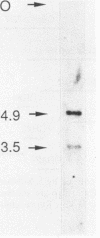
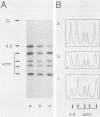
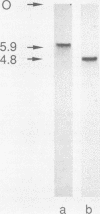
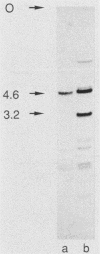
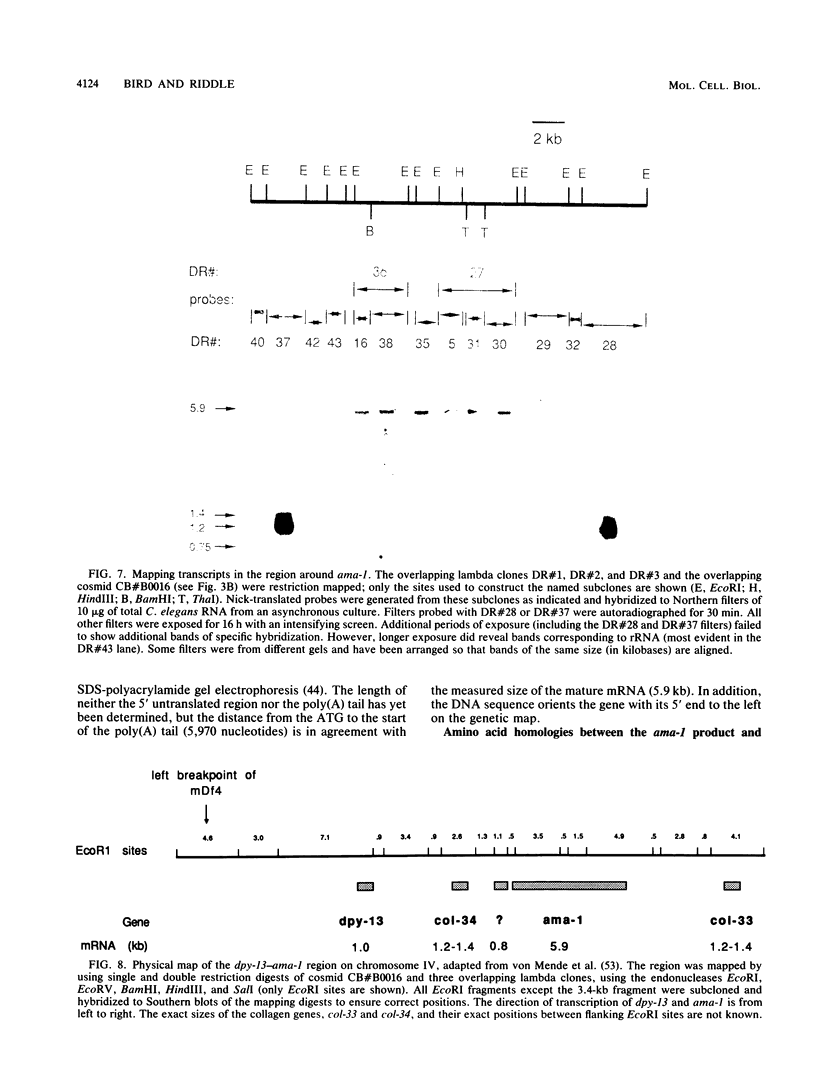
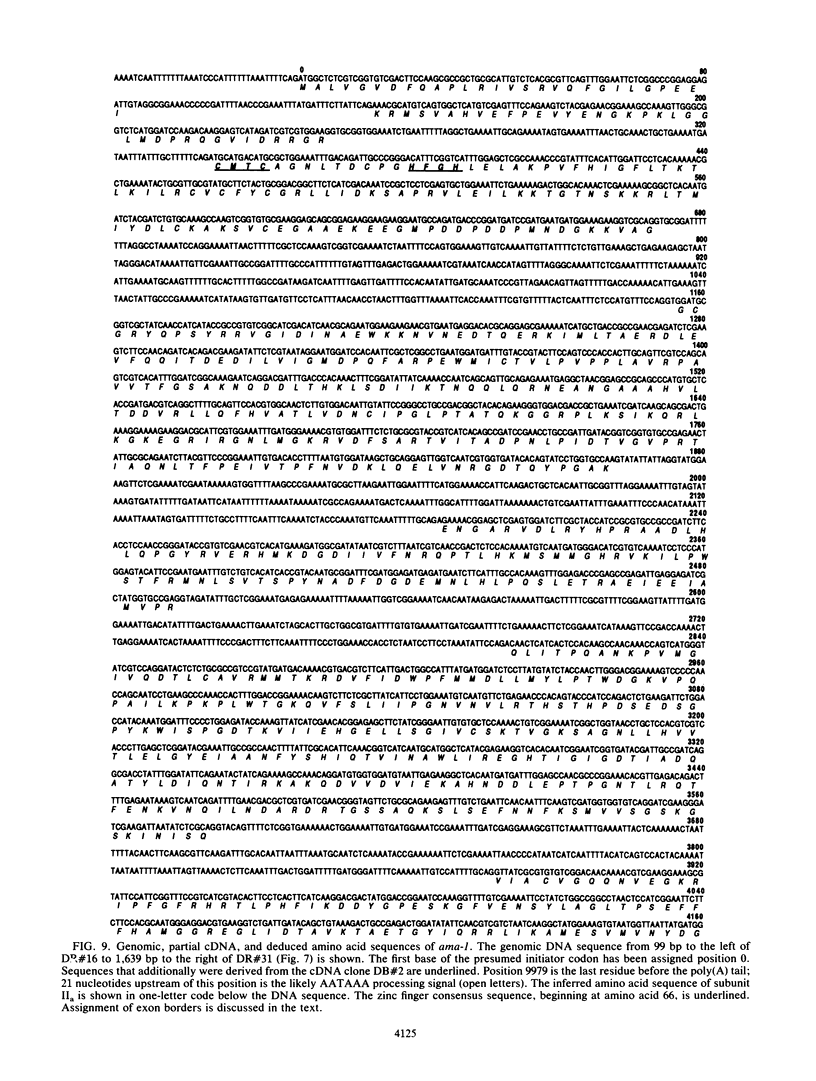
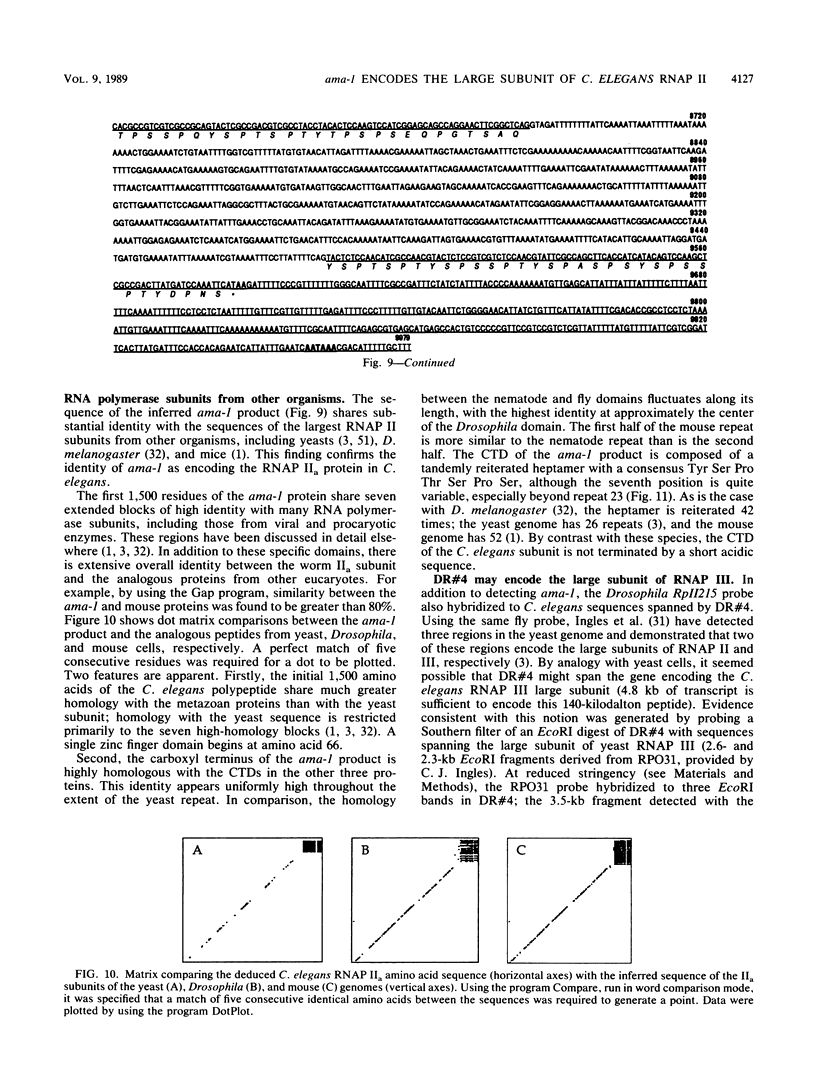
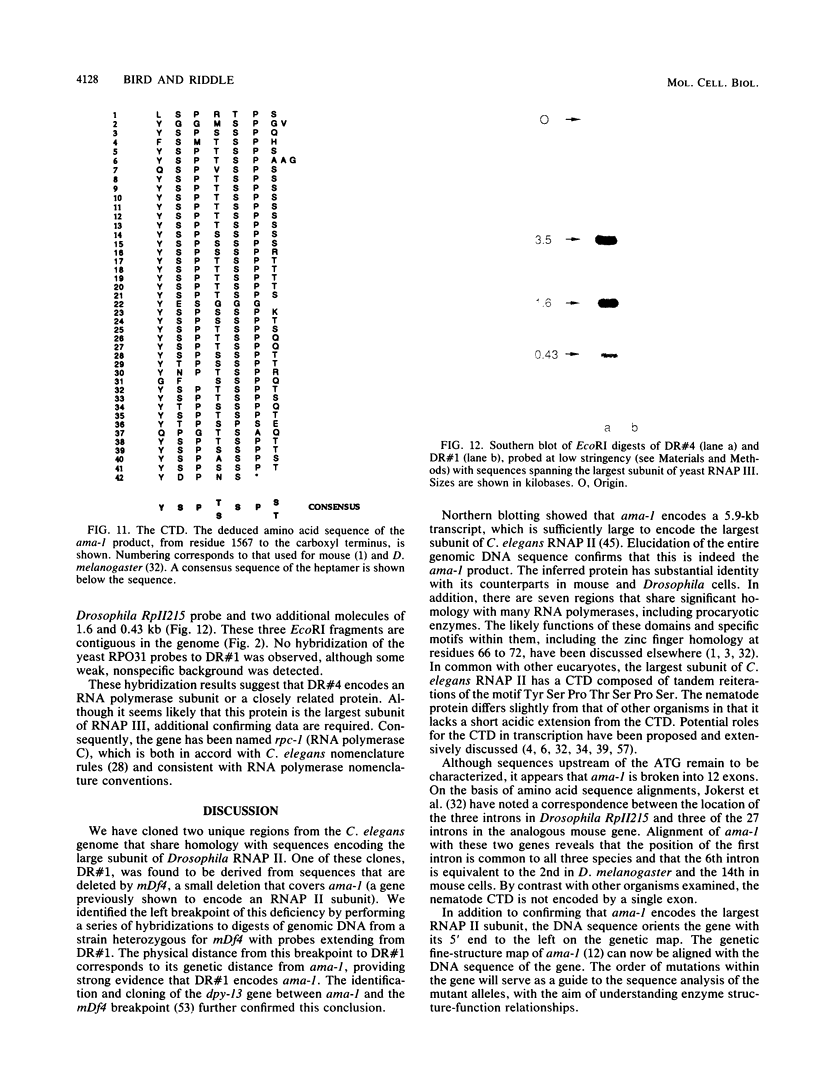
Abstract
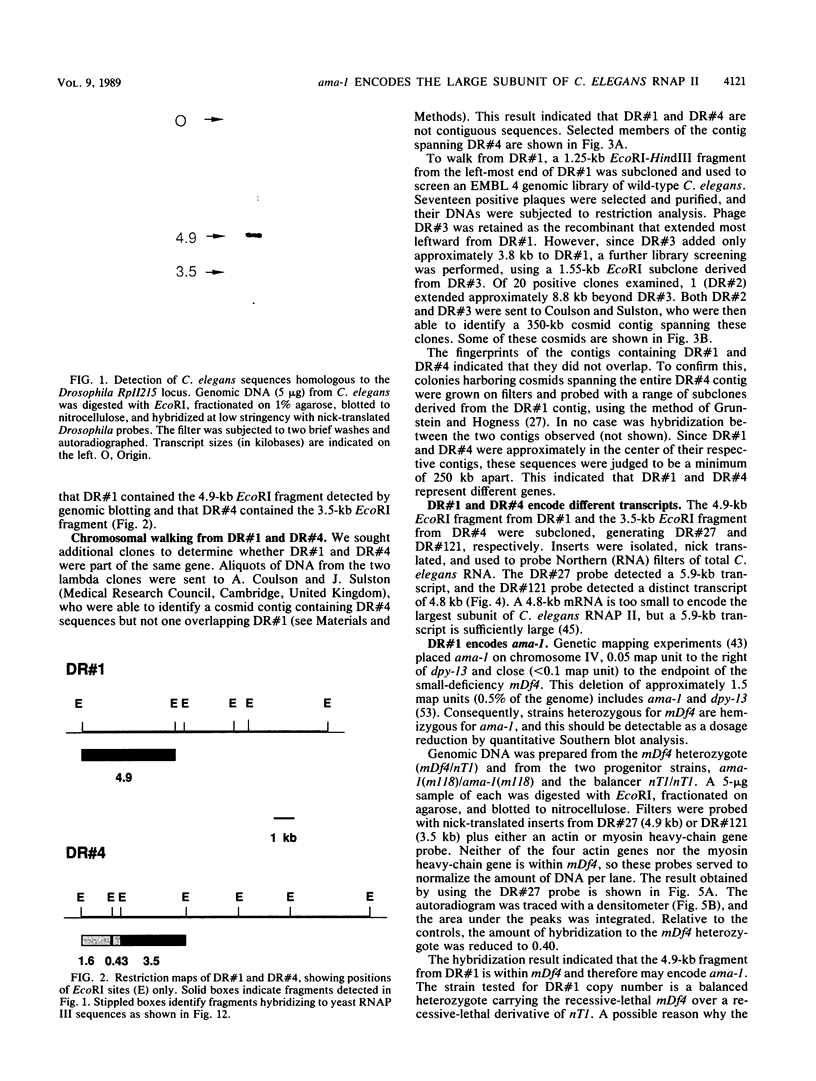
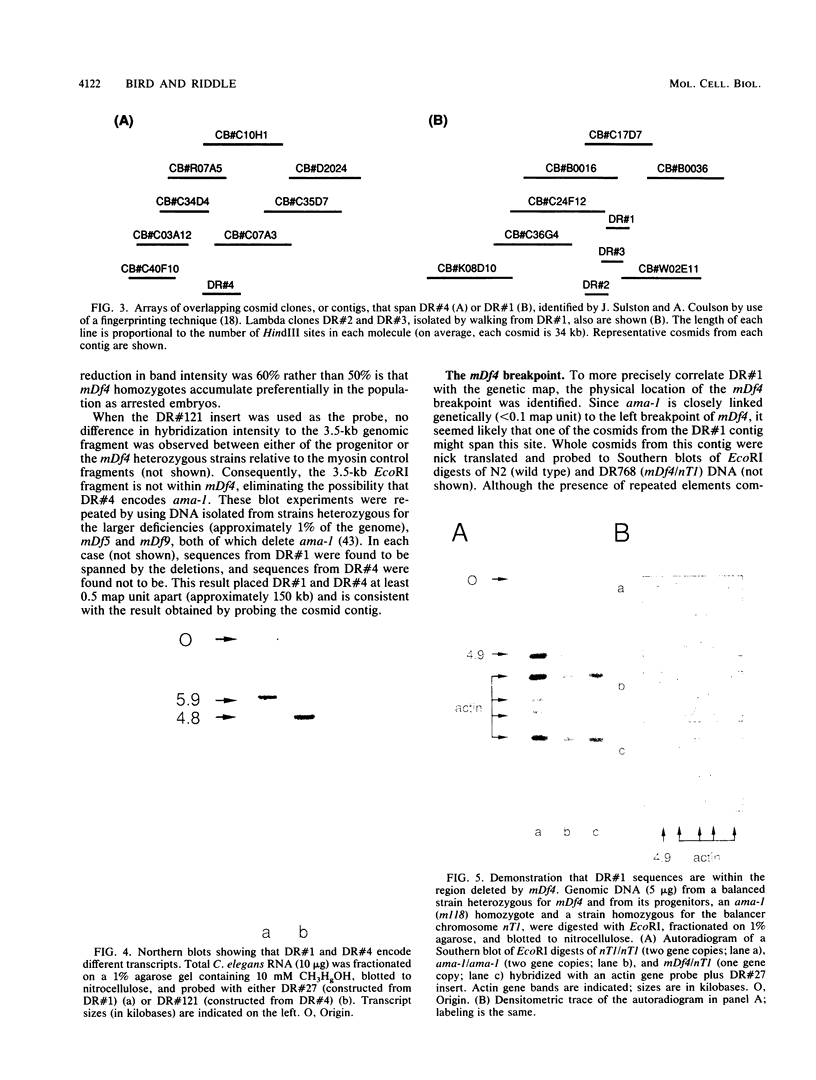
Two genomic sequences that share homology with Rp11215, the gene encoding the largest subunit of RNA polymerase II in Drosophila melanogaster, have been isolated from the nematode Caenorhabditis elegans. One of these sequences was physically mapped on chromosome IV within a region deleted by the deficiency mDf4, 25 kilobases (kb) from the left deficiency breakpoint. This position corresponds to ama-1 (resistance to alpha-amanitin), a gene shown previously to encode a subunit of RNA polymerase II. Northern (RNA) blotting and DNA sequencing revealed that ama-1 spans 10 kb, is punctuated by 11 introns, and encodes a 5.9-kb mRNA. A cDNA clone was isolated and partially sequenced to confirm the 3' end and several splice junctions. Analysis of the inferred 1,859-residue ama-1 product showed considerable identity with the largest subunit of RNAP II from other organisms, including the presence of a zinc finger motif near the amino terminus, and a carboxyl-terminal domain of 42 tandemly reiterated heptamers with the consensus Tyr Ser Pro Thr Ser Pro Ser. The latter domain was found to be encoded by four exons. In addition, the sequence oriented ama-1 transcription with respect to the genetic map. The second C. elegans sequence detected with the Drosophila probe, named rpc-1, was found to encode a 4.8-kb transcript and hybridized strongly to the gene encoding the largest subunit of RNA polymerase III from yeast, implicating rpc-1 as encoding the analogous peptide in the nematode. By contrast with ama-1, rpc-1 was not deleted by mDf4 or larger deficiencies examined, indicating that these genes are no closer than 150 kb. Genes flanking ama-1, including two collagen genes, also have been identified.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn J. M., Jr, Bartolomei M. S., West M. L., Cisek L. J., Corden J. L. Cloning and sequence analysis of the mouse genomic locus encoding the largest subunit of RNA polymerase II. J Biol Chem. 1987 Aug 5;262(22):10695–10705. [PubMed] [Google Scholar]
- Albertson D. G. Mapping muscle protein genes by in situ hybridization using biotin-labeled probes. EMBO J. 1985 Oct;4(10):2493–2498. doi: 10.1002/j.1460-2075.1985.tb03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Allison L. A., Wong J. K., Fitzpatrick V. D., Moyle M., Ingles C. J. The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol Cell Biol. 1988 Jan;8(1):321–329. doi: 10.1128/mcb.8.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei M. S., Corden J. L. Localization of an alpha-amanitin resistance mutation in the gene encoding the largest subunit of mouse RNA polymerase II. Mol Cell Biol. 1987 Feb;7(2):586–594. doi: 10.1128/mcb.7.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei M. S., Halden N. F., Cullen C. R., Corden J. L. Genetic analysis of the repetitive carboxyl-terminal domain of the largest subunit of mouse RNA polymerase II. Mol Cell Biol. 1988 Jan;8(1):330–339. doi: 10.1128/mcb.8.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektesh S., Van Doren K., Hirsh D. Presence of the Caenorhabditis elegans spliced leader on different mRNAs and in different genera of nematodes. Genes Dev. 1988 Oct;2(10):1277–1283. doi: 10.1101/gad.2.10.1277. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggs J., Searles L. L., Greenleaf A. L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullerjahn A. M., Riddle D. L. Fine-structure genetics of ama-1, an essential gene encoding the amanitin-binding subunit of RNA polymerase II in Caenorhabditis elegans. Genetics. 1988 Oct;120(2):423–434. doi: 10.1093/genetics/120.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. L., Whitmore G. F., Siminovitch L. Mammalian cells with altered forms of RNA polymerase II. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3119–3123. doi: 10.1073/pnas.69.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cho K. W., Khalili K., Zandomeni R., Weinmann R. The gene encoding the large subunit of human RNA polymerase II. J Biol Chem. 1985 Dec 5;260(28):15204–15210. [PubMed] [Google Scholar]
- Cochet-Meilhac M., Chambon P. Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins. Biochim Biophys Acta. 1974 Jun 27;353(2):160–184. doi: 10.1016/0005-2787(74)90182-8. [DOI] [PubMed] [Google Scholar]
- Corden J. L., Cadena D. L., Ahearn J. M., Jr, Dahmus M. E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7934–7938. doi: 10.1073/pnas.82.23.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Kramer J. M., Hirsh D. Number and organization of collagen genes in Caenorhabditis elegans. Mol Cell Biol. 1984 Nov;4(11):2389–2395. doi: 10.1128/mcb.4.11.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Klass M. R., Hirsh D. Analysis of the constancy of DNA sequences during development and evolution of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1333–1337. doi: 10.1073/pnas.76.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L. Amanitin-resistant RNA polymerase II mutations are in the enzyme's largest subunit. J Biol Chem. 1983 Nov 25;258(22):13403–13406. [PubMed] [Google Scholar]
- Greenleaf A. L., Borsett L. M., Jiamachello P. F., Coulter D. E. Alpha-amanitin-resistant D. melanogaster with an altered RNA polymerase II. Cell. 1979 Nov;18(3):613–622. doi: 10.1016/0092-8674(79)90116-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R., Brenner S., Hodgkin J., Herman R. K. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979 Sep;175(2):129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Biggs J., Wong J. K., Weeks J. R., Greenleaf A. L. Identification of a structural gene for a RNA polymerase II polypeptide in Drosophila melanogaster and mammalian species. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3396–3400. doi: 10.1073/pnas.80.11.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Guialis A., Lam J., Siminovitch L. Alpha-Amanitin resistance of RNA polymerase II in mutant Chinese hamster ovary cell lines. J Biol Chem. 1976 May 10;251(9):2729–2734. [PubMed] [Google Scholar]
- Ingles C. J., Himmelfarb H. J., Shales M., Greenleaf A. L., Friesen J. D. Identification, molecular cloning, and mutagenesis of Saccharomyces cerevisiae RNA polymerase genes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2157–2161. doi: 10.1073/pnas.81.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokerst R. S., Weeks J. R., Zehring W. A., Greenleaf A. L. Analysis of the gene encoding the largest subunit of RNA polymerase II in Drosophila. Mol Gen Genet. 1989 Jan;215(2):266–275. doi: 10.1007/BF00339727. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The nematode Caenorhabditis elegans. Science. 1988 Jun 10;240(4858):1448–1453. doi: 10.1126/science.3287621. [DOI] [PubMed] [Google Scholar]
- Kim W. Y., Dahmus M. E. The major late promoter of adenovirus-2 is accurately transcribed by RNA polymerases IIO, IIA, and IIB. J Biol Chem. 1989 Feb 25;264(6):3169–3176. [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Expression of the Caenorhabditis elegans collagen genes col-1 and col-2 is developmentally regulated. J Biol Chem. 1985 Feb 10;260(3):1945–1951. [PubMed] [Google Scholar]
- Nonet M., Sweetser D., Young R. A. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987 Sep 11;50(6):909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- Politz J. C., Edgar R. S. Overlapping stage-specific sets of numerous small collagenous polypeptides are translated in vitro from Caenorhabditis elegans RNA. Cell. 1984 Jul;37(3):853–860. doi: 10.1016/0092-8674(84)90420-3. [DOI] [PubMed] [Google Scholar]
- Riva M., Memet S., Micouin J. Y., Huet J., Treich I., Dassa J., Young R., Buhler J. M., Sentenac A., Fromageot P. Isolation of structural genes for yeast RNA polymerases by immunological screening. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1554–1558. doi: 10.1073/pnas.83.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski T. M., Bullerjahn A. M., Riddle D. L. Lethal and amanitin-resistance mutations in the Caenorhabditis elegans ama-1 and ama-2 genes. Genetics. 1988 Oct;120(2):409–422. doi: 10.1093/genetics/120.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski T. M., Riddle D. L. A Caenorhabditis elegans RNA polymerase II gene, ama-1 IV, and nearby essential genes. Genetics. 1988 Jan;118(1):61–74. doi: 10.1093/genetics/118.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford T., Golomb M., Riddle D. L. RNA polymerase II from wild type and alpha-amanitin-resistant strains of Caenorhabditis elegans. J Biol Chem. 1983 Nov 10;258(21):12804–12809. [PubMed] [Google Scholar]
- Sanford T., Prenger J. P., Golomb M. Purification and immunological analysis of RNA polymerase II from Caenorhabditis elegans. J Biol Chem. 1985 Jul 5;260(13):8064–8069. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles L. L., Jokerst R. S., Bingham P. M., Voelker R. A., Greenleaf A. L. Molecular cloning of sequences from a Drosophila RNA polymerase II locus by P element transposon tagging. Cell. 1982 Dec;31(3 Pt 2):585–592. doi: 10.1016/0092-8674(82)90314-2. [DOI] [PubMed] [Google Scholar]
- Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985;18(1):31–90. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Isolation and characterization of an alpha-amanitin-resistant rat myoblast mutant cell line possessing alpha-amanitin-resistant RNA polymerase II. J Biol Chem. 1975 Jul 10;250(13):4825–4831. [PubMed] [Google Scholar]
- Sulston J. E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974 May;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Lee J. M., Weeks J. R., Jokerst R. S., Greenleaf A. L. The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3698–3702. doi: 10.1073/pnas.85.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mende N., Bird D. M., Albert P. S., Riddle D. L. dpy-13: a nematode collagen gene that affects body shape. Cell. 1988 Nov 18;55(4):567–576. doi: 10.1016/0092-8674(88)90215-2. [DOI] [PubMed] [Google Scholar]