Abstract
Insight has been mostly studied from a clinical perspective. Recently, attention moved to cognitive insight or the ability to monitor and correct one's erroneous convictions. Here, we investigated the neuroanatomical correlates of cognitive insight. We administered the Beck cognitive insight scale to 45 outpatients with a schizophrenia diagnosis and 45 age- and gender-matched healthy control subjects who underwent a MRI investigation, including high-resolution volumetric and diffusion tensor imaging sequences. Gray and white matter volume, mean diffusivity and fractional anisotropy were used as dependent variables and were analyzed on a voxel-by-voxel basis with reference to the cognitive insight indexes. Self-reflectiveness was positively related to gray matter volume of the right ventro-lateral prefrontal cortex (VLPFC). No statistically significant results emerged from the DTI analyses, and no significant relationships were found for self-certainty and global cognitive insight. Reduced self-reflectiveness is related to a reduced volume of the VLPFC, an area involved in generating and maintaining in working memory different hypotheses about the self. This line of research focusing on the metacognitive features of insight in schizophrenia can provide relevant information to identify patients who are most vulnerable to lack of insight and develop effective cognitive therapeutic strategies.
Keywords: insight, schizophrenia, metacognitive, awareness, neuroimaging
INTRODUCTION
Insight is a multidimensional concept. In psychosis, the clinical dimension of insight implies the ability to label unusual mental events as pathological, to verbally admit having a mental disorder and to recognize the need for treatment (David, 1990). Lack of insight has detrimental effects on symptom relapse and frequency, adherence to treatment and quality of life (Caton et al., 2006). Many questionnaires have been developed to assess clinical insight (McEvoy et al., 1981). These questionnaires are based on patients' verbal admissions, which might reflect the mere repetition of information received rather than the ability to integrate this information into their own thought processes and to distance from their own erroneous convictions. This is why Beck and colleagues (2004) integrated the clinical dimension of insight with the additional dimension of cognitive insight, defined as the ability to evaluate and modify misperceptions and delusional thinking and openness to integrating corrective information. It is considered to reflect the metacognitive underpinnings of awareness of illness, that is self-monitoring and regulation of one's own performance (Beck et al., 2004; Koren et al., 2004; Gilleen et al., 2010). Thus, cognitive insight complements clinical insight, because it deals with patients' actual ability to process information about their condition.
The Beck Cognitive Insight Scale (BCIS) (Beck et al., 2004) was developed to assess two subdimensions of cognitive insight: (i) self-reflectiveness (SR), or the ability to self-reflect, to question unusual experiences and to consider corrective feedback and (ii) self-certainty (SC), or the unwillingness to modify one's own interpretations and self-overconfidence in making judgments. These factors represent two opposite cognitive attitudes and are considered to affect the overall cognitive dimension of awareness of illness (Gilleen et al., 2010). In patients with a diagnosis of schizophrenia, a lower cognitive insight index indicates a poor realism in monitoring one's own misperceptions and distorted experiences; therefore, it characterizes more impaired subjects (Beck et al., 2004; Pedrelli et al., 2004). Furthermore, in schizophrenia, lower global cognitive insight and SR and higher SC were found related to worse cognitive performances (Buchy et al., 2009; Lepage et al., 2008; Cooke et al., 2010; Orfei et al., 2010). In the light of these considerations, the clinical relevance of cognitive insight is quite promising, because it represents a prognostic factor for identifying patients at risk of poor compliance and developing more efficient therapeutic strategies.
Over the past 20 years, the neuroanatomical correlates of clinical insight were investigated, highlighting inverse relationships with volume of the right frontal cortex (Laroi et al., 2000; Flashman et al., 2001; Sapara et al., 2007), particularly the dorso-lateral prefrontal cortex (DLPFC), the anterior cingulate (Ha et al., 2004; Shad et al., 2004, 2006a) and the temporal areas (Ha et al., 2004; Cooke et al., 2008). Other studies reported no relationship (David et al., 1995; Rossell et al., 2003; Bassitt et al., 2007). On the contrary, to date, the neuroanatomical correlates of cognitive insight have been scarcely studied. Only one study (Buchy et al., 2010) reported that the BCIS composite index was positively correlated with total left hippocampal volume, SC was inversely correlated with bilateral hippocampal volumes and no correlation was found with SR. However, this work, in spite of its relevance, focused on specific regions of interest, to verify specific a priori hypotheses, thus excluding different relevant information and did not include a healthy control group.
The main aim of our study was to investigate the neuroanatomical correlates of cognitive insight in a sample of patients with schizophrenia, using different volumetric–macrostructural and diffusion tensor imaging (DTI)-microstructural neuroimaging approaches. The secondary aim of the present study was to determine whether the areas related to cognitive insight are the same as those involved in mechanisms of schizophrenia. In order to do this, we investigated possible differences in the structural neuroanatomy between patients with schizophrenia and healthy controls (HC). Our main and secondary aims were tested for significance on the whole brain. Considering the high number of statistical tests performed, we used a statistical correction for multiple comparisons in order to avoid false-positive results.
MATERIALS AND METHODS
Subjects
We recruited 60 consecutive outpatients diagnosed with schizophrenia according to the DSM-IV-TR (APA, 2000) criteria. All patients were diagnosed by one senior clinical psychiatrist (G.S.) using the structured clinical interview for DSM-IV-TR (SCID-I/P) (First et al., 2002). Other inclusion criteria were: (i) age between 18 and 65 years; (ii) at least 8 years of education; (iii) no dementia or cognitive deterioration according to the DSM-IV-TR criteria, and a mini-mental state examination (MMSE) (Folstein et al., 1975) score higher than 24, consistent with normative data in the Italian population (Measso et al., 1993); and (iv) suitability for a MRI scan.
Exclusion criteria were: (i) a history of alcohol or drug dependence or traumatic head injury; (ii) any past or present major medical or neurological illnesses; (iii) any other psychiatric disorder or mental retardation diagnosis; and (iv) MRI evidence of focal parenchymal abnormalities or cerebrovascular diseases.
Out of the initial sample of 60 patients, 7 were excluded for comorbid substance use disorders, 3 for cognitive deterioration and 5 for comorbid medical or neurological illnesses. Thus, the final sample consisted of 45 consecutive outpatients. All patients were in a phase of stable clinical compensation. Age at onset was defined as age at first hospitalization or, when possible, age at onset of positive or negative symptoms before the first hospitalization.
The Positive and Negative Syndrome scale (PANSS) (Kay et al., 1987) was administered to rate severity of psychopathological symptoms. PANSS ratings were obtained on all information available pertaining to the last week of the assessment. Extrapyramidal side effects due to current treatment were assessed by the Simpson–Angus Rating scale (SARS) (Simpson and Angus, 1970). The abnormal involuntary movements scale (Guy, 1976) was administered to determine whether tardive dyskenisia was present; however, no patient suffered from this disturbance. All patients were receiving stable oral doses of one or more atypical antipsychotic drugs such as risperidone, quetiapine or olanzapine. Antipsychotic dosages were converted to estimated equivalent dosages of olanzapine by using a standard table (Woods, 2003).
We recruited 45 HC in the same geographical area; they were rigorously matched for age and gender with the patients diagnosed as having schizophrenia. All HC were screened for any current or past diagnosis of DSM-IV-TR axis I or II disorders using the SCID-I and SCID-II (First et al., 2002). A diagnosis of schizophrenia or any other mental disorder in first-degree relatives was also an exclusion criterion.
Our local Ethics Committee approved the study protocol. Written informed consent was obtained from all patients after they received a full explanation of the study procedures.
Insight assessment
Cognitive insight was assessed using the BCIS, a 15-item self-rating instrument. The items are statements that represent cognitive attitudes. Respondents are asked to rate the extent to which they agree with each statement using a 4-point Likert scale ranging from 0 (I do not agree at all) to 3 (I completely agree). Factorial analysis revealed two sub-factors of cognitive insight, namely SR, which concerns willingness to acknowledge fallibility and to consider external feedback (e.g. If somebody points out that my beliefs are wrong, I am willing to consider it), and SC, indicating overconfidence in one's own judgments and resistance to cognitive change (e.g. I can trust my own judgment at all times) (Beck et al., 2004; Pedrelli et al., 2004). By subtracting the SC score from the SR score, an R–C index composite score is obtained, which indicates global cognitive insight.
The scale has proved to be valid and reliable for individuals with schizophrenia, schizoaffective and other psychotic disorders (Pedrelli et al., 2004), major depression and bipolar disorders (Beck et al., 2004; Engh et al., 2007) and healthy subjects (Warman and Martin, 2006; Martin et al., 2010). The BCIS has been translated into various languages and validated (Favrod et al., 2008; Uchida et al., 2009; Kao and Liu, 2010). We administered a validated Italian version of the BCIS (Orfei et al., 2007a).
Image acquisition
All participants underwent the same imaging protocol, which included standard clinical sequences (FLAIR, DP-T2 weighted) and whole-brain T1 weighted and DTI using a 3T Allegra MR imager (Siemens, Erlangen, Germany) with a standard quadrature head coil. All planar sequence acquisitions were obtained in the plane of the Anterior Commissure–Posterior Commissure line. Particular care was taken to center subjects’ head in the head coil and to restrain their movements with cushions and adhesive medical tape. Diffusion volumes were acquired using echo-planar imaging (TE/TR = 89/8500 ms, bandwidth = 2126 Hz/vx; matrix size 128 × 128; 80 axial slices, voxel size 1.8 × 1.8 × 1.8 mm3) with 30 isotropically distributed orientations for the diffusion-sensitizing gradients at a b value of 1000 s mm2 and 6 b = 0 images. Scanning was repeated three times to increase the signal-to-noise ratio. Whole-brain T1-weighted images were obtained in the sagittal plane using a modified driven equilibrium Fourier transform (MDEFT) (51) sequence (TE/TR = 2.4/7.92 ms, flip angle 15, voxel size 1 × 1 × 1 mm3). Two trained neuroradiologists co-inspected the clinical MR sequences (i.e. T1- and T2 weighted and FLAIR images) available for all participants to ensure they had no vascular lesions or any other radiological problems.
Image processing and analyses
High-resolution T1 weighted and DTI images were processed separately to obtain indices of brain macro- and microstructural variations.
First, we applied voxel-based morphometry (VBM) to investigate regional cortical volume alterations in association with the cognitive insight scale by using an extension of statistical parametric mapping 5 (SPM5; Wellcome Department of Imaging Neuroscience, London, UK), specifically, the VBM5.1 Toolbox written by Gaser (http://dbm.neuro.uni-jena.de/vbm) and running in Matlab 2007b (MathWorks, Natick, MA, USA). Images were normalized and segmented into gray matter (GM) and white matter (WM) partitions in the new unified segmentation step (Ashburner and Friston, 2005). Then, they were modulated by the Jacobian determinants for nonlinear warping only, from the normalization step, to preserve volume information. The modulated GM and WM images, on which all analyses were performed, were smoothed with a Gaussian kernel of 8-mm full-width at half-maximum. Subsequently, DTI images were processed using FSL 4.1 (www.fmrib.ox.ac.uk/fsl/). Image distortions induced by eddy currents and head motion in the DTI data were corrected by applying a 3D full affine (mutual information cost function) alignment of each image to the mean no diffusion weighting (b0) image. After these corrections were made, DTI data were averaged and concatenated into 31 (1 b0 + 30 b1000) volumes. A diffusion tensor model was fit at each voxel and generated maps of FA and MD. The FA maps were processed using TBSS (tract-based spatial statistics) (Smith et al., 2006) part of FSL. All subjects’ FA data were aligned into a common space with the nonlinear registration FNIRT tool which uses a b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton, which represented the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto the skeleton by searching perpendicular to the skeleton for maximum FA values in individual subject's FA maps. Statistical comparisons were then restricted to voxels in the WM skeleton.
The MD maps were registered to the Montreal Neurological Institute (MNI) template using a full-affine (correlation ratio cost function) alignment with nearest-neighbour re-sampling and subsequently smoothed using a Gaussian kernel with a full-width half-maximum (FWHM) of 8 mm.
Statistical analyses
The chi-square test was used to compare the patient group with the HC group for the gender variable; unpaired t-tests were performed to compare the two groups for age, educational level and MMSE variables. To determine the significance of the correlations between continuous variables, correlation analyses and Fisher's r to z transformation were performed. The level of statistical significance was defined as P < 0.05.
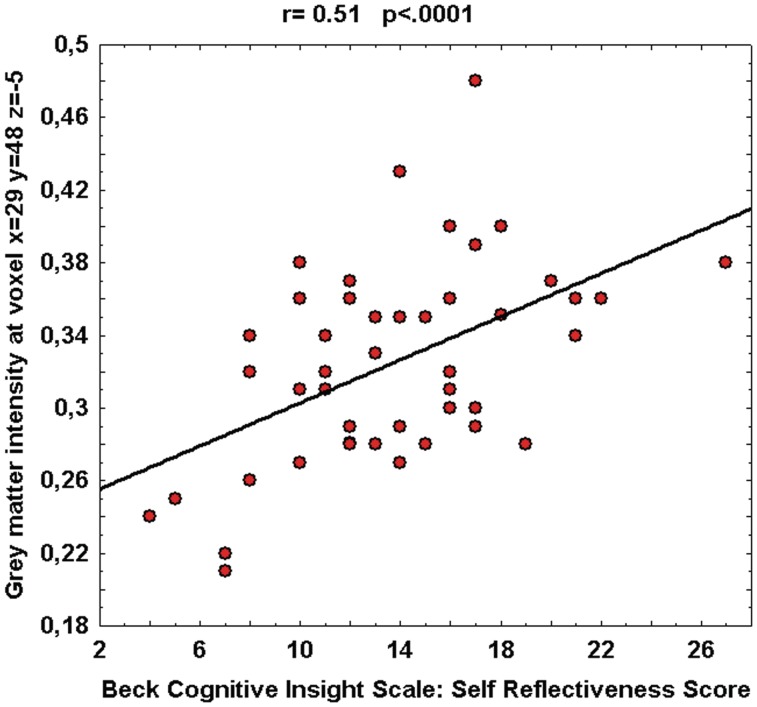
VBM analyses were carried out at the voxel level using SPM5. To identify the brain regions in which patients showed GM or WM volumetric changes correlated with the BCIS scores, distinct multiple regression models were computed using the SR, the SC and the R-C global score as regressors. Age and years of formal education were used as covariates of no interest in the analyses since there is evidence that these variables can play a confounding role in brain volumetric studies (Barnes et al., 2010). Then, for each subject we extracted the signal intensity of the voxel where the statistical peak of the voxel-based correlation was found (MNI coordinates x = 29, y = 48, z = −5), plotted it against the SR score and calculated the Pearson's r coefficient between the two variables (r = 0.51, p < .0001). The scatterplot is shown in Figure 2.
Fig. 2.
Relationship between GM intensity and BCIS-SR score at the statistical peak of voxel-based correlation. Pearson's r coefficient, relative P-value and linear fit (solid black line) are also reported.
To identify the brain regions in which patients showed MD variations in association with BCIS scores, three distinct multiple regression models were computed using the SR, the SC and the R-C scores as regressors and age and years of formal education as covariates of no interest.
We investigated whether those brain regions characterized by a significant correlation between volumetric or DTI parameters and cognitive insight scores were located in areas with significant differences in the comparison between patients and HC. To do this, the areas with significant correlation between BCIS scores and MRI measures were used as binary masks in which a difference between HC and patients was analysed using the t-test, with age and education as nuisance variables.
To avoid type I errors, these analyses were performed using the random fields theory family-wise error (FWE) correction (P < 0.05), which controls the possibility of any false positives across the entire volume (Friston et al., 1996). Finally, the correlation between WM tract-based organization and BCIS scores (using age and education as variables of no interest) was carried out on the skeletonized FA data using Randomise (part of FSL), which carries out permutation-based testing and inference using threshold-free cluster enhancement (TFCE) (Nichols and Holmes, 2002).
RESULTS
The socio-demographic characteristics of the HC and patients with schizophrenia are presented in Table 1.
Table 1.
Sociodemographic and clinical characteristics of 45 patients diagnosed with schizophrenia and 45 healthy comparison subjects
| Characteristics | Schizophrenic patients (n = 45) |
Healthy controls (n = 45) |
|||
|---|---|---|---|---|---|
|
n (%) |
n (%) |
χ2 | df | P | |
| Gender (male) | 29 (64) | 29 (64) | 0.000 | 1 | – |
| Mean ± s.d. | Mean ± s.d. | t | df | P | |
| Age (years) | 38.8 ± 11.4 | 38.8 ± 12.6 | 0.000 | 88 | – |
| Educational level (years) | 12.0 ± 3.0 | 14.6 ± 2.8 | 4.3 | 88 | <0.0001 |
| MMSE | 27.6 ± 1.7 | 29.6 ± 0.8 | 7.0 | 88 | <0.0001 |
| Age at illness onset (years) | 26.4 ± 8.2 | – | – | – | – |
| Illness duration (years) | 12.4 ± 9.7 | – | – | – | – |
| PANSS positive | 22.9 ± 6.3 | – | – | – | – |
| PANSS negative | 19.0 ± 6.6 | – | – | – | – |
| PANSS GP | 47.3 ± 10.7 | – | – | – | – |
| SARS | 5.3 ± 4.9 | – | – | – | – |
| Olanzapine Equivalents (mg/day) | 17.5 ± 21.5 | – | – | – | – |
| BCIS_SR | 13.8 ± 4.7 | ||||
| BCIS_SC | 8.3 ± 4.8 | ||||
| BCIS_R-C | 5.5 ± 6.0 |
df = degrees of freedom; s.d. = standard deviation; MMSE = mini mental state examination; PANSS = positive and negative syndrome scale; GP = general psychopathology; SARS = Simpson–Angus rating scale; BCIS = Beck cognitive insight scale; SR = self-reflectiveness score; SC = self-certainty score; R-C = overall cognitive insight score
The two groups did not differ for sex or age, but differed for educational level and MMSE score.
The SC index, which measures self-overconfidence, was positively related to the PANSS negative symptoms score (r = 0.429, n = 45, P = 0.0029) and negatively to the PANSS composite score (r = −0.325, n = 45, P = 0.0291). No significant relationship emerged between the SR or the R-C indexes, and all the other PANSS scores.
To obtain the precise anatomical localization of our VBM results, we superimposed significant statistical maps emerging from the analyses on the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002), which is available from MRIcron software (http://www.cablati.com/mricro/mricron/index.html); it includes all main gyri and sulci of the cerebral cortex and the subcortical and deep GM structures for a total of 90 anatomical volumes of interest.
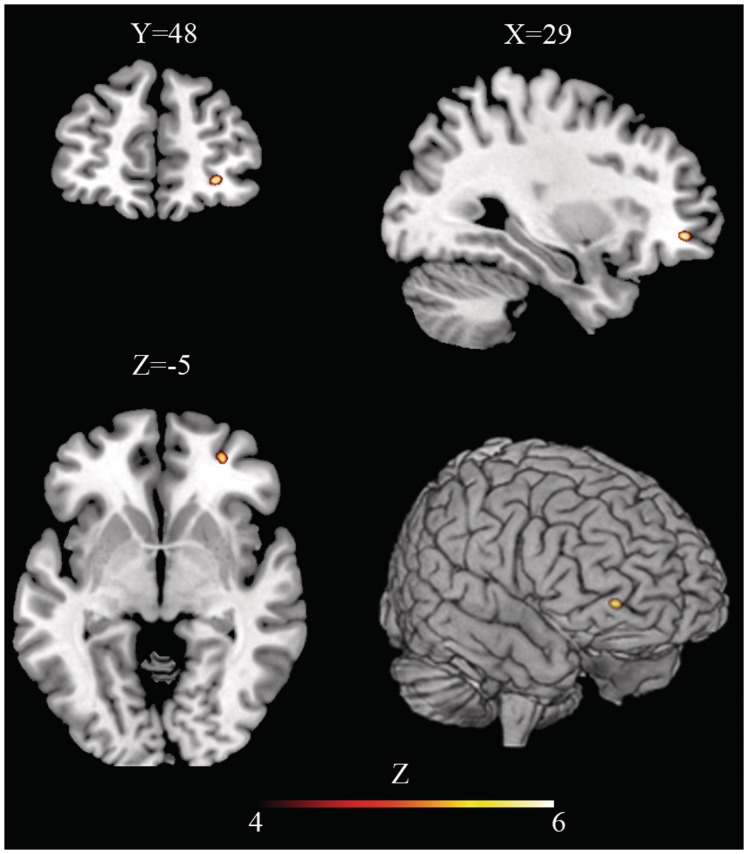
A single cluster (72 voxels) of statistically significant positive correlation [t = 4.83; equivZ = 4.28; P (FWE corrected) = 0.027] between GM volume and the SR subscore was found in the right orbito-frontal areas (Frontal Mid Orbital), including the Brodmann area (BA) 47/11.
Fig. 1.
Structural correlate of self-reflectiveness is located in the right Ventro-Lateral Pre-Frontal Cortex. Axial, coronal and sagittal slices and 3D brain volume rendering show areas of significant (p < 0.05, Family Wise Error corrected) voxel-based positive correlation between regional Gray Matter volume and self-reflectiveness score in patients with schizophrenia diagnosis (z values above the color bar indicate normalized t-values). Y,X,Z-coordinates are reported in Montreal Neurological Institute space. In Figure right is right and left is left.
The scatterplot showed in Figure 2 indicates the relationship between GM intensity and SR score at the statistical peak of the voxel-based correlation (MNI coordinates x = 29, y = 48, z = −5).
No areas of statistically significant correlation between the SC subscore or the overall R-C score and regional GM volume changes were found applying the statistical correction FWE. The same lack of significant correlation between the SC subscore or the overall R-C score and regional GM volume changes was confirmed even when the statistical threshold was lowered to P < 0.001, uncorrected for multiple comparisons.
Further, we aimed at testing the hypothesis that the area, where a statistically significant correlation between GM volume and SR subscore emerged, would show some structural difference between patients with schizophrenia and HC. Thus, this area was considered as a region of interest where we investigated differences of GM volume between HC and patients. The analysis was performed using age and education as nuisance variables and setting the statistical threshold at P < 0.001, uncorrected (given the relatively small number of comparisons performed). No significant difference was found.
Moreover, no area of statistically significant correlation was found between WM macrostructural volume and cognitive insight scores. This lack of significant results was confirmed even when the statistical threshold was lowered to P < 0.001, uncorrected for multiple comparisons.
With regard to DTI analyses, we found no statistically significant correlation between indices of microstructural alteration (i.e. MD and FA maps) and BCIS scores even when the statistical threshold was lowered to P < 0.001, uncorrected for multiple comparisons.
DISCUSSION
The present study aimed at investigating the neuroanatomical correlates of cognitive insight by using multimodal neuroimaging techniques and considering the whole brain. Secondarily, we wanted to determine whether changes in brain structure related to cognitive insight could be traced back to structural abnormalities specific to schizophrenia. Three main results emerged. First, reduced willingness to correct one's distorted perceptions (as indicated by the BCIS SR index) is related to reduced GM volume of the right VLPFC, namely, BA 47/11 or the pars orbitalis. VLPFC plays a key role in several high-level cognitive functions (Plum, 1987; Fuster, 1989), namely in working memory and decision making. With regard to working memory, VLPFC is involved in transferring, maintaining and matching autobiographical and semantic information acquired recently or retrieved from long-term memory (Nyberg et al., 2003; Burianova and Grady, 2007; Marklund et al., 2007). Structural and functional neuroimaging studies showed that in schizophrenia a reduction of volume, a lower activation and hyperconnectivity of the VLPFC are related to deficits in encoding and retrieving verbal material in working memory tasks (Antonova et al., 2004; Scheuerecker et al., 2008; Meda et al., 2009; Ragland et al., 2009). With regard to decision making, very recent works have shown that the right VLPFC plays a peculiar role in the generation of alternative hypotheses in tasks in which individuals are required to generate a response to a problem when a variety of answers are possible (Goel and Vartanian, 2005; Vartanian and Goel, 2005). To note, in schizophrenia a greater activation within the right VLPFC is related to greater impulsivity and to the difficulty in inhibiting incorrect responses, which prevent effective decision-making processes (Kaladjian et al., 2011). In the light of these considerations, our result is very intriguing. In fact, SR is defined as the ability to consider at the same time a number of information, perspectives and alternative hypotheses in order to provide judgments about the self. This ability actually implies verbal working memory and decision-making processes. Thus, our data suggest that in schizophrenia a lower volume of VLPFC is related to a poor ability to consider alternative hypotheses about one's own misperceptions and biases, which in turn impairs a correct awareness of illness.
Second, our data suggest that the relationship between SR and brain structure are confined to the level of volumetric variations of the GM. In fact, no statistically significant association between WM alterations and behavioral measures were found. WM alterations are often described in schizophrenia, which suggests that disturbed communication (‘disconnectivity’) within and between brain regions might be the core pathology of this disorder (Peters et al., 2010). The lack of association between the cognitive insight dimensions and WM alterations found here suggests that the etiopathogenic processes underpinning deficits of self-awareness does not coincide with those specifically underlying the development of schizophrenia. Consistently with this hypothesis, our third result indicates that the VLPFC did not show any structural difference between patients with schizophrenia and HC. Notably, similar levels of cognitive insight dimensions were found in patients with various diagnoses, i.e. schizophrenia, schizoaffective disorders and bipolar disorders (Beck et al., 2004; Pedrelli et al., 2004; Engh et al., 2007). Thus, we can speculate that the neural mechanisms underlying defective SR are not specific for schizophrenia, but rather might be common to different neuropsychiatric disorders. However, further works on the neuroanatomical correlates of cognitive insight should compare various diagnostic groups in order to clarify this issue.
Before concluding, some limitations of our study must be considered. First, the SC index and the global R-C index were not correlated with any structural data in either GM or WM. This is in contrast with the findings of the only previous study (Buchy et al., 2010) on this topic, where SC and R-C indexes were related to hippocampal volume. This difference in findings between the two studies may be due to the different characteristics of the samples. In fact, in our study, patients were at various stages of illness, whereas only first-episode patients were enrolled in Buchy et al.'s study. The effect of these two indexes might be more evident in first-episode patients than in a sample characterized by longer duration of illness, which may imply longer treatment. Last, Buchy's and co-workers limited their analyses to a specific brain structure (i.e. the hippocampus), while our investigation was carried out on a whole brain basis. Second, as our sample was homogeneous with regard to race and recruitment source, our results could be limited in extendibility to different populations. Thus, future research is needed to deepen this issue. Third, the patients in our sample were in a stable phase of illness and were being treated with stable doses of antipsychotics; therefore, we cannot exclude that medication affected cognitive insight. Fourth, we included only nondemented patients with a MMSE score ≥ 24 to avoid a confounding effect on the data because they did not fully understand the items. Therefore, it must be determined whether this selection criterion created a bias. Fifth, the cross-sectional structure of the study might also be a limitation. In fact, a longitudinal study might be more informative in predicting patients' outcomes, including treatment response.
In conclusion, our results showed a significant relationship between the SR subdimension of the BCIS and the volume of the VLPFC, which has a role in generating and maintaining alternative hypotheses about the self for subsequent decision-making. Our data contribute to deepen our knowledge of the metacognitive and self-monitoring underpinnings of insight in patients with schizophrenia. Indeed, the study of cognitive insight is quite promising, as it provides at least two relevant clinical implications. First, it should help identify patients with schizophrenia who are most vulnerable to poor self-awareness and thus most at risk of frequent relapses and low adherence to treatment. Second, it could contribute to the development of specific treatment strategies aimed at improving self-monitoring and flexibility of thought. A final consideration regards the mechanisms of awareness of illness disorders in schizophrenia. In fact, as the motivational hypothesis about defective awareness of illness has progressively faded (Moore et al., 1999; Pia and Tamietto, 2006), data on the neuropsychological and neuroanatomical correlates of clinical insight grew, lack of insight in schizophrenia could be conceived as a neurocognitive deficit, analogously to anosognosia in brain injury and dementia (Babinski, 1914; Shad et al., 2006b; Orfei et al., 2007b, 2008).
Conflict of Interest
None declared.
Acknowledgments
Mariangela Iorio contributed to the image analyses. Claire Montagna contributed to English revision.
This work was supported by Italian Ministry of Health grants (RC06-07-08-09/A).
REFERENCES
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophrenia Research. 2004;70:117–45. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorder, DSM-IV-TR. Washington: 2000. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Babinski J. Contribution à l’étude de troubles mentaux dans l'hémiplegie organique cérébrale. Revue Neurologique. 1914;27:845–7. [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophrenia Research. 2004;68:319–29. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Buchy L, Malla A, Joober R, Lepage M. Delusions are associated with low self-reflectiveness in first-episode psychosis. Schizophrenia Research. 2009;112:187–91. doi: 10.1016/j.schres.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Buchy L, Czechowska Y, Chochol C, et al. Toward a model of cognitive insight in first-episode psychosis: verbal memory and hippocampal structure. Schizophrenia Bulletin. 2010;36:1040–9. doi: 10.1093/schbul/sbp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. Journal of Cognitive Neuroscience. 2007;19:1520–34. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Caton CL, Hasin DS, Shrout PE, et al. Predictors of psychosis remission in psychotic disorders that co-occur with substance use. Schizophrenia Bulletin. 2006;32:618–25. doi: 10.1093/schbul/sbl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophrenia Research. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MA, Peters ER, Fannon D, Aasen I, Kuipers E, Kumari V. Cognitive insight in psychosis: the relationship between self-certainty and self-reflection dimensions and neuropsychological measures. Psychiatry Research. 2010;178:284–9. doi: 10.1016/j.psychres.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A, van Os J, Jones P, Harvey I, Foerster A, Fahy T. Insight and psychotic illness. Cross-sectional and longitudinal associations. British Journal of Psychiatry. 1995;167:621–8. doi: 10.1192/bjp.167.5.621. [DOI] [PubMed] [Google Scholar]
- David AS. Insight and psychosis. British Journal of Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- Engh JA, Friis S, Birkenaes AB, et al. Measuring cognitive insight in schizophrenia and bipolar disorder: a comparative study. BMC Psychiatry. 2007;7:71–8. doi: 10.1186/1471-244X-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrod J, Zimmermann G, Raffard S, Pomini V, Khazaal Y. The Beck cognitive insight scale in outpatients with psychotic disorders: further evidence from a French-speaking sample. Canadian Journal of Psychiatry. 2008;53:783–7. doi: 10.1177/070674370805301111. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;13:255–7. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. New York: Raven Press; 1989. [Google Scholar]
- Gilleen J, Greenwood K, David A. Anosognosia in schizophrenia and other neuropsychiatric disorders: similarities and differences. In: Prigatano GP, editor. The Study of Anosognosia. Oxford: University Press; 2010. [Google Scholar]
- Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cerebral Cortex. 2005;15:1170–7. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Guy W. Abnormal Involuntary Movement Scale (AIMS) In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: U.S. Department of Health Education and Welfare; 1976. pp. 534–7. [Google Scholar]
- Ha TH, Youn T, Ha KS, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Research. 2004;132:251–60. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychological Medicine. 2011;41:291–9. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kao YC, Liu YP. The Beck cognitive insight scale (BCIS): translation and validation of the Taiwanese version. BMC Psychiatry. 2010;10:27. doi: 10.1186/1471-244X-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koren D, Seidman LJ, Poyurovsky M, et al. The neuropsychological basis of insight in first-episode schizophrenia: a pilot metacognitive study. Schizophrenia Research. 2004;70:195–202. doi: 10.1016/j.schres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Laroi F, Fannemel M, Ronneberg U, et al. Unawareness of illness in chronic schizophrenia and its relationship to structural brain measures and neuropsychological tests. Psychiatry Research. 2000;100:49–58. doi: 10.1016/s0925-4927(00)00063-9. [DOI] [PubMed] [Google Scholar]
- Lepage M, Buchy L, Bodnar M, Bertrand MC, Joober R, Malla A. Cognitive insight and verbal memory in first episode of psychosis. European Psychiatry. 2008;23:368–74. doi: 10.1016/j.eurpsy.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Marklund P, Fransson P, Cabeza R, Petersson KM, Ingvar M, Nyberg L. Sustained and transient neural modulations in prefrontal cortex related to declarative long-term memory, working memory, and attention. Cortex. 2007;43:22–37. doi: 10.1016/s0010-9452(08)70443-x. [DOI] [PubMed] [Google Scholar]
- Martin JM, Warman DM, Lysaker PH. Cognitive insight in non-psychiatric individuals and individuals with psychosis: an examination using the Beck cognitive insight scale. Schizophrenia Research. 2010;121:39–45. doi: 10.1016/j.schres.2010.03.028. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Aland J, Jr, Wilson WH, Guy W, Hawkins L. Measuring chronic schizophrenic patients attitudes toward their illness and treatment. Hospital and Community Psychiatry. 1981;32:856–8. doi: 10.1176/ps.32.12.856. [DOI] [PubMed] [Google Scholar]
- Measso G, Caverzeran F, Zappala` G, et al. The mini-mental state examination: normative study of an Italian random sample. Developmental Neuropsychology. 1993;9:77–85. [Google Scholar]
- Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS One. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore O, Cassidy E, Carr A, O'Callaghan E. Unawareness of illness and its relationship with depression and self-deception in schizophrenia. European Psychiatry. 1999;14:264–9. doi: 10.1016/s0924-9338(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, et al. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–7. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Caltagirone C, Spalletta G. Milano: Springer, Italia; 2007a. I disturbi della consapevolezza nelle malattie neuropsichiatriche [Unawareness of illness in neuropsychiatric disorders] [Google Scholar]
- Orfei MD, Robinson RG, Prigatano GP, et al. Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a systematic review of the literature. Brain. 2007b;130:3075–90. doi: 10.1093/brain/awm106. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14:203–22. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Spoletini I, Banfi G, Caltagirone C, Spalletta G. Neuropsychological correlates of cognitive insight in schizophrenia. Psychiatry Research. 2010;178:51–6. doi: 10.1016/j.psychres.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Pedrelli P, McQuaid JR, Granholm E, et al. Measuring cognitive insight in middle-aged and older patients with psychotic disorders. Schizophrenia Research. 2004;71:297–305. doi: 10.1016/j.schres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: What have we learned? Journal of Psychiatric Research. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Pia L, Tamietto M. Unawareness in schizophrenia: neuropsychological and neuroanatomical findings. Psychiatry and Clinical Neuroscience. 2006;60:531–7. doi: 10.1111/j.1440-1819.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- Plum F. Handbook of Physiology: The Nervous System. Bethesda: American Physiological Society; 1987. [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. American Journal of Psychiatry. 2009;166:863–74. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossell SL, Coakes J, Shapleske J, Woodruff PW, David AS. Insight: its relationship with cognitive function, brain volume and symptoms in schizophrenia. Psychological Medicine. 2003;33:111–9. doi: 10.1017/s0033291702006803. [DOI] [PubMed] [Google Scholar]
- Sapara A, Cooke M, Fannon D, et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophrenia Research. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Scheuerecker J, Ufer S, Zipse M, et al. Cerebral changes and cognitive dysfunctions in medication-free schizophrenia—an fMRI study. Journal of Psychiatric Research. 2008;42:469–76. doi: 10.1016/j.jpsychires.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage. 2004;22:1315–20. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia—a pilot study. Psychiatry Research. 2006a;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophrenia Research. 2006b;86:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. 1970;212(Suppl.):11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uchida T, Matsumoto K, Kikuchi A, et al. Psychometric properties of the Japanese version of the Beck Cognitive Insight Scale: relation of cognitive insight to clinical insight. Psychiatry and Clinical Neuroscience. 2009;63:291–7. doi: 10.1111/j.1440-1819.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- Vartanian O, Goel V. Task constraints modulate activation in right ventral lateral prefrontal cortex. Neuroimage. 2005;27:927–33. doi: 10.1016/j.neuroimage.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Warman DM, Martin JM. Cognitive insight and delusion proneness: an investigation using the Beck Cognitive Insight Scale. Schizophrenia Research. 2006;84:297–304. doi: 10.1016/j.schres.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]