Abstract
‘Social brain’ circuitry has recently been implicated in processing slow, gentle touch targeting a class of slow-conducting, unmyelinated nerves, CT afferents, which are present only in the hairy skin of mammals. Given the importance of such ‘affective touch’ in social relationships, the current functional magnetic resonance imaging (fMRI) study aimed to replicate the finding of ‘social brain’ involvement in processing CT-targeted touch and to examine the relationship between the neural response and individuals’ social abilities. During an fMRI scan, 19 healthy adults received alternating blocks of slow (CT-optimal) and fast (non-optimal) brushing to the forearm. Relative to fast touch, the slow touch activated contralateral insula, superior temporal sulcus (STS), medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC) and amygdala. Connectivity analyses revealed co-activation of the mPFC, insula and amygdala during slow touch. Additionally, participants’ autistic traits negatively correlated with the response to slow touch in the OFC and STS. The current study replicates and extends findings of the involvement of a network of ‘social brain’ regions in processing CT-targeted affective touch, emphasizing the multimodal nature of this system. Variability in the brain response to such touch illustrates a tight coupling of social behavior and social brain function in typical adults.
Keywords: affective touch, autistic traits, CT-afferent, fMRI, social brain
Touch enables us to navigate not only the physical world but also the social world. This dual dimensionality of touch has been described as being processed in the brain in a manner similar to pain, via two dissociable dimensions categorized as, sensory-discriminative and motivational-affective (Morrison et al., 2010). Although the perception of discriminative touch, which allows us to perceive pressure, vibration, slip and texture has historically dominated the touch literature (McGlone et al., 2007), neuroscientists have only recently begun to study ‘affective’ or social touch (Francis et al, 1999; Olausson et al., 2002, 2008; Rolls et al., 2003; McGlone et al., 2007; McCabe et al., 2008; Loken et al., 2009; Keysers et al., 2010; Morrison et al., 2010, 2011;Gordon et al., 2011;). This type of pleasant, gentle touch has been linked to a class of slow-conducting, unmyelinated nerves, CT afferents, present only in the hairy skin of mammals, including humans (Loken et al., 2009). Microneurography studies have shown that CT-optimal stroking speeds range from 1–10 cm/s with decreased firing rates for lower and higher speeds (Loken et al., 2009). Interestingly, pleasantness ratings for slow, light touch follow the same pattern as the firing rates for CT-nerves. That is, participants rate gentle touch at 1–10 cm/s as more pleasant than touch of slower or faster velocities (Loken et al., 2009). Such slow, gentle touch is reminiscent of that seen in social interactions, such as those between a parent and a child or intimate partners. Concordant with this observation, and the preservation of this system in patient populations lacking A/B touch receptors (Olausson et al., 2002, 2008), the ‘skin as a social organ’ hypothesis (Morrison et al., 2010) posits that the CT-system represents an evolutionarily conserved mechanism with a direct role in processing social, or affective, touch.
CT afferents project to lamina I of the spinal and trigeminal dorsal horn, which act as a processing station for signals from C-fibers (Sugiura et al., 1986; Craig, 2003). Lamina I neurons continue through the lamina I spinothalamical pathway and project to the insular cortex (Olausson et al., 2002; Craig, 2003). For this reason, and because the insular cortex has been considered a gateway from sensory systems to the emotional system of the frontal lobe (Augustine, 1996; Craig, 2008), initial neuroimaging studies of the brain mechanisms involved in processing CT-targeted affective touch focused on the posterior insula (Olausson et al., 2002; McCabe et al., 2008). Recently, our group (Gordon et al., 2011) used functional magnetic resonance imaging (fMRI) to demonstrate the involvement of several key nodes of the ‘social brain’ in processing such touch. The social brain describes a circumscribed set of brain regions that have evolved to support social cognition. In her seminal writing on this idea, Leslie Brothers (1990) called this set of regions the social brain and included the amygdala, orbitofrontal cortex (OFC) and temporal cortex as its key components (Frith, 2007).
In our original study on affective touch (Gordon et al., 2011), a whole-brain comparison of the response to CT-optimal touch to the forearm relative to the glabrous skin of the palm revealed posterior superior temporal sulcus (pSTS), medial prefrontal cortex (mPFC) and amygdala involvement in processing affective touch, processed by the CT-system. One alternative explanation for these results is that they may not demonstrate something specific to CT-afferents, but instead reflect distinct responses to touch on two discrete body parts. For example, typical adults perhaps code being touched on the arm as more intimate or social than being touched on the palm. Therefore, one goal of the current study was to replicate the finding of ‘social brain’ involvement in processing CT-targeted touch in a slightly different paradigm including two types of touch to the arm. The brushing was performed on the right forearm, which has non-glabrous or hairy skin. The mechanoreceptive innervations of this type of skin include both myelinated (A) and unmyelinated (C) afferents (Vallbo et al., 1999). More specifically, A-beta low-threshold mechanoreceptors have been found to be involved in discriminative touch, while C mechanoreceptors have recently been implicated in subserving emotional touch (see McGlone et al., 2007, for a review). In addition, given findings of social brain dysfunction in autism and other disorders characterized by impairments in social perception and social cognition (e.g. Pelphrey et al., 2004; Pinkham et al., 2008; Kaiser et al., 2010), a primary goal of the current study was to examine the relationship between individual’s social abilities (as measured by autistic traits) and their brain response to affective touch.
One fMRI study to date has examined the brain response to CT-optimal vs non-optimal velocities of touch to the forearm. Morrison and colleagues (2011) reported that slow (CT-optimal) touch was rated as more pleasant than fast touch and elicited greater posterior insular activation. Notably, this study included other conditions of interest and focused on insular response to the different types of touch. The current study aimed to replicate our previous findings of ‘social brain’ engagement and connectivity between frontal and limbic structures during affective touch using CT-optimal and non-optimal velocities to the forearm. We investigated this hypothesis using whole brain GLM contrasts as well as connectivity analyses [i.e. Psychophysiological Interaction (PPI)]. The touch stimuli were gentle strokes with a watercolor brush. Indeed, human touch of a similar velocity could be considered more ‘social’ than brushstrokes. However, fMRI studies have shown that human touch and gentle brushstrokes of the same velocities elicit comparable neural response and pleasantness ratings (Morrison, personal communication). We consider the slow touch to be affective in nature given the previous literature characterizing the response of the CT-system to such gentle touch, which is commonly seen in intimate relationships. Although both fast and slow touch may be considered pleasant, the slow touch may be more ‘affective’ given the higher pleasantness ratings and unique processing by CT-afferents.
We also sought to characterize the relationship between social abilities, touch preferences and the neural response to affective touch. The network of regions implicated in processing CT-targeted touch has been found to play a key role in a variety of social perception and social cognition tasks. These regions are important for detecting biological motion (e.g. Grossman et al., 2000; Saygin, 2007) and complex social processing such as theory of mind or mentalizing (e.g. Gallagher et al., 2000; Frith and Frith 2003, 2006; for review, see Gallagher and Frith, 2003). Notably, these processes and associated neural mechanisms have been consistently implicated as dysfunctional in individuals with autism, a disorder characterized by social impairments. Autistic traits are normally distributed in the general population (Baron-Cohen et al., 2001), reflect individual differences in social cognitive abilities (Losh and Piven, 2007) and correlate with differences in the structure and function of some of the brain regions identified in our previous study on affective touch (Suda et al., 2011; von dem Hagen et al., 2011). Thus, a primary goal of the current study was to examine the relationship between autistic traits and brain mechanisms for processing CT-targeted affective touch. To the extent that the brain mechanisms for processing slow gentle touch reflect a tuning to socially relevant information, we predict that individuals with more autistic traits will show a diminished neural response to such affective information. We also predict that individuals with more autistic traits will report less of a preference for social touch.
MATERIALS AND METHODS
Participants
Nineteen right-handed adults (12 females) ranging in age from 18–26 years (mean = 23.73, s.d. = 2.51), participated in the study. All participants had no known neuropsychological disorder, history of brain injury nor family members with an ASD, and were naïve to the hypothesis under investigation. Written informed consent was obtained for each participant according to a protocol approved by the Yale School of Medicine Human Investigations Committee.
PreScan behavioral ratings and questionnaires
Prior to the scan, participants received the two types of touch (slow and fast brushing) on their right forearm. Brush strokes were administered in the proximo-distal orientation, as in the fMRI paradigm. Participants then rated the pleasantness for each type of touch on a Likert scale (1 = ‘not at all’; 2 = ‘slightly’; 3 = ‘moderately’; 4 = ‘very’ and 5 = ‘extremely’), and were also asked to describe in their own words what each type of touch felt like.
To measure affects and attitudes toward social touch, participants completed the Social Touch Questionnaire (Wilhelm et al., 2001). This 20-item self-report measure assesses comfort and preferences regarding social touch (i.e. ‘I feel comfortable touching people I do not know very well’ and ‘I generally like when people express their affection toward me in a physical way’), with scores ranging from 0 to 80. Lower scores on this measure indicate a preference for social touch, whereas higher scores are associated with rating social touch as unpleasant and reports of avoiding it across a variety of situations.
To measure autistic traits, participants completed the Autism-Spectrum Quotient (AQ; Baron-Cohen et al., 2001), which is a 50-item self-report measure of preferences and tendencies in daily life (e.g. ‘I tend to have very strong interests, which I get upset about if I can’t pursue’; ‘When I talk, it isn’t always easy for others to get a word in edgewise’). Scores range from 0 to 50 and higher scores are associated with more autistic traits. We planned to conduct correlation analyses with these behavioral measures and brain response to affective touch.
Experimental design
Prior to data acquisition, the experimenter measured and marked (with a washable marker) an 8 cm brushing area for both conditions, ranging from the wrist crease toward the elbow on the participant’s right forearm. During an fMRI scan, participants received continuous (i.e. back and forth) brushing to the right forearm in a block design procedure. There were 2 runs of each condition (fast and slow) composed of 8 repetitions of 6-s blocks of touch followed by 12 s of no touch (rest). An additional 6 s of rest separated each block to allow the experimenter to prepare for the next block of touch. Tactile stimuli were slow (8 cm/s) or fast strokes (32 cm/s) administered by a trained experimenter using a 7 cm wide watercolor brush. These speeds correspond to 6 strokes per slow block and 24 per fast block; distances covered in the slow and fast blocks were 48 cm and 192 cm, respectively. This design was identical to that used in our previous study (Gordon et al., 2011), except for fast arm blocks replacing palm blocks. All experimenters were trained prior to data collection and used a visual guide within the scanner to facilitate the administration of the accurate brushing velocity. Participants were instructed to keep their eyes closed for the entirety of the experiment and to focus on the touch. The brusher monitored each participant to ensure that his/her eyes remained closed throughout the duration of the experiment. In addition, an fMRI-compatible eye-tracker (monitored by a research assistant in the control room) was used to confirm that participant’s eyes remained closed. The experiment lasted 10.03 min (602 s) with an initial 10 s of rest that was later discarded from analysis.
Imaging protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. High-resolution T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1230 ms; TE = 1.73 ms; FOV = 256 mm; image matrix 2562; 1 × 1 × 1 mm). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 642; voxel size = 3.4 × 3.4 × 4.0 mm; 34 slices) sensitive to blood oxygenation-leveldependent (BOLD) contrast. Runs consisted of the acquisition of 306 successive brain volumes.
fMRI analysis
Data were preprocessed and analyzed using the BrainVoyager QX 2.0.08 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, linear trend removal and temporal high-pass filtering (GLM with Fourier basis set, using 2 cycles/time course). Functional datasets were co-registered to within-session, T1-weighted anatomical images, which were in turn normalized to Talairach space (Talairach and Tournoux, 1988). Estimated motion plots and cine loops were examined for each participant. An in-house script was used to identify participants for whom, after removing volume acquisitions where movement between two volumes exceeded 1 mm, or integrated movement over 4 volumes exceeded 2 mm, if >25% of the data was removed from the entire experiment, or one experimental condition, a subject would be excluded. The application of this script resulted in the inclusion of all participants.
Initial, general linear model (GLM)-based analyses were conducted for each participant to assess task-related BOLD responses. Regressors were defined as boxcar functions with values of 1 during each condition and 0 otherwise, convolved with a double-gamma hemodynamic response function (HRF). Predictors depicting motion in all six parameters were included as predictors of no interest.
Whole-brain analyses
All group-level analyses were limited to voxels within the MNI brain normalized to Talairach space. This whole brain mask consisted of 1,449,746 (1 × 1 × 1 mm) voxels. Whole brain investigations were conducted using random-effects (RFX) GLM-based analyses. Analyses of each touch condition separately were assessed at a threshold of P < 0.01. This relatively lenient threshold was utilized because it allowed for the identification of a number of regions involved in the each type of touch as preliminary analysis, whereas a direct contrast of the two conditions would serve as a more rigorous assessment of condition specific response(s). In the individual touch condition relative to baseline contrasts, we corrected for multiple comparisons using cluster thresholds determined by the Brain Voyager QX Cluster-level Statistical Threshold Estimator plug-in (Forman et al., 1995; Goebel et al., 2006). After 1000 iterations of a Monte Carlo simulation, the relative frequency of each cluster size was evaluated, and the cluster size corresponding to a corrected threshold of α < 0.05 was determined for each contrast resulting in the use of k = 26 and k = 12 for the fast and slow conditions, respectively. A direct contrast of touch conditions (slow > fast) at a P < 0.01 resulted in robust continuous regions of activation. Therefore, a more stringent threshold than the P < 0.01 used in the baseline contrasts was implemented to discern distinct regions of activation. At a conservative FDR threshold of q < 0.05, implementing a cluster threshold corresponding to alpha < 0.05 resulted in the loss of the mPFC and STS regions as significantly differentiating the slow and fast touch. Given our a priori hypotheses about these regions’ involvement in processing CT-targeted touch (Gordon et al., 2011), we implemented a less conservative threshold to enable identification of ROIs in the slow vs fast contrast that allowed us to explore individual differences related to AQ and STQ. This contrast was assessed at a false discovery rate (FDR) threshold of q < 0.1 (Genovese et al., 2002). As in the initial contrasts, we implemented a cluster threshold corresponding to α < 0.05 after 1000 iterations of a Monte-Carlo simulation, (k = 34).
Functional connectivity analysis
A PPI analysis (Friston et al., 1997) was used to investigate task-related functional connectivity during the two touch conditions. As in our previous study (Gordon et al., 2011), a functionally defined mPFC region was used as a seed for the connectivity analysis, on account of its broad role in social cognition (for review, see Amodio and Frith, 2006). The seed region in the current study was the left mPFC ROI which was functionally defined in the slow > fast contrast described above. A bilateral amygdala and insula mask was used in the PPI analysis to specifically examine task-related functional connectivity in this network of regions following our previous finding of enhanced connectivity during CT-targeted touch (Gordon et al., 2011). The mask regions were anatomically defined using an in-house script based on coordinates from the Talairach database (Lancaster et al., 1997, 2000).
Prior to analysis, the global mean (averaged signal across all voxels) was removed from each volume, a method used to remove physiological artifacts (Fox et al., 2005). PPI regressors for each participant were created by multiplying the preprocessed, normalized time course from the seed region with the difference of the two task regressors convolved with the HRF. This PPI regressor, the two task regressors and the seed region time course were modeled as predictors for each participant, and in turn combined in a multi-participant random-effects GLM analysis. As described above, the multi-participant GLM analysis was limited to voxels within anatomically defined regions of bilateral insula and amygdala. The PPI function was used as the only predictor of interest, and was assessed at a threshold of P < 0.05, k = 2.
RESULTS
Pre-scan behavioral results
Six out of the 18 participants (one participant did not complete pre-scan ratings) rated both types of touch as equally pleasant (Slow mean = 3.78, s.d. = 1.03; Fast mean = 2.83, s.d. = 1.19). A paired samples t-test revealed that pleasantness ratings were significantly higher for the Slow condition, t(17) = 3.449, P = 0.003. Nonetheless, the verbal descriptions highlight the similarity of pleasant ratings of both conditions. Participants described slow touch as ‘soft, soothing’, and ‘like the soft fur of an animal’; they described fast touch as ‘soothing, calming’, and ‘like feathers’.
Eighteen of the 19 participants completed the STQ and 17 of the 19 completed the AQ. Mean STQ score was 27.83 (s.d. = 8.89) with individual scores ranging from 16 to 47. Mean AQ sore was 13.65 (s.d. = 6.11) with individual scores ranging from 6 to 27.
fMRI results
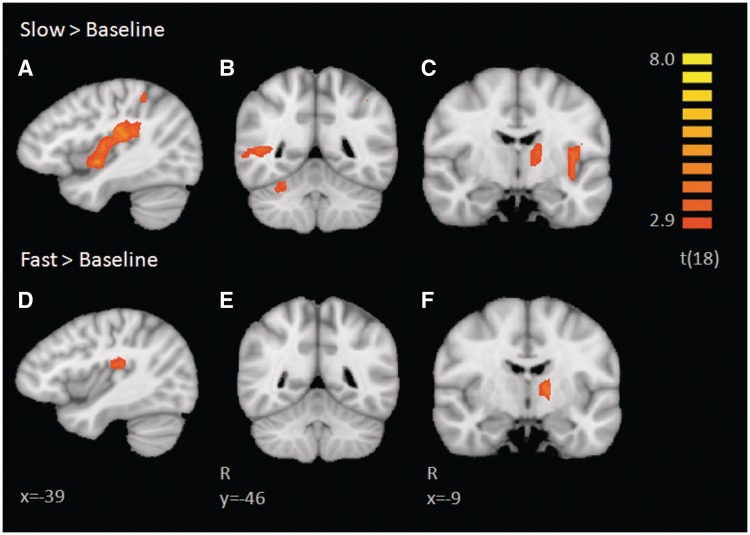
Multi-participant RFX GLM analyses were conducted for Slow > Baseline and Fast > Baseline. Results of these contrasts can be found in Tables 1 and 2, respectively. Both contrasts were assessed at a threshold of P < 0.01 corrected for multiple comparisons with cluster thresholds corresponding to α < 0.05 (Figure 1). Fast touch revealed activation in the right supramarginal gyrus (SMG), left thalamus and left posterior insular operculum. Slow touch revealed activation in the left posterior insula extending into somatosensory cortex, right pSTS, right dorsolateral prefrontal cortex (dlPFC), left intraparietal sulcus (IPS), right SMG, right anterior insula, bilateral cerebellum and left thalamus. These contrasts revealed similar activation in the left thalamus, right SMG and a small portion of overlap in the left posterior insula, with activation being much more robust and extending throughout the insula for slow touch, as illustrated in Figure 1A and D.
Table 1.
Peak coordinates, significance and extent of regions defined in the slow > baseline contrast
| Region of interest | Peak X | Peak Y | Peak Z | t(18) | P-value | Number of voxels |
|---|---|---|---|---|---|---|
| Right supramarginal gyrus | 54 | −28 | 22 | 8.09 | 0.0000 | 8520 |
| Right posterior STS | 54 | −43 | 1 | 4.88 | 0.0001 | 1838 |
| Right dorsolateral PFC | 39 | 44 | 13 | 4.91 | 0.0001 | 578 |
| Right anterior insula | 36 | 23 | 16 | 4.90 | 0.0001 | 869 |
| Right cerebellum | 21 | −61 | −23 | 4.76 | 0.0002 | 1987 |
| Right cerebellum | 18 | −55 | −42 | 5.47 | 0.0000 | 919 |
| Left thalamus | −15 | −10 | 13 | 6.12 | 0.0000 | 774 |
| Left intraparietal sulcus | −39 | −43 | 49 | 3.99 | 0.0009 | 954 |
| Left posterior insula and somatosensory | −48 | −22 | 19 | 7.02 | 0.0000 | 10 382 |
| Left cerebellum | −27 | −55 | −23 | 4.29 | 0.0004 | 403 |
P = 0.01, k = 12.
Table 2.
Peak coordinates, significance, and extent of the regions defined in the fast > baseline contrast
| Region of interest | Peak X | Peak Y | Peak Z | t(18) | P-value | Number of voxels |
|---|---|---|---|---|---|---|
| Right supramarginal gyrus | 54 | −34 | 28 | 5.22 | 0.0001 | 2315 |
| Left thalamus | −15 | −10 | 7 | 5.20 | 0.0001 | 739 |
| Left posterior insula (operculum) | −48 | −22 | 19 | 4.76 | 0.0002 | 2364 |
P = 0.01, k = 26.
Fig. 1.
Brain activations revealed in individual contrasts of slow and fast touch vs baseline. Activation in the left posterior insula is more robust during the slow touch (A, C) relative to the fast touch (D). Similarly, slow touch elicits a right superior temporal sulcus and cerebellar response (B), which is not found in the fast vs baseline contrast (E). Both types of touch elicited activation in the left thalamus (C, F).
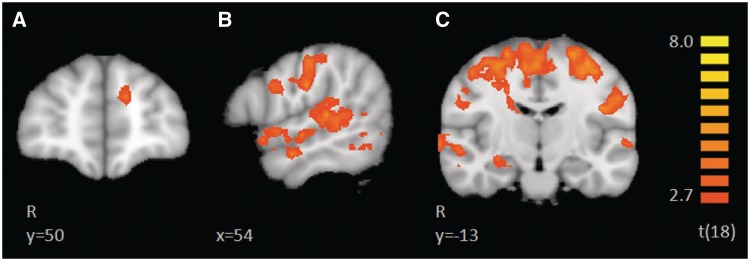
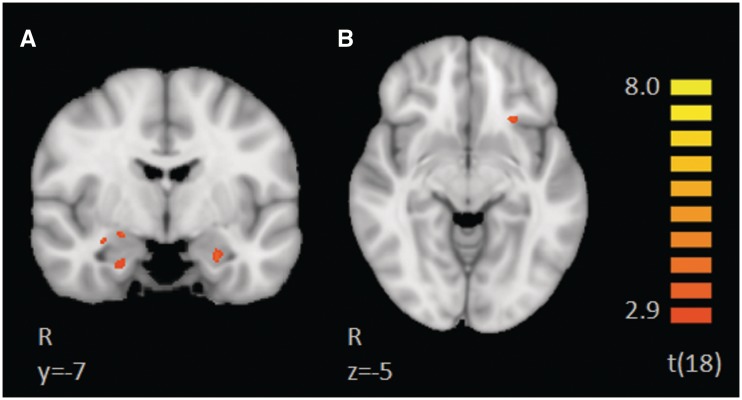
To assess the differences between CT-optimal vs non-optimal touch, we directly compared the BOLD response of slow vs fast touch, at a FDR of q < 0.1, corrected with a cluster threshold of 34. This direct contrast of slow > fast revealed greater activation to Slow touch along the entire right STS (Figure 2B and C) including the right superior temporal gyrus (STG), right amygdala (Figure 2C), right OFC, left mPFC (Figure 2A), left anterior STG, left IPS, left caudate, bilateral pre- and post-central sulcus, encompassing somatosensory regions S1 and S2, bilateral occipital cortex and portions of the cerebellum. Results of this contrast can be found in Table 3. In this contrast, no regions exhibited a greater response to fast > slow touch.
Fig. 2.
Brain activations revealed in the direct contrast of slow > fast. This contrast did not show any regions with greater activation to the fast touch. Regions with a greater BOLD response to slow touch include the left medial prefrontal cortex (A), the entire right superior temporal sulcus (B,C), the right amygdala, and bilateral parietal lobe (C).
Table 3.
Peak coordinates, significance, and extent of the regions defined in the slow > fast contrast
| Region of interest | Peak X | Peak Y | Peak Z | t(18) | P-value | Number of voxels |
|---|---|---|---|---|---|---|
| Right superior temporal sulcus/gyrus | 30 | 14 | −8 | 5.61 | 0.0000 | 9256 |
| Right amygdala | 27 | −13 | −8 | 4.37 | 0.0004 | 1043 |
| Right orbitofrontal cortex | 36 | 32 | 1 | 4.72 | 0.0002 | 1033 |
| Left anterior superior temporal gyrus | −60 | −1 | −2 | 4.91 | 0.0001 | 1387 |
| Left caudate | −15 | 11 | 10 | 5.30 | 0.0000 | 1293 |
| Bilateral pre- and postcentral sulcus | −9 | −22 | 61 | 7.02 | 0.0000 | 64059 |
| Bilateral occipital cortex | 36 | −58 | 10 | 6.22 | 0.0000 | 82848 |
| Cerebellum | 9 | −61 | −35 | 5.54 | 0.0000 | 1129 |
| Left medial prefrontal cortex | −24 | 26 | 28 | 5.04 | 0.0001 | 1716 |
| Left precuneus | −21 | −61 | 40 | 3.84 | 0.0012 | 1151 |
q = 0.1, k = 34.
Correlation analyses
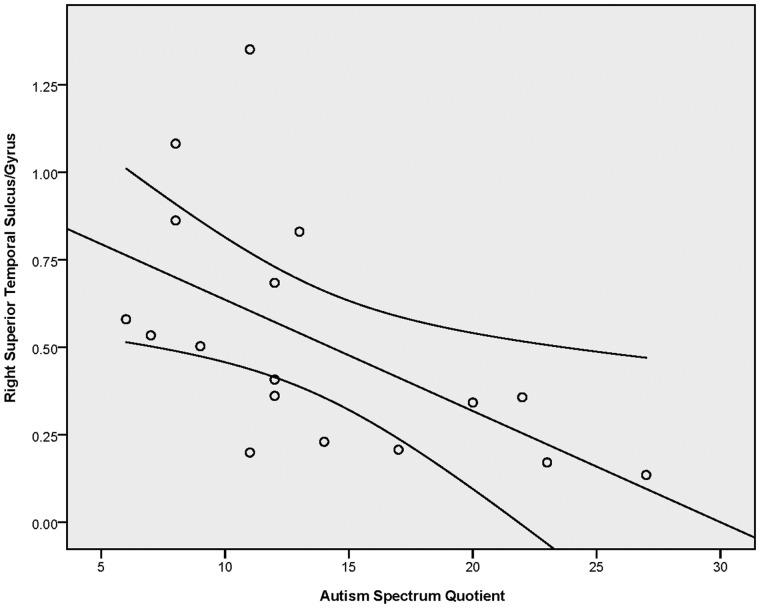
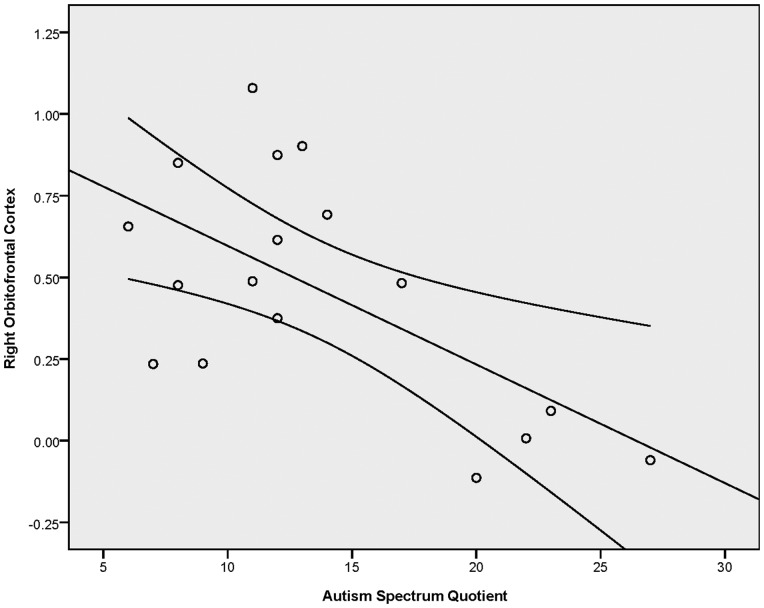
In order to investigate the relationship between autistic traits, as measured by the AQ, and neural response to CT-targeted affective touch, we conducted correlation analyses between AQ scores and the differential response to slow and fast touch in six of the ROIs identified in the Slow > Fast contrast (q < 0.1, k = 34) that have been previously implicated in social processing. Using Brainvoyager, we extracted average betas values (an index of the bold response) per condition for all functional voxels within each ROI for each participant. For each participant, we calculated a difference score reflecting difference in beta values for slow and fast touch (i.e. Slow–Fast). As indicated in Table 4, these analyses revealed negative correlations between the AQ and neural response to CT-targeted affective touch in the right STS and right OFC (see Table 4 for r- and P-values). In these regions, participants with more autistic traits exhibited a diminished differential response to affective touch whereas those with fewer autistic traits had heighted response to the CT-targeted affective touch relative to the fast touch. These correlations are illustrated in Figures 3 and 4.
Table 4.
Results of the correlation analysis for AQ scores and differential response to beta values for slow vs fast touch in the regions defined by the Slow > Fast contrast; correlations between autistic traits and brain response to affective touch
| Region of interest | Pearson correlation | P-value |
|---|---|---|
| Right superior temporal sulcus/gyrus | −0.563 | 0.018* |
| Right orbitofrontal cortex | −0.617 | 0.008** |
| Right amygdale | −0.272 | 0.292 |
| Left medial prefrontal cortex | −0.173 | 0.507 |
| Left anterior superior temporal gyrus | −0.450 | 0.070 |
| Left caudate | −0.417 | 0.096 |
*P < 0.05, ** P < 0.01
Fig. 3.
Results of the correlation analysis between AQ scores and differential beta values to slow and fast touch in the superior temporal sulcus/gyrus region (functionally defined in the slow > fast contrast). Error bars indicate 95% CI.
Fig. 4.
Results of the correlation analysis between AQ scores and differential beta values to slow and fast touch in the orbitofrontal cortex (functionally defined in the slow > fast contrast). Error bars indicate 95% CI.
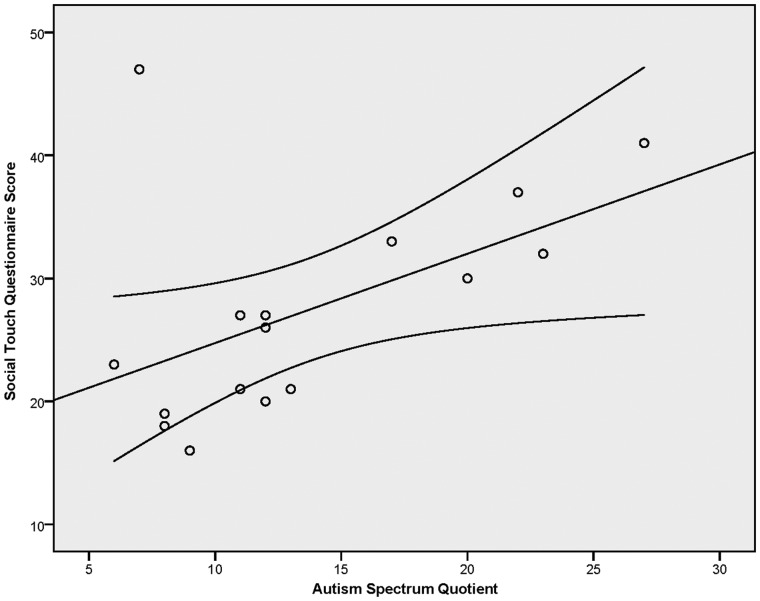
We also investigated the relationship between participants’ self-reported attitudes toward social touch, as measured by the STQ, and the neural response to affective touch. None of these correlations reached significance at the level of P < 0.05. In addition, to determine whether autistic traits are associated with self-reports of negative affect toward, and avoidance of social touch, we conducted a correlation analysis between the AQ and STQ. This analysis revealed a positive relationship between autistic traits and preference for social touch, (r(16) = 0.520, P = 0.039) illustrated in Figure 5. That is, individuals with fewer autistic traits report more positive affect toward and attitudes about social touch. Finally, we conducted post hoc analyses to examine whether pleasantness ratings correlated with brain responses to affective touch. None of these correlations were significant (all Ps > 0.05).
Fig. 5.
Results of the correlation analysis between autistic traits (AQ score) and attitudes toward social touch (STQ score). Error bars indicate 95% CI.
Functional connectivity analysis
We conducted a PPI analysis assessing task-modulated functional connectivity between a Slow > Fast functionally defined mPFC seed region and structurally defined bilateral insula and amygdala. This analysis revealed greater functional connectivity between the mPFC and regions in the right amygdala, left amygdala and left insula during the slow touch condition, as illustrated in Figure 6. Peak coordinates, statistical values, size and anatomical labels for the regions of differential functional connectivity are provided in Table 5.
Fig. 6.
Results of PPI analysis, using a left medial prefrontal cortex seed (functionally defined in the slow > fast contrast) and an anatomically defined bilateral amygdala and insula mask. The figure illustrates coactivation in the left mPFC, bilateral amygdala and left insula during CT-targeted affective, slow touch relative to fast touch, which does not target CT-afferents.
Table 5.
Results of the PPI analysis (P < 0.05, k = 2) using the left medial prefrontal cortex seed, functionally defined in the Slow > Fast contrast, and anatomically defined bilateral insula and amygdala mask
| Region of interest | Peak X | Peak Y | Peak Z | t(18) | P-value | Number of voxels |
|---|---|---|---|---|---|---|
| Right amygdala | 30 | −4 | −11 | 3.08 | 0.006425 | 99 |
| Right amygdala | 21 | −7 | −7 | 2.93 | 0.008883 | 121 |
| Right amygdala | 24 | −7 | −23 | 2.42 | 0.026108 | 86 |
| Left insula | −24 | 20 | −6 | 3.79 | 0.001343 | 58 |
| Left amygdala | −24 | −7 | −17 | 3.11 | 0.006084 | 117 |
DISCUSSION
The results of this fMRI study indicate that key nodes of the ‘social brain’ are specifically involved in processing affective touch, processed by CT-afferents. These nerves, present only in the hairy skin of mammals, respond especially well to slow, gentle touch. A recent study that compared the brain response to CT-optimal touch to the forearm and the glabrous skin of the palm revealed the involvement of the STS, insula, mPFC and amygdala in processing such touch in the forearm only, where CT-afferents are present (Gordon et al., 2011). In order to determine whether the observed differences were specific to the CT-system rather than the stimulation of different body parts, the current study included tactile stimulation of the forearm at CT-optimal and non-optimal velocities. Using whole brain direct contrasts and functional connectivity analyses, we identified a network of regions specifically involved in processing CT-targeted touch. These areas include the STS, OFC, insula, mPFC and the amygdala. An additional novel contribution of the current study is the identification of a relationship between individual’s autistic traits and the neural response to CT-targeted affective touch. Taken together, these findings help to characterize the multimodal nature of the ‘social brain’ and illustrate a tight coupling of social behavior and social brain function. Below, we discuss the implications of these findings for the broader field of social neuroscience and developmental disorders such as autism.
The brain regions found to be specifically involved in processing CT-optimal touch have been implicated in perceiving and interpreting the social world. The STS region plays an important role in understanding the people around us including the visual perception of biological motion (Grossman et al., 2000; Kaiser et al., 2010), intention understanding (Vander Wyk et al., 2009) and theory of mind (Frith and Frith, 2001; Saxe and Kanwisher, 2003). In the auditory domain, the STS has been shown to distinguish between communicative and non-communicative sounds (Belin et al., 2000; Shultz et al., submitted for publication). Our findings highlight the multimodal nature of the STS (Beauchamp et al., 2004; Barraclough et al., 2005) and extend our understanding of this brain region in social perception beyond vision and audition into the tactile domain.
The STS has been associated with processing stimulus intensity and imagination of biological motion; however, the differential response to slow and fast touch in the current study cannot be explained in this way. Beauchamp and colleagues (2008) reported that posterior STS showed an increased response to more intense auditory and tactile stimuli. If activation in this area in our study was due to the intensity of the tactile stimuli, we would predict an increased response in the STS to fast rather than slow touch. If visual imagery of the biological motion of the brusher resulted in STS activation (Grossman and Blake, 2001), we would expect to find comparable activation in this region to both types of touch, yet this region emerged in the slow vs fast contrast. To the extent that participants are imagining the biological motion in the slow and fast conditions, we hypothesize that the differential STS response is driven by the inherent social nature of the slow touch, processed by CT afferents. Finally, the STS has also been implicated in multisensory integration (Amedi et al., 2005; Beauchamp, 2005, 2008). Thus, it is possible that the STS response reflects a greater amount of sensory integration during slow vs fast touch. This speculation is based on the idea that the signals of imagining biological motion and coding of the slow touch as socially relevant combine in the STS region, as multisensory information. But studies of experiencing and imagining such touch are needed to better address the role of imagery in the fMRI results.
The mPFC has been shown to support social–cognitive processes, including self-referential (e.g. Gusnard et al., 2001) and other-inferential (e.g. Mitchell et al., 2005) tasks. Perhaps mPFC activation to slow, gentle touch reflects self-reflection elicited by affective touch (i.e. ‘How does this make me feel?’). Alternatively, or in addition, the engagement of this region may represent the reflecting on the brusher’s mental state elicited by affective touch (i.e. ‘How does this person feel about me? What does this touch mean?’). While it is difficult to determine whether participants were thinking about their own, or the brusher’s mental state, we interpret mPFC activation to slow touch to reflect sensitivity to the inherently social nature of CT-targeted affective touch (see also, Mitchell et al., 2005). It is possible that this region is automatically engaged in processing slow vs fast touch, as the former is reminiscent of meaningful social touch in everyday interactions. More generally, we speculate that the current findings demonstrate that social–cognitive processes may be elicited not only by visual, but also by tactile stimuli.
The OFC has been implicated in decoding and representing primary reinforcers such as taste and touch, guiding behavior, and more broadly in processing reward and emotion (Rolls, 2004). Most relevant to our study is the OFC’s involvement in processing reward (for review, see Rolls, 2000) and pleasant aspects of touch (Francis et al., 1999). Our findings are consistent with the suggestion, originally put forward by McGlone and colleagues (2007), that the OFC may represent the emotional connotation of touch (see also, Rolls, 2000; Rolls et al., 2003). We speculate that the lack of a correlation between pleasantness ratings and OFC response may be due to the limited range of pleasantness ratings. Nonetheless, the slow touch was rated as slightly more pleasant than fast touch; differential OFC activation may reflect the distinct ratings and associated reward of the two types of touch.
As in any fMRI study, it is important to consider the network of regions rather than individual nodes. Not only do the key regions of the ‘social brain’ such as the mPFC and insula support the processing of CT-targeted affective touch, but these regions also show functional connectivity to the amygdala during such touch. The regions identified in our PPI analysis have known connections in humans (e.g. Hampton et al., 2007) and primates (e.g. Baxter et al., 2000). We interpret the findings of differential response to the slow and fast touch in the current study to reflect the involvement of a network of regions working in concert to process CT-targeted affective touch. It has been hypothesize that the amygdala is critically involved in establishing lasting memories of emotional experiences (McGaugh, 2004), accessing motivational or affective value of stimuli (Cardinal et al., 2002) and coding the biological relevance of stimuli to guide behavior and cognition via sensitivity to the motivational, emotional and social meaning of stimuli (Adolphs 2003, 2010; Sander et al., 2003). Perhaps the amygdala codes for the biological relevance of CT-targeted affective touch and alerts other regions in the identified network to the importance of this type of touch.
The current study clarifies a network of regions supporting the processing of affective touch and the functional role of CT-afferents. Nonetheless, there are many issues for future research to examine including top-down and bottom-up influences on the social brain response to CT-targeted affective touch. The current study does not allow us to disambiguate the two. Although participants were told to focus on the touch, we cannot determine the extent to which they were successfully doing so and it is possible, and we suspect likely, that participants were thinking about the brusher during the two touch conditions—perhaps even more so during the slow touch. Indeed, top-down influences have been shown to influence the neural response to touch (McCabe et al., 2008). Therefore, a study that better controls and/or assesses what the participants are thinking about might help to clarify the functional role of the networks identified in the current study. The activation in visual areas during the slow touch is consistent with the interpretation that participants engaged in visual imagery during the CT-targeted affective touch condition, and fits nicely with research suggesting that other sensory modalities, such as sound and touch, can enhance visualization processes (Reiner, 2008). Future studies could examine the similarity in brain mechanisms for thinking about being touched and actually being touched.
Although CT-afferents are present in all neurotypical adults, it is clear from our everyday experiences that there are individual differences in seeking and responding to social touch. The current study illustrates a coupling of social touch preferences, social abilities (operationalized as autistic traits) and brain mechanisms for processing affective touch. It is not surprising, although an exciting novel finding, that individuals with more autistic traits also report an aversion to social touch, as AQ scores positively correlated with our measure of social touch preference (with higher STQ scores indicating less preference for touch). In addition, we identified a negative correlation between autistic traits and brain responses to affective touch, illustrating that individual differences in response to affect touch reflect individual differences in social characteristics. Participants with more autistic traits exhibit less activation to slow, gentle touch in the right STS and the right OFC. Notably, AQ scores negatively correlate with STS response while viewing conversations between two people (Suda et al., 2011), with STS deactivation during rest and with reduced white matter volume in this region (von dem Hagen et al., 2011). A similar negative correlation has also been found in behavioral tasks of biological motion detection (Kaiser and Shiffrar, submitted for publication; Kaiser and Shiffrar, 2010).
Taken together, these AQ correlations point to a disruption in social brain function associated with autistic traits. It is unclear if such disruptions are the result of living a ‘less social life’ or if autistic traits are a result of the associated differences in social brain function. In other words, do individuals with more autistic traits exhibit diminished response to social stimuli (at the level of the brain and behavior) because they have less experience with such information? Or, does dysfunction in social brain circuitry result in the defining features of autism (Kaiser and Pelphrey, 2011)? Alternatively, perhaps the two factors are intertwined from birth, if not before.
The current study adds to the literature on individual differences in autistic traits by demonstrating that people with a greater number of autistic traits exhibit disruptions in the neural mechanisms for processing affective touch. Future studies should examine the role of CT-nerves in the individual differences noted above. For instance, does variability in thresholds and density of CT-nerves correspond to the individual differences in brain activation found in the current study and/or to autistic traits in typical adults? Additionally, although touch processing in autism has received little empirical attention (but see Blakemore et al., 2006; Cascio et al., 2008), as described above, disruptions in the neural mechanisms for processing affective touch have been reported (e.g. Kaiser et al., 2010; Ebisch et al., 2011). The current AQ findings suggest that children with autism, a disorder characterized by social impairments, may show differences in brain mechanisms for processing CT-targeted touch. Further studies are needed to rigorously assess whether or not social dysfunction in autism extends to the tactile domain.
Social interactions in daily life often involve tactile encounters, including touching and being touched by other people. Notably, although we use all of our senses to perceive social cues, being touched by another person is a most intimate exchange; a gentle caress can convey a rich message, perhaps far exceeding that in a facial expression or quality of voice. The current study contributes to a growing literature suggesting that CT-afferent fibers represent an evolutionarily conserved mechanism for processing slow, gentle, affective touch (Morrison et al., 2010). Given the central role of touch in social–emotional development in primates (Harlow and Zimmermann, 1959; Bowlby, 1969) and human infants (Klaus et al., 1970; Barnett, 2005), it is important to study the typical and atypical development of the brain mechanisms for processing CT-targeted affective touch. While the focus in the field has been on the implications of touch, or the lack thereof, in infancy (Stack, 2001), the literature lacks a clear understanding of the mechanisms by which touch plays a critical role in emotional development and social relationships throughout the lifespan. The current study characterizes a network of regions that support the perception of affective touch processed by CT-afferents as well as specific regions of the social brain that show a diminished response to such touch in individuals with more autistic traits. This work sets the stage for future studies to explore the early development of these neural systems and disruptions associated with disorders with pathognomonic social impairments, such as autism.
Acknowledgments
We are grateful to India Morrison for helpful discussions of the results of this study and to Danielle Bolling for comments on an earlier version of this article. We thank Randi Bennett and Jeffrey Eilbott for assistance with brushing and data collection. Funding for this study came from two sources: a Harris Professorship to Kevin Archer Pelphrey and the Yale Autism Center for Excellence from the NIH (grant number: P50 MH081756; PI: K.A.P.).
REFERENCES
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4(3):165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy Science. 2010;119:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedi A, Malach R, Pascual-Leone A. Negative BOLD differentiates visual imagery and perception. Neuron. 2005;48(5):859–72. doi: 10.1016/j.neuron.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Barnett L. Keep in touch: the importance of touch in infant development. Infant Observation. 2005;8(2):115–23. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The Autism Spectrum Quotient (AQ): evidence from Asperger Syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Development Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. Journal of Cognitive Neuroscience. 2005;17(3):377–91. doi: 10.1162/0898929053279586. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20(11):4311–19. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Current Opinions in Neurobiology. 2005;15(2):145–53. doi: 10.1016/j.conb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. NeuroImage. 2008;41(3):1011–20. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41(5):809–23. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–12. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassolit T, Calo S, et al. Tactile sensitivity in Asperger syndrome. Brain and Cognition. 2006;61(1):5. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss. London: Hogarth Press and Institute of Psycho-Analysis; 1969. [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Cardinal, R.N., Parkinson, J.A., Hall, J., Everitt, B.J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews, 26(3), 321–52. [DOI] [PubMed]
- Cascio C, McGlone F, Folger S, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Development Disorders. 2008;38(1):127–37. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of Emotion. New York: Guilford Press; 2008. pp. 272–90. [Google Scholar]
- Ebisch SJH, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Human Brain Mapping. 2011;32(7):1013–28. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10(3):453–59. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith CD. The Social Brain? Philosophical Transactions: Biological Sciences. 2007;362(1480):671–78. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10(5):151–5. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society London, Series B Biological Sciences. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happen F, Brunswick N, Fletcher PC, Frith U. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD. Brain mechanisms for processing affective touch. Human Brain Mapping. 2011 doi: 10.1002/hbm.21480. DOI:10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Res. 2001;41(10–11):1475–82. doi: 10.1016/s0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, et al. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12(5):711–20. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton A, Adolphs R, Tyszka JM, O’Doherty J. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–55. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Zimmerman RR. Affectional responses in the infant monkey. Science. 1959;130(3373):421–32. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, et al. Neural signature of autism. Proceedings of the National Academy of Sciences. 2010;107(49):21223–8. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Shiffrar M. Systematic variation in sensitivity to biological motion in typical adults. J Vision. 2010;7(9):488. [Google Scholar]
- Kaiser MD, Shiffrar M, Pelphrey KA. Socially tuned: brain responses differentiating human and animal motion. Social Neuroscience. doi: 10.1080/17470919.2011.614003. DOI:10.1080/17470919.2011.614003. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11(6):417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Klaus MH, Kennell JH, Plumb N, Zuehlke S. Human maternal behavior at first contact with her young. Pediatrics. 1970;46:187–92. [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Wolof MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience. 2009;12(5):547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Losh M, Piven J. Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(1):105–12. doi: 10.1111/j.1469-7610.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- McCabe C, Rolls ET, Bilderbeck A, McGlone F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Social Cognitive Affective Neuroscience. 2008;3(2):97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh, J.L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27(1), 1–28. [DOI] [PubMed]
- McGlone F, Vallbo AB, Olausson H, Löken L, Wessberg J. Discriminative touch and emotional touch. Canadian Journal of Experimental Psychology. 2007;61(3):173–83. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Morrison I, Löken LS, Olausson H. The skin as a social organ. Experimental Brain Research. 2010;204(3):305–14. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- Morrison I, Björnsdotter M, Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speed. Journal of Neuroscience. 2011;31(26):9554–62. doi: 10.1523/JNEUROSCI.0397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5(9):900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson HW, Cole J, Vallbo A, et al. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neuroscience Letters. 2008;436(2):128–32. doi: 10.1016/j.neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:259–71. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99(1–3):164. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner M. Seeing through touch: the role of haptic information in visualization. In: Gilbert JK, Reiner M, Nakhleh M, editors. Visualization: Theory and Practice in Science Education. Berlin, Germany: Springer: 2008. [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex. 2003;13(3):308–17. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in Neurosciences. 2003;14(4):303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. NeuroImage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saygin AP. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130(9):2452. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Stack DM. The salience of touch and physical contact during infancy: unraveling some of the mysteries of the somaesthetic sense. In: Fogel A, Bremner G, editors. Blackwell Handbook of Infant Development. London: Blackwell; 2001. [Google Scholar]
- Suda M, Takei Y, Aoyama Y, et al. Autistic traits and brain activation during face-to-face conversations in typically developed adults. PLoS One. 2011;6(5):e20021. doi: 10.1371/journal.pone.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234(4774):358–61. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical; 1988. [Google Scholar]
- Vallbo, A.B., Olausson, H., Wessberg, J. (1999). Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. Journal of Neurophysiology, 81(6), 2753–63. [DOI] [PubMed]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. Action understanding in the superior temporal sulcus region. Psychoogical Science. 2009;20(6):771–7. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EAH, Nummenmaa L, Yu R, Engell AD, Ewbank MP, Calder AJ. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cerebral Cortex. 2011;21(3):493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm FH, Kochar AS, Roth WT, Gross JJ. Social anxiety and response to touch: incongruence between self-evaluative and physiological reactions. Biological Psychology. 2001;58(3):181–202. doi: 10.1016/s0301-0511(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neuroscience. 2006;29:259–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]