Abstract
Missing in metastasis (MIM also MTSS1) is an intracellular protein that binds to actin and cortactin and has an intrinsic capacity to sense and facilitate the formation of protruded membranous curvatures implicated in cellular polarization, mobilization and endocytosis. The N-terminal 250 amino acids of MIM undergo homo-dimerization and form a structural module with the characteristic of I-BAR domain. To discern the role of the dimeric configuration in the function of MIM, we designed several peptides able to interfere with MIM dimerization in a manner depending upon their lengths. Overexpression of one of the peptides abolished effectively MIM-mediated membrane protrusions and transferrin uptake. However, a peptide with a high potency inhibiting MIM dimerization failed to impact on its binding to actin and cortactin. Thus, our data indicates that the dimeric configuration is essential for MIM-mediated membrane remodeling and serves as a proper target to develop antagonists specifically against an I-BAR domain-containing protein.
Keywords: MIM, I-BAR, dimerization, actin and cortactin
Introduction
Cellular activities, including migration, intracellular trafficking and endocytosis, require extensively dynamic membranous curvatures that are often ultimately manifested as microprotrusions, invaginations, vesicles and tubules. Accumulated evidence has indicated that initiation and maintenance of these curvatures in mammalian cells is accomplished by the reversible assembly of a series of membrane sculpting or deforming proteins. Many of these proteins contain a common structural domain that was initially recognized in Bin, amphiphysin and Rvs proteins and thus have been used to constitute to a superfamily or BAR family [1–3]. On the basis of the intrinsic curvature of BAR domain, members of the BAR family can be further divided into several subfamilies, including classical BAR, N-BAR, BAR-PH, PX-BAR, F-BAR and I-BAR [4]. While the most BAR domains have a positively charged concave surface, an I-BAR domain displays a convex exterior [5, 6]. Hence, a protein containing an I-BAR domain tends to sense the formation of membrane protrusions rather than invaginations, which are often implemented by other BAR subfamilies. Besides MIM, mammalian genome contains additional four I-BAR genes, encoding IRSP53, ABBA, IRTKS and PINKBAR, respectively [7, 8]. Among them MIM has drawn a particular attention because of its putative role in tumor progression and frequent underexpression in a set of metastatic cells [9–11]. MIM displays typical I-BAR properties when analyzed in vitro and in vivo. Overexpression of MIM leads to increase in the formation of filopodia-like microprotrusions of a variety of cells [12, 13]. On the other hand, depletion of MIM genes in animals impairs cell polarization, chemotactic responses to chemokine, endocytosis of ligand-occupied receptors, and cell-cell interactions in certain tissues [14–16]. While the existing data support a critical role of MIM in membrane deformation, the structural basis for the function of MIM remains unclear.
The ability to induce dynamic membrane remodeling by MIM is presumably attributed to the property of its I-BAR domain. Indeed, it has been reported that the MIM I-BAR binds to PI(4,5)P2-enriched membranes [17], interacts with small GTPase Rac [18], and induces membranous tubules in vitro [17]. However, MIM is distinct from IRSP53 and its closely related I-BAR proteins by containing a serine-rich domain (SRD), a proline-rich domain (PRD) and a C-terminal Wiskott-Aldrich syndrome homology 2 (WH2) motif (Figure 1A). Although the biochemical function of these domains remains elusive, they are associated with at least three intracellular proteins: monomeric actin [12], cortactin [13] and Daam1 [19]. Significantly, all these proteins are well characterized components of cortical microfilament [20, 21], a major cytoskeletal force that drives membrane dynamics. Therefore, MIM could also deform membranes through a process coupling with microfilament-associated proteins.
Figure 1. Analysis of MIM dimerization.
(A) Schematic illustration of murine MIM protein. The regions corresponding to peptide MIM-S1 and MIM-S2 were indicated by darker boxes within the I-BAR domain. SRD, serine-rich domain; PRD, proline-rich domain; and WH2, Wiskott Aldrich syndrome homology region 2. (B) 293T cells were transiently co-transfected with MIM-GFP and MIM-Myc plasmids, lysed and subjected to immunoprecipitation by anti-Myc (9E10). The precipitates as well as a portion of total cell lysates (TCL) were analyzed by Western blot using either anti-GFP or anti-Myc. (C) Cells were co-transfected with MIM-Myc and Flag-MIM. Coprecipitation of MIM-Myc and Flag-MIM was analyzed similarly as (B).
To dissect the specific function of the I-BAR of MIM, it would be necessary to modulate the I-BAR without dramatic disturbance of other motifs. Since MIM I-BAR undergoes a homodimerization, which builds up a zeppelin-like module for membrane binding [5, 22], we thought that it may be useful to develop a specific I-BAR antagonist by targeting the dimerization. In this study, we designed several peptides with inhibitory activities for MIM dimerization and provided evidence for the necessity of I-BAR dimerization in MIM-mediated endocytosis and membrane protrusions. However, a peptide with a strong activity antagonizing MIM I-BAR dimerization did not affect the association of MIM with cortactin and actin, demonstrating a distinct role of the I-BAR domain in membrane remodeling.
Experimental
Chemicals and antibodies
All chemicals unless otherwise indicated were purchased from Sigma Aldrich. Protein-A and protein-G agarose were from Santa Cruz. Glutathione Sepharose 4B was from Amersham. SuperFect was from Qiagen, and GeneJammer was from Stratagene. GFP (green fluorescent protein) antibody was raised against recombinant His-GFP protein as described previously [23]. Anti-Myc monoclonal antibody (9E10) was from BD Biosciences. Anti-cortactin monoclonal antibody (4F11) was from Millipore.
Plasmids
MIM-GFP, MIM-I-BAR-GFP, Flag-MIM, GST-MIM, GST-MIM-I-BAR, His-MIM and MIM-I-BAR-Myc were prepared as described previously [24]. Plasmids encoding Myc, GFP and GST tagged MIM-S1 or MIM-S2 peptides were prepared by ligation of PCR-generated DNA fragments into vectors pcDNA3.1A (Invitrogen), pEGFP-N1 (Clontech), and PGEX4T-2 (Amersham), respectively. The primers used in PCR were listed in the table below.
| Plasmids | 5′-primer | 3′-primer |
|---|---|---|
| pMIM-S1-Myc | 5′-CAGTTAGGATCCATGACCATCATCAGCG-3′ | 5′-CAGTTATCTAGAGATGCTCCGGTGCCGC-3′ |
| pMIM-S2-Myc | 5′-CAGTTAGGATCCATGCGCAAGGCACTC-3′ | 5′-CAGTTATCTAGAGGGCAGCTTGTGAGG-3′ |
| pGST-MIM-S1 | 5′-CAGTTAGGATCCACCATCATCAGCGAC-3′ | 5′-CAGTTAGTCGACCTAGATGCTCCGGTG-3′ |
| pGST-MIM-S2 | 5′-CAGTTAGGATCCCGCAAGGCACTCATC-3′ | 5′-CAGTTAGTCGACCTAGGGCAGCTTGTG-3′ |
| pMIM-S1-GFP | 5′-CAGTTACTCGAGATGACCATCATCAGC-3′ | 5′-CAGTTAGGATCCCGGATGCTCCGGTGC-3′ |
| pMIM-S2-GFP | 5′-CAGTTACTCGAGATGCGCAAGGCACTC-3′ | 5′-CAGTTAGGATCCCGGGGCAGCTTGTGAG-3′ |
Recombinant proteins and peptides
His- or GST-tagged proteins, including His-MIM, GST-MIM-S1, -S2, and GST-MIM-I-BAR, were prepared as described previously [13]. The brief procedure to purify GST-tagged proteins was following. DH5α bacterial cells were transformed by plasmids, and the transformed cells were selected based on the resistance to ampicillin. A single colony of the transformed cells was added to 3 ml of Lennox broth (LB) (Invitrogen) containing 30 μg/ml ampicillin and incubated at 37°C for 8–16 h at a shaking speed of 200 rpm. The culture was further inoculated in 100 ml of pre-warmed LB with 50 μg/ml ampicillin for 1–2 h. When the optical density at 600 nm reached to 0.3 or 0.4, the culture was added with 0.5 mM of isopropyl-beta-D-thiogalactopyranoside. After additional 90 min of incubation, cells were harvested in phosphate buffered saline (PBS), sonicated and centrifuged. The supernatants of lysed cells were incubated with glutathione Sepharose 4B for 2 h at 4°C followed by three times of wash with PBS and stored in PBS containing 0.02% NaN3. If bead-free proteins were needed, GST-tagged proteins were eluted with 10 mM reduced glutathione in 50 mM Tris, pH 8.0, and dialyzed against PBS using Centricon YM-30 filter (Millipore). Protein concentrations were determined by comparing to bovine serum albumin (BSA) in either Protein assay (Bio-Rad) or SDS-polyacrylmide gel electrophoresis (PAGE) followed by Coomassie blue (Bio-Rad) staining. Synthetic peptide MIM-S3, -S4, -S5 and -S6 were commercially prepared by GenScript.
Cell culture
293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Lonza) supplemented with 10% bovine calf serum (Hyclone), 100 units/ml penicillin and 100 μg/ml streptomycin (GIBCO). For DNA-mediated transfection, 1.5×106 cells were seeded in a 100 mm dish in growth medium (DMEM plus 10% of bovine calf serum) without antibiotics and incubated at 37°C and 5% CO2. After 12 h, transfection complexes were prepared by mixing 10 μg DNA, 300 μl DMEM and 60 μl SuperFect or GeneJammer, and incubated at room temperature for 5 to 10 min followed by adding into 3 ml regular growth medium. The mixture was then used to grow cells to be transfected at 37°C for 2–3 h followed by transferring into pre-warmed regular growth medium. After 48 h, the transfected cells were harvested for subsequent analysis.
Immunoprecipitation and Western blot
Cell lysates were clarified by centrifugation at 16,000 g for 5 min. The clarified lysates were added with 25 μl of 50% (v/v) slurry of protein A or protein G Sepharose and 5 μg polyclonal anti-GFP antibody. In some experiments, 1 μg anti-Myc mAb or anti-Flag mAb was used in the reactions. The mixtures were then incubated for 2 h at 4°C on a rotation wheel, and briefly spun down. A portion of the supernatants or the pellets after 3 times of wash were fractionated by SDS-PAGE, transferred to a nitrocellulose membrane and subjected to blot with a proper primary antibody (anti-GFP or anti-Myc) and proper horseradish-peroxidase-conjugated secondary antibody in 5% milk. The antibody reactive substances on the membrane were detected by chemiluminescence and digitally visualized by Kodak ImageStation 2000 using Kodak 1D 3.6 software.
Dimerization analysis
293T cells were cotransfected with pMIM-GFP and pFlag-MIM. After 1 to 2 days of transfection, cell lysates were prepared and then subjected to immunoprecipitation using anti-Flag. Unbound MIM-GFP in the supernatants was detected by Western blot with GFP antibody. In some experiments, cells were co-transfected with pMIM-Myc and pFlag-MIM. In these experiments, Myc and Flag antibodies were used in immunoprecipitation and Western blot, respectively.
To analyze recombinant MIM proteins, GST-MIM-I-BAR at different concentrations was incubated with 25 nM His-MIM for 2 h at 37°C and additional 12 hours at 4°C in 500 μl of PBS supplemented with 20μg/ml BSA, 10 mM PMSF and one pill of Roche protease inhibitor/ml. After incubation, the samples were added with 40 μl of 50% (v/v) glutathione-Sepharose and continued incubation for 30 min at 4°C. The complex of dimerized MIM proteins was precipitated, separated by 10% (v/v) SDS-PAGE and detected by Western blot using polyclonal MIM antibody.
Inhibition of MIM dimerization
Cell lysates derived from 293T cells co-transfected with MIM-GFP (or Flag-MIM) and MIM-Myc were incubated with GST-MIM-S1 or synthetic peptides at different concentrations at 4°C for 2 h. Dimerization of MIM-GFP (or Flag-MIM) with MIM-Myc in the reactions was measured as described above. The intensity of acquired digital bands was quantified by ImageJ software and normalized to percentages as compared to that with the lysate prior to immunoprecipitation and that with the lysate in the absence of antagonists. The normalized values were further plotted as a function of MIM antagonists and used to deduce IC50 using Prism 5 software.
Endocytosis assay
Endocytosis was analyzed as described previously [23]. Briefly, cells were seeded in 12-well plates at the density of 5×104 cells per well and cultured overnight in DMEM supplemented with 10% fetal bovine serum. Prior to analysis the cells were starved in DMEM supplemented with 1% BSA and 20 mM HEPEs at 37°C for 30 min, added with biotin-labeled transferrin (Bio-Tfn) at 10 μg/ml and incubated for 40 min on ice. To initiate endocytosis cells were transferred to a 37°C incubator and incubated for 5 min. Endocytosis was terminated by placing the cells back on ice. The treated cells were lysed, and the total cell lysates were then transferred to a 96-well ELISA plates (Thermo) that had been pre-coated with transferrin antibody and incubated for overnight at 4°C. The plate was then washed and treated with streptavidin-horseradish peroxidase followed by BM blue substrate (Roche). Absorption at 450 nm was determined by a microplate reader (Thermomax).
Immunofluorescence
293T cells were co-transfected with pMIM-GFP and pMIM-S1-Myc. After 24 h of transfection, the cells were trypsinized and seeded onto 6-well plates containing a sterilized fibronectin-coated coverslip and cultured in normal growth medium overnight. The cells were fixed by 4% paraformaldehyde for 20 min and then permabelized by 0.5% Triton X-100 in PBS for 5 min at room temperature. After three washes with PBS, cells were incubated in PBS containing 5% BSA for 30 min and treated with primary antibody for 1 h followed by fluorophore-conjugated secondary antibody for additional 1 h in PBS plus 5% BSA. The stained cells were mounted onto slides, sealed with nail polish and viewed under fluorescence microscope (Nikon TE2000-U) using a 60 x oil objective lens with numerical aperture of 1.40. Digital images were captured by Nikon DXM1200 camera.
Results and Discussion
To analyze MIM dimerization, we prepared two constructs in which MIM was tagged by GFP and Myc epitopes at the C-terminus, respectively, and co-transfected them into 293T cells. Immunoprecipitation with Myc antibody followed by Western blot using GFP antibody demonstrated a stable association between MIM-Myc and MIM-GFP (Figure 1B). We also analyzed cells co-expressing MIM-Myc and Flag-MIM, and verified the presence of a dynamic intramolecular interaction of MIM proteins tagged by different epitopes in cells (Figure 1C).
Crystal studies have revealed that the N-terminal 250 amino acids of MIM are organized into three helices (Figure 2A, top panel, each helix is depicted by two tubes). In the twisted helices the interface for dimerization lies within two areas [5]: the most part of the first helix and the C-terminal part of the third helix. We reasoned that a peptide corresponding to each interface area may have an ability to disrupt MIM dimerization, thereby acting as an effective MIM antagonist. Thus, we designed two peptides MIM-S1 and MIM-S2, which correspond to each interface area, respectively (Figure 2A, middle and bottom panels). We then used their recombinant forms, GST-MIM-S1 and GST-MIM-S2, to pull down the lysates of cells expressing MIM-Myc or MIM-I-BAR-Myc (Figure 2B). While GST-MIM-S1 was able to precipitate effectively MIM-Myc and MIM-I-BAR-Myc, GST-MIM-S2 did so in a much poorer manner under the same conditions (Figure 2C). To verify the interaction within cells, we also prepared MIM-S1 and MIM-S2 tagged by GFP and co-transfected them individually with Flag-MIM into 293T cells. As shown in Figure 2D, MIM-S1-GFP, but not MIM-S2-GFP, co-precipitated readily with Flag-MIM. The poorer ability of MIM-S2 to interact with MIM could be due to a smaller interface area it corresponds to and its less stability as evidenced by significant degradation expressed in either bacterial (Figure 2B) or 293T cells (Figure 2D, data not shown). Since we could not consistently obtain high expression levels of intact MIM-S2, we used MIM-S1 and its derivatives for the rest of the study.
Figure 2. Design of MIM dimerization antagonists.
(A) The structures and sequences of MIM-S1 and MIM-S2. The top panel, a single MIM-I-BAR subunit is shown in a tube format based on Lee’s study [5]. The residues corresponding to MIM-S1 and MIM-S2 are represented as stick balls in deep and light gray colors, respectively. The middle panel, two MIM-I-BAR dimers are depicted in a space-fill format. The areasin the lightest color represent the residues for MIM-S1 and MIM-S2, respectively. The lower panels: the amino acid sequences for MIM-S1 and MIM-S2 are presented. (B) Recombinant GST, GST-MIM-S1 and GST-MIM-S2 proteins were analyzed by SDS-PAGE followed by Coomassie blue staining. Note, GST-MIM-S2 displayed a significant degradation. (C) Lysates derived from 293T cells expressing either MIM-Myc (top panel) or MIM-I-BAR-Myc (lower panel) were subject to pull-down with GST, GST-MIM-S1 and GST-MIM-S2, respectively. The presence of MIM-Myc or MIM-I-BAR-Myc in the precipitates were detected by Western blot using anti-Myc. (D) Lysates of cells co-transfected with different combinations of Flag-MIM, MIM-S1-GFP and MIM-S2-GFP were immunoprecipitated by Flag antibody, and the immunopellets were analyzed by Western blot using GFP antibody. Expression of MIM-S1-GFP and MIM-S2-GFP in total lysates was presented in the lower panel.
To quantify the interaction between MIM-S1 and MIM, GST-MIM-S1 at different concentrations was used to precipitate the lysates of cells expressing either MIM-Myc or MIM-I-BAR-Myc. The MIM proteins remaining in the supernatants after precipitations were measured by Western blot and used as the index for unbound MIM proteins. This analysis estimated that MIM-S1 has a dissociation constant (Kd) of 110 nM for MIM-Myc and 65 nM for MIM-I-BAR-Myc (Figure 3A and B). The apparently higher affinity for MIM-I-BAR-Myc could be due to other domains in full-length MIM (Figure 1A), which may restrict sterically the access to the MIM antagonist. Stable and direct association of MIM-S1 with MIM was further confirmed using purified GST-MIM-S1 and His-MIM in which MIM was tagged by a 6xHis epitope (S-Figure 1).
Figure 3. Quantification of the affinity of GST-MIM-S1 for MIM.
The lysate of cells expressing MIM-Myc (A) or MIM-I-BAR-Myc (B) were incubated with GST-MIM-S1 at the concentrations as indicated. The GST-MIM-S1 binding proteins were isolated by pull-down. The amounts of unbound MIM-Myc or MIM-I-BAR-Myc proteins in the supernatants after precipitation were measured by Western blot using anti-Myc.As the loading control, β-actin in the supernatants was also measured. The data was analyzed by Prism software, which predicted Kd values of 110 nM and 65 nM for MIM-Myc and MIM-I-BAR-Myc, respectively.
We next analyzed whether MIM-S1 peptide would be able to disrupt MIM dimerization. Thus, the lysate of cells co-expressing Flag-MIM and MIM-GFP was incubated with GST-MIM-S1 at different concentrations followed by immunoprecipitation of MIM-GFP. Unpaired Flag-MIM remaining in the supernatant was estimated byWestern blot. As shown in Figure 4A, the presence of GST-MIM-S1 increased markedly the amount of unpaired Flag-MIM, indicative of disruption in MIM dimerization. Quantification estimated a half maximal inhibitory concentration (IC50) of 213 nM for GST-MIM-S1 (Figure 4A). To rule out the possibility that GST in GST-MIM-S1 might have contributed to the inhibition, we prepared a series of synthetic peptides corresponding to the C-terminal part of MIM-S1 (S-Table 1) and analyzed their effect on MIM dimerization. One peptide MIM-S3, which has 33 amino acids, was able to inhibit effectively MIM dimerization with an IC50 of 140 nM (Figure 4B). The peptides ranging from 6 to 20 amino acids within the same region showed a diminished inhibitory activity as their length was reduced (S-Figure 2), suggesting a minimal number of residues necessary for effective competition with MIM dimerization. To verify that the inhibition was not due to unknown cellular factors that might have been co-purified during immunoprecipitation, we also analyzed MIM dimerization using purified recombinant GST-MIM-I-BAR and His-MIM proteins. Under this condition, MIM-S3 peptide displayed a strong inhibitory activity with an IC50 value of approximately 41 nM (S-Figure 3).
Figure 4. Analysis of the activity of MIM-S1 and MIM-S3 to antagonize MIM dimerization.
(A) Lysates of cells expressing Flag-MIM and MIM-GFP were incubated with GST-MIM-S1 for 2 h and subjected to immunoprecipitation with GFP antibody. Unbound Flag-MIM in the supernatants were determined by Western blot using Flag antibody, normalized and plotted as a function of GST-MIM-S1. IC50 was calculated using Prism software. As a control, aliquots of cell lysates were also subjected to Western blot using β-actin antibody. (B) The lysates of cells expressing MIM-Myc and MIM-GFP were incubated with MIM-S3 peptide at varying concentrations as indicated and then subjected to immunoprecipitation using Myc antibody. Unbound MIM-GFP was detected by Western blot using GFP antibody and used to estimate IC50.
We have recently reported that overexpression of MIM-GFP increased the endocytosis of transferrin [23] and were interested in whether MIM-S1 has any influence on MIM-mediated transferrin uptake. Consistent with our previous finding with NIH3T3 cells [23], 293T cells expressing MIM-GFP alone displayed a marked increase in the uptake of transferrin by 30% (Figure 5A). However, the increase was diminished in cells co-expressing MIM-S1-Myc and MIM-GFP. The possible toxicity of MIM-S1-Myc to endocytosis was unlikely because overexpression of MIM-S1-Myc alone in 293T cells, where little endogenous MIM was expressed (data not shown), did not affect the transferrin internalization. Thus, the inhibition manifested by MIM-S1-Myc was specific for the MIM-mediated endocytosis. We also analyzed the effect on membrane deformations mediated by overexpressing MIM-GFP, which provoked the formation of MIM characteristic filopodia-like protrusions (Figure 5B, arrowhead). However, co-expressing MIM-GFP and MIM-S1-Myc reduced significantly the number of those protrusions (Figure 5B, * ) (p<0.0001).
Figure 5. MIM-S1 inhibits MIM-mediated endocytosis and membrane protrusions.
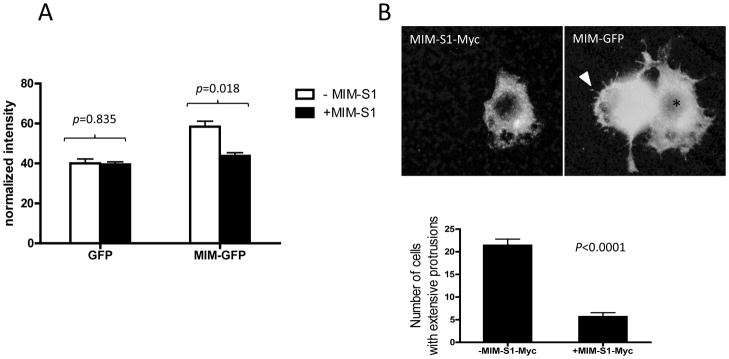
(A) Cells co-expressing MIM-S1-Myc and MIM-GFP or cells expressing GFP only were treated with Bio-Tfn for 5 min. Uptake of Bio-Tfn was analyzed as described in Experimental. While Tfn uptake was markedly increased in cells expressing MIM-GFP, no significant increase was observed in cells expressing both MIM-GFP and MIM-S1-Myc. (B) 293T cells were transiently transfected with MIM-GFP and MIM-S1-Myc, fixed and stained with anti-Myc antibodies. The stained cells were inspected by immunofluorescent microscopy. Original magnification, 600×. A cell showing expression of MIM-GFP only exhibited many filopodia-like long membrane protrusions as indicated by an arrow head. A cell, as indicated by *, expressing both MIM-GFP and MIM-S1-Myc failed to display these MIM characteristic protrusions. Quantification (lower panel) showed that cells co-expressing MIM-GFP and MIM-S1-Myc had about 70% less MIM-mediated membrane protrusions than those expressing MIM-GFP alone.
The critical role of dimerization in the function of other BAR-containing proteins has been also previously described. It has been recently reported that a mutation-mediated disruption of dimerization of endophilin, an N-BAR protein, impairs endocytosis and membrane shaping [25]. The report is essentially consistent with our data presented herein that dimerization is essential for I-BAR mediated membrane deformation. However, dimerization of endophilin also shows a regulatory activity to its SH3 domain, which locates at the C-terminal proximity to its BAR domain because mutations disrupting dimerization impaired the binding of its partners [25]. While MIM does not have an SH3 domain, it contains a PRD and a WH2 domain, which bind to cortactin and monomeric actin, respectively. To analyze whether MIM dimerization has any effect on cortactin and actin binding, we analyzed the association of MIM-Myc with cortactin and actin in the presence of MIM-S3 peptide, which has the most antagonistic activity among all the peptides we have tested. While Myc antibody was able to detect the complex of MIM-Myc with cortactin and/or actin in the lysates of cells expressing MIM-Myc (S-Figure 4), adding MIM-S3 peptide at 500 nM did not apparently interfere with the formation of the complex with either cortactin or actin. Thus, MIM dimerization is dispensable for binding to cortactin and actin.
The inability of MIM-S3 to interfere with cortactin and actin binding is probably due to the fact that PRD and WH2 motifs locate remotely to the I-BAR domain by approximately 360 and 480 amino acids, respectively (Figure 1A). In contrast, the SH3 domain of endophilin is only 41 amino acid apart from its BAR domain, suggesting that dimerization may only influence on the partner that binds to the vicinity of the BAR domain. In this regard, it is worthy to note that there is an SRD motif in juxtaposition to the C-terminus of MIM’s I-BAR (Figure 1A), and that a peptide corresponding to the SRD is reportedly able to bind to Daam1, a formin-like protein that regulates the actin polymerization [19]. Also, IRSP53 and its related proteins PINKBAR and IRTKS contain an SH3 domain adjacent to the C-terminal part of their I-BARs. While it would be interesting to determine whether dimerization could regulate Daam1 or any SH3 binding proteins in other I-BAR proteins, it has been well described that overexpression of an I-BAR itself is potent to induce membrane deformation [12, 17, 26]. Thus, our finding supports the notion that the dimeric MIM-I-BAR is a functional rather than a regulatory motif, which may act in concert with other domains in recruiting membranous curvatures to the locations where actin filaments are actively assembled upon different signaling circuitries. The peptides against MIM dimerization described here would serve as useful tools to distinguish the membrane remodeling function of MIM from those linking to the actin dynamics, which may ultimately provide insight into the mechanics underlying membrane related activities such as intracellular trafficking and cell migration.
Supplementary Material
S-Figure 1. GST-MIM-S1 interacts with recombinant MIM protein.(A) Coomassie blue staining of purified GST-MIM-S1 and His-MIM. Both forms of MIM proteins, in particular His-MIM, display certain degrees of degradation. (B) GST-MIM-S1 at concentrations as indicated was incubated with 90 nM His-MIM at 4°C for 2 h. After the interaction, the samples were subjected to pull down using Ni-IDA beads. The precipitates were separated by SDS-PAGE, and co-precipitated GST-MIM-S1 was detected by Western blot using anti-GST.
S-Figure 2. Short synthetic peptides failed to inhibit MIM dimerization. MIM-S4, MIM-S5 and MIM-6 peptides were analyzed for the ability to inhibit dimerization of MIM-GFP and MIM-Myc as described in the Experimental.
S-Figure 3. MIM-S3 inhibits dimerization of recombinant MIM proteins.(A) Coomassie blue staining of purified His-MIM and GST-MIM-I-BAR proteins. (B) His-MIM (25 nM) was used to dimerize GST-MIM-I-BAR at varying concentrations as indicated. The reactions were precipitated with glutathione beads, and the presence of His-MIM in the pellets was detected by Western blot using MIM antibody. (C) Dimerization of His-MIM (25 nM) and GST-MIM-I-BAR (20 nM) was analyzed in the presence of MIM-S3 at different concentrations. The kd and IC50 values were calculated by Prism software.
S-Figure 4. MIM-S3 does not affect the association of MIM with cortactin and actin. 293T cells were transiently transfected with and without MIM-Myc. The lysates of the transfected cells were subjected to immunoprecipitation with anti-Myc in the presence or absence of MIM-S3 (500 nM). The presence of cortactin and actin in the immunopellets were detected by Western blot using anti-cortactin and anti-β-actin, respectively.
Acknowledgments
We thank Dr. Dan Yu for assisting in endocytosis analysis, immunoprecipitation and Western blot.
Funding
This work was supported by the State Scholarship Fund of the China Scholarship Council (2010609098 to M.C., 20115037 to T.Z.), National Basic Research Program of China (2011CB933503 to M.J.), National Cancer Institute (R01 CA113809 to X. Z.), and Maryland Stem Cell Research Fund (2008-0082 and 2012-0081 to X.Z.)
Abbreviations
- BAR
Bin/amphiphysin/Rvs
- DMEM
Dulbecco’s Modified Eagle Medium
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein (GFP)
- GST
glutathione s-transferase
- I-BAR
inverse BAR
- LB
Lennox broth
- MIM
missing in metastasis
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate buffered saline
- PRD
proline rich domain
- SH3
src homology 3
- SRD
serine rich domain
- Tfn
transferrin
- and WH2
Wiskott-Aldrich syndrome homology 2
Footnotes
Author Contribution
Meng Cao, Tailan Zhan and Xi Zhan conceived the study, designed the experiments and wrote the manuscript. Ming Ji and Xi Zhan are the PIs of the project.
References
- 1.Zimmerberg J, McLaughlin S. Membrane curvature: how BAR domains bend bilayers. Curr Biol. 2004;14:R250–252. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 2.Gallop JL, McMahon HT. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem Soc Symp. 2005:223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 3.Rao Y, Haucke V. Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SH, Kerff F, Chereau D, Ferron F, Klug A, Dominguez R. Structural basis for the actin-binding function of missing-in-metastasis. Structure. 2007;15:145–155. doi: 10.1016/j.str.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PK, Butcher SJ, Lappalainen P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19:95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Pykalainen A, Lappalainen P. I-BAR domain proteins: linking actin and plasma membrane dynamics. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Pykalainen A, Boczkowska M, Zhao H, Saarikangas J, Rebowski G, Jansen M, Hakanen J, Koskela EV, Peranen J, Vihinen H, Jokitalo E, Salminen M, Ikonen E, Dominguez R, Lappalainen P. Pinkbar is an epithelial-specific BAR domain protein that generates planar membrane structures. Nat Struct Mol Biol. 2011;18:902–907. doi: 10.1038/nsmb.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YG, Macoska JA, Korenchuk S, Pienta KJ. MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia. 2002;4:291–294. doi: 10.1038/sj.neo.7900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Liu J, Smith E, Zhou K, Liao J, Yang GY, Tan M, Zhan X. Downregulation of missing in metastasis gene (MIM) is associated with the progression of bladder transitional carcinomas. Cancer Invest. 2007;25:79–86. doi: 10.1080/07357900701205457. [DOI] [PubMed] [Google Scholar]
- 11.Parr C, Jiang WG. Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. Eur J Cancer. 2009;45:1673–1683. doi: 10.1016/j.ejca.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Mattila PK, Salminen M, Yamashiro T, Lappalainen P. Mouse MIM, a tissue-specific regulator of cytoskeletal dynamics, interacts with ATP-actin monomers throughits C-terminal WH2 domain. J Biol Chem. 2003;278:8452–8459. doi: 10.1074/jbc.M212113200. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Liu J, Wang Y, Zhu J, Zhou K, Smith N, Zhan X. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005;24:2059–2066. doi: 10.1038/sj.onc.1208412. [DOI] [PubMed] [Google Scholar]
- 14.Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development. 2010;137:1075–1084. doi: 10.1242/dev.049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saarikangas J, Mattila PK, Varjosalo M, Bovellan M, Hakanen J, Calzada-Wack J, Tost M, Jennen L, Rathkolb B, Hans W, Horsch M, Hyvonen ME, Perala N, Fuchs H, Gailus-Durner V, Esposito I, Wolf E, de Angelis MH, Frilander MJ, Savilahti H, Sariola H, Sainio K, Lehtonen S, Taipale J, Salminen M, Lappalainen P. Missing-in-metastasis MIM/MTSS1 promotes actin assembly at intercellular junctions and is required for integrity of kidney epithelia. J Cell Sci. 2011;124:1245–1255. doi: 10.1242/jcs.082610. [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Zhan XH, Zhao XF, Williams SM, Carey GB, Smith E, Scott D, Zhu J, Guo Y, Cherukuril S, Civin C, Zhan Xi. Mice deficient in MIM expression are predisposed to lymphomagenesis. Oncogene. 2011;14 doi: 10.1038/onc.2011.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bompard G, Sharp SJ, Freiss G, Machesky LM. Involvement of Rac in actin cytoskeleton rearrangements induced by MIM-B. J Cell Sci. 2005;118:5393–5403. doi: 10.1242/jcs.02640. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Komiya Y, Mezzacappa C, Khadka DK, Runnels L, Habas R. MIM regulates vertebrate neural tube closure. Development. 2011;138:2035–2047. doi: 10.1242/dev.058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci USA. 2008;105:210–215. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 2005;24:240–250. doi: 10.1038/sj.emboj.7600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu D, Zhan XH, Niu S, Mikhailenko I, Strickland DK, Zhu J, Cao M, Zhan X. Murine missing in metastasis (MIM) mediates cell polarity and regulates the motility response to growth factors. PLoS One. 2011;6:e20845. doi: 10.1371/journal.pone.0020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhou K, Zeng X, Lin J, Zhan X. Tyrosine phosphorylation of missing in metastasis protein is implicated in platelet-derived growth factor-mediated cell shape changes. J Biol Chem. 2007;282:7624–7631. doi: 10.1074/jbc.M608448200. [DOI] [PubMed] [Google Scholar]
- 25.Gortat A, San-Roman MJ, Vannier C, Schmidt AA. Single Point Mutation in Bin/Amphiphysin/Rvs (BAR) Sequence of Endophilin Impairs Dimerization, Membrane Shaping, and Src Homology 3 Domain-mediated Partnership. J Biol Chem. 2012;287:4232–4247. doi: 10.1074/jbc.M111.325837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodings JA, Sharp SJ, Machesky LM. MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta. Biochem J. 2003;371:463–471. doi: 10.1042/BJ20021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S-Figure 1. GST-MIM-S1 interacts with recombinant MIM protein.(A) Coomassie blue staining of purified GST-MIM-S1 and His-MIM. Both forms of MIM proteins, in particular His-MIM, display certain degrees of degradation. (B) GST-MIM-S1 at concentrations as indicated was incubated with 90 nM His-MIM at 4°C for 2 h. After the interaction, the samples were subjected to pull down using Ni-IDA beads. The precipitates were separated by SDS-PAGE, and co-precipitated GST-MIM-S1 was detected by Western blot using anti-GST.
S-Figure 2. Short synthetic peptides failed to inhibit MIM dimerization. MIM-S4, MIM-S5 and MIM-6 peptides were analyzed for the ability to inhibit dimerization of MIM-GFP and MIM-Myc as described in the Experimental.
S-Figure 3. MIM-S3 inhibits dimerization of recombinant MIM proteins.(A) Coomassie blue staining of purified His-MIM and GST-MIM-I-BAR proteins. (B) His-MIM (25 nM) was used to dimerize GST-MIM-I-BAR at varying concentrations as indicated. The reactions were precipitated with glutathione beads, and the presence of His-MIM in the pellets was detected by Western blot using MIM antibody. (C) Dimerization of His-MIM (25 nM) and GST-MIM-I-BAR (20 nM) was analyzed in the presence of MIM-S3 at different concentrations. The kd and IC50 values were calculated by Prism software.
S-Figure 4. MIM-S3 does not affect the association of MIM with cortactin and actin. 293T cells were transiently transfected with and without MIM-Myc. The lysates of the transfected cells were subjected to immunoprecipitation with anti-Myc in the presence or absence of MIM-S3 (500 nM). The presence of cortactin and actin in the immunopellets were detected by Western blot using anti-cortactin and anti-β-actin, respectively.






