Abstract
Social dysfunction has been recognized as an elementary feature of schizophrenia, but it remains a crucial issue whether social deficits in schizophrenia concern the inter-subjective domain or primarily have their roots in disturbances of self-experience. Social perception comprises vicarious processes grounding an experiential inter-relationship with others as well as self-regulation processes allowing to maintain a coherent sense of self. The present study investigated whether the functional neural basis underlying these processes is altered in first-episode schizophrenia (FES). Twenty-four FES patients and 22 healthy control participants underwent functional magnetic resonance imaging during a social perception task requiring them to watch videos depicting other individuals' inanimate and animate/social tactile stimulations, and a tactile localizer condition. Activation in ventral premotor cortex for observed bodily tactile stimulations was reduced in the FES group and negatively correlated with self-experience disturbances. Moreover, FES patients showed aberrant differential activation in posterior insula for first-person tactile experiences and observed affective tactile stimulations. These findings suggest that social perception in FES at a pre-reflective level is characterized by disturbances of self-experience, including impaired multisensory representations and self-other distinction. However, the results also show that social perception in FES involves more complex alterations of neural activation at multiple processing levels.
Keywords: disturbances of self-experience, functional magnetic resonance imaging (fMRI), multisensory integration, psychosis, self-other distinction
INTRODUCTION
The constitution of an empathic inter-relationship with other individuals is a crucial component of social cognition. From a neurobiological perspective, it has been suggested that multimodal brain regions underlying bodily self-experiences are also involved with the pre-reflective understanding of the feelings and behaviours of other individuals, establishing an inter-subjective link between self and other during social perception (Gallese, 2003a, 2003b; Gallese et al., 2004; Keysers and Gazzola, 2009).
However, it was also argued that distinguishing to whom these behaviours and feelings belong is crucial in order to maintain a coherent sense of self during social perception (Batson et al., 1987; Eisenberg et al., 1989; Banissy et al., 2009). Several authors proposed that social perception does not engage a completely overlapping neural network between self and others' bodily experiences (Gallese, 2003a; Singer et al., 2004; Keysers et al., 2010; Lamm et al., 2011). Furthermore, specific brain regions involved with self-experiences and awareness (Craig, 2002; Farrer et al., 2003; Karnath et al., 2005; Tsakiris et al., 2007) rather appear to differentiate between self and other. For example, posterior insular cortex (pIC) showed opposite activation patterns for first-person tactile experiences (positive modulation) and the observation of affective tactile stimulations in other individuals (negative modulation) (Ebisch et al., 2011). In addition, overlapping activation for experienced and observed tactile stimulations is reported in brain regions that contain multisensory representations, like premotor cortex (PMC) or intraparietal sulcus, integrating motor representations with information from vision, touch, audition and proprioception (Bremmer et al., 2001; Makin et al., 2007; Ebisch et al., 2008).
Social perception may thus emerge as a multifaceted function relying on the dynamic interaction between vicarious processes grounding an experiential understanding of others' feelings, and self-regulation processes (multisensory integration, self-other differentiation), allowing a coherent and unique sense of self (Batson et al., 1987; Cheng et al., 2007; de Waal, 2008).
Schizophrenia is a pervasive and complex neuropsychiatric disorder with prominent deficits in social cognition (Pinkham et al., 2003; Burns, 2006; Derntl et al., 2009). Social impairments may exist independently of neurocognitive impairments, they are related to functional outcome and community functioning, they are present during the prodromal phase of schizophrenia as well as in unaffected family members, and persist when patients are in remission (Couture et al., 2006; Bellack et al., 2007; Addington et al., 2008a; Phillips and Seidman, 2008; Horan et al., 2009; Eack et al., 2010; Fett et al., 2011). However, the exact nature of impaired social abilities in schizophrenia remains a topic of speculation and their underlying causes unknown.
In particular, although a crucial role has been attributed to the self- and its pre-reflective relationship with the external world in schizophrenic pathology since the early 19th century (Bleuler, 1911; Minkowski, 1927), it still remains an open issue whether functional abnormalities underlying the inability to interrelate with others in schizophrenia specifically concern the inter-subjective domain or primarily have their roots in disturbances of self-experience and awareness (Parnas et al., 2002; Gallese, 2003b; Fisher et al., 2008). For example, along with the loss of a coherent sense of self, the relationship and the distinction between self and other may blur (Sass and Parnas, 2003). From a neurobiological perspective, this postulates the concrete question whether patients with schizophrenia may show altered vicarious neural activations or rather aberrant neural processes underlying multisensory integration and differentiation between self and others' bodily experiences during social perception.
Moreover, many studies have described specific impairments in the social perception of affective material in schizophrenia, including first-episode samples (Addington et al., 2008b; Huang et al., 2009; Dickey et al., 2010; Amminger et al., 2011). Often, affective perception was explicitly assessed. It remains unclear whether and how implicit social affective processing is affected in schizophrenia (Linden et al., 2010; Roux et al., 2010).
The present study aimed at investigating these issues by means of functional magnetic resonance imaging (fMRI), focusing in particular on the role of brain regions involved in processing first-person somatosensory stimulation during social perception.
Touch may play a peculiar role in this context, constituting an elementary aspect of self-awareness (Husserl, 1989; Tsakiris et al., 2007). Moreover, somatosensation is considered a crucial component of social perception and empathy (Keysers et al., 2004; Avenanti et al., 2005; Blakemore et al., 2005; Bufalari et al., 2007; Ebisch et al., 2008; Pitcher et al., 2008; Schaefer et al., 2009; Pihko et al., 2010; Wood et al., 2010; Bolognini et al., 2011; Cardini et al., 2011; Meyer et al., 2011; for a comprehensive review see Keysers et al., 2010).
For this purpose, 24 patients with first-episode schizophrenia (FES), a first manifestation of schizophrenia and an important condition to study primary aspects of the pathology without chronicity-related confounds and 22 matched healthy control (HC) participants underwent fMRI scanning during a social perception task. This task required them to watch video clips depicting actors experiencing neutral inanimate touch, or neutral or affective social touch, but without explicitly processing the sensory and affective characteristics of the depicted touch. A tactile stimulation task was added to map brain activation patterns related to first-person bodily experiences.
It could be hypothesized that FES patients during the visual perception of others' tactile experiences show aberrant activation in brain regions involved with first-person tactile experiences, for example, the somatosensory cortices. Alternatively, FES patients may show altered activation patterns in brain regions differentiating between self and other conditions, or in multisensory regions grounding a coherent self-experience, possibly correlating with self-experience disturbances. Moreover, given the abnormalities in the processing of affective material in schizophrenia, altered activation patterns could be expected in particular when affective experiences are implicated.
MATERIALS AND METHODS
Participants
Twenty-four out-patients with FES and 22 matched HC participants were included in the present study. All participating FES patients had a history of a single psychotic episode and all received a diagnosis of schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders - fourth edition (DSM-IV) criteria 6 months after the episode. Nineteen of the HC participants were the same as those reported in our previous study (Ebisch et al., 2011).
FES patients were evaluated by the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996a), rated for symptom severity with the Positive And Negative Symptom Scale (PANSS) (Kay et al., 1987), and evaluated for the presence of basic symptoms (BSs) (Klosterkötter et al., 2001) by means of the Schizophrenia Proneness Instrument (SPI-A) (Schultze-Lutter et al., 2007) by trained psychiatrists. HC participants were evaluated by means of the Structured Clinical Interview for DSM-IV Axis II personality Disorders (First et al., 1996b). Social abilities of the participants were assessed by means of the Empathy Quotient (EQ) questionnaire (Baron-Cohen and Wheelwright, 2004; Lawrence et al., 2004).
The study was approved by the local Ethics Committee. Written informed consent was obtained from all participants after full explanation of the procedure of the study, in line with the Declaration of Helsinki.
Demographic information and participant characteristics are provided in Table 1. Detailed information about participant inclusion is provided in the Supplementary Data.
Table 1.
Demographic information about the FES group and HC group
| Characteristic | FES group (N = 24) | HC group (N = 22) |
|---|---|---|
| Agea (mean ± SD) | 27.3 ± 4.8 | 27.5 ± 3.3 |
| Mean time from psychotic episode (months, mean ± SD) | 8 ± 5 | n.a. |
| Handedness scorea (mean ± SD) | 65.3 ± 18.1 | 69.3 ± 15.8 |
| Male/femalea | 16/8 | 12/10 |
| Diagnosis | (First episode) Schizophrenia | n.a. |
| Intelligence Quotient (mean ± SD) | 100 ± 8.5 | n.a. |
| EQb mean ± SD (cognitive empathyc/emotional reactivityc/social skillsd) | 38 ± 11.4 (12.4 ± 5.4/2.2 ± 5.2/6.3 ± 2.8) | 45.4 ± 9.7 (14.5 ± 4.6/14.6 ± 4.4/8.1 ± 2.3) |
| SCID-II Cluster A | n.a. | Negative |
| SCID-II Cluster B | n.a. | Negative |
| SCID-II Cluster C | n.a. | Negative |
| PANSS positive scale individual scores (mean ± SD) | 16 10 9 14 17 16 21 14 12 16 18 12 10 12 13 11 19 13 8 13 9 10 11 15 (13.3 ± 3.4) | n.a. |
| PANSS negative scale individual scores (mean ± SD) | 8 10 10 9 10 11 12 16 12 24 12 8 10 9 11 9 11 22 9 14 9 8 12 22 (12 ± 4.5) | n.a. |
| PANSS general psychopathology scale individual scores (mean ± SD) | 22 20 20 30 18 24 32 25 22 37 22 22 20 20 23 21 25 25 19 25 20 19 22 35 (23.6 ± 5) | n.a. |
| SPI-A total individual scores (mean ± SD) | 138 28 74 91 115 71 67 5 0 114 40 36 97 17 12 27 42 22 83 82 45 40 49 85 (61.1 ± 38.4) | n.a. |
| Medicatione | 6 Quetiapine, 7 Risperidone, 3 Paliperidone, 4 Aripiprazole, 3 Olanzapine 1 Drug Free | n.a. |
aNo significant differences between the HC and FEP groups.
bSignificant difference between the HC and FEP group (P = 0.02).
cNo significant differences between the HC and FEP group.
dSignificant difference between the HC and FEP group (P = 0.02).
eClorpromazine equivalent mean dose = 422 mg/die SD = 395.5 (calculated on 21 patients because no equivalents are available for paliperidone).
SCID-II, Structured Clinical Interview for DSM-IV Axis II personality Disorders; n.a., not applicable.
fMRI data acquisition and paradigm
For each subject, whole-brain blood oxygen level-dependent (BOLD) contrast fMRI was performed (Philips Achieva 1.5T at the ITAB, Chieti). fMRI data acquisition parameters and procedures were the same as those described in Ebisch et al. (2011). A detailed description can be found in the Supplementary Data.
Four rapid event-related fMRI touch observation runs (social perception task) and one block-design fMRI touch experience run (tactile stimulation task) were acquired for each subject.
The stimuli of the social perception task consisted of 208 randomized video clips of 2400 ms each, representing a touch event according to one of four experimental conditions. In the neutral inanimate condition (BRANCH), a wind-moving palm tree branch, moved by an invisible fan, touched either a male or female hand. In the animate or social conditions, either a male hand touched the back of a female hand or a female hand touched the back of a male hand by means of a neutral social touch (NEUTRAL) or an affective social touch that could have a negative (HIT) or positive (CARESS) valence. In addition to the touch video clips, 31 no touch video clips were randomly inserted in the visual runs, showing either a hand or palm tree branch moving near the hand of the other person, but without touching it. The video clips were separated by a fixation cross at the centre of the screen with a randomized duration (2400, 4800 and 7200 ms). Examples of the visual stimuli and the temporal course of the touch observation runs are visualized in Figure 1.
Fig. 1.

Visual representation of the temporal course of the touch observation runs.
Participants were instructed to watch the video clips attentively during scanning and to mentally count the number of no touch video clips in every run. They had to report the counted number verbally to the experimenter during the break between two runs (mean = 8/run). The no touch trials were not included in the statistical analyses; this task was added to direct participants' attention to the touch during the experiment, without requiring an explicit processing of the sensory and affective characteristics of the depicted touch. fMRI runs with more than two omissions were excluded from data analysis (HC:2/FES:0). There was no significant difference between the HC and the FES group with respect to task performance (P > 0.05).
During the tactile stimulation task, always run after the touch observation runs, the experimenter stimulated the back of either the right or the left hand by means of 1 Hz soft back and forth stroking with a washing glove covering the surface between the wrist and the knuckles.
fMRI data analysis
The fMRI preprocessing procedure was the same as described in Ebisch et al. (2011). A detailed description can be found in the Supplementary Data.
After a percent signal change normalization of the time series from the different runs, the fMRI parameters (β-values) estimated in individual-subject analysis were entered in a second-level voxel-wise random effects group analysis in order to search for activated voxels in relationship with the experimental paradigms (social perception task, tactile stimulation task). The fixation cross was used as baseline in the analysis of task-related BOLD response modulations. Statistical significance was assessed by means of paired t-tests.
The P-value of the statistical maps (P < 0.001 for touch observation, touch experience and conjunction analyses; P < 0.005 for between-group contrasts) and an estimate of the spatial correlation of voxels were used as input in a Monte–Carlo simulation (1000 simulations) to access the overall significance level and to determine a cluster size threshold (k) in order to obtain a significance level of P < 0.05 cluster level corrected for multiple comparisons (Forman et al., 1995).
Conjunction analysis
In order to determine overlapping or differential activation between the social perception task (touch observation) and the tactile stimulation task (touch experience), conjunction analysis was performed [(contrast: any touch observation condition vs baseline) ∩ (tactile stimulation vs baseline)] (Nichols et al., 2005), separately for the HC and the FES group. Overlapping activation was defined as a positive modulation of BOLD response, compared with baseline, by both the social perception and tactile stimulation task, whereas differential activation was defined as a positive modulation of BOLD response by one task, compared with baseline, and a negative modulation by the other task (P < 0.05 corrected, k > 5).
Between-group comparisons: voxel-wise analysis
Group statistical maps of the HC and FES group were compared for the social perception task (any touch observation condition vs baseline) by means of a voxel-wise random-effects analysis. In order to focus specifically on voxels activated by the tactile stimulation task as well as the social perception task, an inclusive mask was created including the voxels that were significantly active during the tactile stimulation task (P < 0.05 corrected, k > 5). This mask was based on significant voxels in either the HC or FES group in order to avoid a bias that may emerge when using a mask based on one of the groups for subsequent between-group contrasts. Voxel-wise between-group contrasts were also performed without a mask (P < 0.05 corrected, k > 8).
Between-group comparisons: pIC regions-of-interest analysis
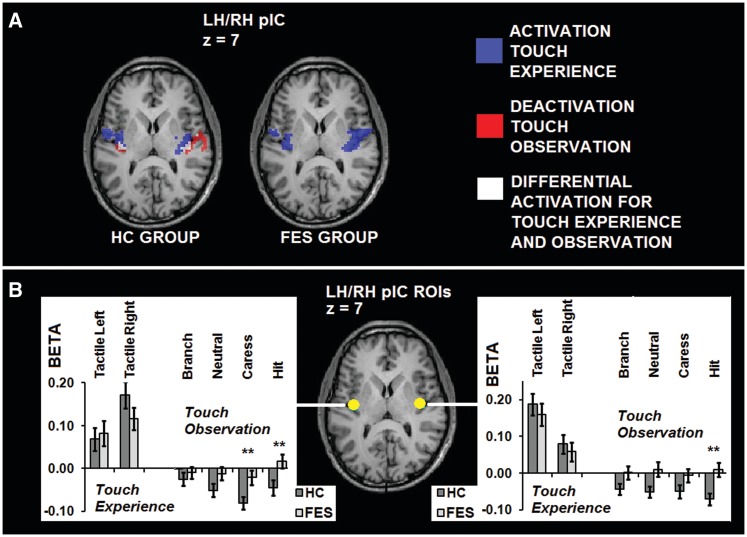
Ebisch et al. (2011) showed that pIC differentiates between self and other conditions when affective experiences are implicated, whereas schizophrenia has been associated with a dysfunctional social perception of affective information as well as an altered self-other distinction, the latter also related to impaired pIC functioning (see ‘Introduction and Discussion’ section). Given that differential activation for touch experience and observation in pIC was clearly present in the HC group, but absent in the FES group (see ‘Conjunction analysis’ under ‘Results’ section; Figure 2A), it was investigated more specifically whether FES patients showed significant alterations concerning the differentiation between self and other during social perception in pIC.
Fig. 2.
(A) Conjunction analysis. Group statistical maps (P < 0.05 corrected) of the HC and FES group depicting positive BOLD modulation clusters for the tactile simulation task (blue), negative BOLD modulation for the social perception task (red; absent in FES) and significant differential activation patterns for touch experience and observation (white; absent in FES) in pIC. (B) ROI-based analysis. Graphs showing activation patterns (β-values) for the different touch experience and observation conditions in the ROIs in pIC, and between-group differences (**P < 0.01).
Independent regions-of-interest (ROI) were created with a 6-mm radius (Poldrack, 2007; Bastiaansen et al., 2011). These ROIs were centred on Talairach coordinates based on structural definitions of pIC (LH pIC: −38, −12, 7; RH pIC: −38, −10, 7; Taylor et al., 2009; Figure 2B). Analysis of variance was performed with diagnosis (HC/FES) as between-subject factor, observation condition (BRANCH/NEUTRAL/CARESS/HIT) as within-subject factor, and the average β-values extracted from the pIC ROIs as dependent variable.
Covariance analysis
β-Values of the individual patients extracted from the brain regions showing differential activation patterns between the HC and FES group were correlated with chlorpromazine equivalence values.
The relationship between BOLD responses during the social perception task in the FES group and symptom severity was investigated by means of voxel-wise covariance analyses between activation patterns for the different touch observation conditions, and PANSS and total SPI-A scores. In order to focus on brain regions also involved in first-person tactile experiences, group statistical maps (P < 0.05 corrected) of the tactile experience task in the HC and FES group were used as an inclusive mask. Covariance group statistical maps were thresholded at P < 0.05 corrected (k > 8), corresponding to a correlation coefficient of r > 0.50.
RESULTS
Conjunction analysis in the HC and FES group
Group statistical maps showed overlapping activation in the HC group between the touch experience and touch observation conditions in left hemisphere (LH) anterior and posterior secondary somatosensory cortex (a/pSII), ventral postcentral gyrus (vPostCG) and anterior superior parietal cortex (aSPC), right hemisphere (RH) ventral PMC (vPMC) and posterior superior temporal cortex (pST), and bilateral occipital–temporal cortex (OT) (Table 2). At an uncorrected statistical threshold (P < 0.001), overlapping activation was detected also in RH pSII and aSPC, and LH mid cingulate cortex (MCC).
Table 2.
Brain regions showing overlapping or differential activation for experienced and observed tactile stimulation in the HC and first-episode psychosis group as detected by conjunction analysis
| Brain region | HC |
First-episode schizophrenia |
||||
|---|---|---|---|---|---|---|
| Talairach coordinates of peak t-value | Cluster size | Peak t-value conjunction | Talairach coordinates of peak t-value | Cluster size | Peak t-value conjunction | |
| LH aSII | −57, −19, 19 | 1304 | 6.308 | −47, −27, 19 | 1608 | 5.638 |
| LH pSII | −45, −28, 22 | 272 | 5.199 | |||
| LH vPostCG | −57, −22, 34 | 1392 | 5.063 | −54, −22, 31 | 503 | 4.880 |
| LH aSPC | −33, −43, −46 | 1582 | 5.667 | −33, −46, 47 | 81 | 4.264 |
| LH TO | −51, −59, −2 | 1656 | 6.544 | – | – | – |
| RH vPMC | 51, 8, 28 | 214 | 4.540 | – | – | – |
| RH pST | 54, −37, 16 | 839 | 5.315 | 59, −30, 19 | 242 | 4.481 |
| RH TO | 51, −54, 4 | 487 | 4.373 | 40, −58, 4 | 456 | 5.117 |
| RH aSPC | 33, −42, 46 | 97 | 4.514 | 30, −42, 48 | 391 | 4.652 |
| RH pSII | 49, −27, 22 | 54 | 4.185 | – | – | – |
| LH MCC | −9, −27, 43 | 108 | 4.064 | – | – | – |
| LH pIC | −37, −17, 11 | 613 | −6.972 | – | – | – |
| RH pIC | 44, −13, 11 | 1266 | −5.299 | – | – | – |
Positive t-values indicate overlapping activation, whereas negative t-values indicate differential activation patterns for the touch experience and observation conditions.
Significant differential activation between the experience and observation of touch in the HC group was found in bilateral pIC (Table 2; Figure 2A and B). Whereas BOLD response in bilateral pIC was increased, compared with baseline, during the experience of touch, BOLD response was decreased, compared with baseline, during the observation of touch.
In the FES group, overlapping activation between the experience and observation of touch was partly similar to the HC group; no overlapping activation was found in LH temporal–occipital junction (TO) and MCC, and RH vPMC and RH pSII (Table 2). The absence of overlapping activation in LH MCC and RH vPMC might be explained by a lack of activation during both the touch experience condition and the touch observation conditions. The absence of overlapping activation in LH TO might be due to a lack of activation for the touch experience condition and in RH pSII due to a lack of activation for the touch observation conditions.
Moreover, no differential activation between the experience and observation of touch was found in bilateral pIC in the FES group, even at P < 0.01 uncorrected. Whereas pIC was positively modulated by the touch experience condition, modulation of activation in pIC during the touch observation conditions was absent in the FES group (Figure 2A and B).
FMRI results regarding the touch experience and observation conditions separately in the HC and FES group are described in detail in the Supplementary Data.
Between-group comparisons: voxel-wise analysis
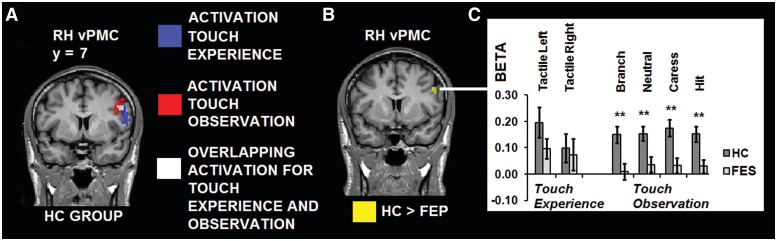
Voxel-wise contrasts including voxels that were also active during the tactile stimulation task yielded significantly increased activation in RH vPMC for the HC group, compared with the FES group, for the social perception task (Talairach coordinates: 50, 7, 32; cluster size = 13). Group statistical maps and graphs of the between-group contrasts within the touch experience mask are shown in Figure 3A–C.
Fig. 3.
(A) Conjunction analysis. Group statistical map (P < 0.05 corrected) of the HC group depicting activation clusters for the tactile stimulation task (blue), social perception task (red) and overlapping activation patterns for touch experience and observation (white; all absent in FES) in vPMC. (B) Masked voxel-wise analysis. Voxels showing significantly reduced activation in the FES group (yellow) in RH vPMC during the social perception task. (C) Graphs showing activation patterns (β-values) for the different touch experience and observation conditions in RH vPMC, and between-group differences (*P < 0.05; **P < 0.01).
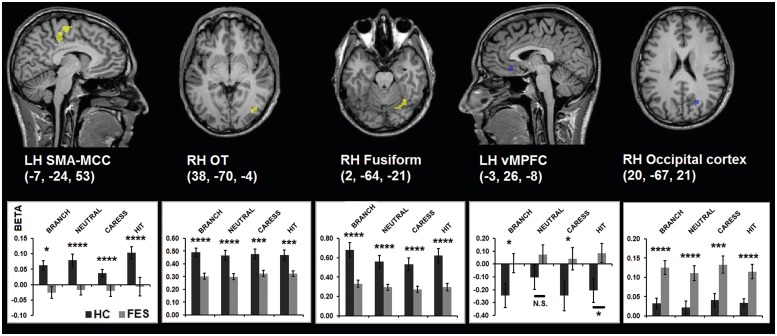
Whole-brain voxel-wise contrasts showed additionally increased activation for the HC group in RH OT, RH fusiform gyrus and LH MCC/supplementary motor area (SMA), whereas increased activation for the FES group, compared with the HC group, was found in LH ventromedial prefrontal cortex (vMPFC) and RH occipital cortex (Figure 4).
Fig. 4.
Whole-brain voxel-wise contrasts. Group statistical maps (P < 0.05 corrected) depicting different neural activation patterns during the social perception task between the HC and FES group in brain regions not activated by the tactile stimulation task, and corresponding graphs representing neural activation (β-values) for the different touch observation conditions and groups (*P < 0.05; ***P < 0.005; ****P < 0.001).
Between-group comparisons: pIC ROI analysis
Between-group comparisons concerning the pIC ROIs demonstrated significant differences between the HC and FES group: a significantly stronger BOLD response decrease was found during the social perception task for the HC group, compared with the FES group [main effect LH pIC: F(1,44) = 5.990, P < 0.01; main effect RH pIC: F(1,44) = 4.975, P < 0.05].
With respect to the individual touch observation conditions, a significantly stronger BOLD signal decrease was found in LH pIC in the HC group, compared with the FES group, specifically for the observation of affective touch, that is, CARESS [F(1,44) = 8.624, P < 0.005] and HIT [F(1,44) = 6.680, P < 0.01]. Concerning RH pIC, between-group comparisons showed a significantly stronger BOLD signal decrease in the HC group, compared with the FES group, for the observation of a HIT [F(1,44) = 6.992, P < 0.01].
Graphs demonstrating the β-values of the individual conditions in both groups in the pIC ROIs are demonstrated in Figure 2B.
Covariance analyses
No significant covariance effect was found for chlorpromazine equivalences in the FES group, suggesting that there was no linear relationship between medication dose and differential activation between the HC and FES group.
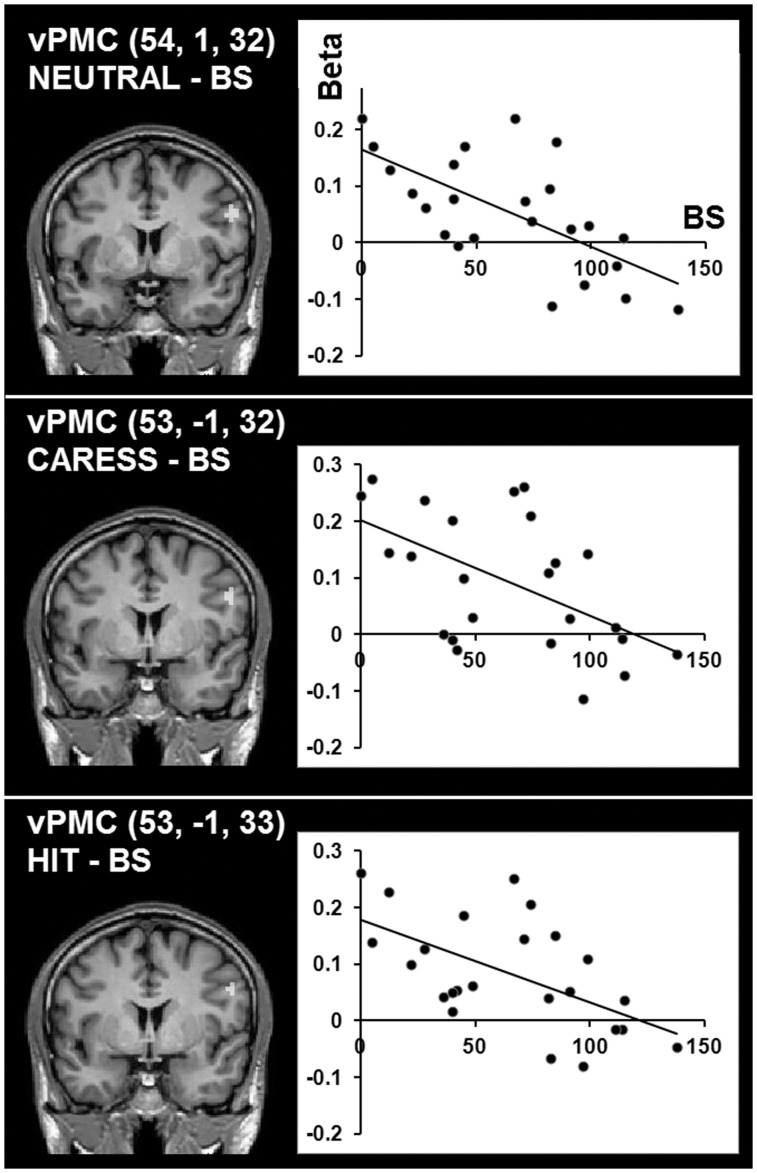
Voxel-wise covariance analyses showed a significant negative relationship between BS and brain activation in the FES group during the observation of a social touch (NEUTRAL/CARESS/HIT; P < 0.05 corrected) in RH vPMC: the higher the total score on the SPI-A, the weaker BOLD response in RH vPMC (Figure 5). No significant correlations were observed for the observation of an inanimate touch (BRANCH) or PANSS scores.
Fig. 5.
Group statistical maps and corresponding scatter plots showing significant correlations (P < 0.05 corrected, r > 0.51) between BS of the FES patients and neural activation (β-values) in RH vPMC during the different social touch observation conditions.
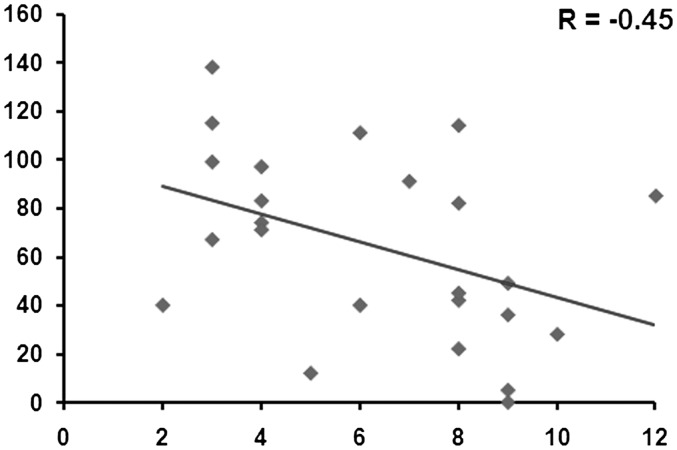
Concerning the EQ questionnaire, significantly lower total EQ scores (P < 0.05) and social skill subscale scores (P < 0.05) were found for the FES group, compared with the HC group (Table 1). A significant correlation was found in the FES group between total SPI-A score and the Social Skill subscale score (r = −0.45, P < 0.05; Figure 6).
Fig. 6.
Correlation between social skill score (x-axis) and BS severity (y-axis).
DISCUSSION
The current study aimed at investigating the neural mechanism underlying pre-reflective social perception in FES. In a previous neuroimaging study, it was shown that a neural network involved in first-person tactile experiences may underpin the sharing of others' bodily feelings on the one hand, and, on the other hand, self-related processes concerning multisensory integration and self-other distinction during social perception (Ebisch et al., 2011). Here, the same experimental paradigm was used to answer the question whether in FES, compared with HC participants, functional abnormalities during social perception of other individuals' affective tactile stimulation specifically concern the intersubjective domain or primarily have their roots in disturbances of self-experience.
vPMC and a coherent sense of self
With respect to brain regions showing overlapping activation for touch observation and experience, a significant difference between the HC and FES group was found for all the touch observation conditions in RH vPMC. BOLD response was significantly weaker in the FES group in all cases.
The coordinates of the vPMC cluster in the present study (50,7,32) are strikingly similar to the coordinates reported by other studies: 52,10,30 (Bremmer et al., 2001); 53,11,12 (Makin et al., 2007). This suggests that this region reflects the putative human homologue of monkey premotor area F4 (Bremmer et al., 2001; Buccino et al., 2001; Galati et al., 2001; Makin et al., 2007; Serino et al., 2011). Indeed, consistent with the multisensory properties of F4, in the present study this region responded to visual as well as somatosensory events in the HC group (Rizzolatti et al., 1981a, 1981b; Bremmer et al., 2001). It has been proposed that this region could be involved in the integration of multisensory information from vision, touch and proprioception onto the motor representations of different body parts (Fogassi et al., 1996a, 1996b; Graziano, 2001; Rizzolatti et al., 2002).
In the present study, activation in vPMC for the experience and observation of touch likely reflects a monitoring/integration of multisensory information, including proprioceptive, visual and tactile self-experiences, related to one's body in different situations. Adequate self-monitoring of multisensory information is crucial for the experience of a coherent sense of self and other (Parnas et al., 2002).
The lack of activation in RH vPMC in the FES group would suggest a disruption of an integrated multisensory representation of the bodily self. Interestingly, activation in this region in the FES group correlated negatively with BS severity: activation in vPMC decreased with augmented symptom severity. This relationship was consistently found between the social touch observation conditions and BS severity, but not with the degree of positive or negative symptoms. BS represent subjective experiential disturbances in the domains of cognition, perception, bodily experience, action and emotion (Huber, 1983; Klosterkotter, 1992). Therefore, the present results support a close link between impaired multisensory representations and a disrupted sense of a coherent self in everyday life (Parnas et al., 2002). A breakdown of self-monitoring has been suggested before in schizophrenia (Frith, 1987; Blakemore et al., 2000; Vinogradov et al., 2008) and vPMC could be a key structure underlying this link.
Such alteration could reasonably lead to the blurring of self boundaries and confusion in the inter-relationship with others (Sass and Parnas, 2003). Indeed, it was reported that schizophrenic patients with high self-monitoring skills had better social skills (Ihnen et al., 1998). This interpretation offers an intriguing hypothesis for further investigations regarding the multisensory properties of vPMC in relationship with self-experience disturbances. Moreover, given that BS remain stable during the entire disease progression, including the prodromal phase of schizophrenia, the study of their relationship with cortical processes and social deficits would be useful also from a clinical point of view, especially in the light of preventive approaches and an early diagnosis of schizophrenia (Addington et al., 2008a; Schultze-Lutter, 2009).
pIC and self-other distinction
A second brain region of interest where differences were identified between the HC and FES group was pIC. In the HC group, BOLD response in pIC was positively modulated, compared with baseline, during first-person experience of touch, but negatively modulated (deactivated), compared with baseline, during the observation of touch in another individual. In contrast, differential activation for first-person touch experiences and the observation of touch in another individual was absent in the FES group; no deactivation was found in pIC during the observation of touch, though normal activation patterns were found in pIC for first-person touch experiences. Deactivation in pIC in the HC group was specific for the observation of social affective touch (Ebisch et al., 2011). Accordingly, significant differences in LH pIC between the HC and FES group were found specifically for the observation of affective social touch, either with a positive (caress) or with a negative (hit) valence in LH pIC. Significant differences in RH pIC between the HC and FES group were found specifically for the observation of a hit.
pIC is considered a central brain region for interoception (Craig, 2002). Thalamo-cortical pathways may provide a direct representation of homeostatic afferent information to pIC that interacts with limbic, somatosensory and motor regions, subserving the awareness of bodily feelings (Augustine, 1996; Saper, 2002; Critchley, 2005; Craig, 2009). Regarding the cutaneous senses, pIC could constitute the primary cortical locus of an interoceptive system regulating threatening (Craig, 2002) or comforting (Olausson et al., 2002; Loken et al., 2009) information from the skin.
In accordance with the idea of pIC as a central cortical node in a system constituting a neural representation of ‘the material me’ (Craig, 2002), empirical evidence also suggests that pIC is involved in self-awareness. A PET study in healthy adults showed a positive relationship between the subjective experience of the rubber hand illusion (RHI: a condition in which an observed rubber hand is subjectively experienced as if it actually were one's own hand; Botvinick and Cohen, 1998) and neural activation in pIC (Tsakiris et al., 2007). pIC has also been related to body part awareness in anosognosia patients with hemiplegia/hemiparesis (Karnath et al., 2005), and to the sense of agency (Farrer et al., 2003).
Finally, fMRI evidence suggests that pIC is involved in social perception. Opposite activation patterns for the experience and observation of touch suggest that pIC differentiates between self and other conditions during social perception when affective experiences are implicated (Ebisch et al., 2011). An inhibitory mechanism at the level of pIC during social perception may facilitate the observer to distinguish at the phenomenal level to whom the observed tactile experience belongs.
Hence, the reduced BOLD suppression in pIC reported here in FES could indicate an impaired differentiation between self and other conditions during social perception. We propose that the absence of deactivation in pIC during the observation of touch in another individual in FES reflects a deficit in the pre-reflective suppression of self-oriented affective arousal, which likely normally contributes to the differentiation between self and other conditions.
Indeed, previous studies in schizophrenia revealed confusion in the attribution of events in the external world to their origin (Bentall et al., 1991; Blakemore et al., 2000; Franck et al., 2001; Vinogradov et al., 2008; Voss et al., 2010). A relationship has been demonstrated between aberrant pIC activation and an impaired sense of agency in schizophrenia (Farrer et al., 2004). Furthermore, the RHI, associated with pIC functioning (Tsakiris et al., 2007), has been found enhanced in schizophrenic pathology (Peled et al., 2000; Morgan et al., 2011).
It remains unclear what may be the dysfunctional neural mechanism underlying the observed reduced suppression of BOLD response in pIC during affective touch observation in FES. Possibly, it could be based on altered connections involved in top–down control processes. Future (functional) connectivity studies are urged to elucidate this issue.
Between-group differences in other brain regions
Some differences could be observed between HC and FES in brain regions not involved in first-person tactile experiences, too. Stronger activation in the HC group during the observation of others' tactile experiences, compared with the FES group, was found in higher order visual areas (ventral fusiform and OT cortex; Downing et al., 2006; Peelen and Downing, 2007) and regions underlying action–perception coupling (MCC/SMA; Vogt, 2005; Dayan et al., 2007). These results may suggest a reduced involvement of brain regions related to the visual and multimodal processing of bodies, animate objects and motion in FES. In contrast, increased activation in the FES group, compared with the HC group, in occipital cortex during the observation of the touch videos, may indicate a stronger involvement of low-level visual cortices in FES patients.
Finally, weaker deactivation was observed in vMPFC in the FES group, compared with the HC group, during touch observation. vMPFC has been associated with the coding of the self-relatedness of stimuli (Northoff, 2007), self-regulation (Heatherton, 2011) and dysfunctional self-processing in schizophrenia (van der Meer et al., 2010). However, since vMPFC often is characterized by task-negative activation patterns (Gusnard and Raichle, 2001), possibly indicating increased self-related processing during periods of rest, it remains to be established whether the observed decreased deactivation in vMPFC in FES may be caused by a reduced activity during periods of rest (fixation cross) or by a failure to suppress vMPFC activity during touch observation (Northoff, 2007). Whereas the former can be interpreted as dysfunctional self-related processes, the latter may suggest that FES patients fail to disengage self-related processes during social perception or to distinguish between self and other conditions.
General discussion and conclusions
In conclusion, the present study supports the hypothesis that social perception at a pre-reflective level in FES is primarily characterized by altered neural activation patterns underlying disturbances of self-experience and self-related processing, concerning both self-other distinction and a multimodal representation of the bodily self. No abnormalities were found in FES with respect to shared activation between experienced and observed touch in somatosensory cortices. Moreover, significant involvement of anterior insula for the hit and caress videos in the HC as well as in the FES group (see Results in Supplementary Data) could be indicative for distinguishing at the neural level the affective visual touch stimuli from the neutral stimuli in both groups (Menon and Uddin, 2010).
Nevertheless, self-reported social skills scores regarding an intuitive understanding of social situations were significantly reduced in the FES group and negatively correlated with BS, reflecting disturbed subjective self-experiences. BS also negatively correlated with neural activation in RH vPMC in the FES group. Therefore, self-experience disturbances in schizophrenia may extend to the social domain as well (Parnas et al., 2002; Gallese, 2003b; Sass and Parnas, 2003). For example, an incoherent sense of one's self-experiences accompanied by the loss of grip on the world may lead to a fading of the very distinction between self and other, and an incapacity to intuitively grasp the meaning of social situations.
Differently from previous studies (Kohler et al., 2000; Phillips et al., 2003; Marjoram et al., 2005; Shean et al., 2005), no relationship was detected between brain activation during social perception, social abilities and positive and negative symptoms. A possible explanation for this is that the included patients had a very recent illness onset and relatively low PANSS scores. Further studies will be needed to investigate patients with more pronounced positive and negative symptoms as well as chronic samples for a better understanding of the relationship between psychotic symptoms, altered social perception, and its progress over time.
There is growing evidence that social cognition deficits are related to social dysfunction in schizophrenia. The present results shed new light on the cortical basis of how self-experience disturbances in schizophrenia pervade the social domain at a relatively early stage. However, the present findings also depict dysfunctional social perception in schizophrenia as a complex impairment at multiple neural processing levels, rather than being confined to brain regions involved with first-person bodily (tactile) experiences.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by the EU grant TESIS (Towards an Embodied Science of InterSubjectivity) and the EU project ROSSI (Emergence of communication in RObots through Sensorimotor and Social Interaction; Grant agreement no. 216125) to V.G.
REFERENCES
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Social functioning in individuals at clinical high risk for schizophrenia. Schizophrenia Research. 2008a;99:119–24. doi: 10.1016/j.schres.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for schizophrenia. British Journal of Psychiatry. 2008b;192:67–8. doi: 10.1192/bjp.bp.107.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, et al. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophrenia Bulletin. 2011 doi: 10.1093/schbul/sbr015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Banissy MJ, Cohen KR, Maus GW, Walsh V, Ward J. Prevalence, characteristics and a neurocognitive model of mirror-touch synaesthesia. Experimental Brain Research. 2009;198:261–72. doi: 10.1007/s00221-009-1810-9. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, et al. Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biological Psychiatry. 2011;69:832–8. doi: 10.1016/j.biopsych.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA, Paduano A. Critical self-reflection and self-perceived altruism: when self-reward fails. Journal of Personality and Social Psychology. 1987;53:594–602. doi: 10.1037//0022-3514.53.3.594. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Green MF, Cook JA, et al. Assessment of community functioning in people with schizophrenia and other severe mental illnesses: a white paper based on an NIMH-sponsored workshop. Schizophrenia Bulletin. 2007;33:805–22. doi: 10.1093/schbul/sbl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall RP, Baker GA, Havers S. Reality monitoring and psychotic hallucinations. British Journal of Clinical Psychology. 1991;30:213–22. doi: 10.1111/j.2044-8260.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128:1571–83. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychological Medicine. 2000;30:1131–9. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox oder Gruppe der Schizophrenien. In: Aschaffenburg G, editor. Handbuch der Psychiatrie. 1911. Spezieller Teil, 4. Abteilung, 1. Hälfte. Leipzig: Deuticke. Translated by Zinkin, J., Lewis, N.D.C. (1950) in: Dementia Praecox or the Group of Schizophrenias. New York: International University Press. [Google Scholar]
- Bolognini N, Rossetti A, Maravita A, Miniussi C. Seeing touch in the somatosensory cortex: a TMS study of the visual perception of touch. Human Brain Mapping. 2011 doi: 10.1002/hbm.21172. doi: 10.1002/hbm.21172 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–96. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–4. [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di RF, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5:77–81. [PMC free article] [PubMed] [Google Scholar]
- Cardini F, Costantini M, Galati G, Romani GL, Làdavas E, Serino A. Viewing one's own face being touched modulates tactile perception: an fMRI study. Journal of Cognitive Neuroscience. 2011;23:503–13. doi: 10.1162/jocn.2010.21484. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;32:S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Dayan E, Casile A, Levit-Binnun N, Giese MA, Hendler T, Flash T. Neural representations of kinematic laws of motion: evidence for action-perception coupling. Proceedings of the National Academy of Science USA. 2007;104:20582–7. doi: 10.1073/pnas.0710033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FB. Putting the altruism back into altruism: the evolution of empathy. Annual Review of Psychology. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, et al. Generalized deficit in all core components of empathy in schizophrenia. Schizophrenia Research. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Dickey CC, Morocz IA, Minney D, et al. Factors in sensory processing of prosody in schizotypal personality disorder: an fMRI experiment. Schizophrenia Research. 2010;121:75–89. doi: 10.1016/j.schres.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cerebral Cortex. 2010;16:1453–61. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, et al. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia Bulletin. 2010;36:1081–8. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Ferri F, Salone A, et al. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. Journal of Cognitive Neuroscience. 2011;23:1808–22. doi: 10.1162/jocn.2010.21551. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Perrucci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V. The sense of touch: embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. Journal of Cognitive Neuroscience. 2008;20:1611–23. doi: 10.1162/jocn.2008.20111. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Miller PA, et al. Relation of sympathy and personal distress to prosocial behavior: a multimethod study. Journal of Personality and Social Psychology. 1989;57:55–66. doi: 10.1037//0022-3514.57.1.55. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Frith CD, et al. Neural correlates of action attribution in schizophrenia. Psychiatry Research. 2004;131:31–44. doi: 10.1016/j.pscychresns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–33. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV Axis I Disorders-Research Version (SCID I, version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996a. [Google Scholar]
- First MB, Gibbon M, Spitzer RL. Structured Clinical Interview for DSM IV Axis II-Personality Disorders (SCID II,Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996b. [Google Scholar]
- Fisher M, McCoy K, Poole JH, Vinogradov S. Self and other in schizophrenia: a cognitive neuroscience perspective. American Journal of Psychiatry. 2008;165:1465–72. doi: 10.1176/appi.ajp.2008.07111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. Coding of peripersonal space in inferior premotor cortex (area F4) Journal of Neurophysiology. 1996b;76:141–57. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Fadiga L, Rizzolatti G. Space coding in inferior premotor cortex (area F4): facts and speculations. In: Lacquaniti F, Viviani P, editors. NATO ASI Series: Multi-Sensory Control of Movement. Dordrecht: Kluwer; 1996a. pp. 99–120. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Franck N, Farrer C, Georgieff N, et al. Defective recognition of one's own actions in patients with schizophrenia. American Journal of Psychiatry. 2001;158:454–9. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]
- Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychological Medicine. 1987;17:631–48. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- Galati G, Committeri G, Sanes JN, Pizzamiglio L. Spatial coding of visual and somatic sensory information in body-centred coordinates. European Journal of Neuroscience. 2001;14:737–46. doi: 10.1046/j.0953-816x.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- Gallese V. The manifold nature of interpersonal relations: the quest for a common mechanism. Philosophical Transactions of the Royal Society A. 2003a;358:517–28. doi: 10.1098/rstb.2002.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003b;36:171–80. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Graziano MS. Is reaching eye-centered, body-centered, hand-centered, or a combination? Reviews in the Neurosciences. 2001;12:175–85. doi: 10.1515/revneuro.2001.12.2.175. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophrenia Research. 2009;107:47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chan RC, Lu X, Ma Z, Li Z, Gong QY. An exploratory study of the influence of conversation prosody on emotion and intention identification in schizophrenia. Brain Research. 2009;1281:58–63. doi: 10.1016/j.brainres.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Huber G. The concept of substrate-close basic symptoms and its significance for the theory and therapy of schizophrenic diseases. Nervenarzt. 1983;54:23–32. [PubMed] [Google Scholar]
- Husserl E. Ideas Pertaining to a Pure Phenomenology and to a Phenomenological Philosophy Second Book: Studies in the Phenomenology of Constitution. Dordrecht: Kluwer Academic Publishers; 1989. [Google Scholar]
- Ihnen GH, Penn DL, Corrigan PW, Martin J. Social perception and social skill in schizophrenia. Psychiatry Research. 1998;80:275–86. doi: 10.1016/s0165-1781(98)00079-1. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? Journal of Neuroscience. 2005;25:7134–8. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bulletin. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology. 2009;19:666–71. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–46. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J. The meaning of basic symptoms for the genesis of the schizophrenic nuclear syndrome. Japanese Journal of Psychiatry and Neurology. 1992;46:609–30. doi: 10.1111/j.1440-1819.1992.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Archives of General Psychiatry. 2001;58:158–64. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Bilker W, Hagendoorn M, Gur RE, Gur RC. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biological Psychiatry. 2000;48:127–36. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, David AS. Measuring empathy: reliability and validity of the Empathy Quotient. Psychological Medicine. 2004;34:911–9. doi: 10.1017/s0033291703001624. [DOI] [PubMed] [Google Scholar]
- Linden SC, Jackson MC, Subramanian L, Wolf C, Green P, Healy D, et al. Emotion-cognition interactions in schizophrenia: implicit and explicit effects of facial expression. Neuropsychologia. 2010;48:997–1002. doi: 10.1016/j.neuropsychologia.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience. 2009;12:547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Zohary E. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. Journal of Neuroscience. 2007;27:731–40. doi: 10.1523/JNEUROSCI.3653-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram D, Gardner C, Burns J, Miller P, Lawrie SM, Johnstone EC. Symptomatology and social inference: a theory of mind study of schizophrenia and psychotic affective disorder. Cognitive Neuropsychiatry. 2005;10:347–59. doi: 10.1080/13546800444000092. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Kaplan JT, Essex R, Damasio H, Damasio A. Seeing touch is correlated with content-specific activity in primary somatosensory cortex. Cerebral Cortex. 2011;21:2113–21. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkowski E. Psychopathologie des schizoïdes et des schizophrènes. Paris: Payot; 1927. La schizophrénie. Translated in part by Cutting, J. Reprinted (1986) in: The Clinical Roots of the Schizophrenia Concept (eds J. Cutting and M. Shepherd). Cambridge: Cambridge University Press. [Google Scholar]
- Morgan HL, Turner DC, Corlett PR, et al. Exploring the impact of ketamine on the experience of illusory body ownership. Biological Psychiatry. 2011;69:35–41. doi: 10.1016/j.biopsych.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Northoff G. Psychopathology and pathophysiology of the self in depression - neuropsychiatric hypothesis. Journal of Affective Disorders. 2007;104:1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1:131–6. [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. The neural basis of visual body perception. Nature Reviews Neuroscience. 2007;8:636–48. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I. Touch feel illusion in schizophrenic patients. Biological Psychiatry. 2000;48:1105–8. doi: 10.1016/s0006-3223(00)00947-1. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophrenia Bulletin. 2008;34:888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihko E, Nangini C, Jousmaki V, Hari R. Observing touch activates human primary somatosensory cortex. European Journal of Neuroscience. 2010;31:1836–43. doi: 10.1111/j.1460-9568.2010.07192.x. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. American Journal of Psychiatry. 2003;160:815–24. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Garrido L, Walsh V, Duchaine BC. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. Journal of Neuroscience. 2008;28:8929–33. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Current Opinion in Neurobiology. 2002;12:149–54. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. I. Somatosensory responses. Behavioural Brain Research. 1981a;2:125–46. doi: 10.1016/0166-4328(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behavioural Brain Research. 1981b;2:147–63. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- Roux P, Christophe A, Passerieux C. The emotional paradox: dissociation between explicit and implicit processing of emotional prosody in schizophrenia. Neuropsychologia. 2010;48:3642–9. doi: 10.1016/j.neuropsychologia.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophrenia Bulletin. 2003;29:427–44. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Human Brain Mapping. 2009;30:2722–30. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: the basic symptom concept. Schizophrenia Bulletin. 2009;35:5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkötter J. 2007. Strumento di valutazione per la propensione alla schizofrenia. Versione per adulti. Trad. Giuliano Aiello. Rome: Giovanni Fioriti Editore s.r.l. Translated by Aiello G. (2011). Rome: Giovanni Fioriti Editore s.r.l. [Google Scholar]
- Serino A, Canzoneri E, Avenanti A. Fronto-parietal areas necessary for a multisensory representation of peripersonal space in humans: an rTMS study. Journal of Cognitive Neuroscience. 2011;23:2956–67. doi: 10.1162/jocn_a_00006. [DOI] [PubMed] [Google Scholar]
- Shean G, Murphy A, Meyer J. Social cognition and symptom dimensions. The Journal of Nervous and Mental Disease. 2005;193:751–5. doi: 10.1097/01.nmd.0000185868.58587.5b. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17:2235–44. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cerebral Cortex. 2008;18:2532–9. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010;133:3104–12. doi: 10.1093/brain/awq152. [DOI] [PubMed] [Google Scholar]
- Wood R, Gallese V, Cattaneo L. Visuotactile empathy within the primary somatosensory cortex revealed by short-latency afferent inhibition. Neuroscience Letters. 2010;473:28–31. doi: 10.1016/j.neulet.2010.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.