Abstract
We examined social cognition in a sample of healthy participants who had prior magnetic resonance imaging (MRI) gray matter volume studies of the orbital frontal cortex (OFC) that was parcellated into three regions: gyrus rectus, middle orbital gyrus and lateral orbital gyrus. These subjects also completed a self-report measure of Machiavelli personality traits, along with psychometric tests of social comprehension and declarative episodic memory, all of which we used as proxy measures to examine various features of social cognition. The data pointed to distinct functional–anatomical relationships highlighted by strong correlations of left lateral orbital gyrus and Machiavellian scores and right middle orbital gyrus with social comprehension and declarative episodic memory. In addition, hierarchical regression analyses revealed statistical evidence of a double dissociation between Machiavellian scores and left lateral orbital gyrus on one hand, and social comprehension with right middle orbital gyrus, on the other hand. To our knowledge, these findings are the first to show evidence linking normal variation in OFC subregions and different aspects of social cognition.
Keywords: orbital frontal cortex, social cognition
INTRODUCTION
In 1846 in Cavendish Vermont, a railroad foreman named Phineas Gage had been laying track when a sharp-pointed 3-cm-thick, 109-cm-long tamping iron rod exploded through his left cheekbone, slicing behind his left eyeball and impaling his frontal lobes. Miraculously, Gage, described as only momentarily stunned, regained consciousness immediately and with the help of his fellow railroad workers walked away from the accident. But his personality and social comportment would never be the same, and the once conscientious and capable foreman was now seen as crass and unpredictable, and as his friends and acquaintances attested ‘Gage was no longer Gage’ (Harlow, 1868 cited in Damasio et al., 1994) (p. 1102).
Fast forward over a century later, Damasio et al. (1994), using measurements from Gage’s skull and modern techniques of computerized neuroimaging, reconstituted the entry facial and exit skull wounds suffered by Gage. Their computerized modeling of Gage’s brain revealed damage confined to prefrontal cortices, particularly for the left hemisphere, the anterior half of the orbital frontal cortex (OFC), the anterior and mesial frontal cortices and for the right hemisphere, anterior and mesial OFC, as well as mesial and polar frontal cortices, but notably supplementary motor area and Broca’s area in the left hemisphere, including Broadmann’s areas 44, 45 and 47 were spared (Damasio et al., 1994). More recent neuroimaging studies emanating from the new and burgeoning school of social brain sciences (e.g. Lieberman, 2007) have greatly extended the neuroanthropological work of Damasio and colleagues (1994). Much of this research has been organized around the construct of social cognition, which is defined as a complex set of representations of internal bodily states, knowledge of self, perceptions of others and interpersonal motivations that is supported by widely distributed networks of diverse brain regions, including the temporoparietal junction, the temporal sulcus and the temporal pole as well as medial prefrontal cortex, especially orbital frontal sections (Adolphs, 2003; Amodio and Frith, 2006). Behrens et al. (2009) proposed that this neural circuitry facilitates social cognition via coordination of two distinct, yet intimately connected brain systems (i) instrumental reward learning whose functions extend to mediating social preference and valuation and (ii) perceptual and cognitive mechanisms underlying theory of mind processes that are engaged when considering the intention of others. The dynamic interplay of these cognitive, motivational and neural processes is thought to lay the groundwork for the development of our perceptions of self and others as well as our social preferences (Blakemore and Griggs, 2007; Dunbar and Schultz, 2007; Hermann et al., 2007).
The OFC, a key hub in this expansive neural circuitry of sociality, is considered among the most polymodal regions of the brain receiving multi-sensory inputs of taste, smell, auditory, visual and somatosensory as well as visceral signals, due to its wide and deep connections to functionally diverse cortical and subcortical regions, including the amygdala, cingulate cortex, insula, hypothalamus, hippocampus, striatum, as well as its neighboring dorsolateral prefrontal cortex (Kringelbach, 2005). The OFC is located between the frontopolar gyri rostrally, the anterior perforated substance caudally, the inferior frontal gyrus laterally and the ventromedial margin of the cerebral hemisphere medially (Duvernoy, 1999; Chiavaras and Petrides, 2000). Its anatomically heterogeneous sulcogyral morphology (Ono et al., 1990; Chiavaras and Petrides, 2000; Nakamura et al., 2007) is thought to be reflective of the rich molecular processes underlying neuronal migration, local neuronal connection, synaptic development, as well as lamination and formation of cytoarchitecture (Rakic, 1988; Armstrong et al., 1995).
In light of such structural and functional diversity, researchers have focused on dividing the OFC into distinct subregions. Duvernoy (1999), for example, used the major orbital sulci to divide the OFC into five subregions of gyrus rectus, and medial, anterior, posterior and lateral orbital gyri. More recently, Nakamura et al. (2008), in an effort to mitigate differences arising from variability of OFC sulcogyral morphology, particularly the H-shaped sulcus (Chiavaras and Petrides, 2000), used a 3D MRI region-of-interest approach to develop a reliable parcellation of the OFC into three subdivisions. Focusing on two of the most stable and reliably imaged sulci as anatomical boundaries—olfactory sulcus and lateral orbital sulcus—Nakamura et al. (2008) divided the OFC into three regions of interest: gyrus rectus, middle orbital gyrus and lateral orbital gyrus, and examined each region in relation to gray matter volume and neuropsychological performance in schizophrenia and age-matched control subjects. Their results lent validity to these OFC subdivisions, pointing to a bilateral reduction in middle orbital gyrus in schizophrenia, with smaller volumes in this subregion correlating with disease-related difficulties in social communication as reflected by increased severity of thought disturbance (Nakamura et al., 2008). Moreover, for healthy participants who served as control comparisons for the schizophrenia sample, social decision making, as assessed by the Iowa Gambling Task, correlated with increased gray matter volume of the right middle orbital gyrus (Nakamura et al., 2008).
Other studies have compared the functional roles of lateral and medial OFC subregions in sociality in non-clinical samples. For example, functional magnetic resonance imaging (fMRI) findings suggest that abstract rewards and punishments, as measured monetarily, may lead to differential engagement of lateral and medial OFC sectors (O’ Doherty et al., 2001; Kringelbach, 2005). These data pointed to heightened activation of the lateral but not medial area of the OFC following a punishing outcome, whereas a rewarding outcome elicited increased activation of the medial but not lateral area of the OFC (O’ Doherty et al. 2001). Spitzer et al. (2007) extended these findings by examining the relationship of individual differences in Machiavellian personality characteristics and fMRI brain activation recorded while pairs of research participants played a social norm compliance game involving an economic exchange. They reported that Machiavelli scores correlated strongly with both heightened lateral OFC activation, especially of the left hemisphere, and norm compliance under the threat of social punishment. Spitzer and colleagues concluded that left lateral OFC may represent an important neural network for social norm compliance, providing neurobiological substrata for judging and evaluating social opportunities and threats—abilities that are central to the development of Machiavellianism.
Elliot et al. (2000), in their review of fMRI studies, also revealed evidence of dissociation between the medial and lateral OFC. They noted medial orbital frontal involvement in a variety of cognitive tasks, including delayed match to sample, guessing, as well as language tests of sentence completion and story comprehension. They suggested that although these tasks impose different cognitive demands, all require for effective performance monitoring, and holding in mind prior reward values in order that current contingencies can be abstracted and future outcomes can be predicted. Elliot et al. (2000) noted that while these mental processes and computations depend on prefrontal lobe circuitry, in general, it is the specific demands that these diverse tasks impose on the monitoring of reward values that account for the differential role of medial orbital frontal activation, with studies suggesting right medial orbital frontal to be especially important in social decision making (Zald and Rauch, 2006; Nakamura et al., 2008)
Taken together, these functional and structural neural imaging studies suggest that medial and lateral OFC regions can be dissociated, both functionally and anatomically. That is, as Elliot et al. (2000) findings suggested, the engagement of the medial OFC in reward learning may have an important role in a diversity of learning tasks of higher order abilities ranging from those that may be described primarily as cognitive to those that extend to social domains related to understanding personal interactions. Moreover, the medial OFC has its strongest connections to the hippocampus and associated areas of the cingulate, retrosplenial and entorhinal cortices and anterior thalamus (Mesulam et al., 1985; Vogt et al., 1987; Morecraft et al., 1992), which would also be consistent with its involvement in higher order cognition, especially declarative episodic memory. On the other hand, while the lateral OFC also has strong connections to brain regions critical for higher order cognition, its links to the inferior parietal lobule and dorsolateral prefrontal cortex (Goldman-Rakic, 1987; Fuster, 1997), may suggest a special role in Machiavellian personality traits related to detecting and evaluating social threats to self-interest (Spitzer et al., 2007).
Based on these fMRI studies, the current study tested for a double dissociation between anatomy, as measured by lateral and middle orbital gyri gray matter, and function, as assessed by a self-report measure of Machiavelli personality traits and psychometric tests of cognition, which include measures of social reasoning and judgment. A pencil-and-paper measure of Machiavellianism, psychometric tests of declarative memory, intellectual abilities, including social reasoning as well OFC gray matter volumes were all acquired from a sample of healthy participants who had served as control subjects in several of our other studies of schizophrenia (Nakamura et al., 2008; Nestor et al., 2010). Based on prior research, we hypothesize a double dissociation of function and anatomy: to wit, variance in self-report Machiavelli personality traits but not in psychometric intellect and memory will be significantly accounted for by gray matter volumes of left lateral orbital gyrus but not right middle orbital gyrus, whereas variance in intellect and memory but not Machiavelli personality traits will be significantly accounted for by gray matter volumes in right middle orbital gyrus but not left lateral orbital gyrus.
METHODS
Participants
Twenty-five healthy right-handed, participants (19 males/6 females) who served as normal comparison subjects for prior MRI studies of veterans with schizophrenia (Nakamura et al., 2008) participated. All participants met Structured Clinical Interview for DSM-IV Axis I Disorders-Non-patient Edition (SCID-NP) criteria of no past or current Axis 1 and/or Axis II disorder (First et al., 1997, 2002). Participants had a mean age (s.d.) of 41.08 (9.10) years and a mean education (s.d.) of 15 (1.98) years. All participants gave informed consent. The self-report and psychometric tests were administered at the Boston VA Medical Center (Brockton, MA Division) and the MRI studies were conducted at the Brigham and Women’s Hospital in Boston, MA, USA. MRI studies and neuropsychological testing were completed over the course of ∼3 months. The research protocol was approved by the Institutional Review Board of the Boston VA Medical Center and Harvard Medical School.
Measures
The Mach IV, a measure of Machiavelli personality traits, consists of 20 statements that participants rate on a 7-point Likert scale (1 = strongly disagree, 4 = no opinion and 7 = strongly agree). This ordinal scale, with a constant arbitrary base of 20 points, generates scores ranging from 40 to 160. A total score of 100 is set to represent the mean (and theoretically neutral) level of Machiavellianism (Christie and Gies, 1970; Sullivan and Allen, 1999). Reliabilities studies have demonstrated the Mach IV to be internally consistent (Paulhus and Williams, 2002) and validity studies have linked high Mach IV scores to measures of subclinical forms of narcissism and psychopathy but not with either functional or dysfunctional types of impulsivity (Jones and Paulhus, 2011).
The Wechsler Adult Intelligence Scale-Third Edition (WAIS-III, Wechsler, 1997a) provides two specific subtests, one non-verbal and timed, Picture Arrangement and the other verbal and untimed, Comprehension, each of which taps abilities related to reasoning and understanding of social concerns. Both subtests have served as proxy measures of social aspects of cognitive intelligence (Rappaport et al., 1968; Kaufman, 1990; Weiner, 1996; Nestor et al., 2010). Moreover, the cartoon format of Picture Arrangement has been used quite extensively in studies of social cognition, particularly those focusing on investigating neuropsychological bases of theory of mind (Sarfati et al., 1997; Happé et al., 1999; Gallagher et al., 2000; Corcoran et al., 1997; Aboulafia-Brakha et al., 2011). Additional evidence supporting the validity of these subtests as a measure of a separable WAIS-III factor of social comprehension comes from findings that Comprehension and Picture Arrangement scores (i) validated two empirically derived subtypes of mentally disordered murderers, a psychotic subtype, diagnosed primarily with schizophrenia and a non-psychotic subtype defined primarily by high levels of psychopathy (Nestor et al., 2002) and (ii) predicted mentally ill, incompetent-to-stand trail defendants (Nestor et al., 1999).
The Wechsler Memory Scale-Third Edition (WMS-III) (Wechsler, 1997b) provides a highly reliable and valid standardized test of declarative episodic memory. Among the WMS-III indexes are immediate memory and general (delayed) memory, each divided into both auditory and visual domains. The Doors and People Test (DPT) was administered as an additional measure of declarative episodic memory (Baddeley et al., 1994). It consists of four subtests: verbal recall (People Test); visual recall (Shapes Test); verbal recognition (Names Test); and visual recognition (Doors Test). The DPT has been used to demonstrate involvement of hippocampus and extra-hippocampal regions in memory impairment in idiographic lesion case studies (Reed and Squire, 1997; Vargha-Khadem et al., 1997; Manns and Squire, 1999; Baddeley et al., 2001) as well in patients with chronic schizophrenia (Nestor et al., 2007).
MRI processing
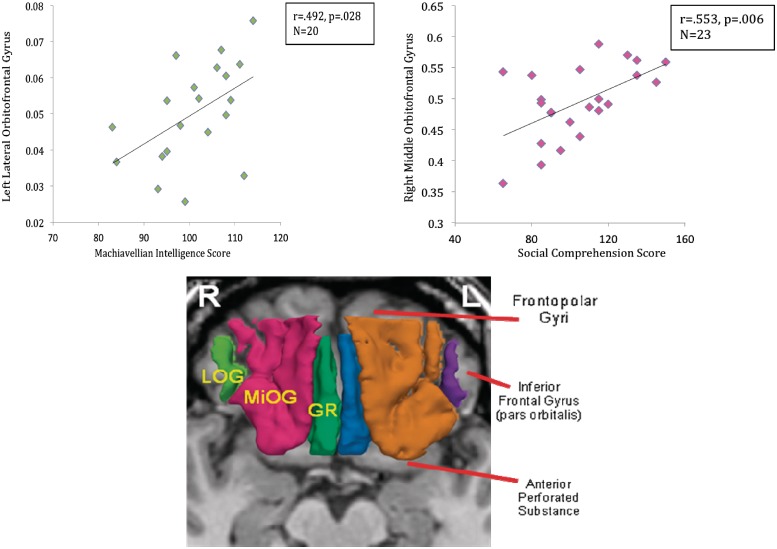
The MRI processing is described in detailed in Nakamura et al. (2008). In brief, MR images were acquired with a 1.5-Tesla General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women’s Hospital in Boston. A 3D Fourier transformed spoiled gradient-recalled (SPGR) acquisition sequence yielded a coronal series of contiguous 1.5 mm images (TE = 5 ms, TR = 35 ms, repetition = 1, nutation angle = 45°, field of view = 24 cm, acquisition matrix = 256 × 256 × 124, voxel dimension = 0.9375 × 0.9375 × 1.5 mm). Next, a double-echo spin-echo yielded 108 contiguous axial double-echo (proton-density- and T2-weighted) slices, with 54 levels, throughout the brain (TE = 30 and 80 ms, TR = 3000 ms, field of view = 24 cm, an interleaved acquisition with 3-mm slice thickness, voxel dimensions = 0.9375 × 0.9375 × 3.0 mm). The T2 information from the double-echo spin-echo axial slices was registered to the SPGR images. An expectation–maximization (EM) segmentation technique (Bouix et al., 2007; Pohl et al., 2007) was used to segment the images into three major tissue classes: gray matter; white matter; and CSF, using both SPGR and T2-weighted MR information as well as spatial priors. This technique was used to obtain Intra-Cranial Contents (ICC) volume. Manual tracing of OFC ROI was performed on non-segmented images to avoid segmentation errors due to susceptibility artifacts which are common in the OFC region. Images were realigned using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt, and resampled into isotropic voxels (0.9375 mm3). This realignment procedure was essential for precise and consistent ROI delineation. 3D information was used to provide reliable delineation of the OFC ROI with a software package for medical image analysis [3D slicer, http://www.slicer.org] on a workstation. Definition and details of the method of region of interest for the OFC are provided in Nakamura et al. (2008). The relative volume is calculated as: absolute volume/intracranial contents (ICC) * 100 (%). Figure 1 depicts image of the OFC divided into three subregions: gyrus rectus, middle orbital gyrus and lateral orbital gyrus.
Fig. 1.
Orbital frontal cortex subregions of the lateral orbital gyrus (LOG; left: purple, right: light green), middle orbital gyrus (MiOG; left: brown, right: red) and gyrus rectus (GR; left: blue, right: green) along with scatter plots of left lateral orbital frontal gyrus and Machiavellianism, and right middle orbital frontal gyrus and social comprehension.
Statistical analyses
Pearson Product Moment correlations tested for associations between scores on Mach IV and psychometric measures of social comprehension and declarative episodic memory with OFC subregion gray matter volumes. We next employed partial correlations as a test for the specificity of the relationship of Mach IV and OFC volume, covarying for scores on psychometric tests. Likewise, we tested for the relationship of social comprehension and OFC volume, covarying for Mach IV scores. Parametric, hierarchical regression analyses were then used to partition the total variance of the dependent variable, test score, among the independent variables, MRI measures of OFC subregions volumes.
To examine the unique contribution of MRI measures to test scores, partial (rp) and semi-partial (rsp), correlations were computed in a series of hierarchical regression analyses, which permitted the evaluation of significant univariate relationships by partitioning total variance of the dependent variable (test score) among independent variables (MRI OFC subregion volumes) (Cohen and Cohen, 1975). The partial correlation squared (rp2) is the proportion of variance of a particular test score (e.g. Mach IV) shared by a specific OFC subregion (e.g. left lateral orbital gyrus), after the effects of the other OFC subregions (e.g. right lateral orbital gyrus) have been removed from both the test score and MRI gray matter volumes (Cohen and Cohen, 1975). This statistic answers the question, ‘What proportion of the remaining test score variance (i.e., that which is not estimated by the other IVs in the equation) is uniquely estimated by this gray matter volume?’ By comparison, the square of the semi-partial correlation (rsp2) estimates the amount of test score variance that is uniquely shared with a particular gray matter subregion volume measure after the effects of all other subregions on that particular brain area have been removed (Cohen and Cohen, 1975). It is semi-partial because the effects of the other independent variables have been removed from the independent variable but not from the dependent variable. In conjunction with other linear regression statistics, partial and semi-partial correlations provide a comprehensive picture of how subregion gray matter volumes of the OFC relate to scores on particular measures of social cognition when collinearity is controlled.
In addition, based on prior fMRI studies, we hypothesized a double dissociation with Mach IV but not social comprehension scores corresponding to gray matter volume of left lateral orbital gyrus but not right middle orbital gyrus gray matter volume, in comparison to social comprehension but not Mach IV scores corresponding to gray matter volume of right middle orbital gyrus but not left lateral orbital gyrus. We test for this hypothesized double dissociation using a pair of hierarchical regression model: for Mach IV as the dependent variable, left lateral orbital gyrus gray matter, entered first as an independent variable, followed by right middle orbital gyrus gray matter. For social comprehension as the dependent variable, right middle orbital gyrus gray matter, entered first as an independent variable, followed by left lateral orbital gray matter. For all regression analyses, the F-to-enter probability was 0.05 and the F-to-exclude probability was 0.1. Significance levels are two tailed.
RESULTS
Table 1 presents the mean scores for the Mach IV, WAIS-III, DPT and WMS-III. Participants showed nearly identical Mach IV [M (s.d.) = 101.58 (13.76)] and WAIS-III Full Scale IQ [M (s.d.) = 101.21 (8.92)] scores, F < 1. Participants had similar scores across WAIS-III indexes of verbal comprehension [M (s.d.) = 103.13 (14.48)], perceptual organization [M (s.d.) = 107.48 (17.56)], working memory [M (s.d.) = 105.74 (15.68)] processing speed [M (s.d.) = 101.87 (14.48)] and social comprehension [M (s.d.) = 105.57 (11.96)]. Likewise, participants had similar scores for WMS-III indexes of immediate [M (s.d.) = 102.86 (17.20)] and delayed [M (s.d.) = 105.48 (14.65)] memory. For the DPT, participants had similar scores across measures of verbal recall for the People Test [M (s.d.) 9.95 (3.39)], visual recall for the Shapes Test [M (s.d.) = 10.15 (3.92)] and visual recognition for the Doors Test [M (s.d.) = 10.25 (3.11)], with higher scores for verbal recognition for the Names Test [M (s.d.) = 13.25 (3.34)].
Table 1.
Self-report and neuropsychological test scores for research participants
| Measures | M (±s.d.) |
|---|---|
| Demographics | |
| Age | 41.08 (±9.10) |
| Education | 15.00 (±1.98) |
| SES | 2.42 (±1.06) |
| Mach IV | |
| Duplicitous | 38.50 (±5.56) |
| Human nature views | 31.10 (±5.62) |
| Morality | 11.40 (±2.64) |
| Total | 101.00 (±8.74) |
| WAIS-IIIa | |
| Verbal comprehension | 103.13 (±14.48) |
| Perceptual organization | 107.48 (±17.56) |
| Working memory | 105.74 (±15.68) |
| Processing speed | 101.87 (±14.48) |
| Social comprehension | 104.56 (±23.93) |
| Full-scale IQ | 105.22 (±16.25) |
| WMS-IIIa | |
| Auditory immediate | 104.32 (±17.56) |
| Visual immediate | 101.05 (±15.22) |
| Auditory delayed | 105.76 (±14.82) |
| Visual delayed | 101.43 (±15.28) |
| Immediate memory | 103.82 (±17.38) |
| Delayed memory | 105.48 (±14.65) |
| Delayed recognition | 105.71 (±14.26) |
| Doors and peopleb | |
| People | 9.95 (±3.39) |
| Shapes | 10.15 (±3.92) |
| Names | 13.25 (±3.34) |
| Doors | 10.25 (±3.11) |
aStandard scores [M (s.d.) = 100 (15)].
bAge-scaled scores [M (s.d.) = 10 (3)].
Note. Values are means ± s.d.’s. Mach IV = Machiavellianism; WAIS-III = Wechsler Adult Intelligence Scale—Third Edition; WMS-III = Wechsler Memory Scale—Third Edition.
Table 2 presents relative volumes for the orbital frontal subregions. A repeated measures analysis of variance (ANOVA) with two within-subject factors of side (left/right) and subregion (gyrus rectus, middle orbital gyrus and lateral orbital gyrus) revealed significant effects for subregion: F(2,48) = 1427.03, P < 0.001, η2 = 0.983 and the interaction of side × subregion: F(2,48) = 8.68, P = 0.001, η2 = 0.266. Paired t-tests indicated a significantly greater right than left gray matter volume for gyrus rectus, t(24) = −3.90, P = 0.001, but greater left than right gray matter volume for middle orbital gyrus, t(24) = 2.50, P = 0.02 in contrast to similar gray matter volume for right and left lateral orbital gyrus, t(24) = −1.38, P = 0.181.
Table 2.
Relative volumes for orbital frontal cortex subregions
| OFC subregion | M (±s.d.) |
|---|---|
| Gyrus rectus | |
| Left | 0.161 (±0.026) |
| Right | 0.174 (±0.026) |
| Middle orbital gyrus | |
| Left | 0.520 (±0.056) |
| Right | 0.495 (±0.056) |
| Lateral orbital gyrus | |
| Left | 0.050 (±0.014) |
| Right | 0.054 (±0.012) |
Note. Values are means ± s.d.’s.
Table 3 shows higher Mach IV scores correlated significantly with increased gray matter volume of the left lateral orbital frontal gyrus (r = 0.492, P = 0.028). This correlation remained significant when controlling for WAIS-III Full Scale IQ (partial r = 0.549, P = 0.018). As shown in Table 3, for the WAIS-III, higher social comprehension correlated significantly with increased volume of left (r = 0.538, P = 0.008) and right (r = 0.553, P = 0.006) middle orbital gyrus as well as with left (r = 0.428, P = 0.041) and right (r = 0.494, P = 0.017) total OFC volume. Higher WAIS-III social comprehension remained significantly correlated with increased gray matter volume of the left middle orbital gyrus when controlling for WAIS-III indexes of working memory (partial r = 0.521, P = 0.013), processing speed (partial r = 0.471, P = 0.027) and perceptual organization (partial r = 0.453, P = 0.034). Likewise, WAIS-III social comprehension remained significantly correlated with right middle orbital gyrus when controlling for WAIS-III indexes of working memory (partial r = 0.484, P = 0.002) and processing speed (partial r = 0.449, P = 0.03) but not for perceptual organization or verbal comprehension. These partial correlations indicated that the relationship of greater left middle orbital gray matter volume and higher WAIS-III social comprehension was independent of scores on WAIS-III index measures of working memory, processing speed and perceptual organization but not for WAIS-III verbal comprehension. By comparison, the right middle orbital gyrus volume and WAIS-III social comprehension correlated positively, independently of WAIS-III indexes of working memory and processing speed, but not for WAIS-III indexes of perceptual reasoning or verbal comprehension.
Table 3.
Pearson correlations of orbital frontal cortex subregions and social cognitive and memory measures.
| Measures | Left |
Right |
||||
|---|---|---|---|---|---|---|
| GR | MiOG | LOG | GR | MiOG | LOG | |
| Mach IV | −0.307 | 0.313 | 0.492* | −0.394 | 0.169 | 0.118 |
| WAIS-III SC | −0.120 | 0.538** | 0.054 | 0.025 | 0.553** | 0.141 |
| WMS-III | ||||||
| Immediate | 0.348 | 0.237 | −0.043 | 0.297 | 0.562** | −0.187 |
| Delayed | 0.294 | 0.318 | −0.147 | 0.176 | 0.445* | −0.170 |
| Recognition | 0.152 | 0.445* | −0.252 | 0.048 | 0.481* | −0.355 |
| Doors and people | ||||||
| People | 0.343 | 0.499* | −0.002 | 0.297 | 0.540* | 0.063 |
| Shapes | 0.030 | 0.369 | 0.053 | −0.063 | 0.477* | −0.074 |
| Names | 0.077 | 0.455* | −0.103 | 0.091 | 0.443* | −0.103 |
| Doors | −0.053 | 0.371 | −0.204 | 0.297 | 0.381 | 0.271 |
*P < 0.05; **P < 0.01
Note. GR= gyrus rectus; LOG = lateral orbital gyrus; Mach IV = Machiavellianism; MiOG = m middle orbital gyrus; WAIS-III SC = Wechsler Adult Intelligence Scale—Third Edition Social Comprehension; WMS-III = Wechsler Memory Scale—Third Edition.
For the WMS-III, higher scores for immediate memory correlated with increased gray matter volume for right middle orbital gyrus (r = 0.562, P = 0.007) and total right OFC volume (r = 0.582, P = 0.005). WMS-III delayed memory correlated with right middle orbital gyrus (r = 0.445, P = 0.043) and total right OFC volume (r = 0.448, P = 0.042). Higher scores on WMS-III auditory delayed recognition correlated with increased gray matter volume in left (r = 0.445, P = 0.043) and right (r = 0.481, P = 0.027) middle orbital gyrus. When controlling for WAIS-III social comprehension, immediate memory but not delayed memory remained significantly correlated with right middle orbital gyrus (partial r = 0.469, P = 0.03) and total right OFC volume (partial r = 0.506, P = 0.019). By contrast, auditory recognition memory no longer correlated with gray matter for either left or right middle orbital gyrus after controlling for WAIS-III social comprehension. Yet when controlling for Mach IV scores, immediate memory remained significantly correlated with right middle orbital frontal gyrus (partial r = 0.563, P = 0.015) and total right OFC volume (partial r = 0.574, P = 0.013). Delayed memory also remained significantly correlated with gray matter volume of right middle orbital frontal gyrus when controlling for Mach IV scores (partial r = 0.482, P = 0.05) as did delayed auditory recognition with left (partial r = 0.550, P = 0.022) and right (partial r = 0.589, P = 0.013) middle orbital gyrus.
For the DPT, left middle orbital gyrus volume correlated with higher scores on recall of people (r = 0.499, P = 0.025) and recognition of names (r = 0.455, P = 0.044), as did right middle orbital gyrus volume correlate with recall of people (r = 0.540, P = 0.014) and shapes (r = 0.477, P = 0.033) and recognition of names (r = 0.443, P = 0.051). Age-scaled scores for recall of people also correlated with total gray matter volume for both left (r = 0.558, P = 0.011) and right (r = 0.591, P = 0.006) OFC and remained significant when controlling for WAIS-III social comprehension for total gray matter volume for both left (partial r = 0.452, P = 0.052) and right (partial r = 0.472, P = 0.041) OFC. Also when controlling for WAIS-III social comprehension, a strong relationship emerged between recall scores for people and left gyrus rectus volume (partial r = 0.616, P = 0.005). When controlling for Mach IV, recall for people remained significantly correlated with left (partial r = 644, P = 0.007) and right (partial r = 0.526, P = 0.036) middle orbital gyrus, and for total gray matter volume for left (partial r = 0.729, P = 0.001) and right (partial r = 0.588, P = 0.017) OFC. In addition, when controlling for Mach IV, significant partial correlations emerged for shape recall and right middle orbital gyrus (partial r = 0.503, P = 0.047) and for recognition of doors and left middle orbital gyrus (partial r = 0.554, P = 0.026) as well as for recognition of names for left (partial r = 0.558, P = 0.025) and right (partial r = 0.547. P = 028) middle orbital gyrus, and for left total gray matter OFC volume (partial r = 0.520, P = 0.039).
We next used hierarchical multiple regression to compare the contributions of orbital frontal subregion volumes to the behavioral measures. For the dependent variable Mach IV scores, we entered the subregion volumes in the following order: left lateral orbital gyrus, left middle orbital gyrus, right lateral orbital gyrus and right middle orbital gyrus. The regression indicated that gray matter volume for the left lateral orbital gyrus produced an R = 0.492, R2 Change = 0.242, F(1,18) = 5.74, P = 0.028 and adding the other subregion volumes did not significantly increase the variance explained. However, both left lateral orbital gyrus (standardized B = 0.709, t = 3.06, P = 0.008) and left middle orbital gyrus (standardized B = 0.776, t = 2.13, P = 0.05) contributed significantly to Mach IV scores, with left lateral orbital gyrus yielding a partial correlation of 0.620 and a semi-partial correlation of 0.588, and left middle orbital gyrus a partial correlation of 0.482 and semi-partial correlation of 0.409. These values indicated that the left lateral orbital gyrus uniquely accounted for ∼38.44 and 34.57% and left middle orbital gyrus, 23.23 and 16.73% of the variance in Mach IV scores.
For the dependent variable, WAIS-IV social comprehension, hierarchical multiple regression analyses with right middle orbital gyrus entered first, followed by left middle orbital gyrus, right lateral orbital gyrus and left lateral orbital gyrus revealed the following: Gray matter volume for only the right middle orbital gyrus produced an R = 0.553, R2 change = 0.306, F(1,21) = 9.27, P = 0.006, with a partial correlation of 0.353 and semi-partial correlation of 0.297. These correlation values indicated that the right middle orbital gyrus uniquely accounted for ∼12.46 and 8.8% of the variance in WAIS-III social comprehension scores.
For the dependent variable, WMS-III immediate memory, with independent variables, right middle orbital gyrus entered first, followed by left middle orbital gyrus, right lateral orbital gyrus and left lateral orbital gyrus, hierarchical regression revealed the following: gray matter volume for only the right middle orbital gyrus produced an R = 0.562, R2 change = 0.315, F(1,20) = 9.22, P = 0.007, with a partial correlation of 0.516 and semi-partial correlation of 0.485. These correlation values indicated that the right middle orbital gyrus uniquely accounted for 30.25 and 28.52% of the variance in WMS-III immediate memory scores.
Last, we employed hierarchical regression to examine the question of a double dissociation between OFC anatomy and sociality: that is, whether gray matter volume of two distinct regions (left lateral orbital gyrus, right middle orbital gyrus) differentially predicted scores on two tasks (Mach IV, WAIS-III social comprehension). For Mach IV, left lateral orbital gyrus gray matter, entered first as an independent variable, predicted Mach IV scores (standardized B = 0.499, t = 2.42 P = 0.027) whereas right middle orbital gyrus gray matter did not (P > 0.35). For social comprehension, right middle orbital gyrus gray matter, entered first as an independent variable, predicted WAIS-III social comprehension (standardized B = 0.552, t = 2.97, P = 0.008), whereas left lateral orbital gray matter did not (P > 0.50). These hierarchical regression results pointed to a double dissociation of anatomy and function, whereby gray matter of left lateral but not right middle orbital gyrus predicted Mach IV but not WAIS-III social comprehension scores, in stark contrast to the right middle but not left lateral orbital gyrus gray matter which predicted WAIS-III social comprehension but not Mach IV scores.
DISCUSSION
The principal question centered on the extent to which individual differences in Machiavelli personality traits and on psychometric measures of social comprehension and declarative episodic memory could be accounted for by variance in OFC gray matter volumes. First, greater gray matter volume for the left lateral orbital gyrus correlated with higher Machiavelli personality traits, as measured by the pencil-and-paper self-report Mach IV. Second, increased gray matter volume in the middle orbital gyrus correlated with several of the psychometric tests of cognition, including declarative episodic memory and IQ measures of social comprehension
These findings suggested individual differences in OFC gray matter may be linked to performance on behavioral measures of various aspects of social cognition. For the Mach IV, the data showed a rather specific relationship of higher Machiavelli personality traits and larger gray matter volume for left lateral orbital gyrus, and to a lesser extent, though statistically insignificant, for left middle orbital gyrus. In fact, hierarchical regression analyses indicated ∼34.57–38.44% of the variance in Mach IV scores could be specifically explained by gray matter volume of left lateral orbital gyrus, with another ∼16.73–23.23% variance in the measurement of Machiavelli personality characteristics uniquely accounted for by left middle orbital gyrus gray matter volume. These values indicate that 51.3–61.67% of the variance in Mach IV scores could be uniquely accounted for by individual differences in gray matter volumes in left lateral and middle orbital gyri.
For psychometric social comprehension, the data suggested that bilateral gray matter volumes of the middle orbital gyrus, but not the left lateral orbital gyrus, as important anatomical sources accounting for individual differences in performance for this measure of social cognition. Approximately 8.8–12.46% variance in social comprehension could be uniquely accounted for by individual differences in gray matter volume in the right middle orbital gyrus. These percentage values proved to be much lower than those that characterized the relationship of Mach IV and left lateral orbital gyrus. This may reflect evidence of a more general relationship between social comprehension and right middle orbital gyrus gray matter than that demonstrated between left lateral orbital gyrus and Machiavelli personality traits.
A key question addressed in the current study centered on whether Machiavelli personality traits, on one hand and social comprehension on the other hand, may each reflect dissociable functions of the lateral and middle orbital gyri, respectively. In fact, the current study employed hierarchical regression as a specific test for a double dissociation between gray matter volumes of the left lateral orbital gyrus and the right middle orbital gyri gray matter with Machiavelli personality traits and psychometric tests of cognition, particularly intelligence tests of social reasoning and judgment. The results lent support for a double dissociation: on one hand, increased gray matter volume of the left lateral orbital gyrus but not the right middle orbital gyrus predicted higher levels of Machiavellianism but not social comprehension. On the other hand, increased gray matter volume of the right middle orbital gyrus but not the left lateral orbital gyrus predicted higher levels of social comprehension but not Machiavelli personality characteristics.
Prior fMRI studies have also examined the question of dissociated functions in the lateral and medial OFC. For example, Spitzer et al. (2007) found that higher levels of Machiavelli traits predicted heightened left lateral OFC activation in subjects’ performance on a norm compliance task under the threat of social punishment. These results complement the current finding linking increased left lateral orbital gyrus gray matter volume and higher levels of Machiavelli personality traits. Likewise, O’ Doherty and colleagues reported heightened activation of the lateral but not medial area of the OFC following a punishing outcome, whereas a rewarding outcome elicited increased activation of the medial but not lateral area of the OFC (O’ Doherty et al., 2001). Elliot and colleagues (2000) in their literature review proposed differential fMRI engagement of medial OFC in a diversity of higher order cognitive tasks, and such a relationship comports well with the current finding linking increased middle orbital gray matter volume with better performance on psychometric tests of social comprehension and declarative episodic memory.
Against this backdrop, the current findings, which point to a double dissociation functions in lateral and medial orbital frontal gyri, suggest that each of these brain regions may make specific contributions to different aspects of social cognition. For example, Machiavelli personality items tap a broad set of social attitudes and strategies that may be described as reflecting a mixture of selfishness and opportunism. For the Mach IV questionnaire, respondents rate their degree of agreement with 20 statements, such as ‘It’s hard to get ahead without cutting corners here and there’. ‘The best way to deal with people is tell them what they want to hear.’ Here the current results suggested the left lateral orbital gyrus as a uniquely important source of the OFC contribution to variation in Machiavelli personality traits. Indeed, the results provided evidence of a rather strong and specific relationship of left lateral orbital gyrus and Machiavelli characteristics, with neither the gyrus rectus nor the middle orbital gyrus gray matter volumes contributing to Mach IV scores. By comparison, for the psychometric measures of social comprehension and declarative memory, respondents perform various mental exercises that call for judgment and reasoning about social dilemmas, as assessed by the WAIS-III Picture Arrangement and Comprehension subtests, or learning and remembering new information such as stories, word pairs, names and faces of people, as measured by the WMS-III and the DPT. And here the current results suggested that gray matter volume in right middle orbital gyrus but not left lateral orbital gyrus accounted for a unique portion of the variance in social comprehension and declarative memory, as assessed psychometrically.
Thus, taken together, these findings help to parse social cognition into distinct but related domains of attitudes, preferences and personality traits, on one hand and information processing abilities, on the other, with each domain influenced by a specific OFC subregion. The pattern emerging from the current findings, then, is one in which individual differences in left lateral orbital gyrus volume influenced social attitudes embodied in Machiavelli personality traits, and variation in right middle orbital gyrus volume corresponded to information processing abilities related to learning and memory in general as well as reasoning and judgment particularly about social matters. The neural processes underlying this division of OFC anatomy and social and cognitive functions are, however, unknown. Researchers have emphasized the OFC as a key site, among other regions, in support of reward learning and instrumental conditioning that includes both non-social and social content (e.g. Behrens et al., 2009). This may reflect that cognitive and social processes are tightly linked, coevolving to favor a host of vital specialized functional adaptations that advanced fitness by promoting effective human transactions (Duchaine et al., 2001). That is, from an evolutionary perspective, the mnemonic abilities related to retrieving information about status, personalities and prior behaviors of individuals may have conferred a selective advantage for solving specific social domain problems, such as perceiving and recalling mental states in predicting the behavior of other people or remembering reputation in detecting cheaters (Duchaine et al., 2001; King-Casas et al., 2005; Wilson, 2007; Nowak and Highfield, 2011).
There are, however, several limitations that influenced the findings of the current study. First is the correlational nature of the study and the relatively small sample size. That is, while the study pointed to rather specific relationships linking OFC subregion volumes with self-report and psychometric tests, these findings were first identified via statistical analyses that entailed computing multiple univariate correlations across a relatively small sample of research participants. The limitation is that as the number of correlation computed increases, so too does the risk of Type1 error. However, to offset this risk, follow-up analyses using hierarchical regression and partial correlation techniques targeted particular univariate relationships. These analyses, which arguably provided greater statistical control than the computing of a series of univariate correlations, in turn offered rather strong evidence for partitioning variance related to individual differences in social cognition into specific OFC subregions. A second limitation is that the study only focused on the OFC, and as such, it is not known how other brain regions may contribute to social cognition. Third, the study relied on standardized measures to assess social cognition, and the extent to which these measures capture the full dimensions of sociality is unclear. In particular, the Mach 4 used here has been employed in other investigations examining the neural underpinnings of social cognition (Spitzer et al., 2007). However, the WAIS-III Comprehension and Picture Arrangement subtests are measures of cognitive intelligence, and their use as proxy measures of social cognition in the current study would benefit from further validation.
In summary, social cognition represents a complex set of mental representations that is supported by widely distributed, functionally diverse, yet tightly connected brain regions, arguably the most prominent of these network cogs is the OFC (Kringelbach, 2005). The OFC itself is an especially rich, diverse and polymodal area, which prompted us to use MRI to define and measure gray matter volume in three distinct anatomical subregions. In addition, the complexity and varied aspects of social cognition led us to employ multiple behavioral measures. These tools helped to tap some of the different dimensions of social cognition, specifically Machiavellianism, social comprehension and the neuropsychological construct of episodic memory. The results supported the parsing of both anatomy and social cognition, as revealed by the double dissociation between left lateral orbital gyrus and Machiavellianism, and right middle orbital gyrus and social comprehension and episodic memory. However, how these local OFC sites contribute to wider based, large-scale brain network computations in support of social behavior remains to be investigated.
Funding
This work was supported by the National Institute of Health (K02 MH 01110 and R01 MH 50747 to M.E.S., R01 MH 40799 and P50 080272 to R.W.M., RO1 MH 63360 to M.N.), National Alliance for Research on Schizophrenia and Depression (to M.K.), the Department of Veterans Affairs Merit Awards (to M.E.S., R.W.M., J.J.L.), and the Department of Veterans Affairs Schizophrenia Center (to R.W.M.). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research (Grant U54 EB005149 to M.E.S.).
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Institute of Health (K02 MH 01110 and R01 MH 50747 to M.E.S., R01 MH 40799 and P50 080272 to R.W.M., RO1 MH 63360 to M.N.), National Alliance for Research on Schizophrenia and Depression (to M.K.), the Department of Veterans Affairs Merit Awards (to M.E.S., R.W.M., J.J.L.), and the Department of Veterans Affairs Schizophrenia Center (to R.W.M.). This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research (Grant U54 EB005149 to M.E.S.).
REFERENCES
- Aboulafia-Brakha T, Christe B, Martory MD, Annoni JM. Theory of mind tasks and executive functions: a systematic review of group studies in neurology. Journal of Neuropsychology. 2011;5(pt. 1):39–55. doi: 10.1348/174866410X533660. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Myers D, Smith CU. What determines the size frequency distribution of beta-amyloid (A beta) deposits in Alzheimer's disease patients? Neuroscience Letters. 1995;187:13–6. doi: 10.1016/0304-3940(95)11325-q. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Emslie H, Nimmo-Smith L. The Doors and People Test. Bury St. Edmunds, UK: Thames Valley Test; 1994. [Google Scholar]
- Baddeley A, Vargha-Khadem F, Mishkin M. Preserved recognition in a case of developmental amnesia. Implications for the acquisition of semantic memory? Journal of Cognitive Neuroscience. 2001;13:357–69. doi: 10.1162/08989290151137403. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324:1160–4. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Bouix S, Martin-Fernandez M, Ungar L, Nakamura M, Koo MS, McCarley RW. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36:1207–24. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore K, Griggs E. Social Policy: An Introduction. New York: Open University Press; 2007. [Google Scholar]
- Chiavaras MM, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. The Journal of Comparative Neurology. 2000;422:35–54. [PubMed] [Google Scholar]
- Christie, R., Geis, F.L., editors. (1970). Studies in Machiavellianism. New York: Academic Press.
- Cohen J, Cohen P. Applied Multiple Regression/Correlational Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1975. [Google Scholar]
- Corcoran R, Cahill C, Frith CD. The appreciation of visual jokes in people with schizophrenia: a study of ‘mentalizing' ability. Schizophrenia Research. 1997;24:319–27. doi: 10.1016/s0920-9964(96)00117-x. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–05. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–7. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Cosmides L, Tooby J. Evolutionary psychology and the brain. Current Opinion in Neurobiology. 2001;11:225–30. doi: 10.1016/s0959-4388(00)00201-4. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. New York, NY: Springer, Wien; 1999. [Google Scholar]
- Elliot R, Dolan RJ, Frith C. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) Washington, D.C.: American Psychiatric Press, Inc; 1997. [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Philadelphia, PA: Lippincott- Raven; 1997. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind'. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind' in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Development. 1987;58:601–22. [PubMed] [Google Scholar]
- Happé F, Brownell H, Winner E. Acquired ‘theory of mind' impairments following stroke. Cognition. 1999;70:211–40. doi: 10.1016/s0010-0277(99)00005-0. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. History of Psychiatry. 1868;4:271–81. [Google Scholar]
- Hermann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–6. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Jones DN, Paulhus DN. The role of impulsivity in the dark triad of personality. Personality and Individual Differences. 2011;51:679–82. [Google Scholar]
- Kaufman AS. Assessing Adolescent and Adult Intelligence. Needham Heights, MA: Allyn Bacon; 1990. [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Baumeister RF, Vohs KD, editors. Encyclopedia of Social Psychology. Thousand Oaks, CA: Sage Press; 2007. [Google Scholar]
- Manns JR, Squire LR. Impaired recognition on the doors and peopletest after damage limited to the hippocampal region. Hippocampus. 1999;9:495–9. doi: 10.1002/(SICI)1098-1063(1999)9:5<495::AID-HIPO2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Principles of Behavioral and Cognitive Neurology. Philadelphia: F.A. Davis; 1985. [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. The Journal of Comparative Neurology. 1992;323:341–58. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–95. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, McCarley RW, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain. 2007;130:693–707. doi: 10.1093/brain/awm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Daggett D, Haycock J, Price M. Competence to stand trial: a neuropsychological inquiry. Law and Human Behavior. 1999;23:397–412. doi: 10.1023/a:1022339130582. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kimble M, Berman I, Haycock J. Psychosis, psychopathy, and homicide: a preliminary neuropsychological inquiry. The American Journal of Psychiatry. 2002;159:138–40. doi: 10.1176/appi.ajp.159.1.138. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Kuroki N, et al. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Research: Neuroimaging. 2007;155:21–8. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Nakamura M, Niznikiewicz M, McCarley RW, Shenton ME. Comparing prefrontal gray and white matter contributions to intelligence and decision making in schizophrenia and healthy controls. Neuropsychology. 2010;24:121–9. doi: 10.1037/a0016981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Highfield R. Altruism, Evolution, and Why We Need Each Other to Succeed. New York, NY: Free Press; 2011. Super cooperators. [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathy CD. New York, NY: Thieme Medical Publishers; 1990. Atlas of the cerebral sulci. [Google Scholar]
- Paulhus DL, Williams KM. The dark triad of personality: Narcissism, Machiavellianism, and psychopathy. Journal of Research in Personality. 2002;36:556–63. [Google Scholar]
- Pohl KM, Bouix S, Nakamura M, et al. A hierarchical algorithm for MR brain image parcellation. IEEE Transactions on Medical Imaging. 2007;26:1201–12. doi: 10.1109/TMI.2007.901433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Progress in Brain Research. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rappaport D, Gill MM, Schafer R. Diagnostic Psychological Testing. 1968. (rev. Ed. R.R. Holt). New York: International University Press. [Google Scholar]
- Reed JM, Squire LR. Impaired recognition in patients with lesions limited to the hippocampal formation. Behavioral Neuroscience. 1997;111: 667–75. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Sarfati Y, Hardy-Bayle MC, Besche C, Widlocher D. Attribution of intentions to others in people with schizophrenia: a non-verbal exploration with comic strips. Schizophrenia Research. 1997;25:199–209. doi: 10.1016/s0920-9964(97)00025-x. [DOI] [PubMed] [Google Scholar]
- Sullivan RJ, Allen JS. Social deficits in schizophrenia defined in terms of interpersonal Machiavellianism. Acta Psychiatrica Scandinavica. 1999;99:148–54. doi: 10.1111/j.1600-0447.1999.tb07213.x. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Hermberger B, Groen G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56:185–96. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. The Journal of Comparative Neurology. 1987;262:256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 3rd edn. San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd edn (WMS-III) San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Weiner IB. Psychodiagnosis in Schizophrenia. New York: Wiley; 1996. [Google Scholar]
- Wilson DS. Evolution for Everyone: How Darwin’s Theory Can Change the Way We Think About Our Lives. New York, NY: Delacorte Press; 2007. [Google Scholar]
- Zald D, Rauch S. The Orbitofrontal Cortex. New York: Oxford University Press; 2006. [Google Scholar]