Abstract
Self-motion perception and the vestibulo-ocular reflex (VOR) were investigated in healthy subjects during asymmetric whole body yaw plane oscillations while standing on a platform in the dark. Platform oscillation consisted of two half-sinusoidal cycles of the same amplitude (40°) but different duration, featuring a fast (FHC) and a slow half-cycle (SHC). Rotation consisted of four or 20 consecutive cycles to probe adaptation further with the longer duration protocol. Self-motion perception was estimated by subjects tracking with a pointer the remembered position of an earth-fixed visual target. VOR was measured by electro-oculography. The asymmetric stimulation pattern consistently induced a progressive increase of asymmetry in motion perception, whereby the gain of the tracking response gradually increased during FHCs and decreased during SHCs. The effect was observed already during the first few cycles and further increased during 20 cycles, leading to a totally distorted location of the initial straight-ahead. In contrast, after some initial interindividual variability, the gain of the slow phase VOR became symmetric, decreasing for FHCs and increasing for SHCs. These oppositely directed adaptive effects in motion perception and VOR persisted for nearly an hour. Control conditions using prolonged but symmetrical stimuli produced no adaptive effects on either motion perception or VOR. These findings show that prolonged asymmetric activation of the vestibular system leads to opposite patterns of adaptation of self-motion perception and VOR. The results provide strong evidence that semicircular canal inputs are processed centrally by independent mechanisms for perception of body motion and eye movement control. These divergent adaptation mechanisms enhance awareness of movement toward the faster body rotation, while improving the eye stabilizing properties of the VOR.
Key points
The semicircular canals of the labyrinths are a source of information for self-motion perception and reflex eye movements.
Prolonged vestibular asymmetric stimulation of standing humans about the earth-vertical axis, made of fast body rotation to one side and slow rotation to the other side, induced different adaptive mechanisms in the perception of body motion and in the vestibulo-ocular reflex (VOR).
Motion perception became progressively more asymmetric, increasing gradually in response to the fast body rotation and decreasing in response to the slow rotation. VOR became gradually more symmetric, decreasing for fast body movement and increasing for slow movement.
These oppositely directed adaptive effects in motion perception and VOR persisted for at least 30 min.
Long-lasting asymmetric stimulation discloses independent brain mechanisms for perception of body motion and eye movement control.
These adaptive mechanisms may enhance awareness toward the side where the body is moving faster, while improving eye stabilizing properties of the VOR.
Introduction
The information originating in the vestibular receptors elicits ocular and postural reflexes and contributes to the perception of body movements. However, whether reflex and perceptual responses undergo the same central processing is a matter of controversy. Research on the immediate responses to rotation stimuli have shown a similar gain both for self-motion perception and for the vestibulo-ocular reflex (VOR) (Mergner et al. 1992, 1998a; Schweigart et al. 2002). During whole body yaw rotation, both self-motion perception and VOR exhibit comparable high-pass transfer properties on increasing stimulus frequency from 0.025 to 0.4 Hz (Mergner et al. 1991, 1998b). These characteristics arise from the mechanical properties of the cupula (Fernandez & Goldberg, 1971; Curthoys et al. 1977; Cohen et al. 1981; Oman et al. 1987) and central integrating mechanism subserving perceptual and oculomotor responses (Guedry & Lauver, 1961; Young & Oman, 1969; Brandt et al. 1974; Vibert et al. 1977; Waespe & Henn, 1977; Robinson, 1981; Hain & Zee, 1992).
Likewise, the similarity of the time constants of decay of post-rotational motion perception and VOR, both in normal subjects (Okada et al. 1999) and in cerebellar patients (Bronstein et al. 2008), would suggest a similar contribution of the so-called velocity storage integrator to both perception and VOR. Thus, even though perceptual and ocular reflex responses do show differences in threshold (Seemungal et al. 2004), rise time and plateau (Sinha et al. 2008), both responses are considered to be driven by the same central mechanism, requiring no additional processing to explain their dynamic characteristics (Bertolini et al. 2011). This view is not universally accepted, though, since studies by Merfeld et al. (2005a,b) on tilt and linear acceleration and by Grabherr et al. (2008) on perceptual yaw rotation, show no evidence for simple filtering on perceptual tilt or an influence of velocity storage on perceptual yaw rotation.
Perceptual and oculomotor processing has also been compared during repetitive and prolonged vestibular stimulation. In this case, both responses progressively decrease during unidirectional and bidirectional constant angular accelerations (Guedry & Lauver, 1961; Guedry & Collins, 1968; Brown & Wolfe, 1969; Barnes, 1995; Clément et al. 2008) and during continuous galvanic vestibular stimulation (St George et al. 2011). Similarly, a parallel response decay is observed after prolonged unilateral rotation, featuring a negative post-rotatory nystagmus (Waespe & Henn, 1977) and an oppositely directed illusion of body rotation (Brandt et al. 1974).
Studies using prolonged but asymmetric vestibular stimuli are, however, scarce, and this is surprising given that such protocols may shed light on related processes of lesion-induced neural plasticity and vestibular compensation following unilateral vestibular lesions (Curthoys & Halmagyi, 1995). Recently, in the course of an investigation on cervicovestibular interactions, we unexpectedly found that repetitive, asymmetric whole body rotations enhanced perceptual responses to the faster rotation and reduced responses to the slower rotation (Panichi et al. 2011). This finding suggested that asymmetric long-term vestibular stimulation might induce adaptive patterns that do not occur for symmetric stimulation. Therefore, here we apply such stimuli to investigate the hypothesis that the vestibular reflex and vestibular perceptual systems are subserved by different central mechanisms. Accordingly, we deliver prolonged asymmetric vestibular stimulation involving faster movements in one direction and slower movements in the opposite.
Perceptual and reflex effects were studied during both short (four cycles) and long sequences of stimuli (20 cycles) to examine adaptation processes to short- and long-lasting stimulation. After-effects were also investigated by studying the responses to a single test stimulus after the adaptation phase, to assess if any stimulus-induced asymmetry remains stored in the CNS. We show that prolonged asymmetric stimulation induces a dramatic and persistent asymmetry in motion perception while minimizing the initial asymmetry in the VOR. Thus, we provide evidence that the sensory signals from the semicircular canals undergo different central processing for self-motion perception and VOR.
Methods
Fourteen healthy subjects aged 20–35 years (eight men; mean age 25.8 years) participated in the study after giving written informed consent. The experimental protocol was in accordance with the Declaration of Helsinki (1964) and was approved by the Ethical Committee of the University of Perugia.
Subjects stood on the centre of a computer-controlled rotating platform, in a completely darkened and acoustically isolated cabin of 100 cm radius. Subjects placed one hand on their chest and the other hand on the pointer used for tracking a remembered earth-fixed visual target. A vertical holder with three horizontal extensions secured the head, shoulders and pelvis to the platform in the primary position (Fig. 1A). A video camera recorded two pairs of reflective infrared markers, placed on the scalp midline on the left and right acromion, respectively. This measured head yaw rotation. When the head markers revealed displacements unrelated to the stimulus the trial was discarded. Roll and pitch head displacements were prevented by a plastic collar.
Figure 1. Motion perception and VOR in response to symmetric and asymmetric whole body yaw rotations.
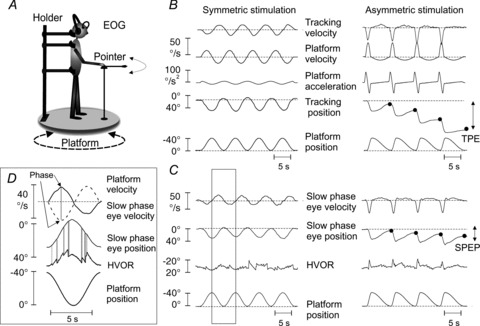
A, schematic drawing of the experimental set-up. B, motion perception during symmetric (left) and asymmetric (right) stimulations. The filled circles indicate the TPE evaluated at the end of each asymmetric oscillation cycle. C, VOR during symmetric (left) and asymmetric (right) stimulations. The SPEP was extracted from the HVOR trace, composed by slow and quick phases. The inset D represents an enlargement of the highlighted part of the traces in C, to show how the SPEP was extracted from the eye nystagmus (HVOR). The grey vertical stripes in D point to the portion of the HVOR trace containing quick phases that were eliminated and substituted by interpolation to obtain the SPEP. On top of the inset, slow phase eye velocity (continuous line) and platform velocity (dashed line) are compared for evaluation of gain (eye and platform peak velocities) and phase (difference in time between eye and platform peak velocities, arrows) of VOR. EOG, electro-oculography; HVOR, horizontal vestibulo-ocular reflex; SPEP, slow phase eye position; TPE, tracking position error; VOR, vestibulo-ocular reflex.
Stimulation protocols
The subjects underwent three different protocols for examining self-motion perception and the VOR. We used yaw rotation about an earth-vertical axis, in a range (amplitude 40°, frequencies from 0.04 to 0.4 Hz) known to activate vestibular receptors (Fernandez & Goldberg, 1971). The sessions for motion perception, VOR and the different protocols were separated by at least 3 day intervals. The sequence was randomly assigned to the subjects. Each protocol was applied four times to each subject in separate and randomized sessions.
Short-lasting asymmetric stimulation (protocol 1)
The stimulation consisted of four continuous asymmetric cycles, delivered for examining short-term effects of asymmetric rotation on the perceptual and ocular reflex responses. The stimulus asymmetry resulted from a combination of sinusoidal half-cycles of the same displacement but different duration (Fig. 1B and C, right panels). Asymmetry was defined as the ratio between the duration of the slow half-cycle (SHC) and the total duration of the cycle × 100. Total cycle duration was 6.6 s, corresponding to a frequency of 0.15 Hz. For 80% asymmetry, the internal frequency of the fast half-cycle (FHC) was 0.38 Hz and that of the SHC 0.09 Hz; for 70% asymmetry, FHC 0.25 Hz, SHC 0.11 Hz; for 60%, FHC 0.19 Hz, SHC 0.125 Hz; 50% indicates that the stimulus was symmetric. In most cases, the cycles were initiated from the centre toward the right side and back to the centre (from 0° to −40°) but, to control for stimulus direction, oscillations were also delivered with the FHC toward the left or with the SHC preceding the FHC (five subjects). As a further control, five other subjects underwent oscillations centred around the initial body position (±20°).
Long-lasting asymmetric stimulation (protocol 2)
This protocol investigated the magnitude and time course of the adaptive effects. The asymmetric stimulation (0.15 Hz, 80% of asymmetry) was simply continued up to 20 cycles and the same variables recorded as for protocol 1. In addition, a control condition of equal duration but featuring symmetric cycles (0.15 Hz) was delivered to five subjects to assess any response adaptation to prolonged symmetric vestibular stimulation.
Probing the after-effects: single-cycle symmetric test stimulation after long-lasting asymmetric stimulation (protocol 3)
A single, sinusoidal symmetric test cycle (±20° around the initial body position, at 0.04, 0.1, 0.2 or 0.4 Hz in different trials) was administered 1 min after the end of the asymmetric conditioning stimulation. Further, to assess the time course of decay of the after-effects, we delivered a single symmetric test cycle (0.04 Hz) every 2.5 min up to 1 h after the end of the asymmetric conditioning stimulation. Subjects were allowed to move freely during the conditioning test intervals. As, in preliminary experiments, we noted that the symmetric test stimulus tended to cancel the effects induced by the asymmetric stimulation, we always delivered an asymmetric cycle immediately after the symmetric test cycle. This prevented the unwanted cancellation, without inducing either attenuation or potentiation of the after-effects, as shown by the comparison with the effect of a symmetrical test stimulus given in isolation at 10 min intervals from the end of the asymmetric stimulation.
Subject instructions and recording techniques
Self-motion perception
Subjects were asked to manually track with a pointer the remembered position of an earth-stationary light spot (diameter 1 cm) initially projected on to the wall of the cabin in front of them, at eye level. The pointer, connected with a precision potentiometer, pivoted on a platform-fixed support, 25 cm in front of the body axis and at 100 cm height from the platform (Fig. 1A). The spot was presented for 30 s before platform rotation onset, and was switched off just before rotation onset. Therefore, during platform oscillations in the dark, subjects pointed toward the remembered earth-fixed target. The instantaneous position of the pointer measured the perceived body position in space (Pettorossi et al. 2004; Siegle et al. 2009). Subjects were given practice sessions consisting of tracking during both symmetric and asymmetric stimulations in the dark. The responses to symmetric and to asymmetric stimulation were considered accurate when the tracking response to the first cycle reproduced the stimulus profile in amplitude, phase and shape. A pause of at least 1 h elapsed between the training and testing sessions to prevent possible carryover effects.
Pointer and platform signals were recorded on a PC for off-line computing of position and angular velocity of the pointer with respect to the platform. The analog waveforms were digitized by a 12-bit A/D card (Labview, National Instruments, Austin, Texas, USA) at a sampling rate of 500 Hz per channel. Parallax error due non-coincidence of the pointer's and body's rotational axes was corrected by the formula:
where θP, θR, θP−θR, D and R are the pointer angle, the real angle (body centred angle), the error due to the parallax, the distance D from the pointer to the body rotation axis, and the distance R between subject and visual target, respectively.
We evaluated the mismatch between the perceived position and target position at the end of the rotation cycles by subtracting the pointer position from the target position. This variable was named tracking error. In case of symmetric stimulation, we expected the tracking error to be zero, whereas after asymmetric stimulation a certain error would be present. According to a previous study (Panichi et al. 2011), the direction of the error was expected in the direction of the SHC, as the perceived rotation was larger during the FHC than during the following SHC. During the analysis, the tracking error of each cycle was accrued with the tracking error of the previous cycle (Fig. 1B) and the cumulative error (after four or 20 cycles) was obtained. Tracking velocity was the first derivative of the position signal and was used for the evaluation of gain and phase of the responses. The gain of the response, to both FHC and SHC was expressed as the ratio of the peak angular velocity of the platform to the peak angular velocity of the tracking, and the phase of the response was expressed as the difference in degrees between peak tracking velocity and peak body rotation velocity.
Protocol 1 (asymmetric stimulation) was repeated in the same subjects, but the instruction was to focus attention only on their own body rotation, instead of on the position of the remembered target. This instruction suppressed eye movements, as shown by the simultaneous recording of electro-oculography (EOG), and allowed us to evaluate self-motion perception without the possible influence of reflex eye movement (Pettorossi et al. 2004). In this case, the final tracking error was the difference between the pointer position and the body position.
Vestibulo-ocular reflex
In this experiment, subjects would not point to the remembered target, but disengaged their attention from any voluntary spatial task by performing a simple mental calculation (counting backwards from 100 in steps of one). Both arms were crossed on the chest. Horizontal eye movements were recorded by binocular, bi-temporal EOG (Biomedica Mangoni, Pisa, Italy) with a bandwidth of 0–500 Hz. All tests were performed in the dark after 30 min of darkness adaptation. Eye displacements from the midline did not exceed 20°, so that recording of eye movements was approximately linear. Slow phase eye velocity was computed after desaccading the EOG by means of custom-made interactive software (Fig. 1, inset D): all fast eye movements, either induced by the stimulation (quick phases of nystagmus) or occasional spontaneous saccades, were identified based on the abrupt change in velocity (Panichi et al. 2011) and eliminated. The data points pertaining to the removed fast responses were reconstructed by interpolation based on values of the preceding and following slow phase data points.
Gain (eye velocity/platform velocity) and phase (difference in degrees between peak eye velocity and peak velocity of body rotation) were measured (Fig. 1, inset D) and the effects of repeated asymmetric stimulation on these parameters quantified. We also measured the cumulative slow phase eye position at the end of the four cycles of oscillation as an index of the effect of asymmetric stimulation on the VOR (this equates to the cumulative tracking error during the perceptual task) (Fig. 1C). We expected that, on symmetric stimulation, the cumulative slow phase eye position would be negligible because of equal ocular displacement in both directions, whereas a substantial cumulative slow phase eye position would be evident during asymmetric stimulation. Before and after each session, EOG signals were calibrated by having subjects look at an earth-fixed target light placed in front of them during 40° sinusoidal symmetric oscillations at 0.15 Hz. The range of error was less than 0.5°.
Statistical analysis
Unless otherwise indicated, all mean values are followed by the s.d. Repeated-measures ANOVA (one-way or two-way) was used for multiple comparisons of tracking and VOR variables. In the two-way ANOVA, we compared: (1) tracking or VOR gain (and phase) between FHC and SHC stimulation across four consecutive asymmetric cycles; (2) tracking error or cumulative eye position between observed and predicted conditions across the percentage of stimulus asymmetry; and (3) tracking or VOR gain of the after-effects between the two directions of the symmetric test stimulus across different stimulus frequencies. When the main effects or the interaction were significant, post hoc analysis was made with the Scheffé test.
The level of significance was set at P < 0.05 for both the ANOVA and post hoc comparison. Before the ANOVA, Levene's test assessed the homogeneity of the variances. In just one case, the epsilon approximation (Greenhouse & Geisser, 1959) was used to compensate for violations of sphericity. Exponential and linear functions were used for fitting tracking error and cumulative slow phase eye position during and after asymmetric stimulation. All fittings were performed by means of the software OriginPro (OriginLab Corporation, Northampton, Massachusetts, USA).
Results
Short-lasting asymmetric stimulation (protocol 1)
The four cycles of asymmetric whole-body rotation combined a FHC to one side and a SHC that returned subjects back to the initial position (Fig. 1).
Self-motion perception
In response to the 80% asymmetric stimulation (to the right and return), the tracking profile during the four cycles was related to the profile of the platform rotation (Figs 1B and 2A). The mean gain and phase of the tracking of the first FHC (internal frequency 0.38 Hz) and of the first SHC (internal frequency 0.09 Hz) were consistent with those reported at similar frequencies by Mergner et al. (1992) and with those observed in our preliminary experiments (see Fig. 2B and Supplementary file 1 and 2). Tracking gain increased up to 1.15 ± 0.28 during the consecutive FHCs, while it decreased to zero during the consecutive SHCs (Fig. 2A and B). The two-way ANOVA, with velocity of half-cycle (FHC and SHC) and cycle number as main factors, showed a difference between FHC and SHC (F(1,26)= 45.9, P < 0.001) and across consecutive cycles (F(3,78)= 17.7, P < 0.001). There was an interaction between half-cycle velocity and cycle number (F(3,78)= 105.3, P < 0.001). The post hoc test indicated that the gain of the response to the FHC significantly increased by cycles 3 and 4, whereas the gain of SHC responses started to significantly decrease at the second cycle (see Fig. 2B). The same analysis, performed on the phase values, showed that phase was affected by the half-cycle velocity (F(3,78)= 134.6, P < 0.001), but was roughly constant across the consecutive cycles (F(3,78)= 0.25, P= 0.89). The interaction was not significant (F(3,78)= 0.21, P= 0.97). Thus, the asymmetric stimulus caused a progressive enhancement of movement perception during FHC and a reduction during SHC.
Figure 2. Effect of asymmetric whole body yaw rotations on motion perception and VOR.
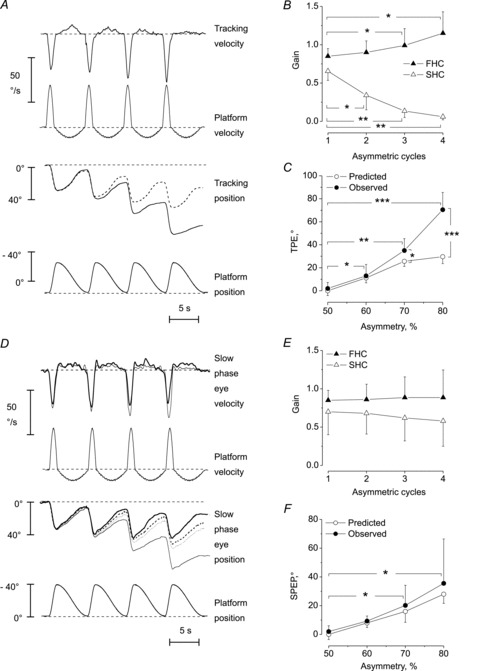
Tracking of perceived body motion (A–C) and VOR (D–F) in response to four-cycle asymmetric stimulation. A, top panel, velocity of tracking progressively increases (see the downward peaks) during the FHCs of the platform and decreases (the small upward peaks) during the SHCs. Bottom panel, the dashed line indicates the position predicted by subsequent ‘copy-and-paste’ addition of the four ‘first cycle’ tracking responses. The difference between the recorded (continuous line) and the predicted tracking traces (dashed line) progressively increases. B, changes in gain of movement perception during the four cycles. Mean and s.d. of gains (tracking peak velocity/platform peak velocity) during the FHC (filled triangles) and during the SHC (open triangles) from the first to the fourth cycle. The gain increases and decreases with the successive cycles, for FHC and SHC, respectively (*P < 0.05, **P < 0.01). C, difference between observed (filled circles) and predicted (open circles) values of mean TPE at the end of the four cycles. The data refer to 0.15 Hz stimulation, at different percentages of stimulus asymmetry, from 50% (sinusoidal) to 80% (maximal asymmetric stimulus). The more asymmetric the stimulus, the greater the TPE and the difference between predicted and observed values (*P < 0.05, **P < 0.01, ***P < 0.001). D–F, eye response to asymmetric stimulation. D, the superimposed traces refer to two subjects showing different effects on the amount of slow phase eye velocity and position increase during the 80% asymmetric stimulation. The cumulative SPEP of a subject (thin line) increases across the cycles more than predicted by linear addition of four ‘first cycle’ responses (dashed line). Conversely, the cumulative SPEP of the other subject (thick line) increases less than that predicted (dotted lines). These positional differences in SPEP are the consequence of different eye velocities of the two subjects (top trace). E, VOR mean gain during the four cycles of asymmetric stimulation in response to FHC (filled triangle) and SHC (open triangle). There are no significant differences between the response to the first and to the following cycles (P > 0.1). F, observed (filled circles) and predicted (open circles) values of the cumulative SPEP after four cycles of stimulation at different degrees of asymmetry. There are no significant differences between observed and predicted values. In both cases, the third and fourth cycles were different (*P < 0.05) compared to the first cycle. FHC, fast half-cycle; SHC, slow half-cycle; SPEP, slow phase eye position; TPE, tracking position error; VOR, vestibulo-ocular reflex.
This finding was confirmed by retrospective questioning: subjects referred that they did not perceive any movement during SHCs 3 and 4. This different perception of body rotation in the two directions of the stimulus led to a remarkably erroneous assessment of the absolute target position at the end of the four asymmetric cycles, with a mean tracking error of approximately 70° toward the left side of the subject (Fig. 2A and C). Each subject showed a tracking error that was relatively constant when tested in each of the four sessions administered on different days with intrasubject variability less than ±5°.
To investigate whether the tracking error was merely due to the dynamic properties of the vestibular system or to an adaptive process, we compared the observed tracking error against the ‘predicted’ error, namely the tracking response of the first asymmetric cycle. This was ‘copied-and-pasted’ after the tracking position at the end of each subsequent cycle (see Fig. 2A and C) to estimate the cumulative effect. After four cycles, the measured values were always greater than those ‘predicted’. With 80% stimulus asymmetry, the predicted tracking error was less than half than that actually observed (approximately 30°vs. 70°, respectively; Fig. 2C). With less asymmetric stimuli, both the observed and predicted tracking errors progressively diminished, as did the difference between these values (Fig. 2C). Two-way ANOVA, with observed and predicted conditions and percentage of stimulus asymmetry as the main factors, showed differences in tracking errors between conditions (F(1,26)= 76.3, P < 0.001) and across asymmetry (80%, 70%, 60%, 50%) (F(3,78)= 84,6, P < 0.001). The interaction was also significant (F(3,78)= 34.2, P < 0.001), as the differences in tracking error between conditions were found only for 80% (P < 0.01) and 70% asymmetry (P < 0.05) (for 60% asymmetry, P= 0.37) (Fig. 2C).
Control experiments to verify the effects of initial direction of FHC (to the left instead of the right), order in the sequence (SHC preceding the FHC) and central orientation of the rotation (±20°) showed no major changes. The tracking errors were 72°± 17, 68°± 19 and 66°± 11, when changing direction, sequence and orientation, respectively. Further, asking the subjects to focus only on their own body rotation and rotate the pointer without tracking any remembered visual target, suppressed eye movements (as in Panichi et al. 2011) but tracking error remained close to 70°. Hence, there were no significant differences in tracking error between control conditions and the main protocol mentioned above (one-way ANOVA, F(4,16)= 0.25, P= 0.90).
Vestibulo-ocular reflex
The mean gain of the slow-phase eye movements of VOR for the first FHC (internal frequency 0.38 Hz) was 0.84 ± 0.13 (Fig. 2D and E) and the mean phase was +1.1 ± 2.1. The mean gain for the first SHC (internal frequency 0.09 Hz) was 0.7 ± 0.18 and the mean phase was +3.1°± 2.9. These values are consistent with those reported at similar frequencies with conventional symmetrical stimuli by Mergner et al. (1998b) and with our own experiments (see Supplementary file 1).
After four cycles, the mean VOR gain showed a slight increase to 0.87 ± 0.32 for FHC and a slight decrease to 0.57 ± 0.36 for SHC. Compared to cycle 1, the gains in cycles 2–4 were not different (Fig. 2E). Two-way ANOVA, with velocity of half-cycle (FHC and SHC) and cycle number as main factors, showed a difference in gain between FHC and SHC (F(1,18)= 153.0, P < 0.001), but not across subsequent cycles (F(3,54)= 0.37, P= 0.61). There was no interaction between half-cycle velocity and cycle number (F(3,54)= 0.72, P= 0.54). The same analysis, performed on the phase values, showed that phase was affected by the half-cycle velocity (FHC and SHC; F(1,18)= 0. 152, P < 0.001), but was roughly constant across consecutive cycles (F(3,54)= 0.15, P= 0.99); the interaction was not significant (F(3,54)= 0.51, P= 0.58). Because of the different gain in the two directions, the cumulative slow phase eye position at the end of the four cycles was shifted by +38°± 31, in the direction opposite to the FHC (Fig. 2D and F). Among subjects, the response was somewhat variable; intrasubject variability (s.d. of the mean) was smaller than 4°, while intersubject s.d. was larger than 30°.
The observed and predicted (using the ‘copy-and-paste’ method) cumulative slow phase eye positions (Fig. 2D and F) were compared for different percentage of asymmetric stimulations. Two-way ANOVA, with observed and predicted conditions and percent of stimulus asymmetry as main factors, showed no difference in the cumulative slow phase eye position between conditions (F(1,18)= 0.58, P= 0.45). On the other hand, the cumulative slow phase eye position was different across asymmetry [F (1.18, 21.35), corrected by the epsilon approximation) = 27.3, P < 0.001]; the interaction was not significant (F(3,54)= 0.63, P= 0.59). All post hoc tests on stimulus asymmetries were significant (P < 0.01). Therefore, increasing asymmetry led to an expected increase in cumulative eye position, while observed and predicted conditions had no effect.
As in the motion-perception study, the slow phase eye position was assessed with additional experiments controlling for stimulus direction, sequence and orientation. The slow phase eye position became 31.6°± 21, 29.5°± 33, 36.1°± 21, for leftward initial rotation, for rotations starting with the SHC, and for body-centred rotation, respectively. These values were not different from those observed in the main condition (35.5°± 31) (Fig. 2F) (one-way ANOVA, F(3,12)= 0.29, P= 0.83). This indicates that the observed VOR effects did not depend on direction, sequence and orientation of the asymmetric oscillation cycles.
Long-lasting continuous asymmetric stimulation (protocol 2)
The subjects who underwent the short-lasting four-cycle stimulation were also administered, in different sessions, a longer-lasting sequence (20 cycles) of asymmetric whole body rotation cycles at 0.15 Hz, 80% asymmetry. The tracking position error progressively increased during the 20-cycle stimulation period (Fig. 3A). The increase was approximately exponential within the first 14 cycles, with a mean time constant of 35.2 s ± 1.9 and then became approximately linear with a slope of 8.3°s ± 1.3 (Fig. 3A). After 10 cycles, the peak velocity of the response to the FHC increased to more than twice that of the first cycle (Fig. 3B). In contrast, peak velocity of the tracking response to the SHC decreased to zero within four cycles and remained zero until the end of the oscillations (Fig. 3B).
Figure 3. Effect of prolonged symmetric and asymmetric rotations on motion perception and vestibulo-ocular reflex.
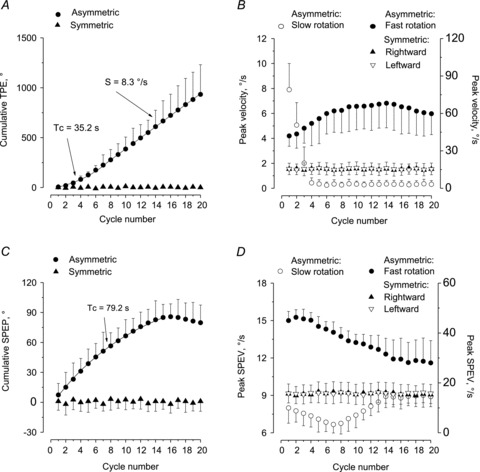
Progressive increase in the TPE (A and B) and decrease in the SPEP (C and D) in response to 20-cycle asymmetric stimulation. A, TPE was evaluated as cumulative displacement of motion perception for asymmetric (circles) and symmetric stimulation (triangle). In the first part of the stimulation, the best fitting of cumulative responses is exponential (Tc) and in the second part it is linear (S). B, peak velocity of the perceptual response to asymmetric stimulation (fast half-cycle: filled circle, right ordinate; slow half-cycle: open circle, left ordinate). The cumulative response to symmetric stimulation is almost zero, being similar for both the rightward (filled triangle) and the leftward (open triangle) response. C, cumulative SPEP for asymmetric (filled circle) and symmetric stimulation (filled triangle). The best fitting of cumulative responses of the first part of the stimulation is exponential. D, SPEV peak in response to fast (filled circles, right ordinate) and to slow stimulation (open circle, left ordinate). SPEV in response to fast half-cycles progressively decreases, while, that in response to slow half-cycles decreases only during the initial period, then progressively increases. Conversely, SPEV during symmetric rightward (filled triangle) and leftward (open triangle) stimulation is not modified. S, slope; SPEP, slow phase eye position; SPEV, slow phase eye velocity; Tc, time constant; TPE, tracking position error.
Conversely, the cumulative slow phase eye position of VOR (directed opposite to the direction of the FHC) increased up to ∼90° and then plateaued with a mean time constant of 79.2 s ± 2.2 (Fig. 3C). In parallel, the eye velocity in response to FHC diminished from ∼45°/s to ∼ 25°/s (Fig. 3D). The eye velocity in response to SHC, after an initial decrease, started increasing after eight cycles. All the values of the following cycles were significantly greater than that of the eighth cycle (one-way ANOVA, F(19,114)= 7.37, P < 0.001; post hoc test between the eighth and ninth cycle, P < 0.05; between the eighth and 20th cycle, P < 0.01).
As a control condition, five subjects underwent 20 cycles of symmetric stimulation (40°, 0.15 Hz). At the end of the 20th cycle, both the tracking position error and the cumulative eye position were negligible, ranging from ±2° to ±4° (Fig. 3A and C). Similarly, the velocity of the tracking and eye responses to symmetric rotation at the first, fourth and 20th cycles were not different for leftward or rightward direction (Fig. 3B and D). Two-way ANOVA, with cycle number and half-cycle direction as main factors, showed no difference in tracking position error across consecutive cycles (F(2,16)= 0.32, P= 0.74) or half-cycle direction (F(1,8)= 0.01, P= 0.93). There was no interaction (F(2,16)= 1.15, P= 0.34). Similar results were found for the cumulative eye position of VOR (for cycle number, F(2,16)= 0.38, P= 0.69; for half-cycle direction, F(1,8)= 0.031, P= 0.91). There was no interaction (F(2,16)= 0.91, P= 0.44). Therefore, at variance with the asymmetric stimulation, symmetric stimulation did not induce any significant adaptation across cycles.
Testing the after-effects with a single cycle symmetric stimulus (protocol 3)
To examine the presence of after-effects of the asymmetric stimulation on motion perception and VOR, we examined the gain of responses to a single symmetric test cycle. This stimulus was given before and 1 min after 14 cycles of asymmetric stimulation, as all asymmetric stimulus-induced effects were fully developed by cycle 14.
After-effect in response to symmetric cycles at different frequencies of stimulation
At 1 min, post-asymmetric stimulation subjects showed an asymmetric response to the two directions of the single symmetric test cycle. The asymmetry was opposite for motion perception and VOR (Fig. 4A and B). For motion perception, the gain of the tracking to the symmetric half-cycle in the same direction as the preceding FHC was higher than the gain in the opposite direction, at all frequencies of stimulation (Fig. 4C). Two-way ANOVA, with direction and frequency as factors, showed an effect of direction (F(1,26)= 406.0, P < 0.0001) and frequency (F(3,78)= 44.5, P < 0.001), but no interaction (F(3,78)= 0.59, P= 0.63). Post hoc comparisons of the pooled direction data at each frequency were significant (all comparisons, P < 0.01) (Fig. 4A and C).
Figure 4. Persistence of the after-effect of motion perception and vestibulo-ocular reflex induced by asymmetric stimulation.
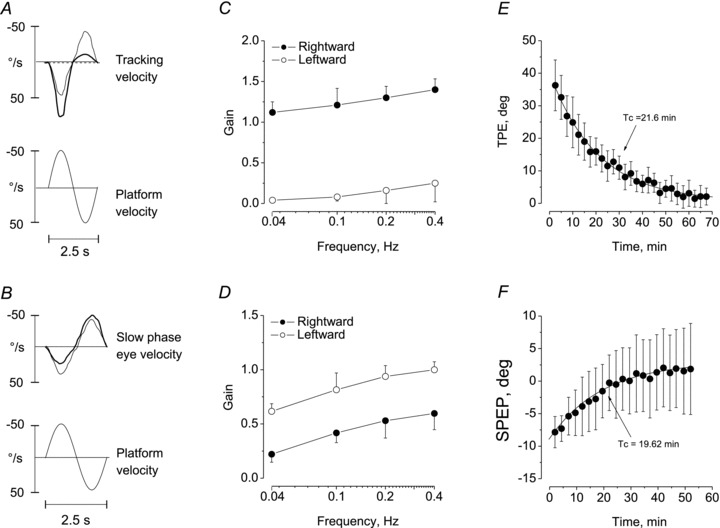
Tracking profile (A, C, E) and vestibulo-ocular reflex (B, D, F) were tested by a single cycle symmetric test stimulus (40°, 0.4 Hz). A, B, (upper traces) velocity of tracking response (A) and slow phase eye velocity (B) before (thin line) and after (thick line) the end of the 14-cycle asymmetric (80% asymmetry, fast half-cycles directed toward the right side). Lower trace, platform velocity during the test stimulus. The responses to the symmetric test cycle are distorted after the asymmetric stimulation, with enhancement of the response to platform rotation toward the right side for the perceptual response and to the left side for the ocular responses. C and D, responses to symmetric stimuli at different frequencies of sinusoidal test (0.04–0.4 Hz) on movement perception and eye responses after 14 cycles of asymmetric stimulation. Means and s.d. of gain in response to the two directions of the symmetric stimulus are reported (rightward direction, filled circles; leftward direction, open circles). The perceptual responses show an increase when subjects are rotated in the same direction of the conditioning fast half-cycle, while the eye responses increased in the opposite direction. E and F, decay of the long-term effect on movement perception (E) and eye responses (F) induced by 14 cycles of conditioning asymmetric stimulation (0.15 Hz, 80% asymmetry), as tested by the single sinusoidal test stimulus (0.4 Hz) repeated every 2.5 min after the end of the conditioning stimulation. Means and s.d. are reported for TPE (E, 14 subjects) and cumulative SPEP (F, 10 subjects). The cumulative SPEP shifts in the opposite direction compared to that of TPE, but the after-effects decayed with a similar time constant. SPEP, slow phase eye position; Tc, time constant; TPE, tracking position error.
VOR gain was also asymmetric (Fig. 4B and D), but had a lower value during rotation in the direction of the preceding FHC and a higher value in the SHC direction. Two-way ANOVA, with direction and frequency as factors, showed a difference across frequencies (F(3,54)= 184.2, P < 0.001) and between directions (F(1,18)= 70.3, P < 0.001). The interaction was not significant (F(3,54)= 0.47, P= 0.71). The differences between each frequency were computed for the pooled direction data. The pooled data were different between each frequency pair (P < 0.01 for all comparisons). Therefore, the absence of direction × frequency interaction for both motion perception and VOR indicated that the after-effects were not dependent on the stimulus frequency.
Persistence of the after-effect
When the symmetric test cycle was administered immediately after the end of the asymmetric stimulation, the perceptual response showed a tracking error of +37°± 8 in the direction of the preceding SHC (Fig. 4E and F). On repeating the stimulus every 2.5 min, this error decreased to zero exponentially with a time constant of approximately 22 min (Fig. 4E). In contrast with the after-effect of motion perception, the cumulative slow phase eye position of the VOR showed an asymmetry of the opposite sign, as the greater amplitude of the response was in the direction of the preceding FHC, causing a positional shift of 9°± 2. However, the positional shift decreased to zero exponentially with a time constant (19.6 min ± 5.2) similar to that of the perceptual responses (Fig. 4F).
Discussion
The main finding of this study is that a prolonged vestibular stimulation consisting of a sequence of asymmetric whole body yaw oscillation cycles induces different adaptive processes in the perception of movement and in the reflex ocular response (VOR).
During the continuous asymmetric oscillations, the perception of body motion in space was enhanced in the direction of the FHC and reduced in the direction of the SHC. The symmetric oscillation only induced a negligible attenuation of movement perception with respect to its initial values. These asymmetric effects were observed regardless of the initial direction of the FHC oscillation (to the right or to the left) and irrespective of the initial half-cycle of the oscillation being fast or slow. Further, the effects were equally robust when both the oscillation cycles were centred across the initial body position and they started from the initial position and returned to it. This indicates that it is the asymmetric shape of the stimulus, and not its absolute orientation with respect to the body's initial position, that induces the observed asymmetric response.
It might be argued that asymmetry in motion perception during asymmetric stimulation might be predicted by the high pass transfer characteristic of the vestibular system, namely enhanced responsiveness to higher than lower frequencies (Fernandez & Goldberg, 1971). However, this was not the case, because position error and gain of the responses for self-motion perception were clearly greater than those predicted (Fig. 2C). This finding can only be explained by the generation of long-term effects in central vestibular circuitry, specifically increasing motion perception towards the fast rotation and decreasing perception during the slow rotation.
The response of the VOR to the repetitive asymmetric stimulation was opposite to the perceptual response, as the stimulation made the VOR symmetric, due to decreased and increased responsiveness to FHC and SHC, respectively. On average, the tendency to symmetrize the stimulus-dependent VOR asymmetry was not significant during the first four cycles, due to initial large intersubject variability, but it became clear and consistent when the stimulation was further prolonged. Incidentally, we found (see Supplementary material 3) that the VOR symmetrizing effect of the repetitive asymmetric stimulations was not due to the eye-in-orbit eccentricity (Robinson et al. 1984) brought about by the quick phases of nystagmus elicited by the FHCs. A recent observation by Anagnostou et al. (2011) also shows that the gain of the VOR remains unaffected by the eye-in-orbit position.
Adaptation and after-effects
Both perceptual and eye movement reflex effects persisted for a long time after the end of the stimulation (approximately 1 h; Fig. 4E and F). Therefore, both are gradually built up during the stimulation and consolidated in the CNS. The after-effects, assessed by a symmetric test stimulus, were similar at all the four frequencies employed for this stimulus. This indicates that both the perceptual and the reflex adaptive processes are not restricted to the frequencies contained in the conditioning stimulus, but only depend on the stimulus asymmetry. This suggests that the adaptation depends on the difference in vestibular activation of one side compared to the opposite, regardless of the stimulation frequency. This frequency independence would suggest a non-specific effect related to a unilaterally dominant activation.
The increasing asymmetry in motion perception contrasts with the reduction reported in previous studies, where unidirectional vestibular stimulation was delivered by repetitive step or sinusoidal or trapezoidal rotation (Guedry & Lauver, 1961; Guedry & Collins, 1968; Brown & Wolfe, 1969; Grunfeld et al. 2000; Clément et al. 2008) and by galvanic stimulation (St George et al. 2011). However, those stimulus patterns are remarkably different from those administered here, where fast rotation toward one side was followed by a less intense stimulation toward the other side. Our asymmetric stimulation will likely cause a different dynamic activation of vestibular neurons during rotation, with the afferent activity being much larger in one side (during FHC) and much less in the other side (during SHC). This activation may imitate unilateral pathological conditions, in which head rotation induces greater afferent activation toward the normal than toward the affected side.
Different central processing of motion perception and vestibulo-ocular reflex
The opposite effects induced by repetitive asymmetric stimulation on self-motion perception and VOR indicate that the central processing of vestibular information diverges considerably within the brain. The sensory signals from the semicircular canals appear to undergo additional neural processing to compute the perception of self-motion, beyond the contribution of the velocity-storage mechanism of the VOR.
Possibly, the reduction of the stimulus-induced asymmetry of VOR occurs at an early stage, namely in the brainstem circuitry controlling the eye movements, while the enhancement in self-motion perception asymmetry is a higher-order phenomenon involving regions where body orientation and movement perception are elaborated. These regions may be widely distributed in the central nervous system. The hippocampus (Sharp et al. 1995) and different areas of the cortex are involved in multisensory processing of vestibular information, including the posterior parietal cortex and the parieto-insular vestibular cortex (Brandt & Dieterich, 1999; Seemungal et al. 2009; Lopez & Blanke, 2011). However, the vestibular nuclei could, at least in part, be responsible for the observed different central processing of both responses. In vitro studies (Grassi et al. 1995; Pettorossi et al. 2011) have shown that repetitive electrical activation of vestibular fibres induces long-term potentiation in the ventral part of the medial vestibular nuclei and long-term depression in its dorsal region. It could be speculated that the ventral pathway showing potentiation may mediate the increase of the asymmetric perceptual response, while the dorsal one, showing depression, may be responsible for VOR symmetrization.
Functional significance of the effects induced by asymmetric whole body rotation
The enhancement of self-motion perception in the direction of the faster body movement suggests an expansion of the dynamic response of the vestibular system. This would be useful to better perceive (as by a contrast-enhancing mechanism) the velocity of body rotation during fast movements and to better extract the information relevant to the ‘impending’ straight-ahead, as may occur during progression along curvilinear trajectories (Grasso et al. 1996; Imai et al. 2001; Courtine & Schieppati, 2003) and ‘on the spot’ pivot turns (Anastasopoulos et al. 2009). Conversely, the decreased sensitivity to slow movements may not necessarily be a functional deficit, because other sensory modalities such as vision and proprioception may substitute for the reduction of vestibular low-frequency responses and provide adequate feedback (Bove et al. 2002; Courtine et al. 2007; Panichi et al. 2011). In contrast, the VOR tends to become symmetric, its gain increasing during the slow rotation. This would assure gaze stability just when body movements are no longer perceived. Others have found a reduced VOR gain during the fast rotation, probably an expression of adaptive effects (Curthoys & Halmagyi, 1995). The findings can be considered in light of the discrepancy commonly observed between subjective vestibular symptoms (perception) and ocular reflex symmetry in patients with labyrinthine damage (Kanayama et al. 1995; Palla et al. 2008; Cousins et al. 2009).
In conclusion, the present study has identified a new type of adaptive mechanism intervening in self-motion perception, different from that of the VOR. This mechanism has been brought to light by the use of asymmetric vestibular stimulation and results in oppositely directed adaptive responses in the perceptual and ocular domain. This divergent central adaptive process may be required for focusing our attention on to the future direction toward which our body is being directed, thanks to enhancement of motion perception toward the more rapid body rotation. On the other hand, VOR symmetrization would be part of a general property of the vestibular system aimed at reducing imbalance in the vestibulo-oculomotor system and compensating for the reduction of movement perception during slow body rotation. These mechanisms, which operate across a wide range of frequency stimulation, may be involved in the adaptive recovery observed after unilateral vestibular damage.
Acknowledgments
This research was supported in part by the Italian Ministry of University and Research (PRIN 2007 no. 2007HTFN9L) and by the Fondazione Cassa di Risparmio of Perugia. We wish to thank Mr D. Bambagioni for technical assistance. The research of A.M.B. is supported by the MRC (UK) grant MR/J004685/1.
Glossary
- FHC
fast rotation half-cycle
- SHC
slow rotation half-cycle
- VOR
vestibulo-ocular reflex
Author contributions
The experiments were performed in the laboratory of V.E.P. at the Università di Perugia. V.E.P.: conception and design of the experiments and drafting the article or revising it critically for important intellectual content. A.M.B.: drafting the article or revising it critically for important intellectual content. M.S.: drafting the article or revising it critically for important intellectual content. A.F.: collection, analysis and interpretation of data. R.P.: collection, analysis and interpretation of data.
M.F.: collection, analysis and interpretation of data. F.M.B.: collection, analysis and interpretation of data. A.K.: collection, analysis and interpretation of data. All authors approved the final version of the manuscript.
Supplementary material
Supplementry Material
References
- Anagnostou E, Heimberger J, Sklavos S, Anastasopoulos D. Alexander's law during high-acceleration head rotations in humans. Neuroreport. 2011;22:239–243. doi: 10.1097/WNR.0b013e3283451769. [DOI] [PubMed] [Google Scholar]
- Anastasopoulos D, Ziavra N, Hollands M, Bronstein A. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res. 2009;193:323–336. doi: 10.1007/s00221-008-1627-y. [DOI] [PubMed] [Google Scholar]
- Barnes G. Adaptation in the oculomotor response to caloric irrigation and the merits of bithermal stimulation. Br J Audiol. 1995;29:95–106. doi: 10.3109/03005369509086586. [DOI] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105:209–223. doi: 10.1152/jn.00154.2010. [DOI] [PubMed] [Google Scholar]
- Bove M, Courtine G, Schieppati M. Neck muscle vibration and spatial orientation during stepping in place in humans. J Neurophysiol. 2002;88:2232–2241. doi: 10.1152/jn.00198.2002. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci. 1999;871:293–312. doi: 10.1111/j.1749-6632.1999.tb09193.x. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dichgans J, Büchele W. Motion habituation: Inverted self-motion perception. Exp Brain Res. 1974;21:337–352. doi: 10.1007/BF00237897. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Grunfeld EA, Faldon M, Okada T. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport. 2008;19:691–693. doi: 10.1097/WNR.0b013e3282fbf9f6. [DOI] [PubMed] [Google Scholar]
- Brown JH, Wolfe JW. Adaptation to constant angular acceleration. Acta Otolaryngol. 1969;67:389–398. doi: 10.3109/00016486909125465. [DOI] [PubMed] [Google Scholar]
- Clément G, Tilikete C, Courjon JH. Retention of habituation of vestibulo-ocular reflex and sensation of rotation in humans. Exp Brain Res. 2008;190:307–315. doi: 10.1007/s00221-008-1471-0. [DOI] [PubMed] [Google Scholar]
- Cohen B, Henn V, Raphan T, Dennett D. Velocity storage, nystagmus, and visuo-vestibular interactions in humans. Ann NY Acad Sci. 1981;374:421–433. doi: 10.1111/j.1749-6632.1981.tb30888.x. [DOI] [PubMed] [Google Scholar]
- Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Eur J Neurosci. 2003;18:177–190. doi: 10.1046/j.1460-9568.2003.02736.x. [DOI] [PubMed] [Google Scholar]
- Courtine G, De Nunzio AM, Schmid M, Beretta MV, Schieppati M. Stance- and locomotion-dependent processing of vibration-induced proprioceptive inflow from multiple muscles in humans. J Neurophysiol. 2007;97:772–779. doi: 10.1152/jn.00764.2006. [DOI] [PubMed] [Google Scholar]
- Cousins SE, Cutfield NJ, Seemungal B, Gresty M, Bronstein A. Vestibular perception after acute vestibular neuritis. Eur J Neurology. 2009;16(Suppl. 3) [Google Scholar]
- Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. [PubMed] [Google Scholar]
- Curthoys IS, Blanks RH, Markham CH. Semicircular canal functional anatomy in cat, guinea pig and man. Acta Otolaryngol. 1977;83:258–265. doi: 10.3109/00016487709128843. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of the peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of the peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Grassi S, Della Torre G, Capocchi G, Zampolini M, Pettorossi VE. The role of GABA in NMDA-dependent long term depression (LTD) of rat medial vestibular nuclei. Brain Res. 1995;699:183–191. doi: 10.1016/0006-8993(95)00895-w. [DOI] [PubMed] [Google Scholar]
- Grasso R, Glasauer S, Takei Y, Berthoz A. The predictive brain: anticipatory control of head direction for the steering of locomotion. Neuroreport. 1996;7:1170–1174. [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Grunfeld EA, Okada T, Jauregui-Renaud K, Bronstein AM. The effect of habituation and plane of rotation on vestibular perceptual responses. J Vestib Res. 2000;10:193–200. [PubMed] [Google Scholar]
- Guedry FE, Collins WE. Duration of angular acceleration and ocular nystagmus from cat and man. II. Responses from the lateral canals to varied stimulus durations. Acta Otolaryngol. 1968;65:257–269. doi: 10.3109/00016486809120967. [DOI] [PubMed] [Google Scholar]
- Guedry FE, Lauver LS. Vestibular reactions during prolonged constant angular acceleration. J Appl Physiol. 1961;16:215–220. [Google Scholar]
- Hain TC, Zee DS. Velocity storage in labyrinthine disorders. Ann NY Acad Sci. 1992;656:297–304. doi: 10.1111/j.1749-6632.1992.tb25216.x. [DOI] [PubMed] [Google Scholar]
- Imai T, Moore ST, Raphan T, Cohen B. Interaction of the body, head, and eyes during walking and turning. Exp Brain Res. 2001;136:1–18. doi: 10.1007/s002210000533. [DOI] [PubMed] [Google Scholar]
- Kanayama R, Bronstein AM, Gresty MA, Brookes GB, Faldon ME, Nakamura T. Perceptual studies in patients with vestibular neurectomy. Acta Otolaryngol. 1995;520:408–411. doi: 10.3109/00016489509125284. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O. The thalamo-cortical vestibular system in animals and humans. Brain Res Rev. 2011;67:119–146. doi: 10.1016/j.brainresrev.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol. 2005a;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt & translation. J Neurophysiol. 2005b;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Mergner T, Siebold C, Schweigart G, Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85:389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- Mergner T, Rottler G, Kimming H, Becker W. Role of vestibular and neck inputs for the perception of object motion in space. Exp Brain Res. 1992;89:655–668. doi: 10.1007/BF00229890. [DOI] [PubMed] [Google Scholar]
- Mergner T, Nasios G, Anastasopoulos D. Vestibular memory-contingent saccades involve somatosensory input from the body support. Neuroreport. 1998a;9:1469–1473. doi: 10.1097/00001756-199805110-00041. [DOI] [PubMed] [Google Scholar]
- Mergner T, Schweigart G, Botti F, Lehmann A. Eye movement evoked by proprioceptive stimulation along the body axis in humans. Exp Brain Res. 1998b;120:450–460. doi: 10.1007/s002210050418. [DOI] [PubMed] [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122:1293–1303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- Oman CM, Marcus EN, Curthoys IS. The influence of semicircular canal morphology on endolynph flow dynamics. An anatomically descriptive mathematical model. Acta Otolaryngol. 1987;103:1–13. doi: 10.3109/00016488709134691. [DOI] [PubMed] [Google Scholar]
- Palla A, Straumann D, Bronstein AM. Vestibular neuritis: vertigo and the high-acceleration vestibulo-ocular reflex. J Neurol. 2008;255:1479–1482. doi: 10.1007/s00415-008-0935-2. [DOI] [PubMed] [Google Scholar]
- Panichi R, Botti FM, Ferraresi A, Faralli M, Kyriakareli A, Schieppati M, Pettorossi VE. Self-motion perception and vestibulo-ocular reflex during whole body yaw rotation in standing subjects: The role of head position and neck proprioception. Hum Mov Sci. 2011;30:314–332. doi: 10.1016/j.humov.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Pettorossi VE, Panichi R, Bambagioni D, Grassi S, Botti FM. Contribution of eye position to movement perception. Acta Otolaryngol. 2004;124:471–474. doi: 10.1080/00016480410017314. [DOI] [PubMed] [Google Scholar]
- Pettorossi VE, Dieni CV, Scarduzio M, Grassi S. Long-term potentiation of synaptic response and intrinsic excitability in neurons of the rat medial vestibular nuclei. Neuroscience. 2011;187:1–14. doi: 10.1016/j.neuroscience.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Robinson DA. In: Handbook of Physiology. The nervous system. Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, Control of eye movements, editors. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1277–1320. [Google Scholar]
- Robinson DA, Zee DS, Hain TC, Holmes A, Rosenberg LF. Alexander's law: its behavior and origin in the human vestibulo-ocular reflex. Ann Neurol. 1984;16:714–722. doi: 10.1002/ana.410160614. [DOI] [PubMed] [Google Scholar]
- Schweigart G, Chien RD, Mergner T. Neck proprioception compensates for age-related deterioration of vestibular self-motion perception. Exp Brain Res. 2002;147:89–97. doi: 10.1007/s00221-002-1218-2. [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Gunaratne IA, Fleming IO, Gresty MA, Bronstein AM. Perceptual and nystagmic thresholds of vestibular function in yaw. J Vestib Res. 2004;14:461–466. [PubMed] [Google Scholar]
- Seemungal BM, Rizzo V, Gresty MA, Rothwell JC, Bronstein AM. Perceptual encoding of self-motion duration in human posterior parietal cortex. Ann N Y Acad Sci. 2009;1164:236–238. doi: 10.1111/j.1749-6632.2009.03772.x. [DOI] [PubMed] [Google Scholar]
- Siegle JH, Campos JL, Mohler BJ, Loomis JM, Bülthoff HH. Measurement of instantaneous perceived self-motion using continuous pointing. Exp Brain Res. 2009;195:429–44. doi: 10.1007/s00221-009-1805-6. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Etkin D, Tzanetos DB. Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci. 1995;15:173–89. doi: 10.1523/JNEUROSCI.15-01-00173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Zaher N, Shaikh AG, Lasker AG, Zee DS, Tarnutzer AA. Perception of self motion during and after passive rotation of the body around an earth-vertical axis. Prog Brain Res. 2008;171:277–281. doi: 10.1016/S0079-6123(08)00639-0. [DOI] [PubMed] [Google Scholar]
- St George RJ, Day BL, Fitzpatrick RC. Adaptation of vestibular signals for self-motion perception. J Physiol. 2011;589:843–853. doi: 10.1113/jphysiol.2010.197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert N, De Waele C, Serafin M, Babalian A, Muhlethaler M, Vidal PP. The vestibular system as a model of the sensorimotor transformations. A combined in vivo and in vitro approach to study the cellular mechanisms of gaze and posture stabilization in mammals. Prog Neurobiol. 1977;51:243–286. doi: 10.1016/s0301-0082(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1977;27:523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Young RL, Oman CM. Model for vestibular adaptation to horizontal rotation. Aerospace Med. 1969;40:1076–1080. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


