Abstract
Porcine ear necrosis was investigated in 23 conveniently chosen farms, consisting of 14 case farms and 9 control farms. Biopsies of lesions and oral swabs from pigs on 11 case farms were examined by histology and bacterial culture. All farms were visited for observations and a survey on management, housing, and the presence of other clinical signs or behavioral vices. Histological examination revealed that the lesions began on the surface and progressed to deeper layers, and that vascular damage did not appear to be the initiating cause. Spirochetes were only rarely observed in histological examination and were not cultured from biopsies and oral swabs. Staphylococcus aureus and Staphylococcus hyicus were cultured from 91% and 66% of samples, respectively. Ear biting and a humid environment were associated with ear necrosis. On some farms large numbers of pigs were affected and lesions were sometimes extensive. The condition appears to be an infectious disease beginning on the surface of the skin; contributing environmental and management factors are likely.
Résumé
Enquête sur la nécrose des oreilles chez les porcs. La nécrose des oreilles porcines a fait l’objet d’une enquête dans un échantillonnage de convenance de 23 fermes, composé de 14 fermes de cas et de 9 fermes témoins. Les biopsies de lésions et des prélèvements oraux faits chez des porcs dans 11 fermes de cas ont été examinés par histologie et culture bactérienne. Toutes les fermes ont été visitées pour recueillir des observations et effectuer un examen de la gestion, du logement et de la présence d’autres signes cliniques ou vices de comportement. L’examen histologique a révélé que les lésions commençaient sur la surface et progressaient vers des couches plus profondes et que les dommages vasculaires ne semblaient pas être la cause initiale. Des spirochètes ont été rarement observés à l’examen histologique et n’ont pas été cultivés dans les biopsies et les prélèvements oraux. Staphylococcus aureus et Staphylococcus hyicus ont été cultivés dans 91 % et 66 % des échantillons, respectivement. Les morsures d’oreilles et un environnement humide étaient associés à la nécrose des oreilles. Dans certaines fermes, un grand nombre de porcs étaient affectés et les lésions étaient parfois importantes. L’affection semble être une maladie infectieuse qui commence à la surface de la peau; des facteurs contributifs liés à l’environnement et à la gestion sont probables.
(Traduit par Isabelle Vallières)
Introduction
Ear necrosis is a common problem on Canadian pig farms and several etiologies have been proposed but not confirmed. The clinical manifestation of ear necrosis has been described as the appearance of open wounds, crust, and bleeding on 1 or both ears (1). Little is understood regarding the risk factors that influence severity and prevalence of ear necrosis. In general, ear necrosis is considered to have little effect on pig performance, at least when the lesions are only mild to moderate (2). However, the unsightly visual appearance could interfere with the sale of affected pigs, and raise welfare concerns.
The etiology of ear necrosis is unknown. Staphylococcus hyicus and Staphylococcus aureus have been suggested as potential causative agents because of the similarity of histopathological lesions and bacterial cultural results between ear necrosis and exudative epidermitis (3–5). The pathogenesis of staphylococcal skin disease involves bacterial production of exfoliative toxins (ETs) which act as “molecular scissors” to cleave the epidermis of mammalian skin (6). For a long time it was thought that S. aureus ETs did not affect the epidermis of pigs (7); however, recent findings have demonstrated the targeting of desmoglein1 in pig skin by S. aureus ETB and ETD (6). Spirochetes have also been considered as potential causative agents of ear necrosis (8) and in 1 recent study spirochetes (genus Treponema) were cultured and identified from ear necrosis lesions (9).
Clinical lesions of ear necrosis need to be differentiated from secondary skin lesions developing occasionally at ear tips as a result of vasculitis associated with systemic diseases such as salmonellosis and classical swine fever (10). An argument has been made that ear necrosis is the result of ischemic injury due to thrombosis of the small dermal capillaries in the tips of the ears as a result of immune-complex deposition, as occurs in porcine circovirus associated disease (PCVAD) (11), or due to mycotoxin-associated vasculopathy (10).
Ear necrosis appears to be influenced by environmental and management factors. Purchased feed, fully slatted flooring without straw, poor air quality and high humidity, and a high stocking density of newly weaned pigs have been found to increase the risk of ear necrosis (12). Dry feed, early weaning, poor housing conditions, fighting, feed and dirt on the tips of ears, and concurrent mange have also been identified as possible risk factors (4,13).
The objectives of this study were to: describe clinical and histopathological lesions of ear necrosis; determine the presence of potential pathogens, particularly Staphylococcus species and spirochetes; and determine herd-level risk factors of ear necrosis.
Materials and methods
A total of 23 conveniently selected farms were used in the study (14 case farms and 9 control farms). To be included as a control farm, the farm owner or manager had to report that the farm had no history of clinical signs of ear necrosis, and the absence of ear necrosis had to be confirmed by the researchers through an on-farm visit. All farms were visited between May 2010 and April 2011, and the observations were conducted by the same individual on all farms. The research was approved by the animal care committee of the University of Guelph.
Investigation of potential causative organisms
Sampling for bacterial culture
Material for diagnostic purposes was collected from 11 of the 14 case farms. On each farm, 9 pigs were selected for testing by choosing 3 pigs from early, mid, and late stages of clinical disease. Early-disease stage groups consisted of pigs with small black scabs on their ears and were from pigs in the youngest affected age group. Mid-disease stage groups were slightly older (1 or 2 wk) and generally exhibited lesions that had progressed to open wounds, thick scabs, and some loss of ear tissue. Late-disease stage groups were the oldest pigs, which generally showed lesions that had resolved but bleeding wounds and thick scabs were still common. Three wedge-shaped ear tissue biopsies and an oral swab were taken from each pig. One biopsy sample was placed in a sterile plastic tube for culture of S. hyicus and S. aureus. Another biopsy sample was placed in a tube with fastidious anaerobe broth (FAB), (LAB 71, Lab M, Heywood, Lancashire, UK) for culture of spirochetes. A third biopsy sample was placed in a 100 mL jar containing 50 mL of 10% formalin solution for histologic examination. For the histology samples, an effort was made to capture the margin of normal and lesional skin. A cotton swab was used to sample the mouth (especially around the gums) of the same pig and was placed in a tube with FAB for culture of spirochetes. All samples were transported in an insulated container and submitted on the day of collection by taking them directly to the Animal Health Laboratory (AHL), University of Guelph, Guelph, Ontario. Bacterial culture was performed and bacteria were identified as S. hyicus and S. aureus by standard laboratory techniques including colony morphology, hemolysis, Gram-stain, catalase reaction, and coagulase reaction. Antimicrobial susceptibility testing with penicillin G, ampicillin, ceftiofur, spectinomycin, sulphonamide, tetracycline, tiamulin, and trimethoprim/sulfamethoxazole was determined by the disk diffusion method (Kirby-Bauer Procedure) defined by the Clinical and Laboratory Standards Institute (14). For the purpose of analyzing the data, isolates classed as intermediate susceptibility were included with the bacteria identified as resistant.
Culture of spirochetes was performed following the method described by Pringle et al (9). Ear tissues and oral samples in FAB were incubated for 24 to 48 h at 37°C in an anaerobic chamber. One drop of the broth culture was added to a 0.22 μm membrane filter placed on fastidious anaerobe agar with 10% horse blood. Following incubation of the agar plate at 37°C for 2 to 3 days in an anaerobic chamber, the bacterial growth under the filter was examined by phase contrast microscopy.
Histopathological examination
Following 24 h fixation in 10% neutral buffered formalin, skin biopsies were processed for histologic examination. Where sufficiently large, biopsies were bisected prior to processing, and all biopsies were processed in total. Serial tissue sections (4 μm) from each biopsy were stained with hematoxylin and eosin (H&E) and Warthin-Starry silver (WS) and were examined using light microscopy by a single pathologist (JD).
Immunohistochemistry (IHC) for Treponema spp. was performed on tissue sections from those cases in which spirochetes were identified by WS stain.
Investigation of risk factors at farm level
All 23 farms were visited and a questionnaire was administered to the producers during a face-to-face interview. Information from the questionnaire included: farm demographics, weaning age, pig flow, cleaning procedures, vaccination and medication regimens, feeding information, and source of pigs. In addition, observation notes for each disease-stage group were completed by the researchers after collecting samples during the visit to the farm. Information collected through on-farm observation included: the prevalence of ear necrosis for each disease stage, a description of the lesions, the presence of clinical signs other than ear necrosis and the presence or absence of behavioral vices. A description of the facilities, noting the stocking density, type of flooring, feeder and waterer type, and room temperature was recorded. A subjective assessment of humidity and air quality in the pen was recorded. On control farms, groups of pigs that were comparable to the age of early, mid, and late disease stages for case farms were chosen for observation.
Data management and statistical analysis
Information from survey questionnaires and observation notes for each stage were entered separately into Epi-data Software (EpiData Entry version 3; The EpiData Association, Odense M, Denmark) and imported to Stata software (Stata Intercooled, version 10; Stata Corporation, College Station, Texas, USA) for further processing and analysis. Initially, data were checked for accuracy, consistency, and missing values.
The average prevalence of ear necrosis in each stage group was estimated as the number of pigs affected with ear necrosis divided by total number of pigs in the pen.
Unconditional association between ear necrosis status of farms and putative risk factors, obtained from the survey and observation notes were evaluated using a chi-squared test or Mann-Whitney U-test, as appropriate (P < 0.05). Variables in the survey were measured at the farm level and the variables in the observation notes were measured for each of the 3 stage groups at farm-level. These were then offered as separate variables in a different stage group modeling. Initially, a total of 16 variables was obtained from the survey and each of 21 variables obtained in early, mid, and late stage groups from the observation notes were examined. Correlation analysis was performed using the Spearman’s rank correlation statistic to identify collinearity among variables. Independent variables associated with the dependent variable at a 20% significance level (P < 0.2) in unconditional screening were considered primary predictors of interest and were included in the multivariable main effects model. Then the presence or absence of lesions of ear necrosis on the farm as the dependent variable was regressed on these primary predictors using multivariable logistic regression. Manual model building, which combined forward selection, was employed in building a multivariable model. Model fitting was evaluated by the Pearson Goodness of fit test.
Results
Investigation of potential causative organisms
Staphylococcal culture
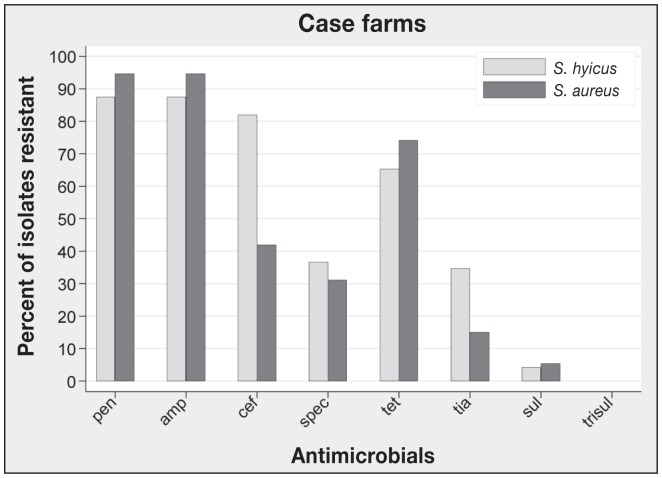
A total of 96 pigs were selected from 11 case farms (mean: 8.7 pigs/farm). The recovery rate of S. hyicus from ear tissue biopsies was 66% and the recovery rate of S. aureus from ear tissue biopsies was 91%. There was no significant difference in the recovery rates (Wald X2P = 0.36). Both S. aureus and S. hyicus were recovered from 58.3% of pigs, whereas S. aureus was recovered alone from 32.3% of the pigs and S. hyicus was recovered alone from 7.3% of the pigs. At the farm level, the recovery rate was 90.9% (10/11) and 100% (11/11) for S. hyicus and S. aureus, respectively, based on at least 1 positive isolate from a farm. The recovery rates of S. hyicus were 76.9%, 66.7%, 66.7%, in early, mid, and late stages, respectively. The recovery rates of S. aureus were 82%, 93.3%, and 90%, in early, mid, and late stages, respectively. Antimicrobial resistance profiles of S. hyicus and S. aureus isolates are presented in Figure 1. The most common resistance pattern of S. hyicus isolates was penicillin G-ampicillin-ceftiofur (27.8%), and the most common resistance pattern of S. aureus isolates was penicillin G-ampicillin-ceftiofur-tetracycline (20.4%).
Figure 1.
Antimicrobial resistance profiles of S. hyicus and S. aureus from clinical cases of ear necrosis.
pen — penicillin G, amp — ampicillin, cef — ceftiofur, spec — spectinomycin, tet — tetracycline, tia — tiamulin, sul — sulphonamide, trisul — trimethoprim-sulfamethoxazole. Number of S. hyicus isolates is 63; number of S. aureus isolates is 87.
Spirochetes
Attempts to culture spirochetes from ear biopsies and oral swabs were unsuccessful. In WS-stained ear biopsies, spirochetes were identified in 5 pigs (5.2%), including 4 pigs (11.1%, 4/36) from the early stage group and 1 pig (3.7%, 1/27) from the mid-stage group. Spirochetes in 4 of 5 WS-positive biopsies were confirmed as Treponema spp. by IHC. Spirochetes were clustered within crusts or pustules, or were located at margins of ulcerated foci in the epidermis. In the spirochete-positive group, 3 pigs were positive for S. hyicus, S. aureus, and spirochetes (3.1%, 3/96); 1 pig was positive for S. aureus and spirochetes (1%, 1/96); and 1 pig was positive only for spirochetes (1%, 1/96). Farm prevalence of spirochetes was 36.4% (4/11).
Histological examination
Biopsies from the majority of affected pigs demonstrated crusting erosive to ulcerative pinnal dermatitis with variably severe neutrophilic dermatitis and large numbers of bacterial cocci in crusts. Lesions in affected pigs consistently involved epidermis, and were typically more severe in epidermis than in underlying dermis. Superficial dermal inflammation was dominated by neutrophils, with fewer lymphocytes and histocytes. In a few cases, vasculitis or overt thrombosis was evident (6/96) and vascular lesions were associated with foci of epidermal ulceration and dermal necrosis/inflammation. Dermal granulation tissue and fibrosis, indicative of lesion chronicity, were present in 25/96 (26%) cases.
Risk factors at the farm level
The average prevalence rates of ear necrosis on case farms were: 31.6% [standard deviation (SD): 32.8], 44.2% (SD: 36.1), and 54.8% (SD: 38.1)] for early, mid, and late stages, respectively. The average ages of each disease stage group on case and control farms are presented in Table 1. The degree of severity varied between farm and within farm. The most severe lesions resulted in the loss of as much as one-third of the ear.
Table 1.
The average ages of pigs showing clinical signs of early, mid, and late stage ear necrosis on case farms (n = 14) and the ages of pigs on control farms (n = 9) that were used in the study
| Early | Mid | Late | Early-Lateb | |
|---|---|---|---|---|
| Case (wk) | 6.6 (3–12)a | 7.7 (5–13) | 10 (5–16) | 3.7 |
| Control (wk) | 5.7 (3–12) | 8.4 (6–12) | 14 (8–20) | 8.6 |
Range.
The mean of age-difference from early to late stage.
The variables in survey questionnaires, which were found to be unconditionally associated with ear necrosis status of the farm under P < 0.2, are presented in Table 2. Based on observation notes, the variables in early, mid, and late stage groups, which were unconditionally associated with ear necrosis status of the farm under P < 0.2, are presented in Table 3. In the multivariable model of observation notes, the variables of perception of high humidity and the presence of ear biting were significantly associated with the presence of ear necrosis (Table 4). No evidence of a confounding effect was found within the variables in the final model. Similarly, no statistically significant interaction effects were identified (P > 0.05). Visual assessment of residuals was performed and this identified an outlier, but no coefficient changes were shown without the outlier.
Table 2.
Descriptive statistics of variables in survey questionnaires found to be unconditionally associated with ear necrosis status of the farm (P < 0.2)
| Variables | Case mean (SD) | Control mean (SD) | P-value |
|---|---|---|---|
| Minimum weaning age (days) | 16.9 (1.15) | 27 (1.72) | 0.05 |
| Average weaning age (days) | 20.7 (0.99) | 28.6 (3.31) | 0.06 |
| Number of sows on farm | 871.1 (287.07) | 357.4 (121.15) | 0.2 |
| Zinc level in feed (ppm) | 191.3 (41.7) | 1009.8 (465.7) | 0.2 |
| Downtime after washing (days) | 1.9 (0.32) | 1.1 (0.4) | 0.2 |
SD — standard deviation.
Table 3.
Descriptive statistics of variables unconditionally associated (P < 0.2) with pigs showing clinical signs of early, mid, and late stage ear necrosis based on observations
| Variables | Compared with | P-value |
|---|---|---|
| High humidity in the pen | Moderate and low humidity in the pen | 0.01 |
| Presence of ear biting | 0.01 | |
| Presence of tail biting | 0.01 | |
| Low availability of drinkers per pig | 0.01 | |
| Presence of scratches | 0.06 | |
| Low availability of feeders per pig | 0.06 | |
| Concrete solid floor | Half slatted and wholly slatted | 0.07 |
| Low availability of space per pig (ft2) | 0.09 | |
| Bowl drinker | Bowl + nipple and nipple drinker | 0.12 |
| High temperature (°C) | 0.15 |
Table 4.
Herd level factors associated with ear necrosis in the final multivariable logistic regression model based on observational data (P < 0.05)
| Variable | OR | OR (95% CI) | P-valuea |
|---|---|---|---|
| Presence of ear biting | 22.34 | 1.09–459.49 | 0.04 |
| High humidity level (versus low) | 52.63 | 2.81–1000 | 0.01 |
| Number of observations: 24 | |||
| AUCb: 0.90 | |||
| AICc: 22.61 BICd: 26.14 |
OR — odds ratio.
Wald test was used to determine statistical differences.
AUC — area under the ROC (receiver operating characteristic) curve.
AIC — Akaike’s Information Criteria.
BIC — Bayesian Information Criteria.
Pearson X2 goodness-of-fit test, P < 0.001.
In the final multivariable model using results of the survey, no variables were significantly associated with the presence of ear necrosis under the 5% significance level.
From the survey, the source of semen and genetic composition of piglets, the breeds of sows and replacement gilts showed a wide variety in both case and control farms. Small purebred breeders and a number of multinational genetic supply companies contributed to supply semen and replacement gilts to both case and control farms. In addition, there was no difference in the types of farm between case and control farms.
Discussion
The low prevalence of spirochetes found by histological examination and the lack of spirochetes identified by culture in the current study is similar to the findings of Richardson et al (3), who also observed spirochetes in only a small number of affected animals. It is possible that spirochetes were present but we were not able to culture them. However, if spirochetes were the primary agent, one would expect their presence to be more prominent in histological examination using silver staining techniques. The evidence from this study suggests that these organisms are more likely secondary invaders rather than primary pathogens.
In contrast, both S. aureus and S. hyicus were frequently isolated from samples of the ear lesions. However these organisms can be commonly isolated from the skin of pigs. In order to determine whether these organisms play a role in the etiology of ear necrosis it will be necessary to confirm that these isolates can produce exfoliative toxins (ETs) and to conduct challenge studies to reproduce the disease. Histological examination showed lesions similar to the lesions of exudative epidermitis such as severe epidermal inflammation with erosion, ulceration, and crust with relatively mild dermal inflammation through all stages of the disease (15). Our attempt to find early changes of gross vesiculation (3) was unsuccessful; possibly, the very early lesions may have been too subtle to be recognized when pigs were chosen for sampling or at the time of the visit there were no pigs available at the very earliest stage.
In the current study, the staphylococcal isolates showed a high degree of resistance to some of the commonly used antibiotics, suggesting that bacterial resistance might lead to poor response to antibiotic treatment.
The low prevalence of vascular lesions in biopsies from the study suggests that ear necrosis does not represent a manifestation of PCVAD (11); the vascular lesions appear to have developed secondarily to epidermal inflammation. In addition, the case farms were not associated with other clinical signs such as depression, respiratory disease, or enteric disease that could be indicative of infections with PCV2 or other systemic diseases. As well, the advent of widespread PCV2 vaccination and significant reduction in PCVAD has not eliminated or reduced the occurrence of ear necrosis in the case herds. The results are consistent with the study by Lang et al (16) who investigated the role of PCV2 and couldn’t detect virus in the lesions when using PCV2 in-situ hybridization testing. Mycotoxins were not investigated but the histological description of the lesions and the lack of other clinical signs associated with mycotoxicosis tend to rule out their involvement.
Ear biting was strongly associated with the presence of ear necrosis in the study. Trauma from ear biting has been suggested to be the triggering factor in allowing infectious agents to invade the skin and cause necrosis (17) rather than being the serious sequela of ear necrosis. However, bloody ear lesions are likely to attract penmates whose chewing could result in the severity of the lesions (18). Therefore, further research on whether ear necrosis leads to biting or biting is a prerequisite for ear necrosis is warranted since the results of such a study would assist the current effort towards the control of ear necrosis.
High humidity was also a risk factor for ear necrosis in the present study which is in agreement with other reports (4,12). This is consistent with bacterial etiology since high humidity may facilitate the buildup of staphylococci or other pathogens. However the assessment of the air quality and whether the barn was humid in this study was subjective. In addition, humidity in a pig barn is highly variable because it is influenced by weather, season, and even activities in the barn such as washing. Therefore this association of high humidity and ear necrosis needs to be considered with caution. Case farms reported an earlier weaning age than control farms and this finding is in agreement with a previous study (13). It has been suggested that when pigs are weaned at an early age they are more apt to engage in behavioral vices such as chewing on penmates and possibly causing mild skin damage; therefore, weaning age could be associated with ear necrosis. The small number of farms used in this study makes it difficult to clearly identify management and environmental risk factors and is a significant weakness of the study.
There were numerous sources of semen, replacement of gilts, and different breeds of sows among the farms in this study. Pigs with floppy ears have been suggested to be more prone to ear biting and possibly more vulnerable to necrosis compared to pigs with upright ears (17). However no such association was noted in this study.
In conclusion, this study provides evidence that ear necrosis is possibly an infectious disease beginning with lesions on the outer skin surface. Spirochetes are not likely a primary cause since they were only occasionally observed during histological examination. Staphylococcus species should be considered to be potential causative agents since they were commonly isolated from ear lesions and the histological description of the lesions are similar to exudative epidermitis which is a disease caused by Staphylococcus species. However at this stage the role of Staphylococcus species is speculative. Further research is necessary to investigate the isolates from cases of ear necrosis and determine the presence of exfoliative toxins. Ear necrosis is in all likelihood a disease that requires multiple contributing factors. A study involving large numbers of case and control farms is required to thoroughly investigate management and environmental factors but in this small study high humidity, early weaning, and the presence of ear biting appeared to be associated with the condition.
Acknowledgments
The research was funded by Ontario Pork, the Animal Health Strategic Investment Fund, Ontario Ministry of Agriculture, Food and Rural Affairs, and the University of Guelph. We are very appreciative of the farmers who participated and Bryan Bloomfield for technical assistance. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Petersen HH, Nielsen EO, Hassing AG, Ersbll AK, Nielsen JP. Prevalence of clinical signs of disease in Danish finisher pigs. Vet Rec. 2008;162:377–382. doi: 10.1136/vr.162.12.377. [DOI] [PubMed] [Google Scholar]
- 2.Busch ME, Jensen IM, Korsgaard J. The development and consequences of ear necrosis in a weaner herd and two growing-finishing herds. Proc Inter Pig Vet Soc Cong. 2010;45 [Google Scholar]
- 3.Richardson JA, Morter RL, Rebar AH, Olander HJ. Lesions of porcine necrotic ear syndrome. Vet Pathol. 1984;21:152–157. doi: 10.1177/030098588402100203. [DOI] [PubMed] [Google Scholar]
- 4.Mirt D. Lesions of so-called flank biting and necrotic ear syndrome in pigs. Vet Rec. 1999;144:92–96. doi: 10.1136/vr.144.4.92. [DOI] [PubMed] [Google Scholar]
- 5.van Duijkeren E, Jansen MD, Flemming SC, et al. Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg Infect Dis. 2007;13:1408–1410. doi: 10.3201/eid1309.061268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishifuji K, Sugai M, Amagai M. Staphylococcal exfoliative toxins: “Molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci. 2008;49:21–31. doi: 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Elias PM, Fritsch P, Mittermayer H. Staphylococcal toxic epidermal necrolysis: Species and tissue susceptibility and resistance. J Invest Dermatol. 1976;66:80–89. doi: 10.1111/1523-1747.ep12481412. [DOI] [PubMed] [Google Scholar]
- 8.Harcourt RA. Porcine ulcerative spirochaetosis. Vet Rec. 1973;92:647–648. doi: 10.1136/vr.92.24.647. [DOI] [PubMed] [Google Scholar]
- 9.Pringle M, Backhans A, Otman F, Sjölund M, Fellström C. Isolation of spirochetes of genus Treponema from pigs with ear necrosis. Vet Microbiol. 2009;139:279–283. doi: 10.1016/j.vetmic.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Cameron R. Diseases of the skin. In: Straw EB, Zimmerman JJ, D’Allaire S, Taylor JD, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publishing; 2006. pp. 179–198. [Google Scholar]
- 11.Zlotowski P, Correa AMR, Barcellos DESN, Driemeier D. Presence of PCV2 in ear lesions in the course of PCVAD in growing pigs in Brazil. Proc Inter Pig Vet Soc Cong. 2008;555 [Google Scholar]
- 12.Busch ME, Wachmann H, Nielsen EO, Baekbo P. Risk factors for ear necrosis and tail lesions in weaners. Proc Inter Pig Vet Soc Cong. 2008;277 [Google Scholar]
- 13.Behavioral problems, including vices and cannibalism. In: Smith WJ, Penny RHC, editors; Leman AD, Glock RD, Mengeling WL, Penny RHC, Scholl E, Straw B, editors. Diseases of Swine. 5th ed. Ames, Iowa: Iowa State University Press; 1981. p. 671. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard-third edition. CLSI document. 2008;28:M31–A3. [Google Scholar]
- 15.Wegener HC, Skov-Jensen EW. Exudative epidermitis. In: Straw BR, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Pub; 2006. pp. 675–679. [Google Scholar]
- 16.Lang C, Volmayr T, Waxenecker F, Hofstetter U. Etiology of the ear necrosis syndrome-investigation of infectious agents. Proc Inter Pig Vet Soc Cong. 2010;43 [Google Scholar]
- 17.Penny RHC, Mullen PA. Ear biting in pigs. Vet Annu. 1976;16:103–110. [Google Scholar]
- 18.Fraser D. Attraction to blood as a factor in tail-biting by pigs. Appl Anim Behav Sci. 1987;17:61–68. [Google Scholar]