Abstract
In the context of chronic childhood emotional maltreatment (CEM; emotional abuse and/or neglect), adequately responding to facial expressions is an important skill. Over time, however, this adaptive response may lead to a persistent vigilance for emotional facial expressions. The amygdala and the medial prefrontal cortex (mPFC) are key regions in face processing. However, the neurobiological correlates of face processing in adults reporting CEM are yet unknown. We examined amydala and mPFC reactivity to emotional faces (Angry, Fearful, Sad, Happy, Neutral) vs scrambled faces in healthy controls and unmedicated patients with depression and/or anxiety disorders reporting CEM before the age of 16 years (n = 60), and controls and patients who report no childhood abuse (n = 75). We found that CEM was associated with enhanced bilateral amygdala reactivity to emotional faces in general, and independent of psychiatric status. Furthermore, we found no support for differential mPFC functioning, suggesting that amygdala hyper-responsivity to emotional facial perception in adults reporting CEM may be independent from top–down influences of the mPFC. These findings may be key in understanding the increased emotional sensitivity and interpersonal difficulties, that have been reported in individuals with a history of CEM.
Keywords: Amygdala, childhood emotional maltreatment, fMRI, mPFC, stress
INTRODUCTION
Childhood Emotional maltreatment (CEM) encompasses any act of commission (i.e. verbal abuse) or omission (i.e. emotional neglect) that is (potentially) harmful or insensitive to the child's emotional development (Egeland, 2009; Gilbert et al., 2009). One in 10 children growing up in Western societies experiences CEM per year (Gilbert et al., 2009) and CEM has been associated with a cascade of negative outcomes on behavioral, emotional, and social functioning (Teicher et al., 2006; Gibb et al., 2007; Egeland, 2009; Gilbert et al., 2009; Spinhoven et al., 2010). For instance, CEM is associated with enhanced emotional sensitivity in adulthood, as evidenced by enhanced negative self-associations, depressive symptoms, and difficulties in interpersonal relationships (Spertus et al., 2003; Egeland, 2009; Gilbert et al., 2009; van Harmelen et al., 2010a; Horner, in press).
In the context of chronic CEM, adequately responding to facial expressions is an important skill. Detecting when a parent is in a bad mood may help a child to avoid a negative confrontation with that parent. However, over time, this adaptive response may lead to a persistent vigilance for negative facial expressions (Gibb et al., 2009). The amygdala is a key brain region involved in the primary processing of emotional faces, and plays a crucial role in salience detection, fear conditioning, and emotional memory (Davis and Whalen, 2001; Bremner et al., 2005; Todorov and Engell, 2008; Onur et al., 2009; Kim et al., 2011). In addition, adverse rearing environments in animals, such as maternal isolation, loss, and isolation rearing induce a cascade of long-term alterations on a behavioral and neurobiological level, with specific effects in the amygdala (Sanchez, 2001; McEwen et al., 2012). For instance, maternal deprivation is associated with a lasting enhancement of contextual and cued fear conditioning (Oomen et al., 2010), and anxious behavior in rats (Eiland and McEwen, 2010).
In humans, acute stress is associated with subsequent amygdala hypervigilance to emotional stimuli (van Marle et al., 2010; Oei et al., in press). In line with the findings of persistent vigilance in animals, greater left amygdala activation during the processing of negative emotional faces was observed in a small sample of youths who experienced severe emotional and physical neglect in foster care or orphanages (Maheu et al., 2010), and in young adults reporting high childhood family stress (including CEM and physical abuse), while classifying the emotion of fearful and angry faces (Taylor et al., 2006). Thus far, however, it is unknown whether CEM in isolation (i.e. without concomitant physical or sexual abuse) is related to enhanced amygdala activation for negative emotional faces. Furthermore, it is unknown whether CEM-related amygdala activation persists into adulthood, i.e. whether adults reporting CEM are characterized by enhanced amygdala response to negative emotional faces.
Using fMRI, an emotional faces task (employing Angry, Fearful, Sad, Happy, Neutral and Scrambled faces) and applying a hypothesis driven region-of-interest (ROI) analysis approach, we examined amygdala functioning in a large sample (N = 135) of unmedicated outpatients (with major depressive disorder (MDD) and/or anxiety disorder (AD)) and healthy controls (HC). We investigated whether patients and HC reporting CEM (n = 60) showed enhanced amygdala reactivity to emotional faces compared to patients and HC reporting No Abuse (n = 75). In line with Maheu et al. (2010), we expected that individuals reporting CEM showed enhanced amygdala response to negative emotional faces (i.e. Angry, Fearful and Sad), but not to happy or neutral faces. In addition, our group recently reported reduced medial prefrontal cortex (mPFC) gray matter volume in adults reporting CEM (van Harmelen et al., 2010b). However, it is unknown whether the reduced mPFC volume in these adults reporting CEM (van Harmelen et al., 2010b) is also related to altered mPFC responsivity. Therefore, we also investigated differences between the CEM and No Abuse group with respect to mPFC reactivity to emotional faces.
In addition, to examine whether altered amygdala and mPFC activation in response to negative emotional faces are related to psychopathology, we investigated whether abnormal amygdala (and/or mPFC functioning) was more apparent in emotionally maltreated individuals with MDD and/or AD compared to HCs reporting CEM.
METHODS
Participants for the NESDA-MRI study
Participants were drawn from the Netherlands Study of Depression and Anxiety (NESDA, N = 2981). A subset of this large observational cohort study (Penninx et al., 2008) were selected to undergo MRI scanning (233 patients and 68 HC). All participants underwent MRI in one of the three participating centers (i.e. Leiden University Medical Center, Academic Medical Center Amsterdam and University Medical Center Groningen). The Ethical Review Boards of each center approved this study. After complete description of the study to the subjects, written consent was obtained.
Inclusion criteria for the NESDA-MRI patients were: current MDD and/or AD (panic and/or social AD) in the last 6 months according to Diagnostical and Statistical Manual-IV criteria. Diagnoses were established using the structured Composite International Diagnostic Interview (Wittchen et al., 1991), administered by a trained interviewer.
Exclusion criteria for patients in the NESDA-MRI study were: the presence of axis-I disorders other than MDD, panic disorder or social AD (except generalized AD); any use of psychotropic medication other than a stable use of selective serotonin reuptake inhibitors (SSRI) or infrequent benzodiazepine use (3 × 2 tablets weekly or within 48 h prior to scanning), the presence of major internal or neurological disorders; dependence or past year abuse of alcohol and/or drugs; hypertension (>180/130 mm Hg); heavy smoking (>5 cigarettes/day); and general MRI contra-indications. Controls had no lifetime depressive or anxiety disorders and were not taking any psychotropic drugs.
In the current study, some additional exclusion criteria were formulated; patients using SSRI (n = 79) were excluded given its potential effect on face processing (Sheline et al., 2001; Fu et al., 2004). Moreover, we excluded 58 participants because of incomplete fMRI data (n = 9), technical difficulties (n = 24), or poor imaging quality (n = 25). See Supplementary Data for additional information on the excluded groups.
CEM was defined as emotional neglect and/or psychological abuse before the age of 16 years, as measured with the NEMESIS trauma interview (see below for more details concerning this interview). Since we did not expect that a single incident of CEM chronically alters brain functioning, we excluded individuals reporting only a single incident of CEM (n = 24). Moreover, also individuals reporting childhood physical or sexual abuse without CEM (n = 5) were excluded. The final sample consisted of 135 participants (see Table 1 for all demographic and clinical characteristics). Individuals who reported emotional neglect or emotional abuse in childhood that had occurred more than once were included in the CEM group (n = 60; 30 individuals reported only emotional neglect, two reported only emotional abuse, and 28 reported both emotional neglect and abuse; 59 individuals reported their biological parents as perpetrators and one subject reported a sibling as the perpetrator). Individuals who did not report emotional, physical, and sexual maltreatment were included in the No Abuse group (n = 75; Table 1).
Table 1.
Clinical and demographic characteristics of participants reporting CEM vs No Abuse
| Variables | No Abuse (n = 75) | CEM (n = 60) | Analysis |
||
|---|---|---|---|---|---|
| F | X2 | P | |||
| Gender (Male/female) | 26/49 | 20/40 | 0.03 | 0.87 | |
| Handedness (Left/right) | 9/66 | 4/56 | 1.09 | 0.30 | |
| Scan centers (AMC/LUMC/UMCG) | 16/32/27 | 14/28/18 | 0.54 | 0.76 | |
| Age, Mean (SE) | 34.91 (1.22) | 38.22 (1.32) | 3.36 | 0.07 | |
| Education, Mean (SE) | 13.32 (0.34) | 12.52 (0.38) | 2.49 | 0.12 | |
| Neuroticism, Mean (SE) | 32.69 (1.26) | 39.53 (0.93) | 14.83 | 0.001 | |
| Recent Life events, Mean (SE) | 0.58 (0.11) | 0.83 (0.16) | 1.88 | 0.17 | |
| Current diagnosis | 8.73 | 0.03 | |||
| MDD | 18 | 14 | |||
| AD | 17 | 17 | |||
| Comorbid MDD and AD | 11 | 18 | |||
| HC | 29 | 11 | |||
| Lifetime diagnosis | |||||
| MDD | 34 | 44 | 10.71 | 0.001 | |
| AD | 32 | 38 | 5.70 | 0.013 | |
| BAI | 9.19(1.22) | 11.38(1.11) | 1.69 | 0.19 | |
| MADRS | 7.64(1.13) | 12.88(1.32) | 9.18 | 0.003 | |
| Concurrent abuse | |||||
| Physical abuse | 0 | 7 | |||
| Sexual abuse | 0 | 11 | |||
| Physical and sexual abuse | 0 | 5 | |||
Values are represented as n unless otherwise specified.
AMC = Amsterdam Medical Center, LUMC = Leiden University Medical Center, UMCG = University Medical Center Groningen, BAI = Beck Anxiety Inventory, MADRS = Montgomery Asberg Depression Rating Scale.
The CEM and No Abuse groups did not differ in years of education, recent life events, anxious symptomatology, gender, handedness, or scan location (Table 1). However, the CEM group was slightly older, had higher neuroticism scores, reported more depressive symptomatology, and consisted of more individuals with a current psychiatric diagnosis.
Childhood maltreatment
Childhood maltreatment was assessed through the NEMESIS trauma interview (de Graaf et al., 2002). In this interview, respondents were asked whether they had experienced emotional neglect, emotional abuse, physical abuse, and/or sexual abuse before the age of 16 years, how often this had occurred (‘never, once, sometimes, regularly, often, or very often’), and what their relationship with the perpetrator was. Emotional neglect was described as: ‘people at home didn't listen to you, your problems were ignored, you felt unable to find any attention or support from the people in your house’. Emotional abuse was described as: ‘you were cursed at, unjustly punished, your brothers and sisters were favored – but no bodily harm was done’.
Our definition of CEM (i.e. emotional neglect and/or emotional abuse before the age of 16 years) is based on the American Professional Society on the Abuse of Children (APSAC; Binggelli et al., 2001; Egeland, 2009; Horner, in press). This definition states that emotional child maltreatment consists of acts of commission (emotional abuse, such as degrading, terrorizing, belittling, blaming, exploiting) and/or omission (emotional neglect such as isolation, rejection, denying emotional responsiveness), which conveys to the child that he/she is worthless, unloved, and unwanted, and are harmful to the child's emotional developmental needs.
Additional questionnaires
Recent life events (past year) were assessed using the List of Threatening Events Questionnaire (Brugha et al., 1985). Neuroticism was measured with the NEO Five-Factor Inventory (Costa and McGrae, 1992). Furthermore, at the day of scanning, depression and anxiety severity (past week) were measured using the Montgomery Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) and Beck's Anxiety Inventory (BAI) (Beck et al., 1988).
The faces task
The faces paradigm was based on the event-related emotional paradigm used by Wolfensberger and colleaugues (2008) that has been found to robustly activate the amygdala. Photographs of angry, fearful, sad, happy, neutral faces, and a control condition (scrambled faces) were presented to all participants. The photographs were selected from the Karolinska Directed Emotional Faces System (Lundqvist et al., 1998) representing standardized facial expressions of emotions expressed by amateur actors. Twenty-four stimuli were selected for each of 5 facial expressions, comprising 12 female and 12 male faces. Each particular face was not presented more than four times. The scrambled faces were presented 80 times. The faces task was presented using E-prime software (Psychological Software Tools, Pittsburgh, PA, USA). In order to reduce anticipatory effects, an event-related design was employed. This entailed a pseudo-random presentation of a total of 200 stimuli against a black background. Each photograph was shown on the screen for 2.5 s, with an inter-stimulus (black screen) interval varying between 0.5 and 1.5 s. The images were projected onto a translucent screen at the end of the scanner bed, visible via a mirror above the participant's head. All participants were instructed to indicate each face's gender by pressing one of two buttons with the index finger of the left or right hand. During the presentation of scrambled faces, participants had to press left or right buttons in conformity with an arrow pointing to the left or to the right. The reaction times were recorded.
MRI acquisition
Imaging data were acquired using Philips 3T MR systems (Best, The Netherlands) located at the University Medical Centers in Leiden, Amsterdam and Groningen, equipped with a SENSE-8 (Leiden and Groningen) and a SENSE-6 (Amsterdam) channel head coil, respectively. For each subject, echo-planar images were obtained using a T2*-weighted gradient echo sequence [repetition time (TR) = 2300 ms, echo time (TE) = 30 ms (Groningen: TE = 28 ms), matrix size: 96 × 96 (Groningen: 64 × 64), 35 axial slices (Groningen: 39 slices), interleaved acquisition, 2.29 × 2.29 mm in-plane resolution (Groningen: 3 × 3 mm), 3 mm slice thickness]. Echo-planar images were scanned parallel to the anterior–posterior commissure plane. To control for differences in scan sites, we added dummy variables for the different scan centers to all analyses. The faces paradigm was part of a functional scanning session utilizing multiple tasks, these results are reported elsewhere.
fMRI data preprocessing
Functional imaging data were preprocessed and analyzed using Statistical Parametric Mapping (SPM)-5 software implemented in Matlab 7.1.0 (www.mathworks.co.uk). Preprocessing, after extensive quality evaluation of the data, consisted of: manually reorienting the functional images to the anterior commissure, slice time correction, image realignment, registration of the T1-scan to the mean echo-planar image, warping to Montreal Neurological Institute (MNI)-space as defined by the SPM5 T1-template, reslicing to 3 × 3 × 3 mm voxels and spatial smoothing using an 8-mm full-width half-maximum Gaussian kernel. Subject movement (>3 mm) resulted in exclusion of the data from further analysis.
fMRI data analysis
fMRI data were analyzed in the context of the General Linear Model. Hemodynamic responses to each stimulus were modeled with a delta function convolved with a synthetic hemodynamic response function. Low-frequency noise was removed by applying a high-pass filter (cut-off 128 s) to the fMRI time series at each voxel. Statistical parametric maps for each comparison of interest were calculated on a voxel-by-voxel basis.
For each subject, contrasts for facial expressions (Angry, Fearful, Sad, Happy, Neutral) vs scrambled faces were computed. We then conducted a facial expressions (Angry, Fearful, Sad, Happy, Neutral) × Group (CEM vs No Abuse) second level analysis, to examine the main effect of task in our ROIs (i.e. amygdala and mPFC). We specified dummy variables for the different scan centers and a weighted dummy for psychiatric status as covariates. In the weighted dummy for psychiatric status (with values 0 or 0.43), the value for the patient group (n = 94) was weighed according to the size of the control group (41/94 = 0.43). In addition, because of findings of age and gender related differences in amygdala response to emotion processing (Iidaka et al., 2002; Wager et al., 2003), we also specified age and gender as covariates. All results are reported in MNI space, and significance threshold for the main effect of task was set at P < 0.05 family-wise error (FWE) corrected for multiple comparisons. To investigate if there were any CEM-related activations outside our predefined ROIs, we performed a whole-brain analysis with at P < 0.05 FWE corrected.
For the amygdala, we extracted activations for the main effect of task (F) using the Marsbar ROI Toolbox, (Brett et al., 2002). The binary mask in MNI space of the left and right amygdala was specified using the Wake Forest University (WFU) Pick Atlas software, SPM Toolbox (http://fmri.wfubmc.edu).
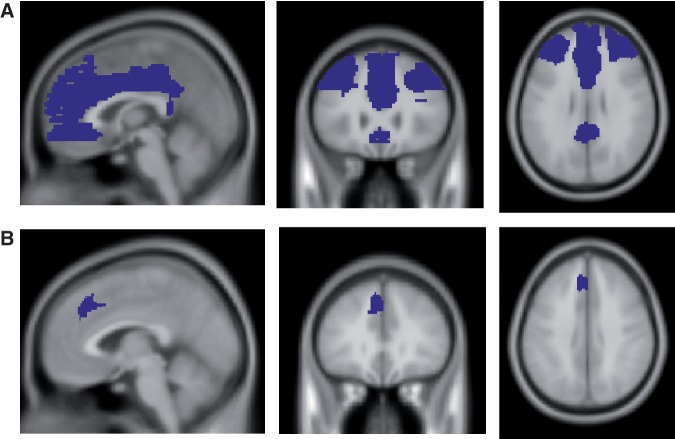
Since the anatomical region of the mPFC is less well defined than the amygdala, group differences (F) in mPFC activations were examined using a facial expressions (Angry, Fearful, Sad, Happy, Neutral) × CEM (CEM vs No Abuse) voxel-wise ROI analysis, while masking for the main effect of task (F). The ROI mask (Figure 1a) was based on the anatomical location of both dorsal and ventral mPFC (including the anterior cingulate cortex) in MNI space (using the WFU Pick Atlas toolbox). Dummy variables for the different scan centers, gender, age, and psychiatric status (weighted) were specified as covariates. Significance for group differences was set at P < 0.001 uncorrected, with spatial extent threshold of five contiguous voxels.
Fig. 1.
ROI Masks for the mPFC. A = Cluster based on anatomical regions of the dorsal, ventral mPFC and the entire ACC. B = Cluster based on structural differences in CEM groups as reported in van Harmelen et al., 2010b.
Based on our previous findings of mPFC volume reductions in CEM in largely the same cohort (van Harmelen et al., 2010b), group differences in activation within this region were also examined by extracting mean activation differences per valence type within this locus using a binary mask that was based on our earlier findings [(x = −11, y = 21, z = 44); see Figure 1b, van Harmelen et al., 2010b].
Following the extraction of Amygdala and mPFC activations (using MARSBAR), we ran correlation analyses to further investigate whether average Amygdala activation for all facial expressions was related to average mPFC activation for all facial expressions, within the No Abuse or CEM group.
SPSS data analysis
Psychometric and performance data were analyzed with SPSS 17. Based on the observed distribution of the data, the appropriate parametric (F) or non-parametric chi-square (χ2) test was performed. Since the CEM group was slightly older (Table 1), age was defined as a covariate in all SPSS analyses (however, all results remained the same when removing age as covariate). In addition, to investigate whether the results are dependent on psychiatric status, in all analyses, we added a weighted dummy for psychiatric status as a covariate. To further examine the exact impact of psychiatric status on emotional face processing in individuals reporting CEM, we finally performed an additional Diagnosis [MDD, AD, co-morbid MDD and AD (CDA), HC] × CEM (No Abuse, CEM) analysis of variance.
Significance was set at P < 0.05, two-tailed, all tests were Bonferroni corrected for multiple comparisons.
RESULTS
Performance on faces task
Performance on faces was assessed using a facial expressions (Angry, Fearful, Sad, Happy, Neutral) × CEM (CEM vs No Abuse) Repeated Measures (RM) Analyses of Covariance (ANCOVA) on the reaction times. Individuals reporting CEM had similar reaction times as individuals reporting No Abuse, F(1,130) = 0.002, P = 0.96, and there was no CEM × facial expressions interaction, F(4,520) = 1.03, P = 0.39. There was a main effect of facial expressions, F(4,520) = 2.41, P = 0.049, Cohen's d (d) = 0.27. Compared to the other faces, participants were faster in labeling the gender of angry faces, all P's < 0.001, independent of maltreatment-status, F(4,520) = 1.03, P = 0.39. Moreover, age had a main effect, F(1,130) = 6.74, P = 0.011, d = 0.45, with older participants having longer reaction times. Psychiatric status did not affect reaction times, F(1,130) = 0.22, P = 0.64.
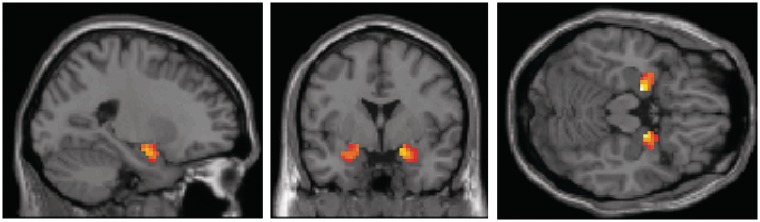
The main effect of task
A facial expressions (Angry, Fearful, Sad, Happy, Neutral) × CEM (CEM vs No Abuse) analysis showed that the task was associated with significant activations in left and right amygdala, x = −18, y = −6, z = −15, cluster size/number of voxels (K) = 236, Z > 8, P < 0.001 and x = 18, y = −6, z = −15, K = 263, Z > 8, P < 0.001; Figure 2 and Table 2. Furthermore, the task was also associated with significant activations in the mPFC, x = 6, y = 36, z = 24, K = 50, Z = 5.52, P < 0.001 and x = 9, y = 48, z = −3, K = 37, Z = 5.04, P < 0.001 (all results are FWE corrected). Other regions that were activated with task (including the right and left fusiform gyrus, the middle occipital gyrus and the superior frontal gyrus) are specified in Table 2. However, facial expressions, (Angry, Fearful, Sad, Happy, Neutral) × CEM (CEM vs No Abuse) whole-brain analysis at P < 0.05 FWE corrected, showed no CEM-related activations in any of the task-related regions outside our ROIs (see also Table 3).
Fig. 2.
Amygdala activation for the main effect of task (emotional vs scrambled faces).
Table 2.
Significant gray matter clusters of the main effect of task
| Clusters | Cluster level, Number of voxels | Coordinates |
|||||
|---|---|---|---|---|---|---|---|
| F | Z-score | P-value | x | y | z | ||
| Right fusiform gyrus | 271 | 411.86 | >8 | <0.001 | 42 | −51 | −21 |
| 145.76 | >8 | <0.001 | 42 | −78 | −12 | ||
| 101.69 | >8 | <0.001 | 24 | −93 | −9 | ||
| Left fusiform gyrus | 115 | 200.84 | >8 | <0.001 | −39 | −51 | −21 |
| Left middle occipital gyrus | 3555 | 198.59 | >8 | <0.001 | −27 | −90 | 9 |
| 186.01 | >8 | <0.001 | 30 | −87 | 12 | ||
| 155.74 | >8 | <0.001 | −24 | −69 | −15 | ||
| Right amygdala | 263 | 175.88 | >8 | <0.001 | 18 | −6 | −15 |
| 77.15 | >8 | <0.001 | 27 | 3 | −21 | ||
| Left amygdala | 236 | 167.89 | >8 | <0.001 | −18 | −6 | −15 |
| 29.29 | 5.22 | <0.001 | −39 | 15 | −24 | ||
| Right superior frontal gyrus | 350 | 75.37 | >8 | <0.001 | 48 | 24 | 24 |
| 74.11 | >8 | <0.001 | 51 | 30 | 18 | ||
| 64.59 | 7.75 | <0.001 | 45 | 9 | 30 | ||
| Left superior frontal gyrus | 83 | 55.29 | 7.19 | <0.001 | −27 | 60 | 9 |
| Right superior parietal lobe | 149 | 53.20 | 7.05 | <0.001 | 54 | −48 | 39 |
| Left superior parietal lobe | 375 | 52.05 | 6.98 | <0.001 | −51 | −48 | 42 |
| 50.92 | 6.90 | <0.001 | −45 | −48 | 48 | ||
| 45.93 | 6.56 | <0.001 | −60 | −39 | 36 | ||
| Right superior temporal gyrus | 117 | 44.10 | 6.43 | <0.001 | 66 | −21 | 9 |
| 26.33 | 4.95 | <0.001 | 63 | −21 | −6 | ||
| 23.50 | 4.66 | <0.001 | 57 | −27 | 18 | ||
| Right superior frontal gyrus | 11 | 33.22 | 5.57 | <0.001 | 24 | 15 | 54 |
| Right anterior cingulate cortex | 54 | 32.65 | 5.52 | <0.001 | 6 | 36 | 24 |
| Right superior frontal gyrus | 51 | 32.33 | 5.49 | <0.001 | 30 | 54 | 21 |
| 28.50 | 5.15 | <0.001 | 21 | 63 | 6 | ||
| Right medial frontal gyrus | 37 | 27.36 | 5.04 | <0.001 | 9 | 48 | −3 |
| 26.27 | 4.94 | <0.001 | 3 | 48 | 6 | ||
Main effect of task (F) at P < 0.05 FWE corrected.
Table 3.
Significant CEM-related gray matter activations outside our ROIs
| Clusters | Cluster level | Coordinates |
|||||
|---|---|---|---|---|---|---|---|
| Number of voxels | F | Z-score | P-value | x | y | z | |
| Left middle temporal gyrus | 35 | 19.52 | 4.23 | <0.001 | −54 | −60 | −3 |
| Left lingual gyrus | 21 | 17.64 | 4.01 | <0.001 | −18 | −72 | −15 |
| Right middle temporal gyrus | 5 | 14.51 | 3.61 | <0.001 | 36 | −81 | 21 |
| Right superior temporal gyrus | 14 | 13.77 | 3.51 | <0.001 | 51 | −30 | 15 |
| 11.44 | 3.17 | <0.001 | 48 | −36 | 21 | ||
| Right lingual gyrus | 9 | 12.58 | 3.34 | <0.001 | 21 | −72 | −12 |
| Left postcentral gyrus | 8 | 12.55 | 3.34 | <0.001 | −57 | −27 | 18 |
Main effect of CEM vs No Abuse (F) whole brain at P < 0.001, uncorrected, with spatial extent threshold of five contiguous voxels.
Amygdala activation in response to emotional faces
We next extracted bilateral amygdala activations for the main effect of task (F), and conducted a facial expressions (Angry, Fearful, Sad, Happy, Neutral) × Lateralization (Left amygdala vs right amygdala) RM ANCOVA with CEM (CEM vs No Abuse) as fixed factor. Lateralization was added as fixed factor to investigate a possible effect of lateralization (Maheu et al., 2010). In line with our expectations, individuals reporting CEM showed enhanced amygdala activation compared to the No Abuse group, F(1,131) = 5.96, P = 0.016, d = 0.43, (Figure 3). There was no main effect of facial expressions, F(4,524) = 0.354, P = 0.83, nor Lateralization, F(1,131) = 0.22, P = 0.64. Moreover, CEM did not interact with facial expressions, F(4,524) = 0.89, P = 0.47, Lateralization, F(1,131) = 1.25, P = 0.26, or facial expressions × Lateralization, F(4,524) = 0.89, P = 0.47. Finally, age and psychiatric status did not have a main effect on amygdala activation, F(1,131) = 1.45, P = 0.23 and F(1,131) = 2.22, P = 0.14. Also when performing a Diagnosis (MDD, AD, CDA, HC) × CEM (No Abuse, CEM) analysis, with Age as covariate, all results remained unchanged, including the main effect of CEM, F(1,126) = 5.26, P = 0.02. Moreover, again, diagnosis did not have a main effect on amygdala activation, F(3,126) = 1.28, P = 0.29, nor did diagnosis interact with CEM, F(3,126) = 0.22, P = 0.89. This absence of a main effect of diagnosis is in line with a recent study in the larger NESDA-MRI sample, were no impact of psychopathology was found on amygdala functioning to emotional and neutral faces (Demenescu et al., 2011).
Fig. 3.
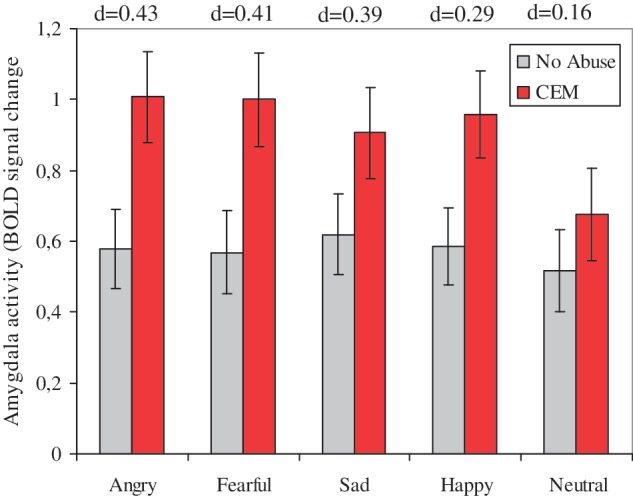
Mean (average left and right) and standard errors of amygdala activation in individuals reporting No Abuse vs CEM. d = Cohen's d for the difference between amygdala activation within the CEM vs No Abuse group.
The CEM group had slightly higher neuroticism and depression severity scores (Table 1). To investigate whether this could potentially explain our findings, we performed two additional RM ANCOVAs. When we added neuroticism as covariate to the analysis, all results remained unchanged (i.e. main effect of CEM, F(1,130) = 5.98, P = 0.02, d = 0.43, and neuroticism had no main effect, F(1,130) = 0.23, P = 0.72), indicating that the findings cannot be explained by the slightly higher neuroticism scores in the CEM group. Also, when we added severity of depression (as measured with the MADRS) as covariate to the analysis, all results remained unchanged [i.e. main effect of CEM, F(1,130) = 5.18, P = 0.02, d = 0.04] and depression severity had no main effect, F(1,130) = 1.04, P = 0.31, indicating that the findings cannot be explained by higher depression levels in the CEM group. In addition, in both the CEM and No Abuse groups, correlation analyses showed no significant relationships between amygdala activations to the emotional and neutral faces and Neuroticism, all P's > 0.15, nor depression, all P's > 0.29.
To exclude the possibility that enhanced amygdala reactivity is driven by a concurrent history of physical and/or sexual abuse in some of the participants (n = 23), we next re-ran the RM ANCOVA while excluding these individuals. In this analysis, all results remained unchanged, including the main effect of CEM, F(1,108) = 5.05, P = 0.03, d = 0.39.
mPFC activation in response to emotional faces
A CEM vs No abuse (F) ROI analysis (Figure 1a) revealed no significant CEM-related activations in the ventral or dorsal mPFC, nor in the entire ACC. These results remained unchanged when mPFC volume was added as a covariate (van Harmelen et al., 2010b). Additionally, when we extracted mPFC activations in the mPFC mask that is based on our previous findings (Figure 1b; van Harmelen et al., 2010b), a RM ANCOVA showed that CEM did not have a main effect on activation in this region either, F(1,131) = 0.01, P = 0.91. Thus, individuals reporting CEM did not differ in mPFC activation in response to emotional facial expressions when compared to individuals reporting No Abuse. Also when performing a Diagnosis (MDD, AD, CDA, HC) × CEM (No Abuse, CEM) analysis, with age as covariate, all results remained unchanged, including that CEM and diagnosis had no main effect on mPFC activation, F(1,126) = 0.001, P = 0.98 and F(3,126) = 1.44, P = 0.24, nor was there a CEM × diagnosis interaction, F(3,126) = 1.29, P = 0.28. In addition, no interactions with facial expressions were found.
Correlation between Amygdala mPFC activations
Following the extraction of Amygdala and mPFC activations (using MARSBAR), we ran correlation analyses to further investigate whether average Amygdala activation for all facial expressions was related to the average mPFC (Figure 1a) activation for all facial expressions, within the No Abuse, or CEM group. However, the test yielded no significant relationship between amygdala and mPFC activations in the No abuse group, r = 0.01, P = 0.92, nor in the CEM group, r = 0.01, P = 0.92. Moreover, a correlational analysis with average amygdala and mPFC activations based on the cluster that we described in van Harmelen et al. (2010b) (Figure 1b) also yielded no significant relationships in the No abuse, r = 0.06, P = 0.59, nor the CEM group, r = 0.14, P = 0.29.
DISCUSSION
This is the first study to show that adults reporting CEM show enhanced amygdala activation in response to emotional facial expressions, independent of psychiatric status, neuroticism, depression severity, and history of concurrent physical or sexual abuse. The amygdala plays a key role in detecting the (emotional or biological) salience of stimuli (Sergerie et al., 2008; Todd and Anderson, 2009; Kim et al., 2011; van Wingen et al., 2011), and in enhancing levels of attention and vigilance toward these stimuli (Davis and Whalen, 2001). Therefore, our results suggest amygdalar hypervigilance toward emotional facial expressions in adults reporting CEM. Moreover, together with similar findings in a small sample of adolescents reporting severe neglect (Maheu et al., 2010), our findings suggest sustained hypervigilance even more than 20 years after the maltreatment took place.
Contrary to our hypothesis, hyper-activation of the amygdala in adults reporting CEM was not restricted to negative facial expressions, but was also found in response to happy and neutral faces, although it should be noted that the effect sizes for the neutral faces are relatively small. Therefore, amygdalar hypervigilance to facial expressions in general, might indicate that individuals with a history of CEM interpret all facial expressions as highly salient. In line with this idea, neglected children are reported to have poor valence discriminatory abilities for different facial emotions (Pollak et al., 2000; Fries and Pollak, 2004; Vorria et al., 2006), and it has been suggested that neglected children may misinterpret all emotional faces as threatening (Pollak et al., 2000). In that respect, happy faces might be interpreted as a mask for more malevolent emotions (Pollak et al., 2000), for example, as being laughed at. Enhanced amygdala activation in response to happy faces could also be indicative of an increased sensitivity toward positive emotional expressions in others (e.g. happy faces might function as safety signal). To disentangle the impact of negative vs positive and neutral faces in individuals reporting CEM, future studies are needed that also asses the subjective ratings of emotional faces besides amygdala activation.
On a neurobiological level, enhanced amygdala responses to all facial expressions may reflect a general noradrenergic sensitization in response to emotional stimuli in individuals reporting CEM. Chronic stress is associated with increased firing of p neurons in the brain stem, and augmented release of noradrenalin in the brain following subsequent stressors (Bremner et al., 1996; Elzinga and Bremner, 2002). Furthermore, enhanced amygdala activation during stress strengthens emotional memory traces and increases fear conditioning (Strange and Dolan, 2004; Joëls and Baram, 2009; Onur et al., 2009). In accordance, in rats, maternal deprivation is associated with a lasting enhancement of contextual and cued fear conditioning (Oomen et al., 2010) and anxious behavior (Eiland and McEwen, 2010).
Another noteworthy result of this study was that enhanced amygdala reactivity to emotional faces in individuals reporting CEM was observed independent of psychiatric status and that no main effect of diagnosis was observed. This finding is in contrast with other studies finding amygdala hyper-reactivity in depressed (Sheline et al., 2001; Anand et al., 2005; Fales et al., 2008) and anxious patients (Straube et al., 2004; Bishop, 2007). It should be noted that our patient sample can be characterized as having relatively mild symptoms, due to the fact that we excluded patients using SSRIs. Possibly, this may have lead to an underestimation of the true effect of psychopathology. However, in a larger sample of the same cohort, in which medicated patients were also included, we recently reported no effect of psychiatric status on amygdala reactivity to emotional faces (Demenescu et al., 2011; see for similar findings; Lawrence et al., 2004; Gotlib et al., 2005; Lee et al., 2008; Almeida et al., 2009; Norbury et al., 2009, and a meta-analysis showing no amygdala hyper-response in depressed patients; Diekhof et al., 2008). Our findings therefore seem to suggest that enhanced amygdala reactivity to emotional faces does not seem to be directly linked to the development of psychopathology in individuals with CEM. Apparently, additional risk factors, such as genetic make-up in itself, or in interaction with exposure to stressful life events during adulthood, or low social support additionally determine who will subsequently develop a depressive and/or AD (Hariri et al., 2002; Kilpatrick et al., 2007).
We did not find support for abnormal mPFC functioning in individuals reporting CEM, nor did we find a significant relationship between Amygdala and mPFC activity. Thus smaller mPFC volume (van Harmelen et al., 2010b) is not related to abnormal mPFC reactivity to emotional facial expressions in adults reporting CEM. Hence, our findings suggests that amygdala hyper-responsivity to emotional facial expressions in individuals with CEM histories may occur independent of the regulatory influences of the mPFC (Fonzo et al., 2010), at least in this gender labeling task which requires minimal cognitive resources (Reddy et al., 2004). However, abnormal mPFC functioning may be observed in tasks posing greater cognitive demands (see, for example, Shin et al., 2006).
This study is not without limitations. First, although a clinically diagnosed PTSD diagnosis was an exclusion criterion, unidentified current or lifetime PTSD symptoms may still have been present in the current sample, which may have influenced our findings. This is not very likely, however, given that the enhanced amygdala responses in individuals reporting CEM was unrelated to psychiatric status. Second, history of childhood maltreatment was retrospectively assessed. In addition, it is important to acknowledge the inherent subjectivity of self-reported CEM. However, it should be noted that, in the current study, neuroticism did not explain enhanced amygdala activation, and in the overall NESDA sample, current affective state did not moderate the association between CEM (as measured with the NEMESIS interview) and lifetime affective disorder (Spinhoven et al. 2010), indicating that recall of CEM in the current sample was not critically affected by current mood state. Furthermore, a recent study showed that depressed women with emotional neglect histories are less prone to produce false memories on the Deese-Roediger, Mcdermott (DRM; Deese, 1959; Roediger & McDermott, 1995) task than depressed women with no emotional neglect and women with any type of maltreatment (Grassi-Oliveira et al., 2011). Finally, our findings are based on a cross-sectional design; therefore, one cannot assume causality. It might be that individuals who have strong amygdala reactions to emotional faces may also have experienced certain behaviors of their parents as more abusive or neglectful. Alternatively, enhanced amygdala reactivity to emotional facial expressions may have been pre-existent and inherited by their parents, whose enhanced amygdala reactivity to emotional faces may have increased their risk to emotionally maltreat their children. Theoretically, longitudinal studies are needed to shed more light on the etiology of our findings, although from an ethical point of view, this is problematic. However, recently, a prospective study in soldiers showed that combat stress exposure is associated with enhanced amygdala responsivity to emotional faces, indicative of a causal role of stress exposure on amygdala hypervigilance. In addition, it appeared that the subjective appraisal of threat, and not the actual exposure, played a key role in amygdala regulation in the aftermath of severe stress (van Wingen et al., 2011).
Taken together, we found that adults reporting CEM show enhanced amygdala response to emotional facial expressions. These findings may represent a persistent hypervigilance for emotional facial expressions in adults reporting CEM. Potentially, during social emotional encounters, enhanced amygdala activation in individuals with CEM might result in strong memory traces and increased fear conditioning in response to emotionally significant stimuli (in this case emotional facial expressions). This may be an important key in understanding the increased emotional sensitivity and difficulties in inter-personal relationships (Spertus et al., 2003; Teicher et al., 2006; Egeland 2009; Gilbert et al., 2009; van Harmelen et al., 2010a; Horner, in press) that has been reported in these individuals.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
A.A. received an investigator-initiated unrestricted research grant from Brystol-Myers Squibb and speakers bureau honoraria from AstraZeneca, Brystol-Myers Squibb, GlaxoSmithKline and Janssen. The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (ZonMw, 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Health and Care Research (IQ Healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos). The principal investigator B.M.E. was funded by a VIDI grant (016-085-353) awarded by the Netherlands Wetenschaps Organisatie (NWO).
REFERENCES
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Philips ML. Elevated amygdala reactivity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry. 2009;67:414–21. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety - psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Binggeli NJ, Hart SN, Brassard MR. The APSAC Study Guides. Vol. 4. Thousand Oaks, CA: Sage; 2001. Psychological maltreatment of children. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney D. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, et al. Positron emossion tomographic imaging of neural correlates of fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2004;34:1–16. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett MAJ-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conferance on Functional Mapping of the Human Brain. 2002 June 2–6, Sendai, Japan. Available on CD-ROM in NeuroImage 16, abstract 497. [Google Scholar]
- Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences - a subset of 12 life event categories with considerable long-term contextual threat. Psychological Medicine. 1985;15:189–94. doi: 10.1017/s003329170002105x. [DOI] [PubMed] [Google Scholar]
- Costa PT, McGrae RR. Professional manual of the revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Psychological Assessments Resources, FL; 1992. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Bijl RV, Smith F, Vollebergh WAM, Spijker J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders. Findings from the Netherlands Mental Health Survey and Incidence Study. American Journal of Psychiatry. 2002;159:620–9. doi: 10.1176/appi.ajp.159.4.620. [DOI] [PubMed] [Google Scholar]
- Demenescu LR, Renken RJ, Kortekaas R, et al. Neural correlates of perception of facial expression in outpatients with depression and anxiety. Psychological Medicine. 2011;41:2253–64. doi: 10.1017/S0033291711000596. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of abberant motivational and affective processing in addiction and mood disorders. Brain Research Review. 2008;59:164–84. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Egeland B. Taking stock: childhood emotional maltreatment and developmental psychopathology. Child Abuse & Negect. 2009;33:22–6. doi: 10.1016/j.chiabu.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2010;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biological Psychiatry. 2010;68:433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries AB, Pollak SD. Emotion understanding in postinstitutionalized Eastern European children. Developmental Psychopathology. 2004;16:355–69. doi: 10.1017/S0954579404044554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CHY, Williams SCR, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment - a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depression and Anxiety. 2007;24:256–63. doi: 10.1002/da.20238. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults information-processing biases for facial displays of emotion. Child Maltreatment. 2009;14:148–56. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. The Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JDE, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–4. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Gomes CF, Stein LM. False recognition in women with a history of childhood emotional neglect and diagnose of recurrent major depression. Consciousness and Cognition. 2011;20:1127–34. doi: 10.1016/j.concog.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Horner G. Emotional maltreatment. Journal of Pediatric Health Care. in press doi: 10.1016/j.pedhc.2011.05.004. doi: 10.1016/j.pedhc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–62. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164:1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioral Brain Research. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–87. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lee BT, Seok JH, Lee BC, et al. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Biological Psychiatry. 2008;32:778–85. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces (KDEF) Stockholm: Karolinska Institute; 1998. [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, et al. preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. New depression scale designed to be sensitive to change. Britisch Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Norbury R, Selvaraj S, Taylor MJ, Harmer C, Cowen PJ. Increased neural response to fear in patients recovered from depression: a 3T functional magnetic resonance imaging study. Psychological Medicine. 2009;40:425–32. doi: 10.1017/S0033291709990596. [DOI] [PubMed] [Google Scholar]
- Oei NY, Veer IM, Wolf OT, Spinhoven PH, Rombouts SARB, Elzinga BM. Stress shifts brain activation towards ventral ‘affective’ areas during emotional distraction. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsr024. doi: 10.1093/scan/nsr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onur OA, Walter H, Schlaepfer TE, et al. Noradrenergic enhancement of amygdala respsonses to fear. Social Cognitive and Affective Neuroscience. 2009;4:199–26. doi: 10.1093/scan/nsn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, et al. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. The journal of Neuroscience. 2010;30:6634–45. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman ATF, Smit JH, et al. Consortium NR. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. International Journal of Methods in Psychiatric Research. 2008;17:121–40. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Developmental Psycholology. 2000;36:679–88. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Reddy L, Wilken P, Koch C. Face-gender discrimination is possible in the near-absence of attention. Journal of Vision. 2004;4:106–17. doi: 10.1167/4.2.4. [DOI] [PubMed] [Google Scholar]
- Roediger HL, III, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1995;21:803–814. [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Developmental Psychopathology. 2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2008;32:811–30. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Spertus IL, Wong R, Halligan CM, Sermetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse & Neglect. 2003;27:1247–58. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Elzinga BM, Hovens JGFM, et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. Journal of Affective Disorders. 2010;126:103–12. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences USA. 2004;101:11454–8. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WHR. Effect of task conditions on brain responses to threatening faces in social phobics: an event related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. American Journal of Psychiatry. 2006;163:993–1001. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Todd RM, Anderson AK. Six degrees of separation: the amygdala regulates social behavior and perception. Nature Neuroscience. 2009;12:1217–8. doi: 10.1038/nn1009-1217. [DOI] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluations of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–12. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, de Jong PJ, Glashouwer KA, Spinhoven P, Penninx BWJH, Elzinga BM. Child abuse and negative explicit and automatic self-associations: the cognitive scars of emotional maltreatment. Behaviour Research and Therapy. 2010a;48:486–94. doi: 10.1016/j.brat.2010.02.003. [DOI] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, van der Wee NJA, et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry. 2010b;68:832–8. doi: 10.1016/j.biopsych.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry. 2009;66:649–55. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16:644–71. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorria P, Papaligoura Z, Sarafidou J, et al. The development of adopted children after institutional care: a follow-up study. Journal of Child Psychology and Psychiatry. 2006;47:1246–53. doi: 10.1111/j.1469-7610.2006.01666.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (Cidi) Britisch Journal of Psychiatry. 1991;159:645–53. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SPA, Veltman DJ, Hoogendijk WJG, Boomsma DI, de Geus EJC. Amygdala responses to emotional faces in twins discordant or concordant for the risk for anxiety and depression. Neuroimage. 2008;41:544–52. doi: 10.1016/j.neuroimage.2008.01.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.