wave pulses (0.2 ms duration) were delivered at a frequency of 0.25 Hz, and stimulus intensity was set at a value nearly three times the perceptual threshold (PT) of the subject, which was able to evoke a small twitch in the target muscle.
In experiment 8, ES was applied to the right supraorbital nerve (SON) with cup electrodes (cathode over the homonymous foramen and anode 2 cm lateral) delivering square electrical pulses of 0.2 ms duration at an intensity three times the R2 threshold (i.e. the lowest intensity that elicited a clear R2 response in at least 5 of 10 trials).
Experimental design
The main experiment was aimed at assessing physiological properties of the corticobulbar projection (experiments 1–3) to lower facial muscles, sensory motor integration and plasticity (experiments 4–7) of face M1. A control experiment (experiment 8) was performed to explore whether paired associative stimulation at 20 ms ISI (PAS20) acts at cortical and/or subcortical level.
Experiment 1. Single-pulse TMS to the left DAO motor cortex
All subjects participated in this experiment, which was aimed at testing bilaterality and symmetry of corticobulbar projections to DAO muscles. Single-pulse TMS was delivered at the left face M1. Ten MEPs were recorded from both cDAO and ipsilateral DAO (iDAO) in two muscle conditions, at rest and during activation at 10% of MVC. The intensity of stimulation was set at 120% of RMT (1.2RMT) in the resting conditions and at 120% of AMT (1.2AMT) in the active conditions. The amplitude and the onset latency of the contra- and ipsilateral MEPs (cMEP and iMEP, respectively) were measured in both resting and active DAO. The MEP peak-to-peak amplitude was measured for each trial and then the single peak-to-peak values were averaged.
Experiment 2. Paired-pulse TMS to the left DAO motor cortex at rest
Eighteen subjects participated in this experiment. The paired-pulse TMS protocol consisted of a subthreshold conditioning stimulus (CS) preceding a suprathreshold test stimulus (TS) by a variety of interstimulus intervals (ISIs), which allowed SICI (2, 3 and 5 ms) and ICF (10 and 15 ms) to be tested. The CS intensity was set at 70% of RMT (0.7RMT) and the TS intensity at 1.2RMT. The six stimulus conditions (TS alone and 5 different ISIs) were each delivered 10 times in a randomized order and responses recorded from relaxed cDAO and iDAO. Mean amplitude of the conditioned MEP was expressed as a percentage of the averaged test MEP.
Experiment 3. Paired-pulse TMS to the left DAO motor cortex during voluntary muscle activation
To evaluate whether SICI and ICF also operate when the DAO is active, paired-pulse TMS was delivered to the left face M1 of all 25 subjects, during voluntary DAO contraction at a level of 10% of MVC. The TS intensity was set at 1.2AMT. Two CS intensities, 70 and 80% of AMT (0.7AMT and 0.8AMT, respectively), were used in two different blocks. The former CS is comparable to the 0.7RMT relative conditioning intensity and the latter is comparable to 0.7RMT as absolute conditioning intensity.
To evaluate the effect of muscle activity and of recording side on SICI and ICF, data collected in the resting and active muscle states were compared. The TMS parameters and data collection were those reported in experiment 2.
Experiment 4. Short-afferent inhibition in cDAO MEPs at rest
The presence of SAI was tested in 15 right-handed subjects, by pairing the ES of the mandibular branch of the right facial nerve with magnetic stimulation of the left face M1. Electrical stimulation was set at an intensity of three times PT. Given that stimulation of trigeminal afferents induces a clear suppression of voluntary EMG activity in perioral muscles (Pavesi et al. 2000), this experiment was performed only at rest. Transcranial magnetic stimulation was set at an intensity of 110% of RMT (1.1RMT) because the high RMT of some subjects did not allow prolonged stimulation. Given that this TMS intensity was insufficient to evoke clear ipsilateral MEPs in most of the subjects, the analysis was focused on the contralateral muscle only. Interstimulus intervals between ES and TMS, delivered in a randomized order, were 5, 10, 15, 20, 25 and 30 ms. For each stimulus condition, 10 responses were recorded from the right DAO at rest. Mean amplitude of the conditioned MEP was expressed as a percentage of the averaged test MEP.
Experiment 5. Long-afferent afferent inhibition in cDAO MEPs at rest
In seven subjects who participated in experiment 4, the presence of LAI was tested using the same experimental procedure and data collection as experiment 4. Interstimulus intervals between ES of the mandibular branch of the right facial nerve and TMS of the left face M1 were 150 and 200 ms. Mean amplitude of the conditioned MEP was expressed as a percentage of the averaged test MEP.
Experiment 6. Effects of PAS20 on the cDAO MEPs at rest
Effects of PAS were investigated in detail only in the resting conditions, because preliminary experiments performed during muscle contraction showed no significant effects in the active DAO, according to data shown in hand muscles (Stefan et al. 2000). The same subjects who participated in experiment 4 were enrolled in this experiment. The PAS intervention was administered by pairing ES of the right facial nerve (3 × PT) with TMS (1.1RMT) of the left face M1 using an ISI of 20 ms between ES and TMS. This time interval was chosen because it corresponds to the peak latency of the DAO MEP plus 5 ms, according to the criterion used by Stinear & Hornby (2005) for leg muscles. Two hundred pairs of stimuli were administered, and the subjects were instructed to keep facial muscles relaxed, to count the stimuli and to concentrate on the DAO muscle. Twenty MEPs were collected from the resting cDAO before and 0 (T0), 10 (T10), 20 (T20) and 30 min (T30) after PAS delivery. Effects of administration of PAS20 were measured by comparing the mean peak-to-peak amplitude (in millivolts) of the test MEP (baseline) with that of MEPs collected at each time interval tested after the PAS intervention.
Experiment 7. Effects of PAS intervention at 10 ms ISI (PAS10) on the cDAO MEPs at rest
Fourteen of the 15 subjects who had participated in experiment 6 were also enrolled in experiment 7. At least 1 week elapsed between the two experiments. The PAS protocol was delivered as described for experiment 6, but 10 ms ISI was used between ES of the right facial nerve and TMS of left face M1 (PAS10). The effects of PAS10 intervention were assessed in the resting cDAO, by comparing the amplitudes of averaged (n = 20) baseline MEPs with averaged MEPs collected at T0, T10, T20 and T30 from PAS intervention.
Experiment 8. Effects of PAS20 on the BR and on the BR recovery cycle
In six subjects who participated in experiment 6, effects of PAS20 on R1 and R2 as well as on the R2 recovery cycle were investigated in the relaxed orbicularis oculi. The R1 and R2 components of the BR were recorded from the right orbicularis oculi, following ES of the ipsilateral SON. The R2 recovery cycle was assessed at ISIs of 250, 500 and 1000 ms (5 trials for each ISI, in a randomized order). Pairs of SON stimuli were given randomly every 20–40 s to minimize habituation.
The raw BR recordings were DC corrected, rectified and averaged offline, and the R1 and R2 areas were measured. For the recovery cycle analysis, the ratio of R2 conditioned/unconditioned area was calculated for each ISI. An R2 recovery index was then calculated in each subject as the mean of R2 area ratio values at 250 and 500 ms ISIs. These parameters (R1 area, R2 area, R2 recovery cycle and R2 recovery index) were assessed before PAS20 delivery (baseline), immediately after (T0) and after 20 min (T20) from PAS20 administration. Only T0 and T20 post-PAS intervals were tested, because the entire battery of SON stimuli lasted nearly 15 min, thus making it impossible to test T10 and T30 post-PAS intervals.
Statistical analysis
Statistical analysis was performed with SPSS 18 software (SPSS Inc., Chicago, IL, USA). In the analysis performed with repeated-measures ANOVA, compound symmetry was evaluated by testing the sphericity with Mauchly's test. The Greenhouse–Geisser correction was used to compensate for non-spherical data. A P value <0.05 was considered significant. Unless otherwise stated, values are expressed as means ± SD.
In experiment 1, differences between MEP latencies and amplitudes elicited by single-pulse TMS were assessed using Student's paired t test, for both resting and active states.
In experiments 2 and 3, the effects of side (ipsilateral and contralateral), ISI (2, 3, 5, 10 and 15 ms) and activity (0.7RMT, 0.7AMT and 0.8AMT) on mean amplitude of the conditioned MEP (expressed as a percentage of the averaged test MEP) were assessed by ANOVA using a model of repeated measures. In the case of significant values, Student's paired t test was used for post hoc analysis, applying the Bonferroni correction for multiple comparisons when needed.
In experiments 4 and 5, repeated-measures ANOVA and post hoc Student's paired t test were performed to evaluate the effects of ISI (5, 10, 15, 20, 25 and 30 ms for SAI, and 150 and 200 ms for LAI) on mean amplitude of the conditioned MEP (expressed as a percentage of the averaged test MEP).
In experiments 6 and 7, repeated-measures ANOVA and post hoc Student's paired t test were performed to evaluate the effects of time (0, 10, 20 and 30 min) and PAS intervention (PAS20 and PAS10) on mean peak-to-peak MEP amplitude (in millivolts).
In experiment 8, repeated-measures ANOVA and post hoc Student's paired t test were used to evaluate the effect of PAS on DAO MEP and on R1 and R2 ratio areas, with time (baseline, 0 and 20 min) and measure (MEP, R1 and R2) as within-subject factors. Repeated-measures ANOVA and post hoc Student's paired t test were used to test the effect of ISI (250, 500 and 1000 ms) and time (baseline, T0 and T20) on R2 area. The relationship between PAS20 effects on DAO MEPs and on the recovery index of R2 (expressed as a ratio of baseline values) was investigated using two-way repeated-measures ANOVA, with measure (DAO MEP and R2 recovery index) and time (T0 and T20) as within-subject factors and Student's paired t test as a post hoc analysis in the event of any significant interactions.
Results
Transcranial magnetic stimulation over the left face M1 evoked bilateral and asymmetric motor potentials in both the relaxed and active depressor anguli oris muscle. The optimal coil position for eliciting MEPs in the cDAO was 3.8 ± 1.9 cm anterior and 7.5 ± 1.5 cm lateral from the vertex. In some subjects, MEPs were polyphasic, with at least three identifiable components (Fig. 1), namely a short-latency peripheral wave (onset <6 ms), due to direct facial nerve stimulation, which was visible only in the ipsilateral muscle, a bilateral medium-latency wave (onset 9–15 ms) followed by a non-consistent long-latency wave (onset >20 ms). Small changes of the coil orientation affected earlier and long-latency components, with varying effects from reduction to disappearance, without altering the medium-latency wave amplitude. We focused our analysis on medium-latency waves (Fig. 2), which have been proved to be of corticobulbar origin (Meyer et al. 1994; Rödel et al. 1999; Dubach et al. 2004).
Figure 1. Motor responses with short (S), medium (M) and long (L) latency recorded from resting and active depressor anguli oris (DAO) muscles following single-pulse transcranial magnetic stimulation (TMS) of the left facial motor cortex, in a representative subject.
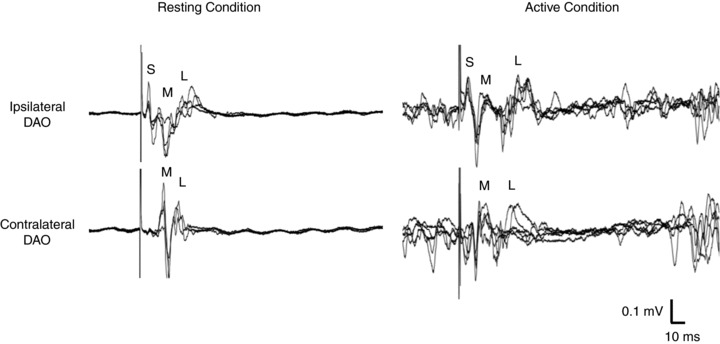
In both resting and active muscle states, the S-wave is present only ipsilaterally to the stimulation side, with no volume conduction to the contralateral muscle. By contrast, M- and L-waves are detected bilaterally. Each trace reports 5 superimposed trials. The TMS intensities were 120% of resting motor threshold (RMT) in the resting conditions and 120% of active motor threshold (AMT) in the active conditions (10% of maximal voluntary contraction).
Figure 2. Motor-evoked potentials (MEPs) recorded from resting and active DAOs of a representative subject, following single-pulse TMS of the left facial motor cortex.
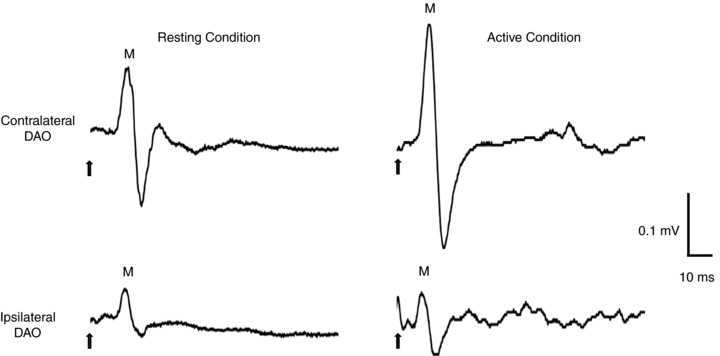
The ipsilateral MEP is smaller and delayed when compared with the contralateral MEP, in both resting and active conditions (10% of maximal voluntary contraction). Each trace is the average of 10 single trials. The TMS intensities were 120% of RMT in the resting conditions and 120% of AMT in the active conditions. Arrows indicate the time of stimulus delivery. M, medium-latency wave.
Experiment 1. Single-pulse TMS to the left DAO motor cortex
At rest, some subjects had a high motor threshold, which made the TMS intensity required to elicit clear motor responses uncomfortable. In the relaxed DAO, a single-pulse TMS of the left face M1, using 1.2RMT as TS, evoked bilateral motor potentials in most of the subjects. Motor-evoked potentials were detected contralaterally in 18 of 25 subjects and ipsilaterally in 14 of 18 subjects, the response being absent in three subjects and hidden by the stimulus artifact in one subject. During voluntary activation of the muscles, a TS of 1.2AMT evoked clear responses in the cDAO of all 25 subjects, while in the iDAO clear MEPs were discernible from the background EMG activity in only 16 of 25 subjects. Mean RMT (n = 18) was 49.9 ± 6.4% MSO, and mean AMT (n = 25) was 46.6 ± 8.5% MSO. The MEP onset, peak latency and amplitude values are shown in Table 1. Students paired t test, performed in the subjects with bilateral responses, showed a significant difference between iMEP and cMEP onset latencies, with contralateral latencies being shorter than ipsilateral latencies, in both the resting (P < 0.001) and active muscle conditions (P = 0.017). On the contrary, peak latencies did not differ significantly. Student's paired t test also showed that cMEPs were significantly bigger than iMEPs, both at rest (P < 0.001) and during voluntary contraction (P = 0.005).
Table 1.
Latencies and amplitudes (means ± SD) of motor potentials evoked in the depressor anguli oris (DAO) muscles during single-pulse transcranial magnetic stimulation of left face M1 with the muscle at rest and during volitional contraction
| Conditions | MEP | Contralateral DAO | Ipsilateral DAO | P value |
|---|---|---|---|---|
| Rest (1.2RMT) | Onset latency (ms) | 10.8 ± 0.6 | 12.0 ± 0.7 | <0.001 |
| Peak latency (ms) | 14.6 ± 0.9 | 14.9 ± 1.4 | n.s. | |
| Amplitude (mV) | 0.19 ± 0.11 | 0.08 ± 0.02 | <0.001 | |
| Active (1.2AMT) | Onset latency (ms) | 9.3 ± 1.1 | 10.9 ± 2.8 | 0.017 |
| Peak latency (ms) | 13.7 ± 1.9 | 14.5 ± 3.2 | n.s. | |
| Amplitude (mV) | 0.34 ± 0.23 | 0.18 ± 0.13 | 0.005 |
Abbreviations: 1.2AMT, 120% of active motor threshold; 1.2RMT, 120% of resting motor threshold; MEP, motor-evoked potential; and n.s., not significant.
Experiment 2. Paired-pulse TMS to the left DAO motor cortex at rest
In these experiments, the mean CS intensity was 0.7RMT, corresponding to 35.0 ± 4.4% MSO. Note that because the contralateral and ipsilateral muscles were recorded simultaneously, mean peak-to-peak amplitudes of unconditioned (test) MEPs (Table 1) were smaller on the ipsilateral side. In relaxed hand muscles, Sanger et al. (2001) found that SICI is less effective when the test MEP is small. Likewise, in the present data we found that SICI was less powerful in the iDAO than the cDAO.
This was borne out in the detailed statistical analysis. Two-way repeated-measures ANOVA comparing side (iDAO versus cDAO) and ISI (2, 3, 5, 10 and 15 ms) showed a significant effect of ISI (P < 0.001) and a significant interaction between side and ISI (P = 0.006). Given that the mechanisms of SICI and ICF are known to differ, we conducted a second analysis on each period separately. Two-way ANOVA performed for SICI (2, 3 and 5 ms) demonstrated a significant effect of side (P = 0.007) and ISI (P < 0.001) and a significant interaction (side × ISI, P = 0.003). This interaction indicates that the time course of SICI is different on the contralateral versus ipsilateral sides. This was confirmed by Student's paired t tests showing that SICI at 2 ms (P = 0.003) and 3 ms (P < 0.001), but not at 5 ms, was stronger in the cDAO. Nevertheless, analysis of each side separately showed that there was significant inhibition at both 2 and 3 ms in the two sides (2 ms, P < 0.001 for both iDAO and cDAO; and 3 ms, P < 0.001 for cDAO and P = 0.004 for iDAO). Two-way ANOVA performed for ICF (10 and 15 ms) demonstrated a significant effect only for ISI (P < 0.001). Post hoc analysis showed a significant facilitation at 10 ms (P = 0.013 for cDAO and P = 0.004 for iDAO) and 15 ms (P = 0.001 for both cDAO and iDAO), but there was no difference between ipsi- and contralateral effects (Fig. 3A).
Figure 3. Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) assessed in the cortical representation of the DAO, in the resting and active conditions.
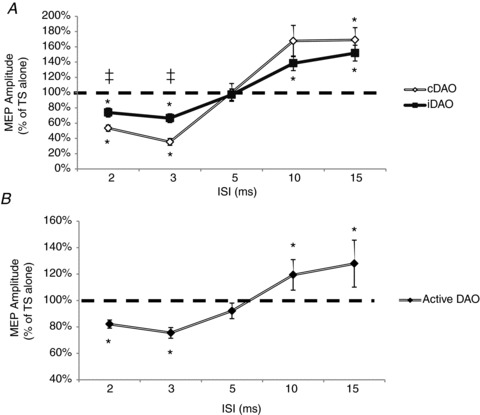
A, mean data obtained from 14 subjects at rest are reported. Paired TMS of the left facial motor cortex [control stimulus (CS) 0.7RMT] induced significant inhibition [interstimulus intervals (ISIs) of 2–3 ms] and facilitation (ISIs of 10–15 ms) of ipsi- and contralateral MEPs. At SICI ISIs, a significantly (‡P < 0.01) stronger effect was observed on the contralateral (cDAO) than on the ipsilateral projection (iDAO). In contrast, at ICF ISIs, MEP facilitation did not differ significantly between sides. B, mean data from 16 subjects in the active conditions are reported. The MEP amplitudes at the two CS intensities (0.7AMT and 0.8AMT) and on the two sides were averaged. A significant inhibition at 2–3 ms and a significant facilitation at 10–15 ms were observed. Ordinates indicate MEP amplitude expressed as a percentage of unconditioned MEP induced by the test stimulus (TS alone), taken as 100% (horizontal dashed line). *P < 0.05. Error bars represent means ± SEM.
Experiment 3. Paired-pulse TMS to the left DAO motor cortex during voluntary muscle activation
In the active conditions (10% of MVC), two different CS were used in two different blocks. In the first block, a CS of 0.7AMT was used, which corresponded to a mean intensity of 32.6 ± 5.9% MSO. In the second block, a CS of 0.8AMT was used, corresponding to a mean intensity of 37.3 ± 6.8% MSO. Mean amplitudes of unconditioned (test) MEPs are reported in Table 1. As in the preceding section, test MEP amplitudes were smaller on the ipsilateral than on the contralateral side; despite this, analysis showed that there was no difference in the depth of SICI during active contraction on the two sides.
Three-way ANOVA with CS (0.7AMT versus 0.8AMT), SIDE (ipsilateral versus contralateral) and ISI (2, 3, 5, 10 and 15 ms) as within-subject factors, showed a significant effect of ISI (P < 0.001) but no significant interactions between ISI and CS, ISI and side, or CS × side × ISI, suggesting that the two CS had the same effect on iMEP and cMEP.
As a result of this lack of interaction between CS and side, averaged MEPs at the two intensities and on the two sides were compared by means of a one-way ANOVA, with ISI as main factor. Results showed a significant effect of ISI (P < 0.001), and planned post hoc Student's t test showed a significant (P < 0.001) inhibition at 2 and 3 ms and a significant (P = 0.001) facilitation at 10 and 15 ms (Fig. 3B).
Comparison of SICI and ICF in resting and active states in the DAO motor cortex
A final question that can be asked about the data obtained in experiments 2 and 3 is whether SICI (or ICF) is different in relaxed versus active states. Visual comparison of Fig. 3A and B suggests that effects are smaller in the active than in the relaxed state. In this set of experiments, we used the same TMS intensities for CS. We directly compared SICI in 14 of the subjects from experiments 2 and 3 who had bilateral MEPs in resting and active states. The RMT and AMT mean intensities were 49.9 ± 6.4% at rest and 43.6 ± 6.4% when active. Control stimulus intensity was 34.9%, which was equivalent to both 0.7RMT and 0.8AMT. Test stimulus intensities were 59.7 ± 7.5% MSO and 52.1 ± 7.1% MSO (equivalent to 1.2RMT and 1.2AMT, respectively). Three-way ANOVA performed for SICI, with ISI (2, 3 and 5 ms), side (cDAO versus iDAO) and activity (rest versus active) as within-subject factors, demonstrated a significant effect of ISI (P < 0.001), side (P = 0.019) and activity (P = 0.01) and significant interactions between ISI and side (P = 0.01), ISI and activity (P < 0.001) and ISI × side × activity (P = 0.03). This suggests that the effect of voluntary contraction differed between contralateral and ipsilateral projections at specific time intervals. Post hoc Student's paired t test showed that SICI was less powerful during voluntary contraction of cDAO at ISIs of 2 ms (P = 0.008) and 3 ms (P = 0.002). Three-way ANOVA performed for ICF (10 and 15 ms) showed no significant interactions (ISI × side × activity; Fig. 4).
Figure 4. Comparison of SICI and ICF in the lower facial motor cortex in resting and active conditions.
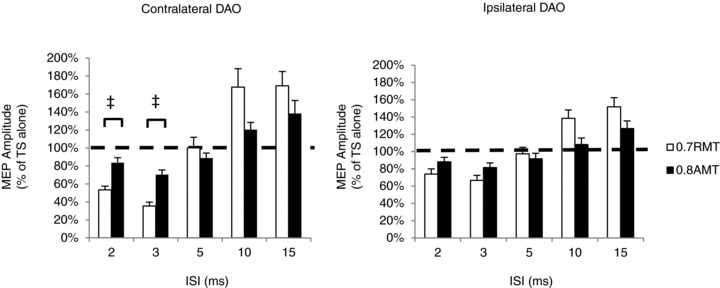
Data from contralateral and ipsilateral DAO muscles as well as SICI and ICF intervals were analysed separately (n = 14 subjects). The extent of SICI and ICF was significantly different in the resting and active muscle states (0.7RMT versus 0.8AMT) at different ISIs depending on the ipsi- and contralateral projection. Ordinates indicate MEP amplitude expressed as a percentage of unconditioned MEP induced by the TS alone, which was taken as 100% (horizontal dashed line). ‡P < 0.05. Error bars represent means + SEM.
Experiment 4. Short-afferent inhibition in cDAO MEPs at rest
In 15 subjects, the effects of a conditioning ES of the right facial nerve on motor potentials induced in the relaxed DAO by TMS of the left face M1 were investigated at ISIs of 5, 10, 15, 20, 25 and 30 ms. Mean PT intensity was 1.03 ± 0.36 mA and mean ES intensity was 3.07 ± 1.12 mA, able to evoke an M-wave in the right DAO. Mean RMT was 52.3 ± 7.6% MSO, and TS intensity mean value was 57.5 ± 7.6% MSO. One-way ANOVA showed no significant effects on the conditioned MEP amplitude, at any interval tested (Fig. 5A).
Figure 5. Effects of stimulation of the right facial nerve on motor potentials induced in the DAO by TMS of the left facial motor cortex.
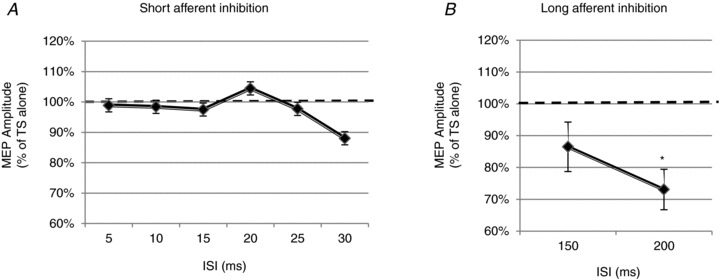
A, the graph shows mean data obtained from the relaxed cDAO of 15 subjects. Facial nerve electrical stimulation preceded single-pulse TMS of the left facial motor cortex by time intervals of 5, 10, 15, 20, 25 and 30 ms. No evidence of significant short-afferent inhibition of the conditioned MEP was observed at any ISI tested. B, the graph shows mean data obtained from the relaxed cDAO of 7 subjects. Long-afferent inhibition was tested by pairing facial nerve stimulation with a TMS pulse at ISIs of 150 and 200 ms. A significant inhibition at 200 ms ISI was observed. Ordinates indicate MEP amplitude expressed as a percentage of the unconditioned MEP induced by the TS alone, taken as 100% (horizontal dashed line). *P < 0.05. Error bars represent means ± SEM.
Experiment 5. Long-afferent afferent inhibition in cDAO MEPs at rest
In seven subjects, the effects of a conditioning ES of the right facial nerve on MEPs in relaxed cDAO were investigated at ISIs of 150 and 200 ms. Mean PT intensity was 1.2 ± 0.39 mA and mean ES intensity was 3.0 ± 0.53 mA. Mean RMT was 47.4 ± 4.9% MSO and the mean TS intensity was 52.7 ± 5.8% MSO. One-way ANOVA showed a significant effect of ES on the MEP amplitude (P < 0.033). Post hoc Student's paired t test showed a significant inhibition at 200 ms ISI (P = 0.035; Fig. 5B).
Experiment 6. Effects of paired associative stimulation at 20 ms ISI on the cDAO MEPs at rest
Fifteen subjects participated in this experiment, which tested whether a PAS20 protocol was effective in producing long-lasting changes in excitability of the facial motor cortex. Two hundred paired stimuli of the right facial nerve and of the left face M1 at 20 ms ISI led to an increase in amplitude of the resting MEP amplitude, with an average enhancement of 34%. Figure 6A shows two averaged MEPs recorded from the relaxed DAO, before and 20 min after PAS20 administration. The mean size of the MEP rose from a baseline value of 0.15 ± 0.13 mV to a post-PAS value of 0.20 ± 0.15 mV (mean of T0–T30 intervals; P = 0.001). One-way repeated-measures ANOVA (within-subject factor of time) showed a significant effect of time (baseline, T0, T10, T20 and T30) on the resting MEP size after PAS20 (P = 0.018). Post hoc Student's paired t test revealed a significant difference between baseline and MEPs collected from 0 to 30 min after PAS20 delivery (T0, P = 0.008; T10, P = 0.004; T20, P = 0.002; and T30, P = 0.042; Fig. 6B).
Figure 6. Effects of PAS20 intervention on the facial motor cortex.
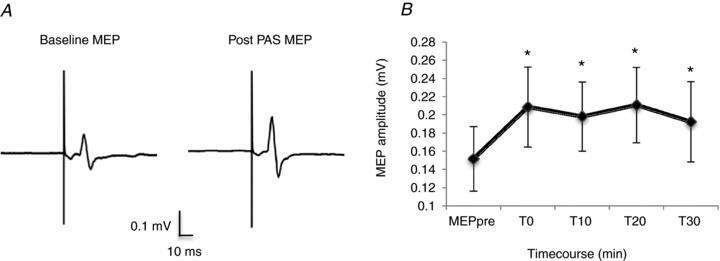
A, the left panel reports the averaged motor-evoked potential recorded from the relaxed right depressor anguli oris muscle following single-pulse TMS of the left facial motor cortex, before PAS20 intervention (baseline MEP). The right panel shows MEP changes observed 20 min after PAS20 intervention. The PAS20 protocol consisted of pairing 200 stimuli (electrical stimulation of the right facial nerve and TMS of the left facial motor cortex) with an ISI of 20 ms. The intensity of electrical stimulation was 3 times subject perceptual threshold; TMS intensity was 1.1RMT. Each trace is the average of 20 single trials. B, the graph reports data from 15 subjects showing the time course of mean MEP changes, compared with the baseline (MEP pre), observed after 0, 10, 20 and 30 min from the PAS20 intervention. *P < 0.05. Error bars represent means ± SEM.
Experiment 7. Effects of PAS intervention at 10 ms ISI on the cDAO MEPs at rest
To test whether the effect of PAS20 was specific to the time interval between stimuli, we also tested for PAS effects at an ISI of 10 ms (PAS10) in 14 subjects. In comparison with baseline, post-PAS MEPs were increased in 13 of 15 subjects after PAS20 and in six of 14 subjects after PAS10 (Fig. 7A).
Figure 7. Effects of PAS10 and PAS20 interventions on the magnitude of MEPs recorded from the DAO, at rest.
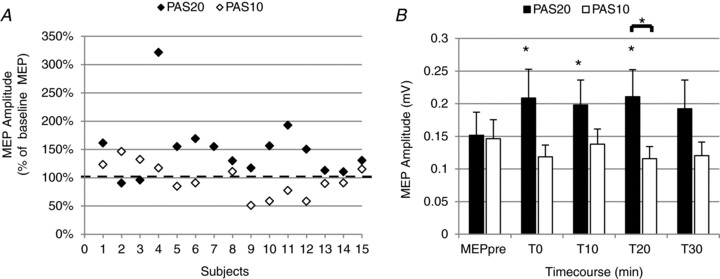
A, the graph shows, for each subject, average MEPs recorded from the relaxed DAO following PAS20 (filled diamonds, n = 15 subjects) and PAS10 interventions (open diamonds, n = 14 subjects). Post-PAS, the MEP amplitude was calculated as the mean of MEPs collected at T0–T30. Ordinates indicate MEP amplitude expressed as a percentage of baseline MEP, which was taken as 100% (horizontal dashed line). Note that, compared with baseline, MEP size was enhanced in 86.6% of subjects after PAS20 and in 42.8% of subjects after PAS10 intervention. B, the histogram shows the time course of effects of PAS20 (filled columns) and PAS10 (open columns) in 14 subjects. Amplitudes of baseline MEP (MEP pre) and of MEP collected after 0, 10, 20 and 30 min are reported for PAS20 and PAS10 protocols. Compared with baseline, a significant facilitatory effect was observed only after PAS20 at all time points tested, whereas no significant effects were detected after PAS10. Comparing MEP amplitudes in the two experimental conditions, a significant difference was detected only 20 min after PAS intervention. *P < 0.05. Error bars represent means + SEM.
When comparing effects of PAS20 and PAS10, two-way repeated-measures ANOVA (within-subject factors of ISI and time) revealed a significant ISI × time interaction (P = 0.008), suggesting that the time course of the PAS effect differed at the two ISIs. One-way ANOVA (within-subject factor of time) showed a significant effect of time (baseline, T0, T10, T20 and T30) only for PAS20 (P = 0.017) but not for PAS10. Student's paired t test showed that PAS20 facilitated MEPs up to 20 min post-PAS (T0, P = 0.007; T10, P = 0.003; and T20, P = 0.005), whereas PAS10 was ineffective at any time point (Fig. 8B). Student's paired t test showed a significant difference in post-PAS MEP amplitudes at T20 (P = 0.024; Fig. 7B).
Figure 8. Simultaneous EMG recording from the relaxed orbicularis oculi (O oculi), nasalis (Nas) and DAO muscles during ipsilateral stimulation of supraorbital and facial nerves, in a representative subject.
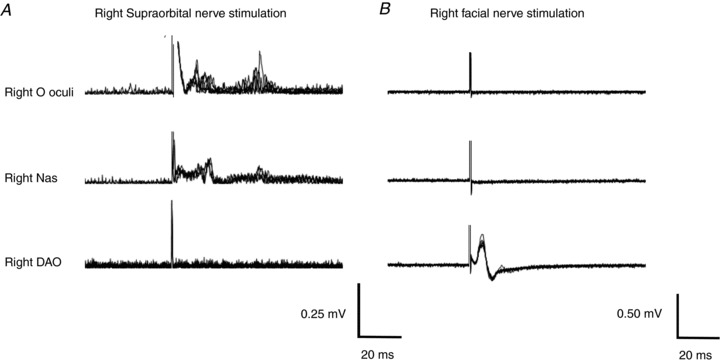
A, electrical stimulation of the supraorbital nerve (pulse duration 0.2 ms, intensity 9 mA) induced clear R1 and R2 responses of the blink reflex in the orbicularis oculi and small conducted R1 and R2 responses in the Nas; by contrast, neither R1 nor R2 responses were seen in the DAO. B, electrical stimulation of the facial nerve (pulse duration 0.2 ms, intensity 3 mA) showed an electrical stimulation-induced motor potential, which was clearly visible in the DAO but not in the Nas and orbicularis oculi. Each trace reports 10 superimposed trials. Each trial was DC corrected, and traces in A were also rectified.
Experiment 8. Effects of PAS20 on blink reflex and blink reflex recovery cycle
Electrical stimulation of the right SON evoked in the orbicularis oculi of all subjects (n = 6) an early R1 response and a late R2 response; there was no response in DAO (Fig. 8A). Likewise, ES of the right facial nerve evoked no reflex responses in the orbicularis oculi (Fig. 8B). Mean R2 threshold was 4.2 ± 1.5 mA and mean ES of the SON was 10.7 ± 2.9 mA. Mean R1 and R2 areas were 0.00195 ± 0.000535 and 0.004809 ± 0.002633 mV s, respectively. Repeated-measures ANOVA, with time (baseline, 0 and 20 min) and measure (DAO MEP, R1 and R2) as within-subject factors, showed a significant interaction (time × measure; P = 0.008). Post hoc one-way ANOVA, performed for DAO MEP, R1 and R2 separately, showed a significant effect of time on MEP amplitudes (P = 0.019) but no significant effect on R1 and R2 areas (Fig. 9). Student's paired t test showed a significant facilitation of MEP at T0 (0.035) and T20 (P = 0.004) and a significant difference between PAS20 effects on MEP and R2 at T20 (P = 0.028).
Figure 9. Effects of PAS20 intervention on the blink reflex and on MEPs evoked by TMS of the left facial motor cortex in the contralateral DAO.
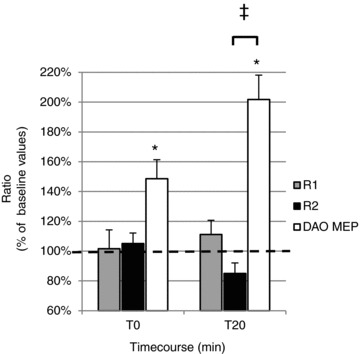
The histogram reports data from 6 subjects in whom long-lasting effects induced by PAS20on the area of the first (R1) and second (R2) areas of the blink reflex as well as on the amplitude of DAO MEPs were measured after 0 min (T0) and 20 min (T20) from PAS20 delivery. Post-PAS effects on R1 and R2 areas and on MEP amplitude were expressed at T0 and T20 as a percentage of the pre-PAS (baseline) value. A significant post-PAS facilitatory effect was observed only for DAO MEP amplitude at all time points tested (*P < 0.05), whereas no significant effects were detected in R1 and R2 areas. Comparing PAS long-lasting effects on MEP and blink reflex, a significant difference was found between DAO and R2 at the T20 time point (‡P < 0.05). Error bars represent means + SEM.
We also studied the effect of PAS20 on the R2 recovery cycle. At baseline, the mean R2 conditioned areas relative to the unconditioned R2 areas were 0.33 ± 0.12 at 250 ms, 0.67 ± 0.24 at 500 ms and 0.87 ± 0.1 at 1000 ms. One-way repeated-measures ANOVA showed a significant effect of ISI (P < 0.001), and post hoc Student's paired t test showed a significant reduction of the conditioned R2 area at 250 ms ISI (P < 0.001). Two-way ANOVA with ISI (250, 500 and 1000 ms) and time (baseline, T0 and T20) performed for R2 area ratios showed a significant effect of ISI (P < 0.001) but no significant effect of time or interaction (ISI × time) on R2 recovery cycle (Fig. 10A).
Figure 10. Effects of PAS20 intervention on the blink reflex recovery cycle, R2 recovery index and MEPs evoked by TMS of the left facial motor cortex in the contralateral DAO.
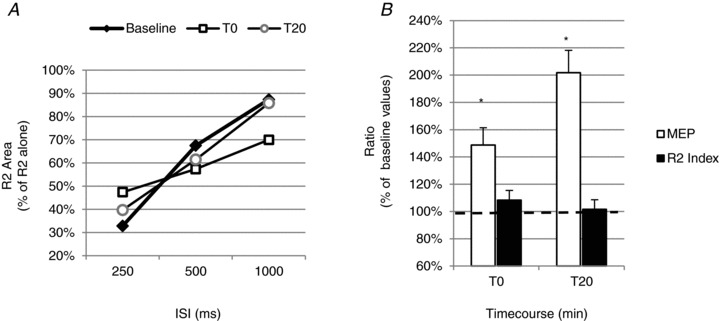
Graphs show mean data obtained from 6 subjects. A, the blink reflex recovery cycle was tested by pairing electrical stimuli given to the supraorbital nerve at ISIs of 250, 500 and 1000 ms. The ratio of the R2 conditioned area/unconditioned area was calculated for each ISI. The R2 recovery cycle was measured before PAS20 (baseline, filled diamonds) and after 0 min (T0, open squares) and 20 min (T20, open circles) from PAS delivery. Compared with baseline, no significant change of R2 recovery cycle was observed at any time point tested, for all ISIs. B, effects of the PAS20 protocol on the amplitude of DAO MEPs and on the R2 recovery index (calculated as the average of the R2 ratio at 250 and 500 ms) are reported in the graph. The DAO MEP and R2 recovery index ratios at T0 and T20 are expressed as a percentage of baseline values. A significant facilitatory effect of the PAS protocol was observed on DAO MEPs at both T0 and T20, whereas no significant changes were observed in the R2 recovery index at any time point tested. *P < 0.05. Error bars represent means + SEM.
Before the PAS protocol, the mean R2 recovery index, expressed as the average of R2 area ratios at 250 and 500 ms ISIs, was 0.5 ± 0.15 (n = 6). Two-way repeated-measures ANOVA, with measure (DAO MEPs and R2 recovery index) and time (T0 and T20) as within-subject factors, showed a significant effect of time (P = 0.14) and a significant interaction (measure × time; P = 0.036). Post hoc Student's paired t test analysis showed a significant effect of PAS protocol on DAO MEPs, whereas there was no significant effect on the R2 recovery index (Fig. 10B).
Discussion
The present data extend previous work on cortical representation of the facial muscles by showing that: (i) TMS applied to the facial motor cortex evokes bilateral and asymmetric responses in the depressor anguli oris muscle; (ii) MEPs are present in the majority of individuals at rest as well as during activation; (iii) SICI and ICF are present bilaterally at rest as well as during voluntary activation; (iv) when MEPs are conditioned by a single electrical stimulus to the facial nerve delivered at short latencies (5–30 ms), there is no SAI; (v) in contrast, at longer latencies (>150 ms) a significant LAI is present; and (vi) repeated paired associative stimulation at an interval of 20 ms leads to long-lasting, spatially specific, LTP-like facilitation of corticobulbar excitability.
Corticobulbar projections to DAO muscles excited by single-pulse TMS over the facial motor cortex
A number of TMS studies have evaluated the motor cortical projection to a range of facial muscles, including frontalis (Cruccu et al. 1990), orbicularis oculi (Benecke et al. 1988; Cruccu et al. 1997; Liscić & Zidar, 1998; Kobayashi et al. 2001; Sohn et al. 2004; Paradiso et al. 2005), nasalis (Rösler et al. 1989; Dubach et al. 2004), orbicularis oris (Cruccu et al. 1990, 1997; Liscić & Zidar, 1998; Rösler et al. 1989; Sohn et al. 2004; Yildìz et al. 2004, 2007; Triggs et al. 2005), mentalis (Benecke et al. 1988; Cruccu et al. 1990; Werhahn et al. 1995; Kobayashi et al. 2001) and depressor anguli oris muscles (Rösler et al. 1989; Meyer et al. 1994; Rimpiläinen et al. 1992; Paradiso et al. 2005). Some of these found no ipsilateral response in lower facial muscles (Cruccu et al. 1990; Kobayashi et al. 2001; Paradiso et al. 2005), which would be consistent with anatomical studies in primates that show only a contralateral projection from M1 face area to the ventral facial nucleus (Jenny & Saper, 1987; Morecraft et al. 2004). Others, however, suggested that the cortical projections to lower facial muscles are bilateral, although with a contralateral predominance (Benecke et al. 1988; Meyer et al. 1989, 1994; Werhahn et al. 1995; Urban et al. 1997; Liscić & Zidar, 1998; Rödel et al. 2000; Yildiz et al. 2004, 2007; Triggs et al. 2005). Our data are similar to these. Ipsilateral responses are not due to volume conduction from EMG in contralateral facial muscles, because they can be recorded from single motor units with needle electrodes (Benecke et al. 1988; Werhahn et al. 1995; Rödel et al. 2000). Consistent with this, we found that the S-wave, due to direct magnetic stimulation of the facial nerve, was detected only ipsilaterally on the stimulated side (Fig. 1), and the M-wave induced by facial nerve electrical stimulation did not spread to other cranial muscles (Fig. 8B). In addition, R1 and R2 responses induced in the orbicularis oculi muscle by the SON electrical stimulation did not appear in the EMG resting activity of DAO (Fig. 8A), suggesting that spread from other facial muscles is unlikely. We conclude that stimulation over the face area of M1 gives rise to bilateral excitation of facial motoneurones innervating muscles of the lower face.
The origin of the ipsilateral response is unclear, especially as it has not been observed by all groups. It has a 1–2 ms longer onset latency and a higher threshold and is not present in all individuals. A latency difference of up to 2.0–2.5 ms between ipsi- and contralateral responses has been reported in several upper and lower facial muscles by many authors (Benecke et al. 1988; Cruccu et al. 1990; Liscić & Zidar, 1998; Triggs et al. 2005). Activation of transcallosal input to the opposite hemisphere can be ruled out, because it involves a longer conduction delay of 5–10 ms (Meyer et al. 1994); furthermore, transection of the corpus callosum has no effect on ipsilateral facial responses to intracortical stimulation in the cat (Guandalini et al. 1990). Bilateral activation of both hemispheres can also be excluded, because we used a figure-of-eight-shaped coil, which induces a focal magnetic field that is unable to spread to the other hemisphere (Rösler et al. 1989).
It has been reported previously that asymmetry of onset latency (ipsilateral > contralateral) is related to amplitude asymmetry (ipsilateral < contralateral) as well as to stimulus strength (Benecke et al. 1988). Thus, the longer latency of the ipsilateral response in the present data may well be the result of its smaller amplitude. This could only be resolved by studying the onset latencies of individual motor units to simulation of each hemisphere.
Occurrence of ipsilateral responses may be due to the presence of a small ipsilateral projection in humans that either does not exist or has not been detected in primates (Jenny & Saper, 1987; Morecraft et al. 2004). However, another possibility comes from work by Morecraft et al. (2004), who found that the area immediately adjacent to the premotor cortex (the facial area of the ventral lateral premotor cortex) gives rise to bilateral but predominantly contralateral outputs to the facial nuclei. It is possible, particularly using a 9-cm-diameter figure-of-eight coil, that our stimulus could have activated this projection and that this could be the source of the ipsilateral responses. If so, the presence of SICI ipsilaterally would also suggest that at least one aspect of inhibitory intracortical circuitry in premotor cortex could be shared with M1. The explanation might also account for the absence of ipsilateral responses in the study by Cruccu et al. (1990), because they used a smaller and more focal TMS coil. Kobayashi et al. (2001) suggest that lack of an ipsilateral response could have been because they did not apply stimuli at an intensity strong enough to avoid discomfort and the undesirable contamination of responses due to peripheral trigeminal and facial nerve activation’. Finally, it is possible that Paradiso et al. (2005) failed to find ipsilateral MEPs because they were primarily concerned with innervation of upper facial muscles recorded at the same time. The fact that the ipsilateral response was absent in some individuals in the present work might be due to reduction or loss of lower face ipsilateral cortical representation in adults, which may occur with maturation after fetal and early postnatal life, when lower facial muscles are likely to be innervated bilaterally and symmetrically, as reported in previous studies (Duchowny & Jayakar, 1993).
Short-interval intracortical inhibition and intracortical facilitation in the lower facial muscles
Short-interval intracortical inhibition and ICF were present bilaterally in the DAO, in agreement with reports from other bilaterally innervated muscles, such as the tongue (SICI and ICF; Muellbacher et al. 2001a; Baad-Hansen et al. 2009), digastric (SICI; Jaberzadeh et al. 2007) and masseter muscles (SICI; Ortu et al. 2008a), suggesting that the contralateral and ipsilateral corticobulbar projections are controlled by similar interneuronal pools. This is in contrast with data obtained in upper facial muscles, where SICI and ICF are present only contralaterally (Paradiso et al. 2005). In addition, we could observe SICI and ICF during active tonic contraction of the muscles. However, it is difficult to be sure whether SICI/ICF is more effective in contralateral than ipsilateral muscles. One reason is that the test MEPs had different amplitudes in the two muscles. In limb muscles at rest, SICI is more difficult to obtain when the test MEP is small (Sanger et al. 2001; Roshan et al. 2003; Ortu et al. 2008b), which could explain why SICI was smaller in iDAO than cDAO. In addition, in the present experiments, the intensity of the conditioning stimulus was set relative to motor threshold of the cDAO and not the iDAO. Again, this could potentially have reduced effectiveness of SICI measured in iDAO. We conclude that although SICI/ICF is present bilaterally, it is unclear whether it is more powerful on one side than the other.
In contrast, it may be easier to draw conclusions from comparison of active and relaxed states. In this case, SICI was more powerful in the relaxed state even though the test MEPs were smaller than in the active state. Given that SICI is usually more powerful when the test MEP is large (at least in hand muscles), this finding in DAO suggests that volitional activity may depress SICI, in agreement with data obtained in other craniofacial muscles (Jaberzadeh et al. 2007) and upper limb muscles (Ridding et al. 1995; Zoghi et al. 2003). There is one proviso to this conclusion. Although the test MEPs were larger in the active conditions, the test intensity was lower in terms of MSO (120% of RMT versus 120% of AMT). Garry & Thomson (2009) suggested that the best comparison between active and relaxed states should use the same intensity of test pulse because this is likely to evoke a similar distribution of I-waves in the descending corticospinal volley. If this was the case for corticobulbar projections to DAO then it would result in less SICI during activity compared with rest. However, the issue can only be resolved with certainty by direct recordings of corticobulbar output, which have never been performed to date in humans.
Interestingly, during muscle voluntary contraction the amount of SICI was not significantly different in ipsi- and contralateral muscles and did not depend on CS intensity, although the post hoc analysis (data not shown) showed a better SICI effect with lower CS (70% of AMT) and a larger ICF with higher CS (80% of AMT), consistent with data obtained in tongue muscles (Muellbacher et al. 2001a) and in upper and lower limb muscles (Chen et al. 1998). The presence of SICI and ICF in the active state contrasts with previous data obtained by Paradiso et al. (2005), who could detect them only in the contralateral DAO during voluntary activation. Differences in the stimulation parameters used in the study of Paradiso et al. (2005) and in our study (intensity of the conditioning stimulus was 95% of AMT versus 70% and 80% of AMT, respectively) and the stimulation site over the scalp (hot spot for the orbicularis oculi muscle versus hot spot for the cDAO, respectively), may contribute to the contrasting results.
Sensorimotor interaction and cortical plasticity in the facial district
This is the first demonstration that the excitability of facial motor cortex is facilitated for at least 30 min following a PAS intervention with an interval of 20 ms between stimulation of the facial nerve and TMS of the cortex. Despite this, there was no short-latency afferent inhibition between stimulation of the facial nerve and TMS of face M1 at any of the intervals tested. This is unlike the data in the hand area of M1, where pairing of median nerve stimulation and TMS at an ISI of 20 ms not only produces a prominent PAS-induced facilitation but also a clear SAI at the same interval (Tokimura et al. 2000). The different results in the face may relate to the fact that our peripheral stimulus may not have produced a sufficiently intense or synchronous afferent volley to evoke SAI, whilst it was sufficient to produce facilitation after PAS. The mandibular branch of the facial nerve contains no sensory fibres, so that no synchronous afferent volley would be conducted back to the cortex. However, the intensity of the electrical stimulus that we used (3 × motor threshold) was sufficient to activate cutaneous receptors in the skin overlying the nerve as well as receptors activated by the evoked muscle twitch. This sensory afferent volley evoked in the trigeminal nerve would be more dispersed than after direct nerve stimulation. Given that the time course of SAI in hand muscles is precise and short lasting, the effect produced by a dispersed afferent volley on the excitability of facial M1 may have been too small to observe. Indeed, SAI cannot be observed in leg muscles after stimulation of the big toe (Bikmullina et al. 2009), perhaps because of similar problems with dispersed conduction, in this case due to the large distances involved.
The afferent volley that failed to evoke SAI in cDAO could nevertheless produce significant inhibition at a latency of 200 ms. This would be consistent with the idea that the pathways mediating these phenomena are different. In hand muscles, SAI is thought to involve paucisynaptic corticocortical connections between the primary somatosensory cortex (SI) and M1 (Tokimura et al. 2000), whilst at longer latencies, peripheral input may also activate contralateral SI, contralateral posterior parietal cortex and bilateral secondary somatosensory (SII) cortices (Forss et al. 1994), all of which could be involved in inhibition of M1 at latencies of >100 ms (Chen et al. 1999; Sailer et al. 2002). Short-afferent inhibition and LAI are differentially affected in Parkinson's disease, again suggesting that they are mediated by different structures (Sailer et al. 2003). In hand muscles, LAI at ISIs of 200 ms is thought to be of cortical origin, because spinal cord excitability is unchanged at this interval (Chen et al. 1999; Classen et al. 2000). Given that the excitability of trigeminal and facial motoneurones following stimulation of cutaneous and muscular trigeminal inputs is fully recovered at ISIs of 100–200 ms (Cruccu et al. 2001), we may conclude that LAI, reported here for the first time in the craniofacial district, is of cortical origin.
In the hand, PAS effects using digital nerve stimulation are as large as those evoked using median nerve stimulation (Stefan et al. 2000), indicating that activity in muscle afferent fibres is not necessary for a PAS effect. In the present case, there are no spindles in the muscles of the lower face (Stål et al. 1990), yet PAS can be produced by repeated pairing of peripheral and TMS stimuli. We suggest that, as for the hand, the effect is likely to be due to an LTP-like phenomenon, given its relatively long duration compared with the short time period required by PAS administration (13 min in our protocol).
In addition, the effect was sensitive to the ISI between the peripheral and central stimuli, being absent at 10 compared with 20 ms. The PAS20 protocol used in our experiments was not effective in two of our 15 subjects. This may simply reflect the normal variability of response to the PAS protocol (e.g. Muller-Dahlaus et al. 2008) or the use of a non-optimal ISI. The most consistent LTP-like effects induced using the PAS technique were obtained when the ISI between the peripheral and central stimuli was optimized to the individual somatosensory-evoked potential (Stefan et al. 2000; Ziemann et al. 2004; Mrachacz-Kersting et al. 2007; Roy et al. 2007). Trigeminal somatosensory-evoked potentials have been poorly studied because of many signal-related problems, which have led the trigeminal somatosensory-evoked potentials to be less popular than upper and lower limb somatosensory-evoked potentials. There is no unanimous agreement on recording procedures, yet and both the latency values and the origin of the trigeminal somatosensory-evoked potential remain controversial (Bennett et al. 1987; Van Loven et al. 2001). For this reason, in our study we based the choice of ISI 20 ms on the MEP peak latency (15 ms on average) plus 5 ms, as done by Stinear & Hornby (2005) in the lower limbs.
There was no overall effect of facial PAS using an ISI of 10 ms; it was inhibitory in most of our subjects and slightly facilitatory or ineffective in the others. Although investigation of possible long-term depression was not one of the aims of the present study, the results suggest that cortical output to lower facial muscles might be susceptible to inhibition after a PAS intervention. Further studies using a greater range of ISIs are necessary to clarify whether it is possible to evoke long-term depression in the facial district.
It is well known that the nervous system can undergo plastic changes at multiple levels and, although the focus of investigation in this field is the cortex, great attention has more recently been paid to subcortical levels, namely to the brainstem (Crupi et al. 2008; Bologna et al. 2010). In our protocols, PAS20 appeared to have no effects on excitability at the brainstem level, because it did not alter either the R1 and R2 responses of the blink reflex or the R2 recovery cycle. These data support the view that LTP-like facilitation of corticobulbar excitability described occurs within the cerebral cortex.
The influence exerted on facial muscles by emotional inputs was not taken into consideration in this study, which would have required a diverse and more complex experimental design. However, the present data contribute to the clarification of some important aspects of facial motor system physiology and report an LTP-like plasticity in the facial motor cortex, which has never been described before.
Conclusion
In conclusion, we confirm the bilateral and asymmetric nature of the corticobulbar projections to lower facial muscles, with the ipsilateral projection being weaker than the contralateral corticobulbar pathway. We also demonstrate that in the M1 area projecting to lower facial motoneurones, SICI and ICF operate not only at rest but also during voluntary contraction. This finding is of particular importance in the light of pathophysiological studies aimed at understanding the mechanisms of motor disorders (such as cranial dystonia or hemifacial spasm) characterized by an almost continuous muscle activity that makes difficult, if not impossible, to study intracortical circuits at rest. Finally, we show, for the first time, an LTP-like phenomenon in the facial motor cortex, indicating that the facial motor cortex is prone to plastic changes of its excitability.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Mr Paul Hammond and of Dr Francesca Ginatempo and express thanks to Professor Giovanni Sotgiu for assistance with the statistical analysis. The experiments were performed at the laboratories of neurophysiology of the Sobell Department of Neurophysiology and Motor Disorders, Institute of Neurology, University College London, London, UK and of the Department of Biomedical Sciences, University of Sassari, Sassari, Italy. Dr Giovanna Pilurzi was funded by a doctoral research fellowship (XXV cycle) awarded by the Ministero Istruzione Universit e Ricerca (MIUR) (Italy) and was supported by Fondazione Banco di Sardegna Sassari (Italy). Dr Alkomiet Hasan was supported by the Deutsche Forschungsgemeinschaft (DFG grant HA 6091/1-1). Dr Tabish Aziz Saifee is funded by a doctoral research fellowship awarded by the National Institute of Health Sciences (NIHS) (UK) (DRF-2009-02-121).
Glossary
- 0.7AMT
70% of active motor threshold
- 0.7RMT
70% of resting motor threshold
- 0.8AMT
80% of active motor threshold
- 1.1RMT
110% of resting motor threshold
- 1.2AMT
120% of active motor threshold
- 1.2RMT
120% of resting motor threshold
- AMT
active motor threshold
- BR
blink reflex
- cDAO
contralateral depressor anguli oris muscle
- cMEP
contralateral motor-evoked potential
- CS
conditioning stimulus
- DAO
depressor anguli oris muscle
- ES
electrical stimulation
- face M1
facial primary motor cortex
- FDI
first dorsal interosseous muscle
- ICF
intracortical facilitation
- iDAO
ipsilateral depressor anguli oris muscle
- iMEP
ipsilateral motor-evoked potential
- ISI
interstimulus interval
- LAI
long-afferent inhibition
- LTP
long-term potentiation
- M1
primary motor cortex
- Mass
masseter muscle
- MEP
motor-evoked potential
- MSO
maximal stimulator output
- MVC
maximal voluntary contraction
- Nas
nasalis muscle
- O oculi
orbicularis oculi muscle
- PAS
paired associative stimulation
- PT
perceptual threshold
- R1
first component of the blink reflex
- R2
second component of the blink reflex
- RMT
resting motor threshold
- SI
primary somatosensory cortex
- SII
secondary somatosensory cortex
- SAI
short-afferent inhibition
- SICI
short-interval intracortical inhibition
- SON
supraorbital nerve
- TMS
transcranial magnetic stimulation
- TS
test stimulus
Author contributions
Conception and design of the experiments: G.P., J.C.R. and F.D. Collection, analysis and interpretation of data: G.P., A.H., T.A.S., J.C.R. and F.D. Drafting the article or revising it critically for important intellectual content: G.P., A.H., T.A.S., E.T., J.C.R. and F.D. All authors approved the final version for publication.
References
- Baad-Hansen L, Blicher JU, Lapitskaya N, Nielsen JF, Svensson P. Intra-cortical excitability in healthy human subjects after tongue training. J Oral Rehabil. 2009;36:427–434. doi: 10.1111/j.1365-2842.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Schönle P, Conrad B. Transcranial magnetic stimulation of the human brain: responses in muscles supplied by cranial nerves. Exp Brain Res. 1988;71:623–632. doi: 10.1007/BF00248756. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Wastell DG, Barker GR, Blackburn CW, Rood JP. Trigeminal somatosensory evoked potentials. A review of the literature as applicable to oral dysaesthesias. Int J Oral Maxillofac Surg. 1987;16:408–415. doi: 10.1016/s0901-5027(87)80076-0. [DOI] [PubMed] [Google Scholar]
- Bikmullina R, Bäumer T, Zittel S, Münchau A. Sensory afferent inhibition within and between limbs in humans. Clin Neurophysiol. 2009;120:610–618. doi: 10.1016/j.clinph.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Bologna M, Agostino R, Gregori B, Belvisi D, Manfredi M, Berardelli A. Metaplasticity of the human trigeminal blink reflex. Eur J Neurosci. 2010;32:1707–1714. doi: 10.1111/j.1460-9568.2010.07446.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallet M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell J, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Berardelli A, Inghilleri M, Manfredi M. Functional organization of the trigeminal motor system in man. A neurophysiological study. Brain. 1989;112:1333–1350. doi: 10.1093/brain/112.5.1333. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Berardelli A, Inghilleri M, Manfredi M. Corticobulbar projections to upper and lower facial motoneurons. A study by magnetic transcranial stimulation in man. Neurosci Lett. 1990;117:68–73. doi: 10.1016/0304-3940(90)90121-o. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Inghilleri M, Berardelli A, Romaniello A, Manfredi M. Cortical mechanisms mediating the inhibitory period after magnetic stimulation of the facial motor area. Muscle Nerve. 1997;20:418–424. doi: 10.1002/(sici)1097-4598(199704)20:4<418::aid-mus3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Truini A, Priori A. Excitability of the human trigeminal motoneuronal pool and interactions with other brainstem reflex pathways. J Physiol. 2001;531:559–571. doi: 10.1111/j.1469-7793.2001.0559i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crupi D, Ghilardi MF, Mosiello C, Di Rocco A, Quartarone A, Battaglia F. Cortical and brainstem LTP-like plasticity in Huntington's disease. Brain Res Bull. 2008;75:107–114. doi: 10.1016/j.brainresbull.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Dubach P, Guggisberg AG, Rösler KM, Hess CW, Mathis J. Significance of coil orientation for motor evoked potentials from nasalis muscle elicited by transcranial magnetic stimulation. Clin Neurophysiol. 2004;115:862–870. doi: 10.1016/j.clinph.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Duchowny M, Jayakar P. Functional cortical mapping in children. Adv Neurol. 1993;64:149–154. [PubMed] [Google Scholar]
- Forss N, Hari R, Salmelin R, Ahonen A, Hämäläinen M, Kajola M, Knuutila J, Simola J. Activation of the human posterior parietal cortex by median nerve stimulation. Exp Brain Res. 1994;99:309–315. doi: 10.1007/BF00239597. [DOI] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res. 2009;193:267–274. doi: 10.1007/s00221-008-1620-5. [DOI] [PubMed] [Google Scholar]
- Guandalini P, Franchi G, Spidalieri G. Low threshold unilateral and bilateral facial movements evoked by motor cortex stimulation in cats. Brain Res. 1990;508:273–282. doi: 10.1016/0006-8993(90)90406-2. [DOI] [PubMed] [Google Scholar]
- Ito T, Ostry DJ. Somatosensory contribution to motor learning due to facial skin deformation. J Neurophysiol. 2010;104:1230–1238. doi: 10.1152/jn.00199.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberzadeh S, Pearce SL, Miles TS, Türker KS, Nordstrom MA. Intracortical inhibition in the human trigeminal motor system. Clin Neurophysiol. 2007;118:1785–1793. doi: 10.1016/j.clinph.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jenny AB, Saper CB. Organization of the facial and corticofacial projection in the monkey: a reconsideration of the upper motor neuron facial palsy. Neurology. 1987;37:930–939. doi: 10.1212/wnl.37.6.930. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Theoret H, Mottaghy FM, Gangitano M, Pascual-Leone A. Intracortical inhibition and facilitation in human facial motor area: difference between upper and lower facial area. Clin Neurophysiol. 2001;112:1604–1611. doi: 10.1016/s1388-2457(01)00632-0. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatki BG, Stegeman DF, Jonas IE. A surface EMG electrode for the simultaneous observation of multiple facial muscles. J Neurosci Methods. 2003;123:117–128. doi: 10.1016/s0165-0270(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Liscić RM, Zidar J. Functional organisation of the facial motor system in man. Coll Antropol. 1998;22:545–550. [PubMed] [Google Scholar]
- Meyer B-U, Britton TC, Benecke R. Investigation of unilateral facial weakness: magnetic stimulation of the proximal facial nerve and of the face-associated motor cortex. J Neurol. 1989;236:102–107. doi: 10.1007/BF00314405. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Werhahn K, Rothwell JC, Roericht S, Fauth C. Functional organization of corticonuclear pathways to motoneurones of lower facial muscles in man. Exp Brain Res. 1994;101:465–472. doi: 10.1007/BF00227339. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louise JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:178–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: new insights from neuroscience. Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kresting N, Fong M, Murphy BA, Sinkjaer T. Changes in excitability of the cortical projections to the human tibialis anterior following paired associative stimulation. J Neurophysiol. 2007;97:1951–1958. doi: 10.1152/jn.01176.2006. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Boroojerdi B, Ziemann U, Hallett M. Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol. 2001a;18:550–558. doi: 10.1097/00004691-200111000-00005. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen LG, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001b;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JF, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187:467–475. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Giaconi E, Tolu E, Rothwell JC. Intracortical modulation of cortical-bulbar responses for the masseter muscle. J Physiol. 2008a;586:3385–3404. doi: 10.1113/jphysiol.2008.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol. 2008b;586:5147–5159. doi: 10.1113/jphysiol.2008.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso OG, Cunic DI, Gunraj AC, Chen R. Representation of facial muscles in human motor cortex. J Physiol. 2005;567:323–336. doi: 10.1113/jphysiol.2005.088542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavesi G, Macaluso GM, Marchetti P, Cattaneo L, Tinchelli S, de Laat A, Mancia D. Trigemino-facial reflex inhibitory responses in some lower facial muscles. Muscle Nerve. 2000;23:939–945. doi: 10.1002/(sici)1097-4598(200006)23:6<939::aid-mus15>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Prior MM, Stinear JW. Phasic spike-timing-dependent plasticity of human motor cortex during walking. Brain Res. 2006;1110:150–158. doi: 10.1016/j.brainres.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimpiläinen I, Karma P, Eskola H, Hakkinen V. Magnetic facial nerve stimulation in normal subjects. Acta Otolaryngol (Stockh) 1992;492:99–102. doi: 10.3109/00016489209136821. [DOI] [PubMed] [Google Scholar]
- Rödel R, Laskawi R, Markus H. Transcranial cortical magnetic stimulation of lower-lip mimetic muscles: effect of coil position on motor evoked potentials. ORL J Otorhinolaryngol Relat Spec. 1999;61:119–125. doi: 10.1159/000027655. [DOI] [PubMed] [Google Scholar]
- Rödel R, Laskawi R, Markus H. Motor potentials of lower-lip mimetic muscles and distal arm muscles to cortical transcranial magnetic stimulation: the possibility of one-dimensional separation of two cortical representation areas. ORL J Otorhinolaryngol Relat Spec. 2000;62:96–99. doi: 10.1159/000027724. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Hess CW, Heckmann R, Ludin HP. Significance of shape and size of the stimulating coil in magnetic stimulation of the human motor cortex. Neurosci Lett. 1989;100:347–352. doi: 10.1016/0304-3940(89)90711-8. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Roy FD, Gorassini MA. Peripheral sensory activation of cortical circuits in the leg motor cortex of man. J Physiol. 2008;586:4091–4105. doi: 10.1113/jphysiol.2008.153726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy FD, Norton JA, Gorassini MA. Role of sustained excitability of the leg motor cortex after transcranial magnetic stimulation in associative plasticity. J Neurophysiol. 2007;98:657–667. doi: 10.1152/jn.00197.2007. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle BJ, Adachi K, Avivi-Arber L, Lee J, Nishiura H, Yao D, Yoshino K. Neuroplasticity of face primary motor cortex control of orofacial movements. Arch Oral Biol. 2007;52:334–337. doi: 10.1016/j.archoralbio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Voller B, Dimyan M, St Clair Gibson A, Hanakawa T, Leo-Sarmiento FE, Jung HY, Hallet M. Cortical control of voluntary blinking: a transcranial magnetic stimulation study. Clin Neurophysiol. 2004;115:341–347. doi: 10.1016/j.clinph.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Stål P, Eriksson PO, Eriksson A, Thornell LE. Enzyme-histochemical and morphological characteristics of muscle fibre types in the human buccinator and orbicularis oris. Arch Oral Biol. 1990;35:449–458. doi: 10.1016/0003-9969(90)90208-r. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesh E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–385. doi: 10.1093/cercor/bhi116. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Hornby TG. Stimulation-induced changes in lower limb corticomotor exicitability during treadmill walking. J Physiol. 2005;567:701–711. doi: 10.1113/jphysiol.2005.090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson P, Romaniello A, Arendt-Nielsen L, Sessle BJ. Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res. 2003;152:42–51. doi: 10.1007/s00221-003-1517-2. [DOI] [PubMed] [Google Scholar]
- Svensson P, Romaniello A, Wang K, Arendt-Nielsen L, Sessle BJ. One hour of tongue-task training is associated with plasticity in corticomotor control of the human tongue musculature. Exp Brain Res. 2006;173:165–173. doi: 10.1007/s00221-006-0380-3. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs WJ, Hacibeh G, Sringer U, Bowers D. Lateralized asymmetry of facial motor evoked potentials. Neurology. 2005;65:541–544. doi: 10.1212/01.wnl.0000172916.91302.e7. [DOI] [PubMed] [Google Scholar]
- Urban PP, Beer S, Hopf HC. Cortico-bulbar fibers to orofacial muscles: recordings with enoral surface electrodes. Electroencephalogr Clin Neurophysiol. 1997;105:8–14. doi: 10.1016/s0924-980x(96)96584-4. [DOI] [PubMed] [Google Scholar]
- Van Loven K, Jacobs R, Van Hees J, Van Huffel S, van Steenberghe D. Trigeminal somatosensory evoked potentials in humans. Electromyogr Clin Neurophysiol. 2001;41:357–375. [PubMed] [Google Scholar]
- Werhahn KJ, Classen J, Benecke R. The silent period induced by transcranial magnetic stimulation in muscles supplied by cranial nerves: normal data and changes in patients. J Neurol Neurosurg Psychiatry. 1995;59:586–596. doi: 10.1136/jnnp.59.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Yildiz N, Yildiz S, Ertekin C, Aydogdu I, Uludag B. Changes in the perioral muscle responses to cortical TMS induced by decrease of sensory input and electrical stimulation to the lower facial region. Clin Neurophysiol. 2004;115:2343–2349. doi: 10.1016/j.clinph.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Bademkiran F, Yildiz N, Aydogdu I, Uludag B, Ertekin C. Facial motor cortex plasticity in patients with unilateral peripheral facial paralysis. NeuroRehabilitation. 2007;22:133–140. [PubMed] [Google Scholar]
- Ziemann U, Iliać TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol. 2003;550:933–946. doi: 10.1113/jphysiol.2003.042606. [DOI] [PMC free article] [PubMed] [Google Scholar]


